1. Introduction
Highly productive crop cultivation systems that can meet the food demands of the ever-increasing human population in a sustainable way are the major challenge for agriculture [
1,
2]. During the previous century, high productivity was based on heavy fertilization, with detrimental consequences such as freshwater and soil pollution, land degradation, and an overall negative environmental impact. Alternative cultivation systems that can restrict chemical fertilizer use by replacing it with more sustainable nutrient sources are required. Aquaponics is a promising solution that addresses all the above-mentioned issues by turning waste into resource under the circular economy concept [
3,
4]. The technique combines fish production in recirculating aquaculture systems (RAS) and crop cultivation through hydroponics [
5]. Wastes from RAS, such as products of fish metabolism and uneaten feed represent a rich nutrient source directed to plants, which by their absorption, clean the water that can return to fish. The link between these two sub-systems is nitrogen conversions performed by nitrifying bacteria (
Nitrosomonas spp. and
Nitrobacter spp.), which transform fish-produced ammonia to nitrate suitable for plants [
6].
Nutrients are the central aspect of aquaponics systems, with several studies attempting to analyze this multifaceted issue in terms of nutrient recovery rates [
7], influence of system’s pH on their availability [
8], and optimization of fish stocking for adequate nutrient release to sustain crop productivity [
9]. Even though aquaponics is, by design, a versatile system in terms of fish rearing conditions, fish feed type and rate, species selection, and water physicochemical adjustments, certain nutrient deficiencies are a ubiquitous characteristic of all tested systems [
5]. Notably, potassium (K) and iron (Fe) are routinely found in sub-optimal concentrations for plant nutrition [
10,
11]. These essential nutrients play a major role in plant metabolism and normal functioning of the photosynthetic apparatus; thus, they are indispensable for the maximization of crop yield and quality of final products [
12]. Many studies from the last decade tackled system-imposed nutrient deficiencies by extensive fertilizer supplementation to meet elemental concentration targets set for soilless crop cultivation systems, including a series of macro- and micronutrients [
13,
14]. Nevertheless, this approach constitutes a strong intervention in nutrient recovery, potentially disrupting the equilibrium among the plants–fish–bacteria sub-systems. Beyond the possible consequences in symbiosis function and viability and fish welfare, these chemical additions compromise the main advantage of aquaponics, i.e., its sustainability and low environmental footprint. In our previous work with aquaponics-grown lettuce, we introduced the concept of minimal nutrient supplementation [
15]. The effects of Fe and K supplementation on crop function and growth, as well as the performance of no-input control treatment, were focused on, the latter with the aim of identifying the system’s constrains. Additionally, the need for a thorough assessment of plant functional parameters in aquaponics research was stressed (see also [
16]). Going beyond solely yield determination, this approach can efficiently identify the system’s limitations and weak points and propose optimization measures.
The minimal supplementation approach was followed in a spinach (Spinacia oleracea var. Virofly)–red tilapia (Oreochromis spp.) co-cultivation in a laboratory-scale aquaponic system in the present study. The aim was to: (i) identify the system’s bottlenecks in terms of nutrient supply and (ii) scrutinize the role of Fe and Fe+K inputs in alleviating limitations posed by the system on spinach function and crop/fish growth. Several functional parameters were monitored throughout the experimental period, specifically focusing on photosynthesis, i.e., gas exchange, state, and efficiency of the photosynthetic apparatus, along with the determination of chlorophyll (chl) content and light-use efficiency. The functional profile was complemented by leaf elemental analysis and measurements of antioxidant activity, while the assessment of several spinach and tilapia growth characteristics completed the picture of crop and fish responses.
2. Materials and Methods
2.1. Experimental Design
The experimental process took place at the University of Thessaly, School of Agricultural Sciences, Aquaculture Laboratory, Section Aquaponics, Greece. In total, 45 spinach seedlings (
Spinacia oleracea var. Virofly) at the stage of four true leaves and 90 red tilapias (
Oreochromis spp.) were used. The co-cultivation was performed using nine autonomous laboratory-scale aquaponic systems (
Figure 1). Each aquaponic system was considered a replicate for plants and fish, and three replicates/treatment were used, with treatment referring to the three different nutrient solutions, as follows:
Control: aquaponic water without further supplementation.
Fe: aquaponic water enriched with iron.
Fe+K: aquaponic water enriched with iron and potassium.
The amount of Fe and K added was calculated according to nutrient target concentrations for spinach and leafy greens in hydroponic cultivation and specifically reached 40 μmol L
−1 for Fe and 11 mmol L
−1 for K [
17,
18]. The supplemented chemical forms were Fe-DTPA (GEOLIX EPE, Chelated Iron DTPA 11%) and potassium sulphate (HONEYWELL FLUKA, K
2SO
4). The first external nutrient input was performed five days after the transplantation of spinach plants to the aquaponic unit to avoid possible transplantation stress. The cultivation period lasted for 45 days until plants reached the marketable size.
The laboratory’s environmental conditions were managed by an integrated climate control system (Opticlimate, models 15,000 PRO3 and PRO4) that maintained the room temperature at 21.84 ± 0.09 °C and the relative humidity at 57.13 ± 0.61%.
2.2. Aquaponic Systems
Each autonomous aquaponic system (
Figure 1) had a 135 L total water capacity and consisted of three vertically arranged sub-units: a raft hydroponic unit (54 L and 0.18 m
2 area), a fish rearing tank (54 L), and a sump filter (27 L), all made of glass material and interconnected with pipes (5 L). The entire surface of all glass tanks was covered by plastic layers (black on the inside and white on the outside) to prevent algae development.
The water flowed from the fish tank to the sump filter via gravity. The sump filter was separated into the mechanical filter and the biological filter. The first one provided mechanical filtration through a plastic net covered with thick layers (10 cm) of fiberglass and sponges (EHEIM, coarse foam filter pad) to withhold solid fish wastes and uneaten feed. The biological filter was made up of 4 L ceramic rings (SERA, siporax, 15 mm), 2 L bioballs (36 mm), and 1 L cylindrical substrate K1 Kaldness media (11 mm) colonized by nitrifying bacteria (PRODIBIO, Biodigest) that carried out the nitrification process. A pump (SUNSUN, 22 W, 1000 L h−1, 0.55 kg) was placed in the last part of the sump filter to ensure the continuous flow of filtered water to the plant unit (Q = 182 L h−1), and the circulation turnover of the aquaponic water was adjusted to be seven times per hour.
2.3. Nutrient Solution
Water physicochemical parameters (temperature, pH, and dissolved oxygen) were regularly monitored during the experiment using multimeter sensors (HACH, HQ40d), with the sampling point being at the middle of the fish tanks. They were kept constant to compromise the needs of plants, fish, and bacteria as follows; the water temperature at 22.75 ± 0.03 °C using heaters (AQUAEL, Ultra heater 100 W) and the dissolved oxygen at 8.50 ± 0.02 mg L−1 using air pumps (HAILEA, ACO-328, 70 L min−1). Daily water exchange with non-chlorine water was performed in small amounts (corresponding to 5%) to both stabilize pH at neutral values (7.44 ± 0.01) and replace the water lost by plants’ evapotranspiration and the process of removal of fish solids.
Weekly monitoring of water nutrient concentrations was performed at the inlet point of the raft hydroponic unit. After being filtered by glass fiber syringe filters (0.7 μm), the collected water was immediately measured photometrically (HACH, DR3900) for iron and nitrate concentrations using pre-weighted reagents (HACH, Iron TPTZ Method 8112; Cadmium Reduction Method, 8039). Potassium concentration was measured by a flame photometer (JENWAY, PFP7) following the corresponding potassium standard curves. The amounts of Fe and K needed to reach the target concentration were calculated, diluted in 2 L of non-chlorine water, and added at the sump filters.
2.4. Tilapia Rearing Conditions and Growth Performance
Red tilapias (
Oreochromis spp.) resulted from reproduction in the premises of the Aquaponics laboratory and were reared for six months before the experiment. All experimental procedures were conducted according to the guidelines of EU Directive 2010/63/EU regarding the protection of animals used for scientific purposes and were applied by FELASA accredited scientists (functions A–D). In total, 90 tilapias were acclimated for 15 days in the system tanks before the experiment began. After this period, the juveniles’ weight and length were measured, and they were equally distributed based on their weight among the nine aquaponic systems. To estimate the exact number of fish per system, the equation of carrying capacity of an aquarium proposed by Hirayama [
19] was used, which is derived from the rates of pollution and possible purification in a closed culture system or aquarium. For the calculations, the oxidizing capacity of the filter and the pollution load were measured. In each system, 10 red tilapias were introduced, with 5.37 ± 0.09 g initial body weight and 7.00 ± 0.04 cm length (4.4 kg m
−3).
Fish were fed ad libitum six days a week and two times a day (10:00 and 16:00) with a commercial fish feed that contained 47.5% crude protein, 6.5% crude fat, 2.0% crude fiber and 6.0% moisture (Tetra, Tetra discus granules, 2 mm). The daily feed consumption was calculated by weighing the amount of fish feed before and after daily meals (g day
−1). The feces was daily removed from the fish tanks by siphoning and the mechanical filter was daily cleaned with tap water. At the middle and end of the experiment, the fish were anesthetized with Tricaine methansulfonate (MS 222, 5 mg L
−1) to measure the exact weight and length of each individual and estimate the following growth parameters:
2.5. Spinach Cultivation
Spinach seeds were germinated in seed trays with soil and perlite (1:1, v/v) in a greenhouse. The seedlings with four true leaves were brought to the laboratory for a three-day acclimatization to the environmental conditions before the commencement of the experiment. During that period, the plants were irrigated with tap water without fertilization. In total, 45 plants (15 plants/treatment) with equal height and number of leaves were selected and randomly distributed among the nine aquaponic systems (28 plants m−2). Before the transplantation process, spinach roots were carefully washed with tap water to completely remove the soil without damaging the roots.
A floating sheet of polystyrene (2.5 cm thick) was placed in each hydroponic unit and hosted five net pots (Hydrofarm, 8.3 × 6.6 × 5.6) filled with lava grains (0.7 cm). The positions of the plants were carefully selected to ensure the homogeneity of the light environment and enough space for their growth (five plants per unit). At the top of the aquaponic systems, HPS lamps (Sylvania, 400 W) were placed to provide artificial light for plants with a mean photosynthetic photon flux density (PPFD) 427.56 ± 3.99 μmol m−2 s−1 (SKYE, PPFD meter). A timer was used to control the photoperiod, which was set at 10 h light:14 h dark. To avoid thermal stress, small fans were placed close to the hydroponic units to ensure optimal temperature for spinach growth, which was regularly checked with a thermocouple (CONSORT, T651) at the leaves level.
2.6. Plant Growth, Physiology and Biochemical Analysis
The experiment was designed to last until plants reached the marketable size, so at least a 45-day cultivation period was expected. However, the low performance of the control plants forced us to terminate their presence in the experiment, considering that this fact per se is an important result and describes the system bottlenecks. Thus, two harvests were conducted. The first one was at D20 (Day 20) when the control plants were harvested, whilst the Fe and Fe+K groups were robust and continued with the experiment. The final harvest of Fe and Fe+K was performed at D45.
2.6.1. Growth Performance
On D20 and D45, the diameter of the rosette and the total leaf area per plant were assessed for all plants. For this, photographs of plants with a reference of 1 cm were taken at the same height above each plant, and they were subsequently processed with image analysis performed by the free software ImageJ (Open-source software, ImageJ.net/ver. ImageJ 1.51j). At the same experimental dates, the leaf specific mass (LSM, g dm−2) was measured on 5 mature leaves per treatment. LSM was calculated from the dry mass (80 °C for 24 h) of leaf samples with known surface area. At D20, the control plants were harvested and the dry weight of their aerial part was measured (after 48 h at 80 °C). At this time-point, the aerial dry weight for the Fe and Fe+K groups was estimated from LSM and leaf area measurements for comparison with control. The final dry weight of leaves in Fe and Fe+K treatments at D45 was measured after the drying process described above.
2.6.2. Total chl Content
SPAD measurements of three leaves per plant (45 replicates/treatment) were carried out on a weekly basis using the portable chlmeter SPAD 502 (Konica Minolta, Tokyo, Japan). The equivalence of chl a+b content (μg cm
−2) was calculated via the equation of a standard curve of SPAD values against the actual chl concentration of the certain SPAD-measured leaf spots. The procedure for the latter included the cutting and extraction of leaf disks with an acetone solution (80%) and small amounts of calcium carbonate and pure sand. The samples were centrifuged (4000 rpm for 10 min), and the absorbance was read at 720, 663, 646, and 470 nm with a dual-beam spectrophotometer (SHIMADZU UV 1900 UV-VIS Spectrophotometer, Duisburg, Germany). The concentrations of chl a and chl b were calculated using the equations of Lichtenthaler and Wellburn [
20].
2.6.3. Photochemical Reflectance Index (PRI)
Leaf reflectance, measured in two narrow wavelength bands centered close to 531 nm and 570 nm, was used for PRI determination, which is calculated as PRI = (R
531nm − R
570nm)/(R
531nm + R
570nm), where R is the reflectance at each wavelength [
21]. The measurements were performed with a portable instrument PlantPen PRI 210 (Photon Systems Instruments, Drásov, Czech Republic) that directly recorded the PRI values. The measurements took place once a week on one mature leaf per plant (15 replicates/treatment) at two time points during the day: in the dark before lights turned on in the morning and at midday (five hours after lights went on). The data were extracted and processed with FluorPen Software (Photon Systems Instruments, Drásov, Czech Republic).
2.6.4. Fluorescence of chl a In Vivo
On a weekly basis, chl a in vivo fluorescence was monitored using a Handy PEA+ fluorimeter (Hansatech Instruments Ltd., King’s Lynn, UK) on one mature leaf per plant (15 replicates/treatment). The measurements were performed before the turning on of the lights in the morning to assess the fully dark-adapted state of PSII. The transients of chl
a fluorescence were recorded by illuminating the leaves with 3000 μmol photons m
−2 s
−1 for two sec; the excitation energy was provided by a red LED array and centered at 650 nm. The fluorescence signal was recorded at five time points (T1—50 μsecs, T2—100 μsecs, T3—300 μsecs, T4—2 msecs and T5—30 msecs). The OJIP transients were analyzed with PeaPlus Software v.1-13 (Hansatech Instruments Ltd., King’s Lynn, UK). The estimated parameters, derived according to the JIP test proposed by Strasser et al. [
22], are the following:
Initial value of the fluorescence, Fo = F50μs
Fluorescence values at 300 μs, 2 ms, and 30 ms: F300μs, FJ, FI
Maximum value of fluorescence, FM
Area between fluorescence induction (OJIP) curve to FM
Normalized total complementary area Sm = Area − FV
Variable chl fluorescence, FV = FM − Fο
Maximal quantum yield of PSII photochemistry FV/FM
Initial slope of relative variable chl fluorescence curve, Mo = 4 (F300μs − Fο)/(FM − Fο)
Relative variable fluorescence (point J), VJ = (F2ms − Fο)/(FM − Fο)
Relative variable fluorescence (point I), VI = (F30ms − Fο)/(FM − Fο)
Maximum quantum yield of primary photochemistry, φPo = TRο/ABS = FV/FM = 1 − Fο/FM
Quantum yield of electron transport to intermediate acceptors, φEo = ETο/ABS = φPo·ψEο = 1 − FJ/FM
Quantum yield of electron transport to final acceptors, φRo = φPο·ψEο·δRο = 1 − FI/FM
Probability that a trapped exciton moves an electron into the electron transport chain to intermediate acceptors, ψEο = ETο/TRο = 1 − VJ
Probability that a trapped exciton moves an electron into the electron transport chain from intermediate receptors to final acceptors of PSI, δRo = REο/ETο = (1 − VI)/(1 − VJ)
Yield of reactive centers of PSI, 1 − VI
Relative yield of final acceptors e− of PSI, 1/VI
Absorption flux (for PSII antenna chls) per reaction center (RC), ABS/RC = (Mo/VJ)·FM/(FM − Fo)
Trapped energy flux per RC, TRo/RC = Mo/VJ
Dissipated energy flux per RC, DIo/RC = (Mo/VJ)·(Fo/FV)
Index of total photosynthetic efficiency, PItotal = (RC/ABS)·(φPo/1 − φPo)·(ψEο/1 − ψEο)·(δRo/1 − δRo)
2.6.5. Light Response Curves (LCs)
The photosynthetic LCs were performed at ten-day intervals on six mature leaves per treatment. A portable photosynthesis system (LI-6400 XT, LI-COR, Lincoln, NE, USA) was used with an LED light source (6400-02B), attached on the top of the chamber to illuminate the samples. The dependence of net photosynthesis (A
n) on PPFD was measured at nine intensity steps (1200, 1000, 800, 600, 400, 200, 100, 50, and 0 μmol m
−2 s
−1), the duration of each step being 3 min. The conditions within the leaf chamber were kept constant during the measurements at 450 ppm CO
2 (using the 6400-01 CO
2 Injector) and a temperature of 22 °C. All LCs were performed from 9:00 to 12:00. The data were analyzed with a modified non-rectangular hyperbola for the estimation of maximum photosynthetic rate (A
max), the quantum yield of photosynthesis (ϕ, mole CO
2 per mole PPFD incident on the surface of the leaf), and for the dark respiration (Rd) [
23].
2.6.6. Spinach Nutrient Content
The analysis of leaf nutritional state was performed on D20 for the control group and at the final harvest (D45) for Fe and Fe+K treatments on three samples per treatment (one pooled sample from each aquaponic system). For the extraction, the samples were digested at 30 °C for two hours with a total solution volume of 4.4 mL, which contained 1.94 mL H
2SO
4, 2.82 mg Se, 82.13 mg Li
2SO
4, and 1.94 mL 30% H
2O
2 [
24]. Subsequently, when the samples reached room temperature, they were diluted with distilled water (50 mL) and analyzed with an ICP-OES spectrophotometer (SPECTRO Analytical Instruments GmbH, Kleve, Germany) for the determination of macronutrient (N, P, K, Ca, Mg expressed as % dry weight) and micronutrient (Fe, Zn, Mn, Cu expressed as ppm dry weight) content.
2.6.7. Antioxidant Activity
The antioxidant activity of spinach leaves was evaluated by means of total phenolic concentration and the DPPH assay. For the former, the Folin–Ciocalteu method was used [
25]. Dry samples pooled from five plants/aquaponic system, resulting in three replicates/treatment, were extracted in 6 mL 50% methanol through incubation in a water bath at 40 °C for 1 h. The 50 mL extracts reacted with the Folin–Ciocalteu reagent and sodium carbonate solution at room temperature for 2 h. The samples were measured photometrically (SHIMADZU UV 1900 UV-VIS Spectrophotometer, Duisburg, Germany) at 760 nm. A standard curve was used to transform absorbance readings to gallic acid equivalence per g leaf dry weight (g GAE g
−1).
The antioxidant activity was also estimated with the DPPH assay (2,2-diphenyl-1-picryhydrazyl) as an indicator of leaf radical scavenging activity. The protocol described by Goupy et al. [
26] and Hayes et al. [
27] was used with few modifications. In total, six samples per treatment were analyzed on the experimental days 20 and 45. Two-hundred-fifty milligrams of liquid-nitrogen-frozen spinach leaves were extracted in a mill with methanol (25 mL) for 30 s. Then, the samples were shaken at 1050 rpm in the dark for 20 min and, subsequently, were centrifuged at 2218×
g for 10 min. The supernatants were 1:1 diluted with methanol, and 2 mL of this sample was mixed with 2 mL of DPPH (freshly prepared, 100 μM). After 30 min of incubation in the dark, the mixture was measured at 517 nm against a control sample of methanol-DPPH with a double-beam spectrophotometer (SHIMADZU 1900 UV-VIS Spectrophotometer, Duisburg, Germany). The results of the assay were expressed as mg ascorbic acid g
−1 fresh weight of leaves, according to the equation of a standard curve prepared with various concentrations of ascorbic acid.
2.7. Statistical Analysis
The analysis of the data was performed with one-way ANOVA followed by Tukey’s post-hoc test after checking the homogeneity and normal distribution of samples with the Shapiro–Wilk and Levene’s tests. A non-parametric Kruskal–Wallis test, followed by a post-hoc Dunn’s test, was used in the cases when ANOVA prerequisites were not met. The significance level was set at p ≤ 0.05, and the statistical analyses were performed with the JASP v.0.16 software (JASP Team 2021 Computer Software).
3. Results
The weekly measurements of water nutrient concentrations are presented in
Table 1. The nitrate concentration was similar throughout the experiment in all aquaponic units and was close to 100 mg L
−1. As for the iron, no statistically significant differences were recorded between Fe and Fe+K groups, with the only exception being the lower Fe concentration on D28. On the contrary, the control group faced zero values of iron in the water already from the beginning until the harvest of this group on D20. As expected, the potassium content was low and similar in control and Fe groups, which had not received any K supplementation, unlike the Fe+K group where the enhanced K concentration was maintained almost stable at close to optimum.
Fish growth parameters were assessed at two time-points, on D20 and D45. Although control plants were harvested on D20 and, thus, removed from the aquaponics units, fish farming continued until the end of the experiment (
Table 2). Fish weight was doubled between the two measurement dates, and the calculated WG was almost 2.5-fold increased; however, no statistically significant differences were recorded among treatments. Additionally, fish length and daily food consumption were similar among treatments. High survival percentage, as well as high SGR and optimal FCR, were evident in all treatments and time points of assessment.
The growth performance of spinach plants is presented in
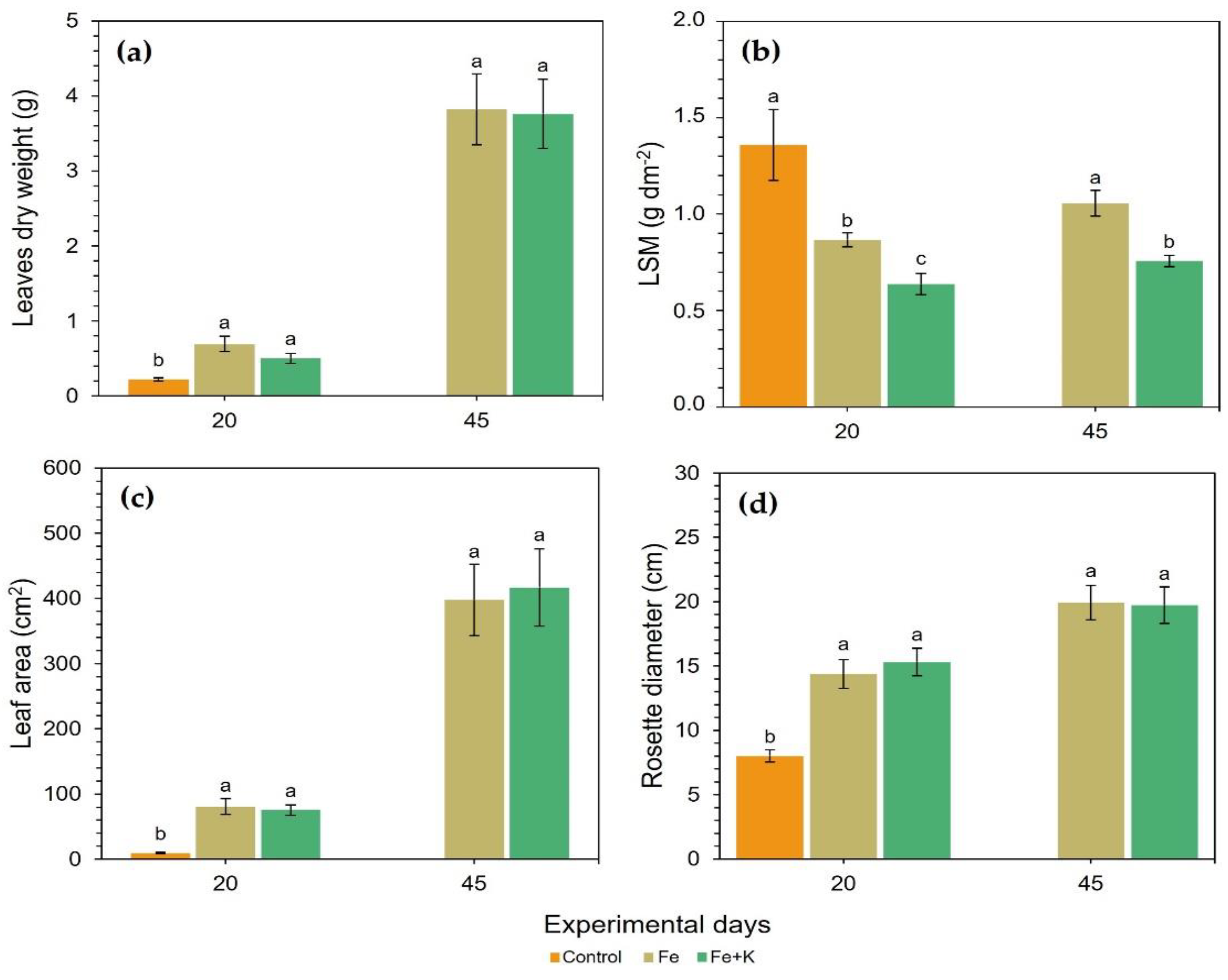
Figure 2 for both harvests. Control plants showed remarkable inferiority compared with the other two treatments in terms of dry aerial biomass accumulation, total leaf area, and rosette diameter, i.e., the expansion of plant in the horizontal axis. At D20, Fe and K supplementation increased the leaf area of spinach by 8.7 and 8.2 times for the treatments Fe and Fe+K, respectively, and doubled the rosette diameter in comparison with the control plants. In all the above-mentioned growth parameters, the Fe and Fe+K groups exhibited similar values. The LSM was the only leaf feature that differentiated all three treatments, with thicker leaves appearing in control plants, followed by Fe plants, while Fe+K group presented the lower LSM values; all these differences were statistically significant. The same pattern of between-treatments differences was followed at the final harvest at D45, where Fe maintained a significantly higher LSM compared to Fe+K, yet the value in each group did not change much after the D20.
The total chl content showed small but statistically significant differences among groups in the first measurement, with control plants outweighing all the others (
Figure 3a). However, extensive chlorosis of control plants appeared after the first week (D10, visual examination, and D14 in
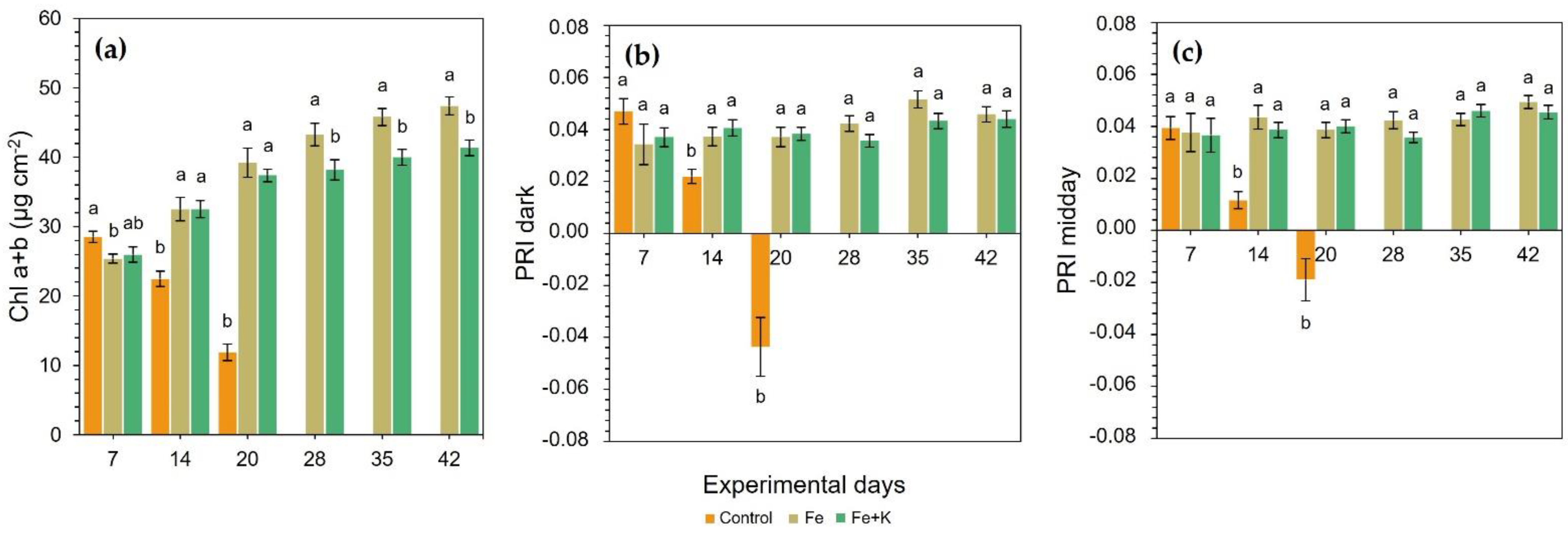
Figure 3a) and sharply deteriorated until D20, reaching 75% reduced chl content compared with Fe and Fe+K treatments. Due to this fact, the harvest of this group was deemed necessary at that time-point. From D28 until the end of the experiment, the Fe group significantly outperformed Fe+K plants in the concentration of total chl.
A similar downward trend with chl was also recorded in both dark and mid-day PRI for control plants (
Figure 3b,c). An early and significant reduction was noticed on D14, and moreover, negative RPI values were obtained in dark and midday PRI on D20, just before the harvest of this group. The Fe and Fe+K treatments sustained stable and similar PRI values throughout the experiment.
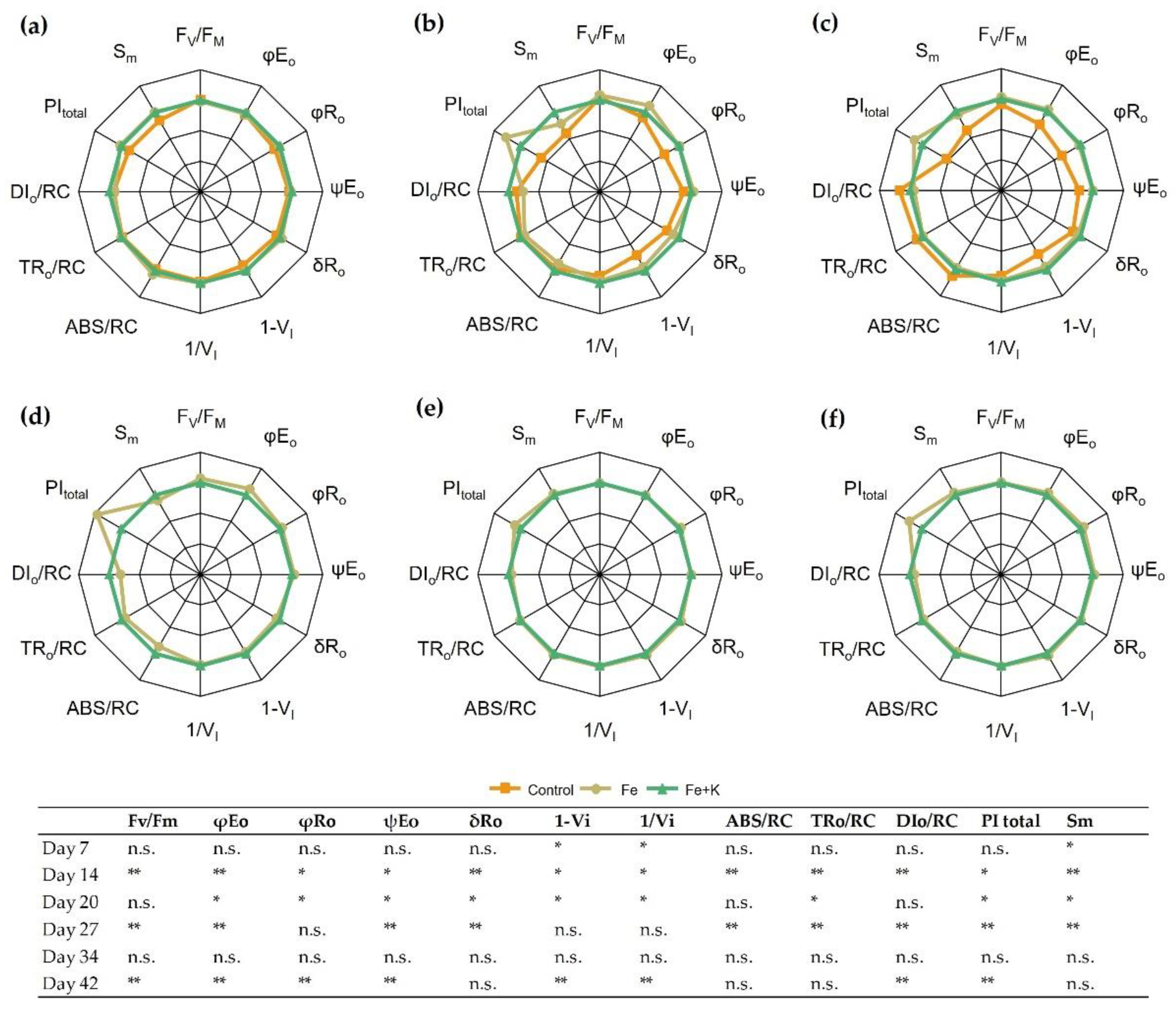
The parameters of in vivo chl a fluorescence in the dark-adapted state are presented in
Figure 4. During the first week of cultivation (D7), the control treatment resulted in lower values in the PSI-related parameters, such as the yield of reactive centers (1−V
I) and the relative yield of final e
− acceptors (1/V
I). On D14, the negative effects of the control treatment were obvious in all fluorescence parameters. Statistically significant decreases were evident in the quantum yield of electron transport to intermediate acceptors (φE
o) and to the final acceptors (φR
o), whose kinetics relate to PSII. Furthermore, parameters such as the probability that a trapped exciton moves an electron into the electron transport chain to intermediate acceptors (ψE
ο) and from intermediate to final acceptors of PSI (δR
o) showed a significant inferiority in the control spinach compared with the other two treatments. In addition, the relative pool size of total electron carriers (S
m) was 3.2 and 3.7 times lower in the control group than Fe and Fe+K plants, respectively. On D20, the picture was complemented with increases in trapped (TR
o) and dissipated (DI
o) energy per reaction center in control plants. In the second half of the experiment, the Fe group exhibited a higher photosynthetic efficiency (PI
total) over the Fe+K group, mainly due to higher quantum yield of electron transport, as depicted in φE
o, φR
o, and ψE
o in the final measurement (D42).
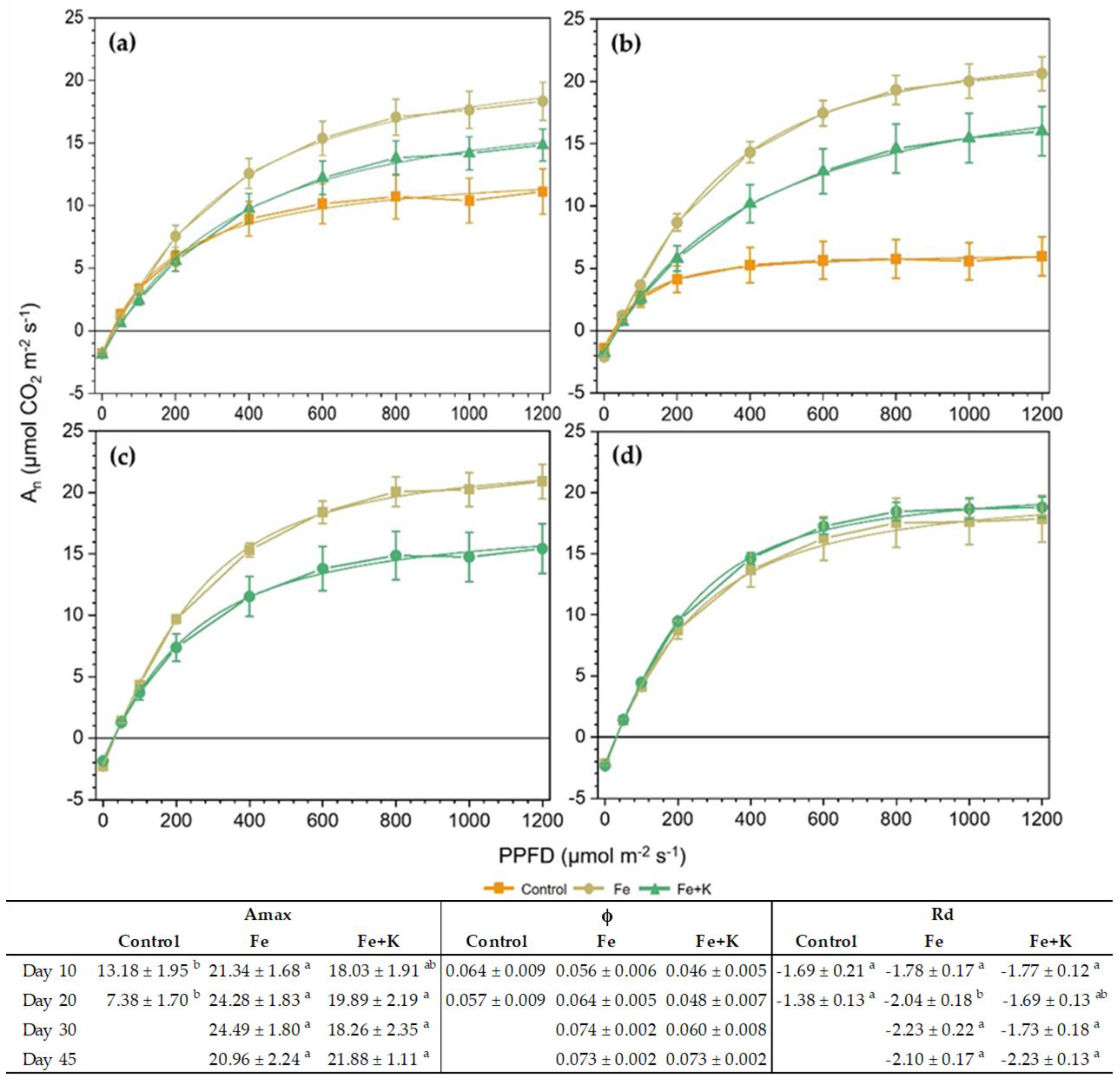
The light-response curves taken on a ten-day basis are presented in
Figure 5. An early indication of the photosynthetic performance inferiority of control plants appeared after ten days of cultivation and was depicted in significantly lower Amax (
Figure 5a) compared to the Fe group. The differences were maximized in D20, where 3.3- and 2.7-times lower Amax was recorded in Control compared with the Fe and Fe+K groups, respectively, combined with significantly decreased Rd. At this date, the Amax of the control plants was almost half its value at D10 and, interestingly, was achieved at low PAR, i.e., already from 200 μmol m
−2 s
−1. Concerning the differences between Fe and Fe+K, the former group showed a non-significant but clear trend for enhanced Amax and Rd, which was eliminated at the final measurement of D45. Both spinach groups maintained high and almost stable values of their photosynthetic response to light throughout the experimental period. The quantum yield of photosynthesis (ϕ) extracted from these curves showed no significant differences among treatments in all measurements.
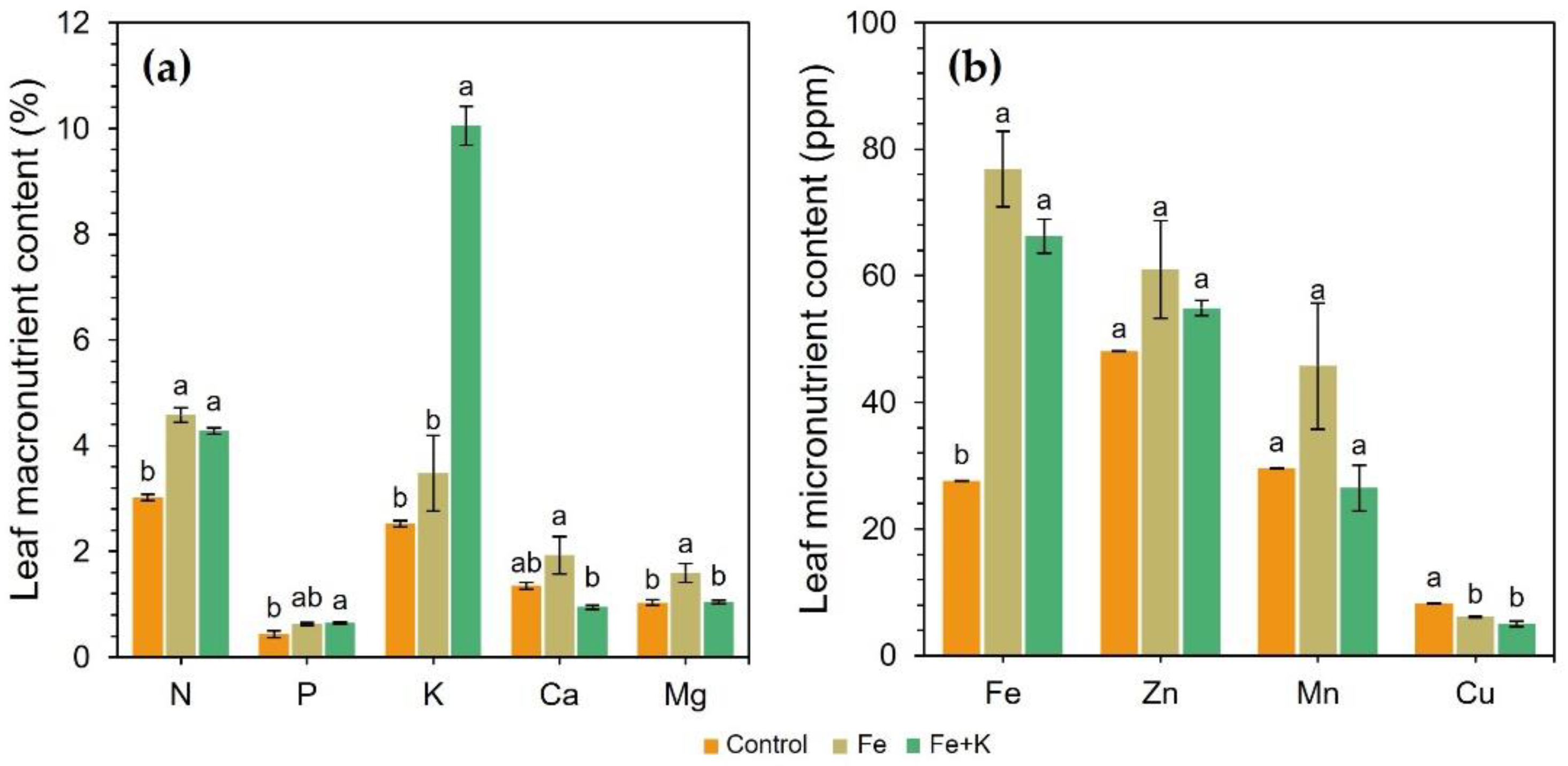
The nutrient composition of the spinach leaves is presented in
Figure 6. Control treatment caused a severe deterioration of spinach nutritional state in terms of N, P, K, Fe, and Zn concentrations. In the remaining nutrients, the control and Fe+K plants showed similar contents. Only leaf K and Mg content statistically differed between the Fe and Fe+K groups. Fe+K-treated plants had 4.0 and 2.9 times more K than the Control and Fe groups, yet significantly less Mg than Fe-treated plants. The latter plant group also showed a non-significant trend for higher concentrations of Ca, Zn, and Mn compared to the other two treatments. The Fe addition in both corresponding groups resulted in a 2.8- and 2.4-times increased iron uptake compared to control.
Spinach antioxidant activity showed that Fe and Fe+K plants differed in terms of total phenolic content at the final harvest (D45) (
Table 3). Prior to this point, total phenolic concentrations were comparable across treatments. In addition, the ascorbic acid equivalents resulting from the DPPH assay was stable during the experiment and similar among treatments.
4. Discussion
One of the principal advantages of the aquaponic systems is that the demand for nitrogen and phosphorus by plants can be met exclusively with nutrients derived from fish farming due to the water recycling process [
28]. The available amount of these nutrients for plant absorption is related to the number of reared fish, crop species, input rate, and quantity of fish feed [
29]. In aquaponic systems, fish metabolic wastes also contain nutrients such as Fe and K that could be utilized by plants; however, the concentrations are significantly lower and unbalanced compared to hydroponic systems and, thus, inadequate to support optimal plant growth [
30]. In the current study, the transformation of ammonia to nitrate concentration fluctuated close to 100 mg L
−1 and was equal among the treatments and almost stable throughout the experiment. A sufficient NO
3 concentration in the water solution for spinach in aquaponics varies from 80 to 200 mg L
−1 [
31,
32]. The same concentration of nitrate for aquaponic spinach cultivation was recorded by Petrea et al. [
33]. In their work, quick downward and upward trends in NO
3-N concentration were recorded and related to plant absorption differential rates, yield of nitrifying bacteria within the biological filter, and quantity of fish feed administered. In the present study, Fe and K concentrations in nutrient solution were maintained close to the target values in the corresponding treatments to meet the plant’s needs, while control plants faced almost zero Fe concentrations. This is in accordance with many aquaponics-relevant works, evidencing that close to zero Fe levels is an important bottleneck of this cultivation system, especially for leafy greens such as spinach, rocket, and lettuce [
15,
17,
34].
Tilapia growth performance did not change with further supplementation of Fe and K, and a high survival rate (97–100%) was recorded for all the treatments. The benefit of using the tilapia species in aquaponics lies on its adaptability to a wide range of abiotic conditions and the maintenance of high survival rates even with large fertilizer inputs [
35,
36,
37]. In the present study, an initial fish stocking density of 4.4 kg m
−3 was selected to meet the plants’ needs, and the calculated daily fish feed input per growing area during the experiment varied from 18 to 32 g m
−2. According to Lennard [
38], an amount of 13 g of fish feed m
−2 day
−1 for tilapia covers the nutrition needs for lettuce at a cultivation density of 25 plants m
−2. As for the SGR of the current work, the values varied from 3.4 to 4.7% day
−1, and the higher values were obtained during the first half (0–20 days) of the experiment. These SGRs were higher than those of Rayhan et al. [
39] (2.6–3.7% day
−1) for juvenile tilapia reared under different stocking densities in aquaponics with Indian spinach (
Basella alba). Stathopoulou et al. [
34], working with Fe and K supplementation in a rocket–tilapia system, documented that inputs have no effects on fish survival and growth, while causing no remarkable histological alterations.
The growth and functional responses of spinach to the three treatments were directly related to the nutritional state of leaves, thus, they will be hereafter discussed in combination. The close to zero levels of iron in association with low levels of potassium in the water had a negative effect on control spinach growth. After a 10-day cultivation period, plants showed interveinal chlorosis in the youngest leaves, while necrosis of leaf tissues was observed a few days later. These symptoms are indicative of iron deficiency [
30,
40], the severity of which was confirmed by tissue elemental analysis. The latter also revealed significantly decreased N, P, K, and Zn concentration in control leaves compared with the other two treatments. Subsequently, the early harvest of this group was deemed necessary since plants showed no signs of recovery. The extensive nutrient stress in the control spinach caused a remarkable deterioration of all growth parameters assessed on D20. The accumulation of biomass in leaves, rosette diameter, and total leaf area were significantly lower in control plants, reaching an 8-fold decrease in the latter parameter compared with the other plant groups. On the contrary, the control treatment resulted in increased LSM, denoting thicker leaves and a more compact mesophyll. It is well documented that changes in the investment of biomass per unit area may be driven by nutrient stress and are mainly ascribed to alterations in leaf density [
41]. Under low nutrient availability at levels that can restrict growth, the arrestment of cell expansion results in smaller leaves and higher LSM [
42]. Lettuce growth response in a similar experimental setup corroborates the findings of the present study, since a two-fold decrease in the final yield of zero-input control compared to the Fe+K group was reported [
15]. A recent work by Levine and Mattson [
43] showed that the addition of K at a range of 58–244 mg L
−1 in hydroponics favored the fresh weight, leaf surface area, and specific leaf area of spinach in comparison with zero levels of this element. Furthermore, the use of special feed additives tailored for aquaponics is being studied to meet the needs of plant nutrition and to provide optimal nutrition to the cultured aquatic animals [
44]. For example, using fish feed with iron–amino acid complex supplementation for Nile tilapia, goldfish, and African catfish maximized spinach growth performance, and the optimal Fe content was 20–30 g Fe kg
−1 fish feed, with its concentration in the nutrient solution fluctuating from 2.24 to 2.90 mg L
−1 [
45].
The extensive chlorosis of control plants, obvious already from D14, was deteriorated on D20. The sharply decreasing content of total chl may be directly attributed to nutrient deficiency, especially Fe, N, and Mg [
46,
47]. A linear relationship between leaf Fe and chl concentration was established in hydroponics [
48], probably arising from the fact that Fe is a constituent of enzymes participating in the chl biosynthetic pathway. It seems that the time-point of D20 is crucial in shaping the chl biosynthesis/degradation course in no external input aquaponics, since a similar response was reported for lettuce [
15] and rocket (Tsoumalakou, unpublished data [
49]) grown in analogous supplementation schemes. Low chl levels cannot sustain efficient photosynthetic rates, inducing decrease in light-use efficiency (LUE), i.e., the efficiency with which the absorbed photosynthetically active radiation is used to produce biomass [
50]. In the control spinach leaves, the above-mentioned relationship was confirmed by the pronounced downtrend of PRI, which directly correlates with LUE, often used as its predictor [
50]. Apart from constantly lower values of PRI in the control compared with the other two plant groups, negative values were also recorded in both dark and midday measurements of D20. This result indicates a severe and chronic stress imposed in photosynthetic LUE that cannot be recovered during the night, probably because it is a combined result of both reduced chl content and functional impairment of the photosynthetic apparatus. The latter is confirmed by gas exchange and fluorescence measurements discussed below and also by the evidenced positive correlation of PRI with relative photosynthetic rates [
51], quantum yield of photosystem II, as well as with its negative correlation with non-photochemical quenching [
52]. Fe and Fe+K supplementation sustained stable and similar PRI values throughout the experiment, though Fe treatment significantly favored total chl concentration in the second half of the experiment, which was depicted in the plants’ photosynthetic performance.
The chl
a in vivo fluorescence measurement revealed a pronounced functional impairment of the photosynthetic apparatus of nutrient-deficient control plants, apparent from D14. PSII photochemistry was severely affected, resulting in significantly lower quantum yields, efficiency of electron transport, and energy fluxes in comparison with Fe and Fe+K supplementation treatments. The decreased quantum yield of electron transport along the route to final acceptors may be correlated to the 3-fold reduced relative pool size of total electron carriers (S
m) assessed in the control compared to Fe and Fe+K groups. The time-course of the above-mentioned reductions corresponds to increasing limitations in electron flow along PSII, a fact that also holds for PSI-related events. Interestingly, a previous work of our group with lettuce in the same experimental setup shows that PSI of control lettuce plants remains unaffected by nutrient deficiency, unlike PSII, which suffers similar down-regulation with spinach [
15]. In the present study, limitations to photochemistry collectively resulted in decreased total photosynthetic efficiency, as described by the index PI
total in the control spinach. On the contrary, Fe and Fe+K supplementation induced an up-regulation of PSII activity, as reflected in all fluorescence parameters throughout the experiment. However, it must be highlighted here that Fe-treated plants outperformed Fe+K in term of PI
total, thus, succeeding in enhanced photochemical performance. The inferiority of the control plants in photochemical efficiency may be partly explained by early chl loss, as also suggested by Jardim et al. [
53], who connected the lower effective and maximum quantum yield of PSII and enhanced non-photochemical quenching with decreased chl levels and leaf K content. Additionally, the poor photochemical efficiency of the control may be related with the well documented suboptimal development of the photosynthetic apparatus under nutrient deficiency, which affects both photosystems [
46]. In parallel, photoinhibitory damage to PSII due to Fe and K deficiency may also play a role [
46,
47], as was clearly depicted in the light-response curves of photosynthesis. Finally, K, Fe and Mn deficiencies were considered the cause of significant reductions in maximum yield of PSII photochemistry in aquaponics-grown basil compared with hydroponics in an early work of Roosta [
54].
The photosynthetic LCs give valuable information about the photosynthetic performance and capacity of the plant at a given time-point of the experiment, reflecting the treatment impacts not only on the instantaneous gas exchange but also on the state and potential of the photosynthetic machinery [
55]. LCs performed during spinach cultivation captured the change in photosynthetic response, i.e., acclimation, to various nutrient environments. Fe-treated plants showed the superior photosynthetic performance throughout the experiment, yet without statistically significant differences from Fe+K group. Contrarily, the overall picture of the control plants illustrated the poor performance and potential of photosynthesis. In this way, LCs delineated and incorporated the adverse effects of nutrient deficiency on the state of photosynthetic apparatus, shown by fluorescence data, and on chl content. Several studies have demonstrated the Fe-deficiency-imposed reductions of net photosynthesis, stomatal conductance, and transpiration rate [
15,
48]. Additionally, the down-regulation of ribulose-1,5-bisphosphate carboxylase (Rubisco) and decreases in cytochrome
b6/
f are linked to iron deficiency in leaves [
48,
56].
The antioxidant activity, as depicted in leaf total phenolic content and DPPH scavenging capacity of control plants, remained at the same levels with the other two treatments at D20, although the detrimental effects on photosynthetic function and growth were obvious. Similar results were reported by Jin et al. [
57] for spinach, which showed comparative levels of phenolics and DPPH scavenging activity under mild Fe-deficient and Fe-sufficient treatments. Nevertheless, the accumulation of reactive oxygen species and the subsequent oxidative damage is well evidenced under Fe- and K-deficiency and, moreover, exacerbated by the prolonged exposure to stress [
58]. In the present work, the only significant difference was found in the final harvest, where K supplementation reduced the phenolic content of spinach leaves. Our results do not agree with the unchanged polyphenolic content under severe K-deprivation found in peanut [
58]. An increase in Fe concentration of the hydroponic nutrient solution from 2 to 5–15 mg L
−1 leads to an increase in total phenolic content in the “Corvair” spinach variety [
59]; however, in our case. both Fe and Fe+K treatments received the same Fe inputs.