Abstract
The aim of this study was to determine the effects of high CO2 with the constant O2 level on the postharvest quality of fig cv. Bursa Siyahi. For this purpose, the atmospheric compositions of 3% O2 + 10% CO2 (PA-1), 3% O2 + 15% CO2 (PA-2), 3% O2 + 20% CO2 (PA-3), and 21% O2 + 0.03% CO2 (RA) were tested under a palliflex controlled atmosphere (PA) storage system at 0°C for 28 days. At the end of the storage, weight loss increased during the storage period, but this increase slowed down in all tested PAs compared to RA. PA-1 and PA-2 delayed softening while PA-3 accelerated this process. There were no side effects in fruits stored under PAs for taste. The lowest total microorganism and decay rates were found in PA-2 and PA-3. The fig fruits stored under PAs had higher sugar and organic acid contents compared to the figs stored under the RA. Respiration rate decreased in all PAs compared to the RA. Ethylene productions increased with senescence in all atmospheres, but PA-3 inhibited this increase. Consequently, 15% CO2 (PA-2) can be used to maintain postharvest quality of Bursa Siyahi fresh fig for 28 days at 0 °C.
1. Introduction
Turkey provides approximately 24% of the world total fig production with 320,000 tons [1]. A large part of this production is considered dried fruit; however, the demand for fresh consumption has shown an increasing trend in the past decade. Bursa Siyahi is the dominant fresh consumption cultivar and fresh fig exportation of Turkey is based on this cultivar. This cultivar is highly attractive and demanded due to its delicious taste, flavor, and biochemical properties as well as attractive colors [2]. However, it is highly perishable due to its delicate fruit characteristics such as soft fruit tissue, thin peel, and ostiole-end opening. The expected postharvest life of fresh fig is limited to generally one or two weeks in ambient atmosphere at 0 °C [3].
The most common postharvest problems of fresh fig are water loss, softening, peel cracking, ostiole leakage and internal/external decays. Fig fruit is extremely sensitive to various pathogens, such as Alternaria spp., Botrytis spp., Fusarium spp., Aspergillus spp., Penicillium spp., and Rhizopus spp. [2,4,5,6], that cause economic losses especially in shelf-life at high temperatures. Karabulut et al. [7] reported that postharvest losses can reach up to 30–50% in shelf-life following 5–7 days of cold storage or transportation, especially when fruits are harvested during the rainy season.
Controlled atmosphere (CA) storage including low O2 and high CO2 have positive effects to maintain fruit quality and disease control in many horticultural commodities [8]. On the other hand, extremely high CO2 levels may have an unfavorable effect on texture and promote off-flavor development [9]. Fresh fig fruit tolerates a high level of CO2 in the storage atmosphere like other berries. Colelli et al. [10] tested the atmosphere enrichment with 15 and 20% CO2 in the Mission fig variety at different storage temperatures for 28 days and highlighted that high CO2 levels decreased the decay incidence and increased postharvest life of figs up to 2–3 weeks. Lowering O2 levels can positively affect the postharvest quality of fresh fig as in high CO2 [11]. Tsantili et al. [12] reported that low O2 (2%) reduced O2 uptake, ethylene production and maintained firmness of Mavra Markopoulou fig fruit stored for 29 days at −1 °C. Regulating atmospheric compositions, especially in berry fruit including fig, provides positive response to high CO2 levels and can be used an alternative to a non-chemical approach to maintain postharvest quality of fresh fig during the storage, transportation, and marketing.
There were a few studies conducted with CA storage on different fresh fig cultivars. Türk et al. [13] tested CA storage of the Bursa Siyahi fig variety at 0 °C containing different CO2 and O2 levels (3% O2 + 3% CO2, 5% O2 + 5% CO2, 10% O2 + 5% CO2, 20% O2 + 2% CO2, and 0.03% O2 + 21% CO2). As a result of this study, CA storage containing 3% O2 + 3% CO2 and 5% O2 + 5% CO2 were recommended for the Bursa Siyahi fig cultivar for four weeks of storage due to their positive effects on overall external appearance and taste. Similarly, CA storage (6% O2 + 17% CO2) was also tested for the Brown Turkey, Kadota, and Mission fig varieties stored at 0 °C for 19 and 31 days. In this study, CA storage reduced and delayed the decay incidence for all cultivars [14]. In another study, Bahar and Lichter [15] studied different CA conditions (5, 10, 15% CO2 with 5% O2) on Ottomanit fresh fig. This study revealed that CA storage including 5% O2 and 5% CO2 maintained firmness, integrity, and decay control compared to the other atmospheric compositions and control.
Harvested fresh figs are generally stored under ambient atmosphere for up to two weeks. Storage duration can be prolonged up to four weeks under CA; however, this is not possible due to some challenges in practice. For example, loading the storage room and atmosphere adjustment take a long time due to the short harvest season and rapid fruit circulation after harvest. At this point, the palliflex storage system can be a solution to overcome these challenges in the storage of fresh fig. In this storage system, fruit are stored on a pallet basis, and the atmosphere of each pallet can be monitored and controlled independently. Higher CO2 levels combined with a low O2 level and the palliflex storage systems were not tested previously in this cultivar. For these reasons, this study was focused to investigate the effects of high CO2 levels with low O2 on the fruit quality of Bursa Siyahi fig cultivars stored under the palliflex storage system.
2. Materials and Methods
2.1. Fruit Material and Storage Experiment
Bursa Siyahi fresh fig fruit were harvested at fully colored (L*: 30.60; C*: 9.7; h°: 221.58) maturity stage from a commercial orchard in Kestel (Bursa, Turkey) (Figure 1). After harvest, the fruit were pre-cooled and then immediately transferred to the postharvest laboratory of Akdeniz University Antalya, Turkey, using a refrigerated truck at 0 °C. Then, the fruit without any visual defects were selected and used in the experiment.

Figure 1.
Skin (a) and flesh (b) color of fig fruit (cv. Bursa Siyahi) at harvest stage.
For establishment of different atmospheric compositions in the palliflex system, fruit were divided into four groups. The first group of fruit was stored in 3% O2 + 10% CO2 (PA-1), the second group was stored in 3% O2 + 15% CO2 (PA-2), the third group was stored 3% O2 + 20% CO2 (PA-3), and the last group was stored in 0.03% CO2 + 21% O2 (regular atmosphere (RA)). Then, all groups of fruit were stored at 0 °C with 90–95% RH for 28 days and sampled at 0, 7, 14, 21, and 28 days of storage. Palliflex storage system specially designed for laboratory scale was used for storage. For this purpose, a 12 m2 cold room was designed to include 10 pallets with a computer-controlled system. The desired atmosphere conditions in the pallets were set up by a flow-through system, mixing N2, CO2, and air via pressure regulators. The atmosphere composition in the pallet was adjusted to the desired level within 15 min. The O2 and CO2 levels in each pallet were automatically controlled and monitored by a computer system (My Fruit Light, Van Amerongen CA Technology B.V., The Netherlands) for 1 h interval to ensure the proper composition.
2.2. Quality Analyses
The fruit were marked and weighed at the beginning of the experiment to determine an initial weight. They were then weighed at the end of each storage time, and the results were calculated as the percent loss from the initial weight. The firmness of fruit was determined by using a penetrometer (Chatillon DFI 10, Largo, FL, USA), measuring the peeled equatorial region on three different sides of the fruit. Fruit firmness was shown as average of these measurements and results were given as Newton (N). To determine the total soluble solids (TSS), fruit samples were homogenized for 5 min by using a hand blender and a 10 g sample was added to 40 mL distilled water and the mix was homogenized for 2 min. Extracts were centrifuged at 3000× g for 5 min at 20 °C (Sigma 2-16K, Osterodo am Harz, Germany). The amount of TSS content was measured by a digital refractometer (HI96801, Hanna, Rhode Island, USA) and the findings were represented as a percentage (%). The purees were diluted ten times with distilled water and used to measure titratable acidity (TA) according to the method described by Bahar and Lichter [15]. The TA was expressed as a percentage of citric acid. For taste evaluation, six panelists (three men and three women, 25–50 years old) tasted fruit, and the data were categorized using a five-point hedonic scale described by Selcuk and Erkan [16]. Decay incidence was recorded by counting the number of fruits with visible symptoms and expressed as a percentage of total fruits. The total microorganisms were examined according to the method described by Cantín et al. [5]. To measure the total microorganism, 1 cm2 pieces were taken from the surface of the fruits and put into microtubes. Then, 1 mL of sterile water was added onto the samples, and they were vortexed for 30 s and 100 µL of the suspension was placed in a Petri dish containing potato dextrose agar (PDA) medium. Microorganisms were counted after incubation for 5 days at 25 °C. The respiration rate and ethylene production of fruit were determined by using a gas chromatography (GC) equipped with thermal conductivity and flame ionization detectors (Thermo Electron S.p.A., Strada Rivoltana, Milan, Italy). To determine respiration rate, 500 g fruit for each treatment was placed in 3 L gas tight jars that were specially designed with a cover seal for an hour at 20 °C. A 1 mL gas sample was taken out from head space of jars with gas-tight syringe and injected into thermal conductivity detector of the system. The calibration was done by using external CO2 standard (1000 ppm). GC conditions were as follows: Supelco 80/100 alumina F1 column, 65 °C oven temperature, 35 °C detector temperature, 45 mL min−1 hydrogen flow, 400 mL min−1 dry air flow during the 4 min analysis time. The GC was calibrated with external ethylene standard (25 ppm) for ethylene production. Then, similarly, 1 mL gas sample injected to flame ionization detector following GC conditions: GS-GasPro 113-4362 capillary column, 130 °C oven temperature, 275 °C detector temperature, 35 mL min−1 hydrogen flow, 350 mL min−1 dry air flow during the 2 min analysis time.
Respiration rate and ethylene production were calculated using Equation (1):
where A is the sample area (ppm)/standard area (ppm), Vj is the jar volume (L), Vp is the product volume (L), t is the time (hour) and g is the fruit weight (kg).
The contents of sugar (glucose and fructose) and organic acid (citric and malic) were determined using the same extracts. For this purpose, fruit were crushed with hand blender and 5 g of sample was immersed in 45 mL of distilled water and homogenized with an ultraturrax (T-25, Ika-Labortechnik, Staufen, Germany). This mixture was extracted for 30 min in an ultrasonic water bath at 40 °C. The samples were centrifuged at 4000× g for 5 min at room temperature after incubation. Finally, the supernatant was put into vials after passing through a 0.45 µm membrane filter (Macherey-Nagel, Düren, Germany). The sugar contents were determined according to a method described by Sturm et al. [17] using high performance liquid chromatography (HPLC) (Shimadzu, LC-20 AD, Kyoto, Japan) equipped with Refractive Index Detector (RID). The analysis was carried out using a Rezex RCM-monosaccharide column (300 × 7.8 mm; Phenomenex, Torrance, CA, USA) with a flow of 0.6 mL min−1, and column temperature was maintained at 65 °C. Twice distilled water was used as mobile phase. Organic acids were determined using ICE ORH-801-organic acid column (300 × 6.5 mm, Transgenomics, Ohama, USA) with a flow 0.6 mL min−1, and column temperature was maintained at 65 °C. For the mobile phase, 0.125 mM H2SO4 was used.
2.3. Experimental Design and Statistical Analysis
The experiment was conducted using completely randomized design with three replicates, and each replication included 22 fruits. The data were analyzed by using the SAS 9.0 statistical software (SAS Inst., Cary, NC, USA) and means were compared using Duncan’s multiple range test (p ≤ 0.05). A comparison of taste scores was performed using a nonparametric ANOVA and multiple comparison of Kruskal–Wallis test (p ≤ 0.05).
3. Results and Discussion
3.1. Weight Loss
Weight loss gradually increased with storage time in all atmospheric compositions. Minimum weight loss was determined in PA-1 (2.68%) followed by PA-2 (2.73%), PA-3 (3.00%), and RA (3.64%) after 28 days of cold storage (Figure 2).
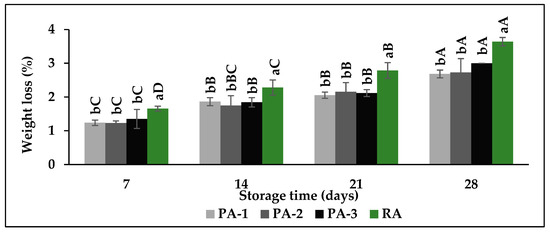
Figure 2.
Effects of different atmospheric compositions on fruit weight loss in fresh fig cv. Bursa Siyahi. Different uppercase letters indicate significant differences for each atmosphere composition during storage and different lowercase letters indicate significant differences between atmosphere compositions for each sampling date at p ≤ 0.05 by Duncan’s multiple range test. Data are the mean ± SE of three replicates. PA-1: 3% O2 + 10% CO2; PA-2: 3% O2 + 15% CO2; PA-3: 3% O2 + 20% CO2; RA: 21% O2 + 0.03% CO2.
These findings clearly illustrated that storage atmospheres except for RA have positive effects to inhibit weight loss; however, there were no statistical differences among atmospheric compositions. The potential of CA storage including low O2 and high CO2 level is effective in preventing weight loss due to its slowdown effects on fruit metabolism [8]. Similarly, CA storage slowed down weight loss compared to control in the Ottomonit fig variety stored for 30 days. Although the weight loss reached 14.4% in control fruit, 5% O2 + 5% CO2 limited this weight loss in 4.4% [15]. Considering the previous studies, it can be said that the palliflex storage system for fig fruit inhibited weight loss compared to RA. This inhibiting effect can be caused by cultivar, maturity stage, and the pallet cover, which is not permeable to water and gas.
3.2. Fruit Firmness
Fruit firmness is an extremely important characteristic for consumers’ acceptance of fig fruit, and the soft ones are generally preferred by consumers; however, too-soft fruit may not be appropriate for handling and transportation. The initial fruit firmness was 6.18 N at harvest. Fruit firmness gradually decreased with storage time in all atmospheric compositions. During the first 7 days of storage, PA-1 and PA-2 maintained firmness while PA-3 and RA stored fruit softened faster (Figure 3). At the end of the storage, the highest decrease in firmness reached up to 52.3% in PA-3 followed by 46.8% in RA in comparison to initial firmness. The highest fruit firmness was determined in PA-1 (3.82 N) and PA-2 (3.37 N) at the end of the storage. Fruit softening is linked to cell-wall carbohydrate metabolism and pectin substrate, and the polygalacturonase (PG) enzyme is important in this process [18]. A similar mechanism has also been molecularly revealed in fig fruit [19]. Teixeira et al. [20] reported that CA storage with 5% O2 and 20% CO2 increased fruit softening in guava fruit during 28 days of storage.
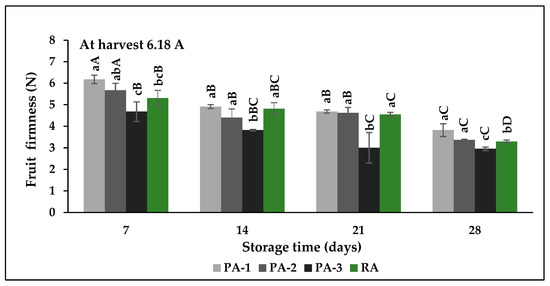
Figure 3.
Effects of different atmospheric compositions on fruit firmness in fresh fig cv. Bursa Siyahi. Different uppercase letters indicate significant differences for each atmosphere composition during storage and different lowercase letters indicate significant differences between atmosphere compositions for each sampling date at p ≤ 0.05 by Duncan’s multiple range test. Data are the mean ± SE of three replicates. PA-1: 3% O2 + 10% CO2; PA-2: 3% O2 + 15% CO2; PA-3: 3% O2 + 20% CO2; RA: 21% O2 + 0.03% CO2.
This situation was explained by a negative correlation between high CO2 and solubilization of pectic compounds. Villalobos et al. [21] stated that fruit softening varies according to the atmospheric compositions, and modified atmosphere packaging (MAP) slowed softening in the Cuello Dama Blanco, Cuello Dama Negro, and San Antonio fig cultivars. Similarly, an atmosphere of 5% CO2 is more effective than 10% and 15% CO2 to delay softening in Ottomonit fig [15]. On the other hand, there were no significant differences between the atmospheric compositions (RA and 6% O2 + 17% CO2) on the firmness of Brown Turkey and Kadota, while a significant effect was found in Mission fig [14].
3.3. Decay Rate and Total Microbial Count
Fresh fig is a highly perishable fruit due to its pomological characteristics, and the postharvest decay rate can reach up to 50% in a short period of time under unfavorable storage conditions. Therefore, most fig fruits are generally consumed as fresh in regions close to the producing areas. Fresh figs are highly affected by pathogens, especially in unsuitable storage conditions [22]. Successful postharvest management in fresh fig is dependent on avoiding mechanical damage and as rapidly as possible getting into the cooling chain. In this study, the palliflex storage was highly efficient to control deterioration during the first 2 weeks of storage and there was no decay observed. After this period, the decay rate was lower on the fruit stored at different atmospheric compositions compared to fruit stored RA. In both 21 and 28 days of storage, the highest decay rate was on the fruit stored under RA. Microbial activity increased during the storage in all atmospheres and the highest total microbial count was recorded on the fruit stored under RA with 14.87 cfu mL−1. However, PA-2 and PA-3 were found highly effective to control microbial count (Table 1). Fungal decay caused by some pathogens such as Alternaria spp., Botrytis spp., Fusarium spp., Aspergillus spp., Penicillium spp., and Rhizopus spp. [2,4,5] is the main cause of rapid and extensive postharvest losses in fresh fig. Lowering O2 and increasing CO2 level are known as an effective treatment to control anaerobic and aerobic microbial growth due to their toxicity effects. The efficiency of inhibition by high CO2 on microorganisms has also been reported for strawberries [23].

Table 1.
Effects of different atmospheric compositions on decay rate and microbial count of fresh fig fruit (cv. Bursa Siyahi).
Many studies have revealed that CA storage can inhibit decay development and microbial count during the storage. Serradilla et al. [24] reported that 5% O2+ 10% CO2 and 8% O2 + 10% CO2 inhibited the growth of bacteria and molds in cherries stored for 15 days. Colelli et al. [10] reported that 15 and 20% CO2 enriched atmospheres resulted in reduction of decay incidence in Black Mission fig. The inhibition effects of modified atmosphere with low O2 and high CO2 on decay incidence and microbial count were also observed on fresh fig [25]. Similarly, microperforated films delayed fungal deteriorations and physiological disorders in San Antonio and Banane figs [26]. In other studies, it was reported that the low O2 level can maintain firmness and decrease respiration and ethylene production, and thus control the deterioration of fresh figs during storage [11,12].
3.4. Total Soluble Solids (TSS), Titratable Acidity (TA) and Taste
The effects of different atmospheric compositions and storage times on the total soluble solid content (TSS) were found statistically significant (p ≤ 0.05). The TSS contents of fresh fig on the 14th day in storage were higher than their initial value at harvest (Table 2). However, TSS content decreased during the storage period due to the fruit senescence and respiration. At the end of the storage, PA-1 and PA-2 had higher TSS content compared to other storage atmospheres (Table 2). A similar trend was also reported for long-term storage of Bursa Siyahi fig fruit during CA storage at 0 °C [13]. Villalobos et al. [26] reported that fruits covered with microperforated film, which can provide a low O2 high CO2 atmosphere, resulted in higher TSS content than control treatment due to decreasing respiration rate and delaying ripening. Titratable acidity (TA) also showed fluctuations during the storage period. Palliflex storage atmospheres (PA-1, PA-2 and PA-3) provided a slight increase in TA content during the first 14 days of storage while RA did not show any changes. After this storage period, TA content decreased in all storage atmospheres. At the end of storage, TA contents in the PA-2 (0.25%) and PA-3 (0.25%) atmospheres were higher than PA-1 (0.23%) and RA (0.19%) stored fruit. After comparison to the initial TA content, it can be clearly said that there were no changes on the fruit stored in PA-2 and PA-3. In a study, chitosan-coated fresh fig had higher TA content than the uncoated fruit due to the effects, which create a gas barrier around the fruit and so slow down metabolism [27]. On the other hand, low O2 and high CO2 caused no off flavor and they provided more stable taste than the fruit stored in RA. Fruit stored under controlled atmospheres had a higher taste score than the fruit under RA after 7 days of cold storage; however, there were no significant differences among PA-1, PA-2, and PA-3. The taste score of the fruit stored in RA decreased after 14 days of storage while the taste score of fruit stored in PA-3 decreased at the end of the storage. PA-2 and PA-1 maintained their initial taste score during the storage period. Waghmare and Annapure [28] reported that irradiation and MAP (5% O2 + 10% CO2) and their combinations positively affected the taste of Poona fig fruit stored at 5 °C for 15 days.

Table 2.
Effects of different atmospheric compositions on total soluble solid (TSS), titratable acidity (TA), and taste 1 of fresh fig fruit (cv. Bursa Siyahi).
Similarly, Ayhan and Karacay [29] reported that active MAP, including 20% CO2 with low and high O2, had a higher taste score and overall appearance in Bursa Siyahi fig fruit compared to the control. MAP was effective to maintain initial taste and texture in Black Mission fig at both 1 °C and 25° C [30].
3.5. Sugar and Organic Acid Contents
The fructose is the major sugar in fresh fig followed by glucose and sucrose. At harvest, the fructose and glucose contents of fig fruit were determined as 9.65 and 7.43 g 100 g−1, respectively (Table 3). Similarly, Çalişkan and Polat [31] reported that fructose and glucose are the dominant sugars in fresh fig fruit grown in the Mediterranean basin. Fructose and glucose content gradually increased during the first 14 days of storage in all atmospheres and then decreased at the end of storage. The fructose contents of the fig fruit stored under PA-1 and PA-3 were higher than the fruit stored under PA-2 and RA. While glucose content of the fruit stored under RA did not change at 7 and 14 days of cold storage, the glucose contents of fruits stored under different atmospheres increased after 7 days of storage. After this period, glucose content decreased on the 14th and 21st days, depending on atmospheric compositions. At the end of 28 days of storage, the glucose contents of the fruit stored under atmospheric compositions were higher than RA; however, no statistical differences were found among the atmospheric compositions. In this study, sugar content first increased and then decreased. This decrease can be the result of senescence and sugar used in respiratory metabolism. The glucose and fructose contents can show different patterns based on cultivars, maturity stage, and storage conditions. Fructose and glucose content increased during the 30 days of cold storage at 0.5 °C plus 1 day at 20 °C in Masui Dauphine and Banane fig cultivars, whereas Bongresi showed similar pattern with our results [32].

Table 3.
Effects of different atmospheric compositions on fructose, glucose, citric and malic acid content of fresh fig fruit (cv. Bursa Siyahi).
Citric and malic acids are the most abundant organic acids in fig fruit. There were no significant differences among the atmospheric compositions during the 7, 14, and 21 days of storage. At the end of the 28 days of storage, the highest citric acid content was found in PA-2 and PA-3, whereas the lowest citric acid was in RA. Malic acid content showed fluctuation during the storage period. It decreased in PA-1 and RA compared to their initial concentration at harvest; however, malic acid content in PA-2 and PA-3 did not change during the storage period. At the end of the storage, the highest malic acid content was in PA-2 and the lowest was in RA. Trad et al. [33] reported that citric acid is the most abundant organic acid following malic acid in fresh fig fruit as determined in this study The decrease in malic and citric acid contents of medlar fruit was significantly prevented by CA storage [16].
3.6. Respiration Rate and Ethylene Production
The respiration rate of fresh fig at harvest was 37.38 mL CO2 kg−1 h−1 and sharply decreased in all storage atmospheres after 7 days of storage (Figure 4a). This situation can be the result of low temperature effects on the respiration rate. Allegro et al. [34] reported that the initial respiration rate of Dottato figs was 28.0 ± 2.5 mL CO2 kg−1 h−1 and decreased to 6.8 ± 1.8 mL CO2 kg−1 h−1 in the fruit stored at 4 °C after 72 h. A similar decreasing trend after storage was also reported in fig fruit belonging to the cultivars Cuello Dama Blanco, Cuello Dama Negro, and San Antonio [21]. In this study, at the 14th and 21st days of storage, the highest respiration rate was found in the fruit stored in RA, while the lowest rates were found those stored in PA-1 and PA-2. At the end of the storage period, fig fruit stored under different atmospheric compositions had lower respiration rates compared to the fruit stored in RA; however, there were no significant differences among the PA-1, PA-2, and PA-3.
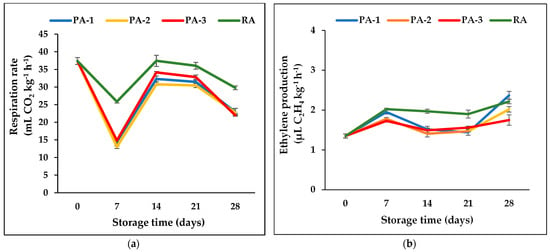
Figure 4.
Effects of different atmospheric compositions on respiration rate (a) and ethylene production (b) in fresh fig cv. Bursa Siyahi. Data are the mean ± SE of three replicates. PA-1: 3% O2 + 10% CO2; PA-2: 3% O2 + 15% CO2; PA-3: 3% O2 + 20% CO2; RA: 21% O2 + 0.03% CO2.
The ethylene production of the Bursa Siyahi fig fruit was 1.35 µL C2H4 kg−1 h−1 and increased after 7 days of storage in all tested atmospheres (Figure 4b). While the highest ethylene production was in fruit stored at RA (2.2 µL C2H4 kg−1 h−1), the lowest was in PA-2 and PA-3 (1.78 and 1.73 µL C2H4 kg−1 h−1), respectively. After 14 and 21 days of storage, ethylene production slightly decreased. At the end of the storage, ethylene productions increased with senescence in all atmospheres, but PA-3 inhibited this increase.
In previous studies, it was reported that CA storage slowed down respiration rate and ethylene production in strawberry [35] and blueberry [36]. Modified atmosphere decreased respiration rate and ethylene production, and thus storage life extended [37]. In other studies, enriched CO2 levels suppressed ethylene production in Masui Dauphine [38] and Mission [10] fig cultivars.
4. Conclusions
Fresh fig has a limited postharvest life and commonly suffers from diseases, softening, and weight loss. A high level of CO2 has been effective in preventing weight loss, decay development, and micro-organism growth in figs as well as slowing down metabolic activities such as respiration rate and ethylene production. However, an atmosphere containing 3% O2 + 20% CO2 accelerated the softening. It was concluded that a 3% O2 + 15% CO2 containing atmosphere can be used to maintain postharvest fruit quality and to ensure reduced product loss in the Bursa Siyahi fig cultivar under palliflex storage. Depending on pre- and postharvest factors, fig varieties give different responses to atmospheric compositions. For this reason, the biochemical properties and molecular mechanisms of the fig fruit stored under variable atmospheric composition should be investigated in the future.
Author Contributions
Conceptualization, A.D. and M.E.; methodology, A.D. and M.E.; software, A.D.; formal analysis, A.D.; investigation, A.D.; writing—original draft preparation, A.D.; writing—review and editing, M.E.; visualization, A.D.; supervision, M.E.; project administration, M.E.; funding acquisition, M.E. All authors have read and agreed to the published version of the manuscript.
Funding
The chemicals and necessary equipment were funded by TUBITAK, grant number 217O016 and The Scientific Research Projects Coordination Unit of Akdeniz University, grant number FBA-2017-2608.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors gratefully thank the support of Alanar Fruit Company (Turkey) for providing the fruit materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Food and Agriculture Organization Statistics Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 25 December 2022).
- Doğan, A.; Erkan, M. Ficus carica: Production, Cultivation and Uses; Zeynel, D., Ed.; Nova Science Publishers: New York, NY, USA, 2022; pp. 185–208. [Google Scholar]
- Crisosto, H.; Ferguson, L.; Bremer, V.; Stover, E.; Colelli, G. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Yahia, E., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 134–158. [Google Scholar]
- Doster, M.A.; Michailides, T.J. Fungal decay of first-crop and main crop figs. Plant Dis. 2007, 91, 1657–1662. [Google Scholar] [CrossRef]
- Cantín, C.M.; Palou, L.; Bremer, V.; Michailides, T.J.; Crisosto, C.H. Evaluation of the use of sulfur dioxide to reduce postharvest losses on dark and green figs. Postharvest Biol. Technol. 2011, 59, 150–158. [Google Scholar] [CrossRef]
- Dogan, A.; Cat, A.; Catal, M.; Erkan, M. First report of Alternaria alternata causing postharvest decay in fig (Ficus carica L. cv. Bursa Siyahi) fruit in Turkey. J. Biotechnol. 2018, 280, S84. [Google Scholar] [CrossRef]
- Karabulut, O.A.; Ilhan, K.; Arslan, U.; Vardar, C. Evaluation of the use of chlorine dioxide by fogging for decreasing postharvest decay of Fig. Postharvest Biol. Technol. 2009, 52, 313–315. [Google Scholar] [CrossRef]
- Thompson, A.K.; Prange, R.K.; Bancroft, R.D.; Puttongsiri, T.; Beaudry, R.M. Controlled Atmosphere Storage of Fruit and Vegetables, 3rd ed.; CAB International: Oxfordshire, UK, 2008; ISBN 9781786393739. [Google Scholar]
- Cantín, C.M.; Minas, I.S.; Goulas, V.; Jiménez, M.; Manganaris, G.A.; Michailides, T.J.; Crisosto, C.H. Sulfur dioxide fumigation alone or in combination with CO2-enriched atmosphere extends the market life of highbush blueberry fruit. Postharvest Biol. Technol. 2012, 67, 84–91. [Google Scholar] [CrossRef]
- Colelli, G.; Mitchell, F.G.; Kader, A.A. Extension of postharvest life of Mission figs by CO2 enriched atmospheres. Hortscience 1991, 26, 1193–1195. [Google Scholar] [CrossRef]
- Dogan, A. Effects of different oxygen levels with high-carbon dioxide atmosphere on postharvest quality of fresh fig under palliflex storage systems. Horticulturae 2022, 8, 353. [Google Scholar] [CrossRef]
- Tsantil, E.; Karaiskos, G.; Pontikis, C. Storage of fresh figs in low oxygen atmosphere. J. Hortic. Sci. Biotechnol. 2003, 78, 56–60. [Google Scholar] [CrossRef]
- Türk, R.; Eriş, A.; Özer, M.H.; Tuncelli, E.; Henze, J. Research on the CA storage of Fig cv. Bursa Siyahı. Acta Hortic. 1994, 368, 830–839. [Google Scholar] [CrossRef]
- Crisosto, G.; Bremer, V.; Dollahite, S.; Crisosto, C.H.; Stover, E.; Ferguson, L. Effect of Controlled Atmosphere Storage on the Quality of Three Fresh Fig Cultivars. 2009. Available online: http://figs4fun.com/Links/FigLink291.pdf (accessed on 26 December 2022).
- Bahar, A.; Lichter, A. Effect of controlled atmosphere on the storage potential of Ottomanit fig fruit. Sci. Hortic. 2018, 227, 196–201. [Google Scholar] [CrossRef]
- Selcuk, N.; Erkan, M. The effects of modified and palliflex controlled atmosphere storage on postharvest quality and composition of ‘Istanbul’ medlar fruit. Postharvest Biol. Technol. 2015, 99, 9–19. [Google Scholar] [CrossRef]
- Sturm, K.; Koron, D.; Stampar, F. The composition of fruit of different strawberry varieties depending on maturity stage. Food Chem. 2003, 83, 417–422. [Google Scholar] [CrossRef]
- Giongo, L.; Poncetta, P.; Loretti, P.; Costa, F. Texture profiling of blueberries (Vaccinium spp.) during fruit development, ripening and storage. Postharvest Biol. Technol. 2013, 76, 34–39. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, Z.; Chen, S.; Vainstein, A.; Ma, H. Proteome and transcriptome analyses reveal key molecular differences between quality parameters of commercial-ripe and tree-ripe fig (Ficus carica L.). BMC Plant Biol. 2019, 19, 146. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.H.A.; Júnior, L.C.C.; Ferraudo, A.S.; Durigan, J.F. Quality of guava (Psidium guajava L. cv. Pedro Sato) fruit stored in low-O2 controlled atmospheres is negatively affected by increasing levels of CO2. Postharvest Biol. Technol. 2016, 111, 62–68. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; López, C.M.; Pereira, C.; Córdoba, M.D.G. Preservation of different fig cultivars (Ficus carica L.) under modified atmosphere packaging during cold storage. J. Sci. Food Agric. 2016, 96, 2103–2115. [Google Scholar] [CrossRef]
- Üstün, H.; Doğan, A. The effect of different UV-C illumination doses on postharvest quality of fresh Fig. Gıda 2022, 47, 744–753. [Google Scholar]
- Allende, A.; Marín, A.; Buendía, B.; Tomás-Barberán, F.; Gil, M.I. Impact of combined postharvest treatments (UV-C light, gaseous O3, super atmospheric O2 and high CO2) on health promoting compounds and shelf-life of strawberries. Postharvest Biol. Technol. 2007, 46, 201–211. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Villalobos, M.C.; Hernández, A.; Martín, A.; Lozano, M.; de Guía Córdoba, M. Study of microbiological quality of controlled atmosphere packaged ‘Ambrunés’ sweet cherries and subsequent shelf-life. Int. J. Food Microbiol. 2013, 166, 85–92. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Ansah, F.; Amodio, M.L.; Serradillas, M.J.; Colelli, G. Application of modified atmosphere packaging with moisture absorber to extend the shelf life of ‘Domenico Tauro’ breba fruit. Acta Hortic. 2017, 1173, 365–370. [Google Scholar] [CrossRef]
- Villalobos, M.C.; Serradilla, M.J.; Martín, A.; Ruiz-Moyano, S.; Pereira, C.; Córdoba, M.G. Use of equilibrium modified atmosphere packaging for preservation of ‘San Antonio’ and ‘Banane’ breba crops (Ficus carica L.). Postharvest Biol. Technol. 2014, 98, 14–22. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef]
- Waghmare, R.B.; Annapure, U.S. Integrated effect of radiation processing and modified atmosphere packaging (MAP) on shelf-life of fresh Fig. J. Food Sci. Technol. 2018, 55, 1993–2002. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, Z.; Karaçay, E. Preservation of the Bursa Siyahı fresh fig under modified atmosphere packaging (MAP) and cold storage. Int. J. AgriSci. 2011, 1, 1–9. [Google Scholar]
- Martínez-Damián, M.T.; Cruz-Arvizu, O.; Cruz-Alvarez, O. Effect of modified atmosphere packaging on nutraceutical quality and overall appearance of figs stored at 1 °C. Not. Bot. Horti. Agrobot. Cluj-Napoca 2020, 48, 2292–2305. [Google Scholar] [CrossRef]
- Çalişkan, O.; Polat, A.A. Phytochemical and antioxidant properties of selected fig (Ficus carica L.) accessions from the eastern Mediterranean region of Turkey. Sci Hortic. 2011, 128, 473–478. [Google Scholar] [CrossRef]
- Byeon, S.E.; Lee, J. Differential responses of fruit quality and major targeted metabolites in three different cultivars of cold-stored figs (Ficus carica L.). Sci. Hortic. 2020, 260, 108877. [Google Scholar] [CrossRef]
- Trad, M.; Bourvellec, L.B.; Gaaliche, B.; Renard, C.M.G.C.; Mars, M. Nutritional compounds in figs from the Southern Mediterranean region. Int. J. Food Prop. 2014, 17, 491–499. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Almenar, E.; Hernández-Muñoz, P.; Lagarón, J.M.; Catalá, R.; Gavara, R. Controlled atmosphere storage of wild strawberry fruit (Fragaria vesca L.). J. Agric. Food Chem. 2006, 54, 86–91. [Google Scholar] [CrossRef]
- Falagan, N.; Miclo, T.; Terry, L.A. Graduated controlled atmosphere: A novel approach to increase “Duke” blueberry storage life. Front. Plant Sci. 2020, 11, 221. [Google Scholar] [CrossRef] [PubMed]
- Bouzo, C.A.; Travadelo, M.; Gariglio, N.F. Effect of different packaging materials on postharvest quality of fresh fig fruit. Int. J. Agric. Biol. 2012, 14, 821–825. [Google Scholar]
- Mathooko, F.M.; Sotokawa, T.; Kubo, Y.; Inaba, A.; Nakamura, R. Retention of freshness in fig fruit by CO2 enriched atmosphere treatment or modified atmosphere packaging under ambient temperature. J. Jpn. Soc. Hortic. Sci. 1993, 62, 661–667. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).