Analysis of Changes in Herbaceous Peony Growth and Soil Microbial Diversity in Different Growing and Replanting Years Based on High-Throughput Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling Selection
2.2. Determination of Plant Morphological Indices Root Activity
2.3. Determination of Soil Physicochemical Properties
2.4. Determination of Soil Enzyme Activities
2.5. DNA Extraction and PCR Amplification
2.6. Illumina Miseq Sequencing and Data Processing
2.7. Statistical Analysis
3. Results
3.1. Changes in Morphological Indices
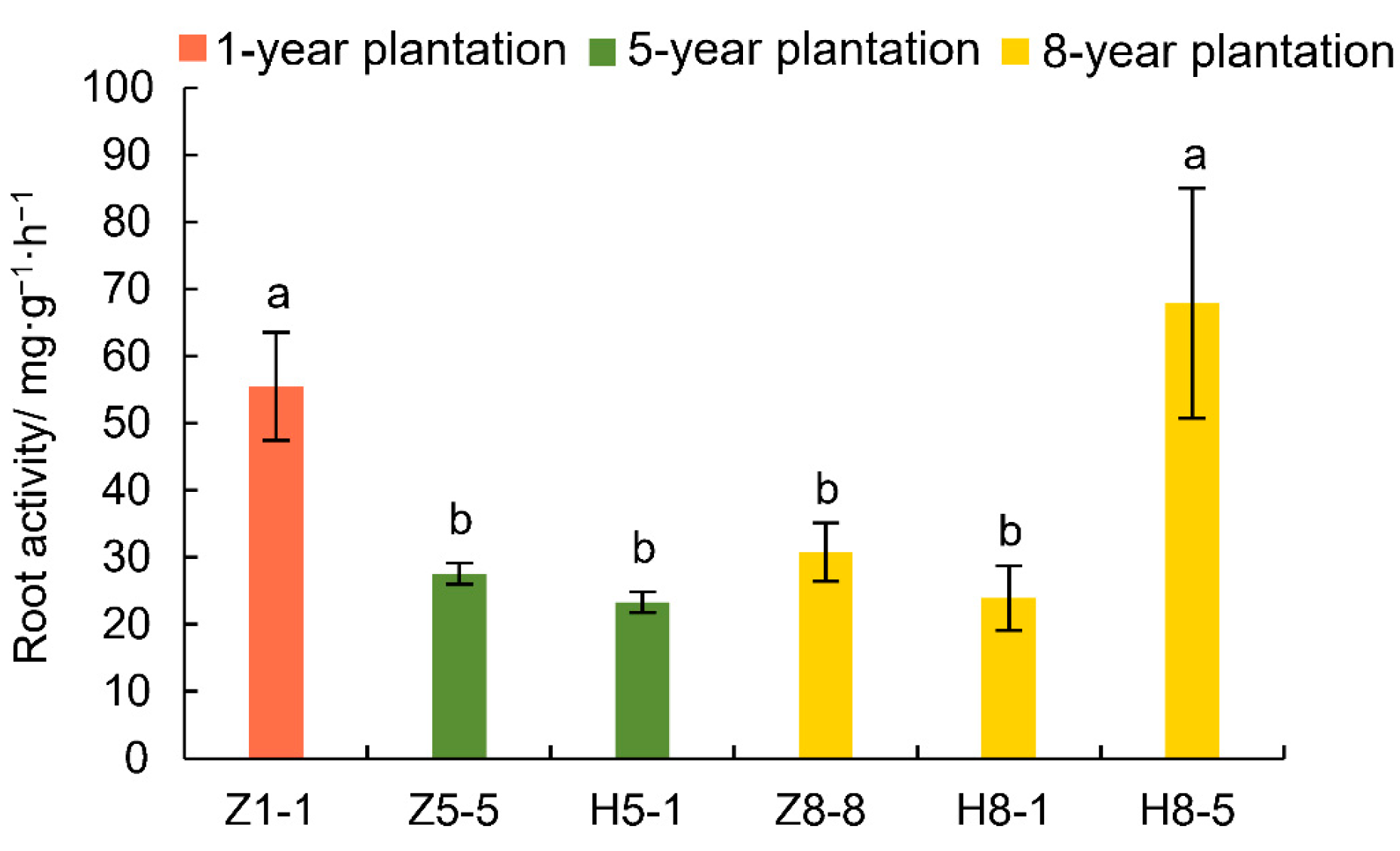
3.2. Changes in Root Activity
3.3. Changes in Soil Physical and Chemical Properties
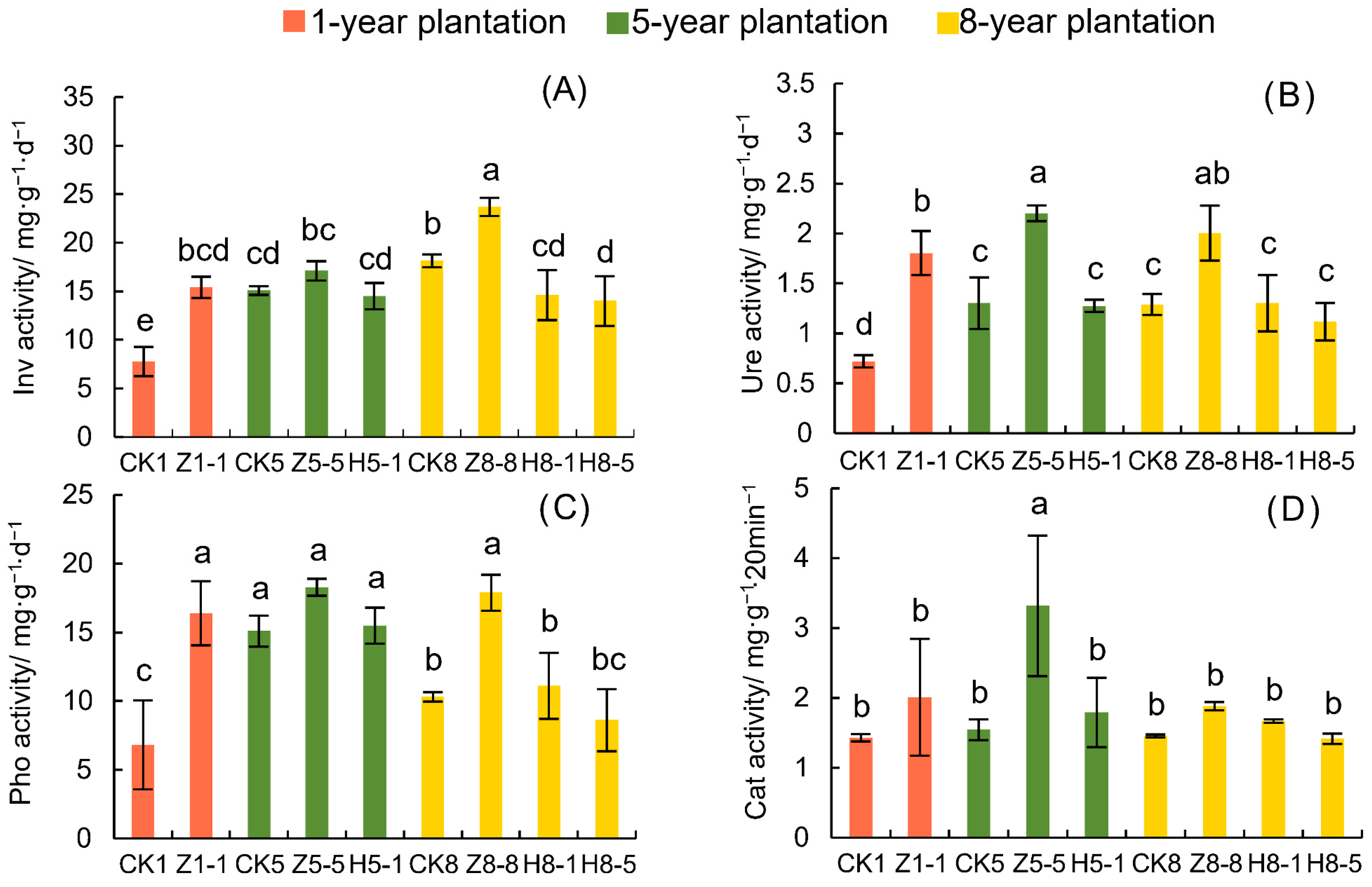
3.4. Changes in Soil Enzyme Activity
3.5. Changes in the Soil Microbial Community
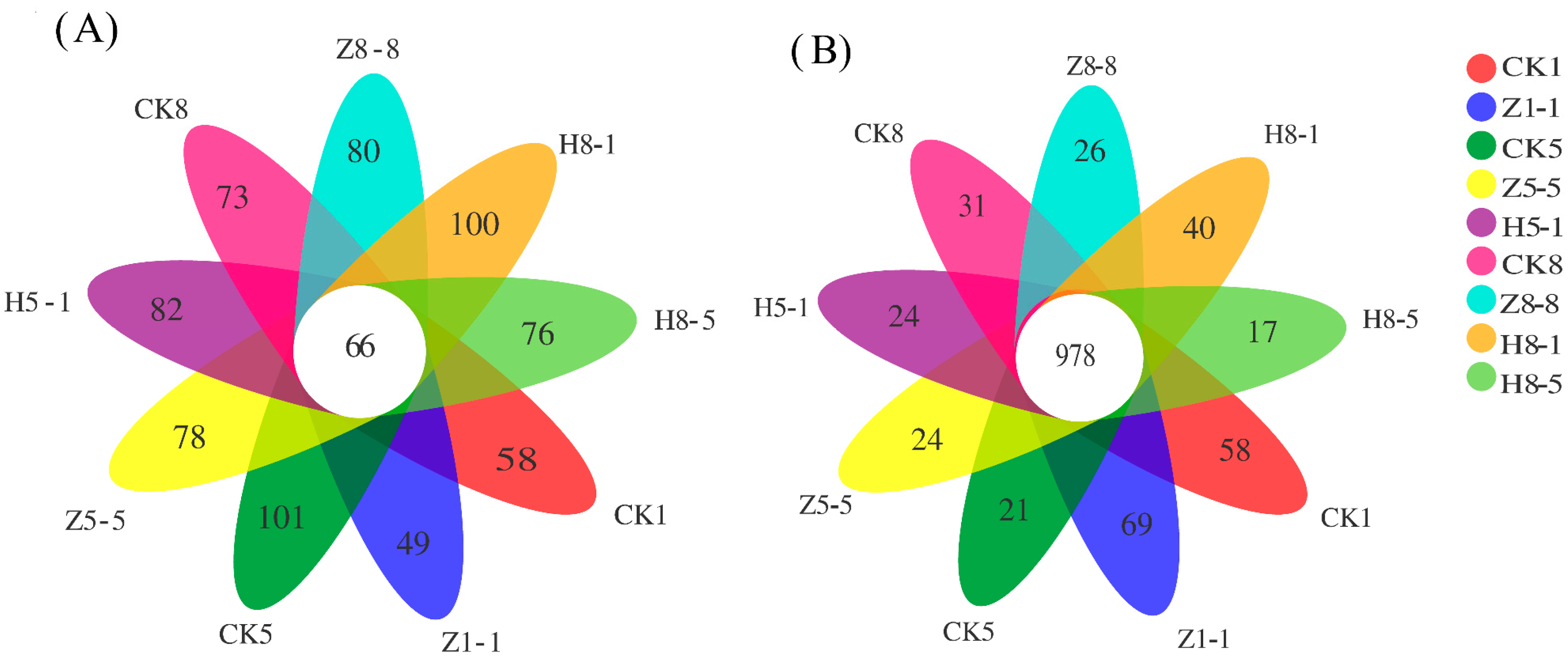
3.5.1. Changes in the Quantity of OTUs in the Soil Microbial Community
3.5.2. Changes in the α-Diversity Indices of Soil Microbial Communities
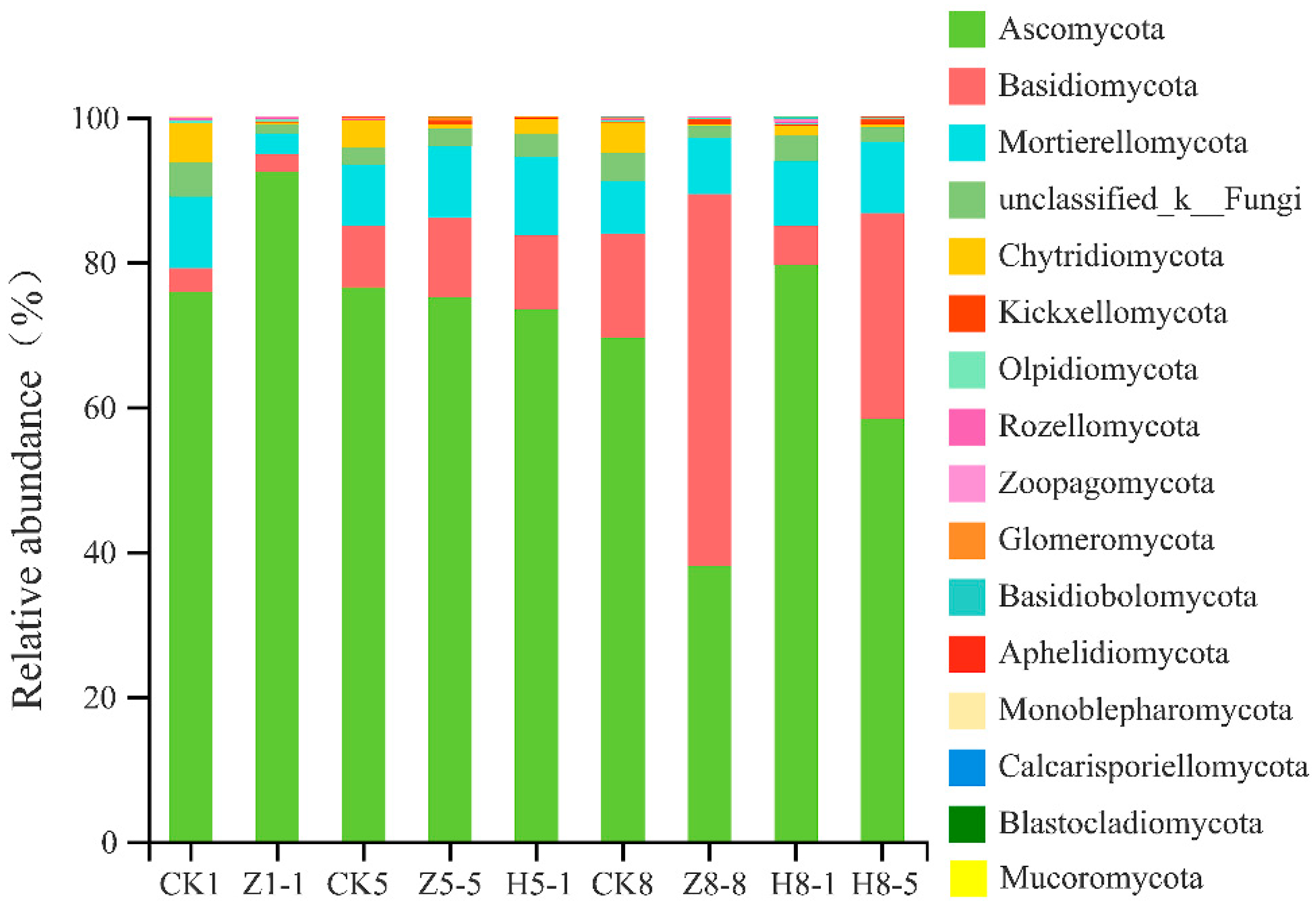
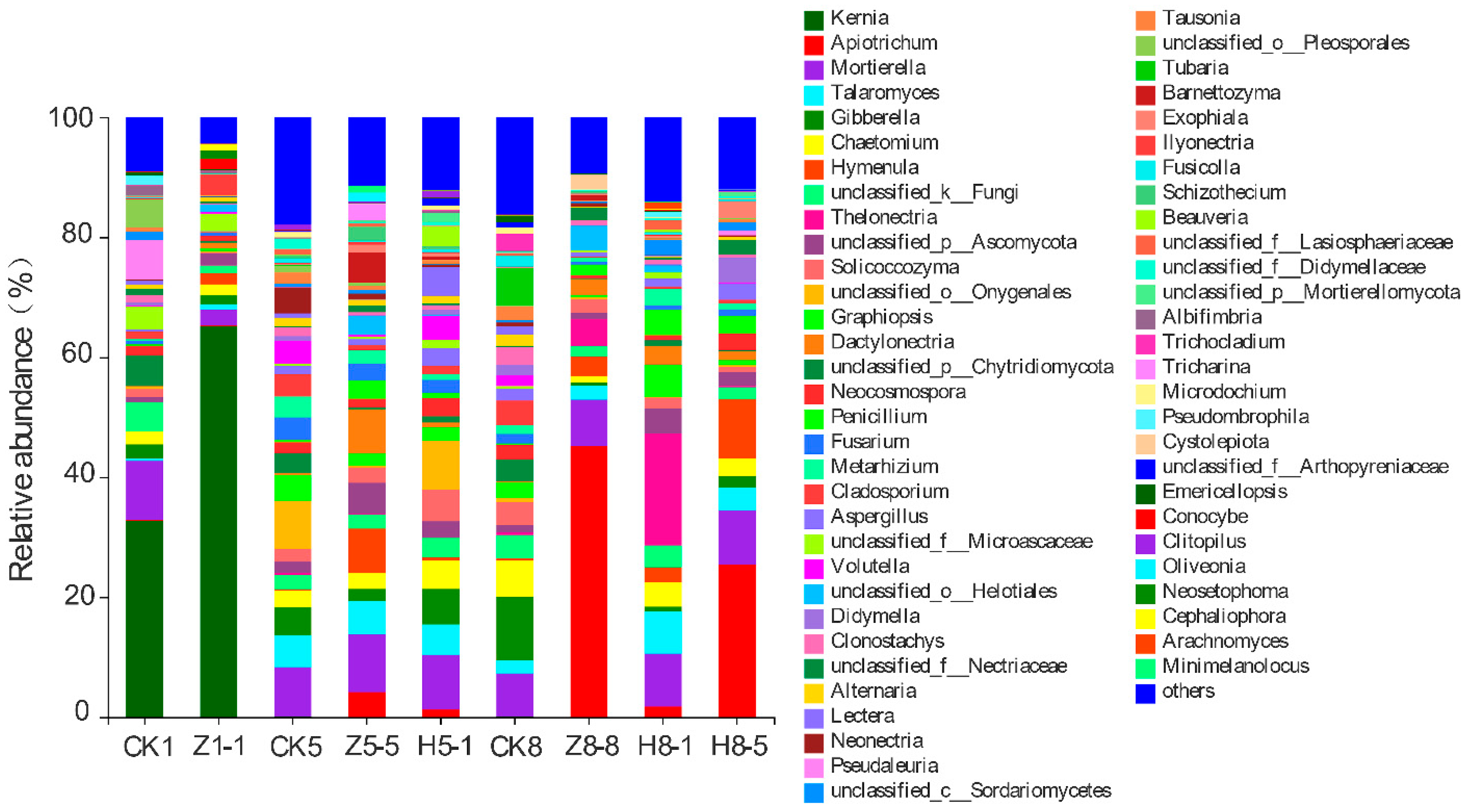
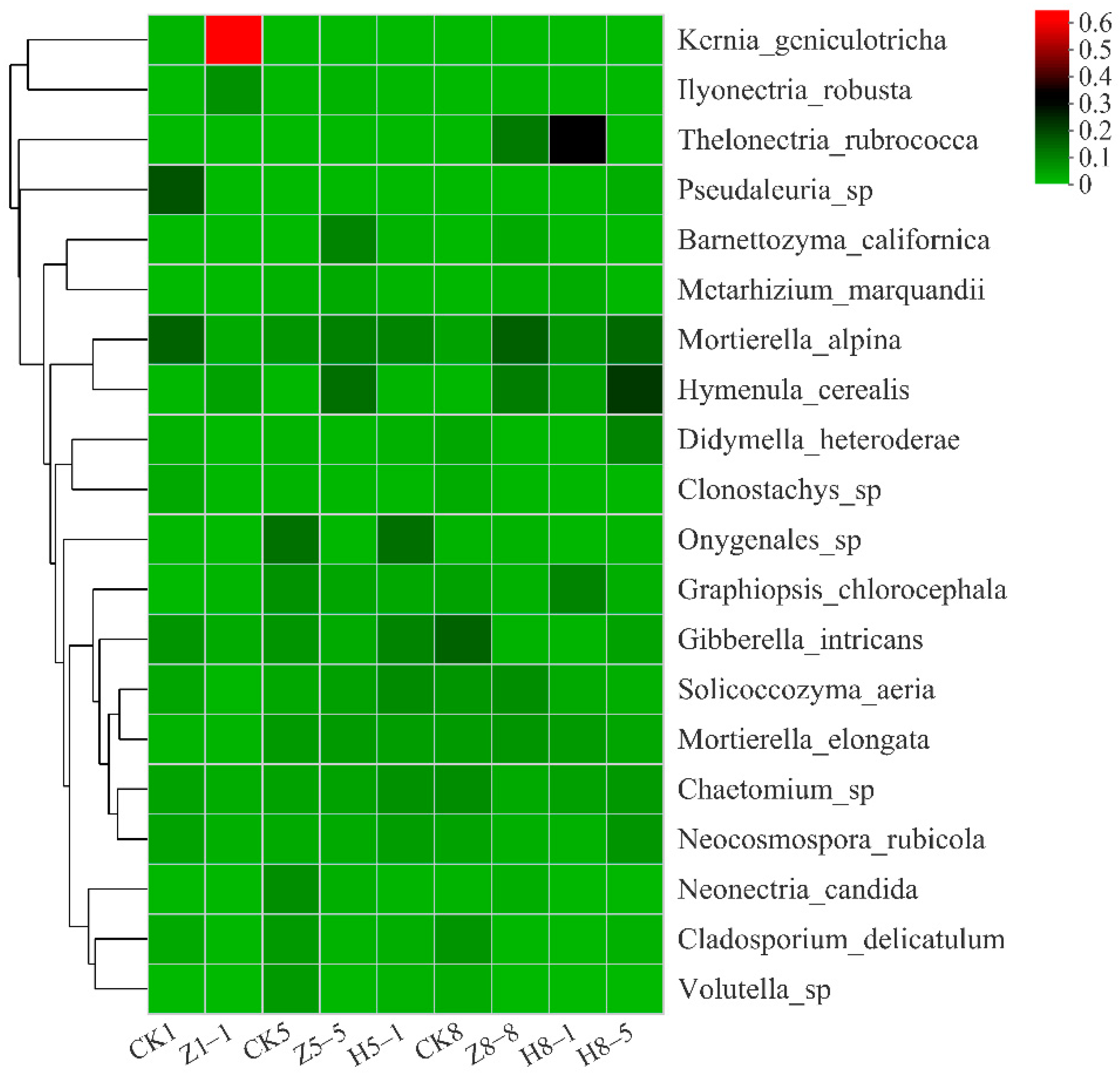
3.5.3. Changes in Soil Fungal Community Composition
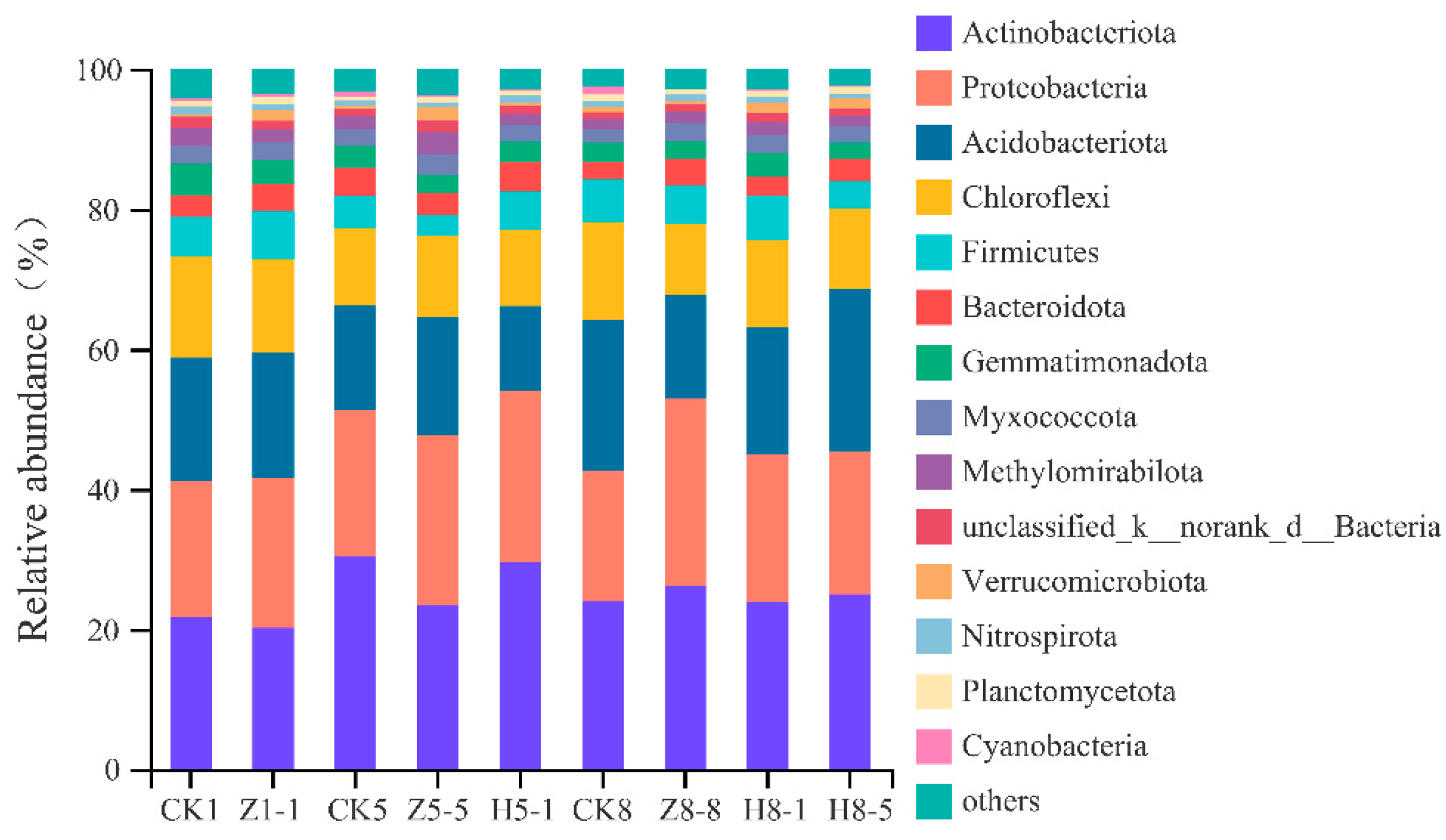
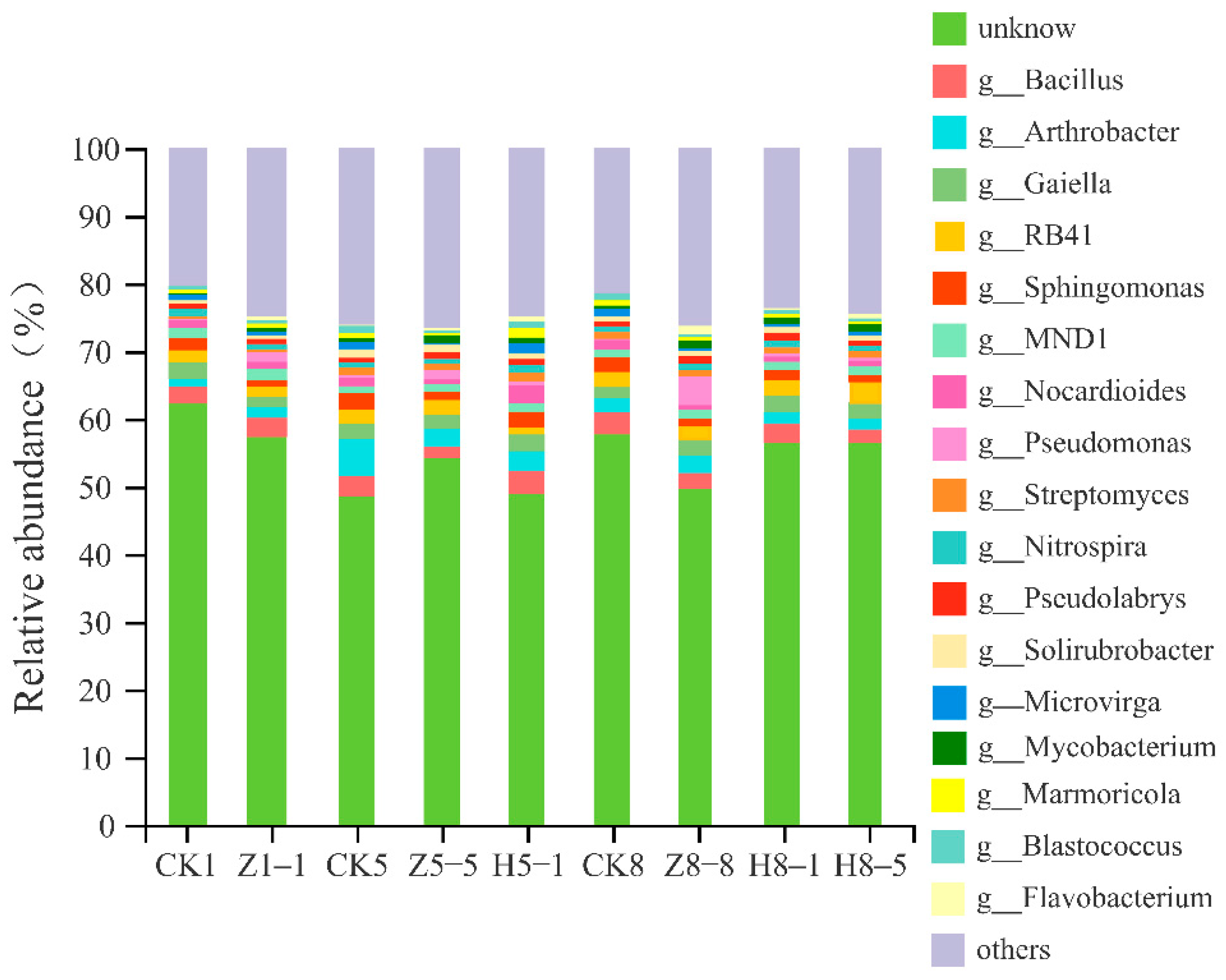
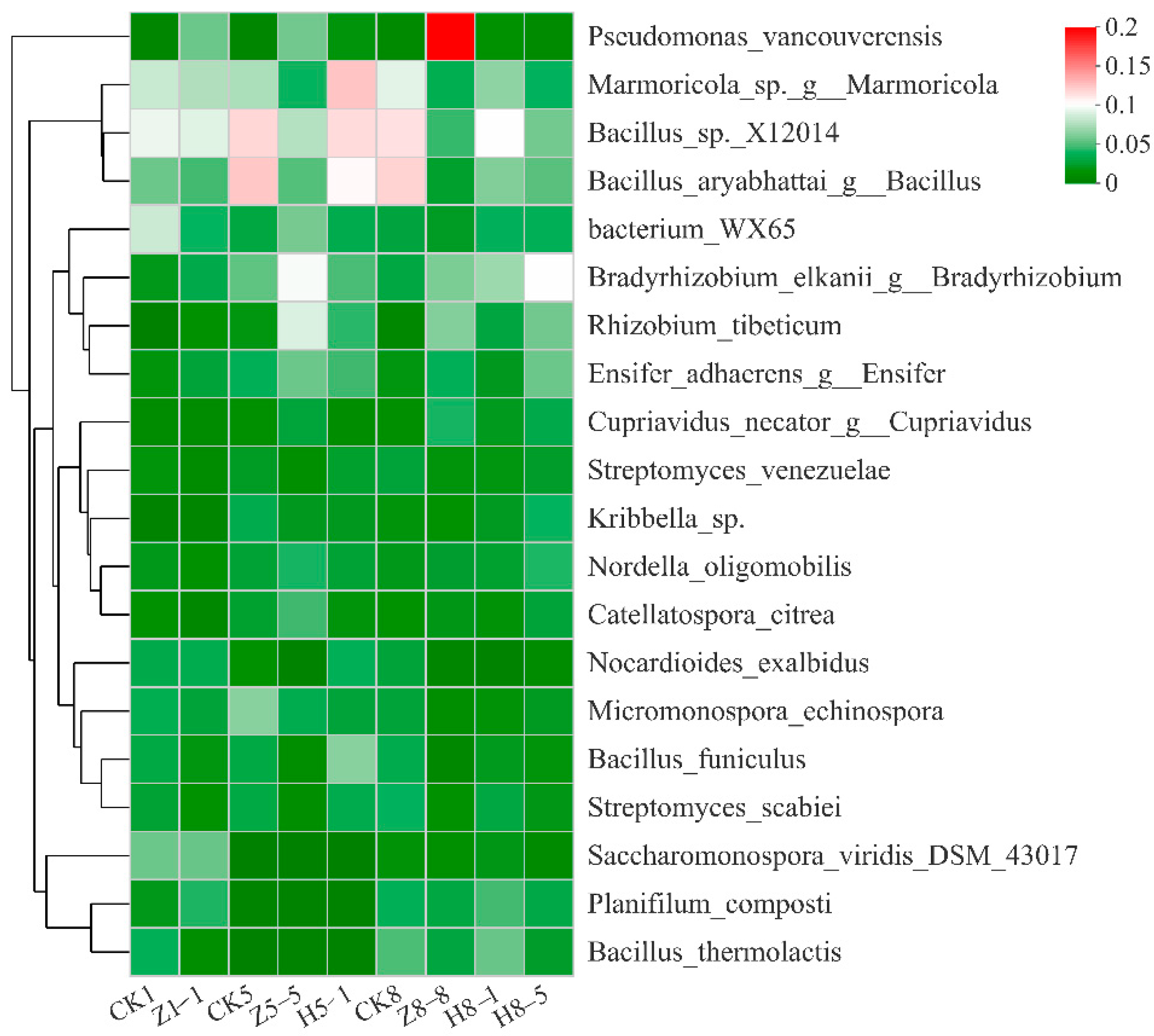
3.5.4. Changes in Soil Bacterial Community Composition
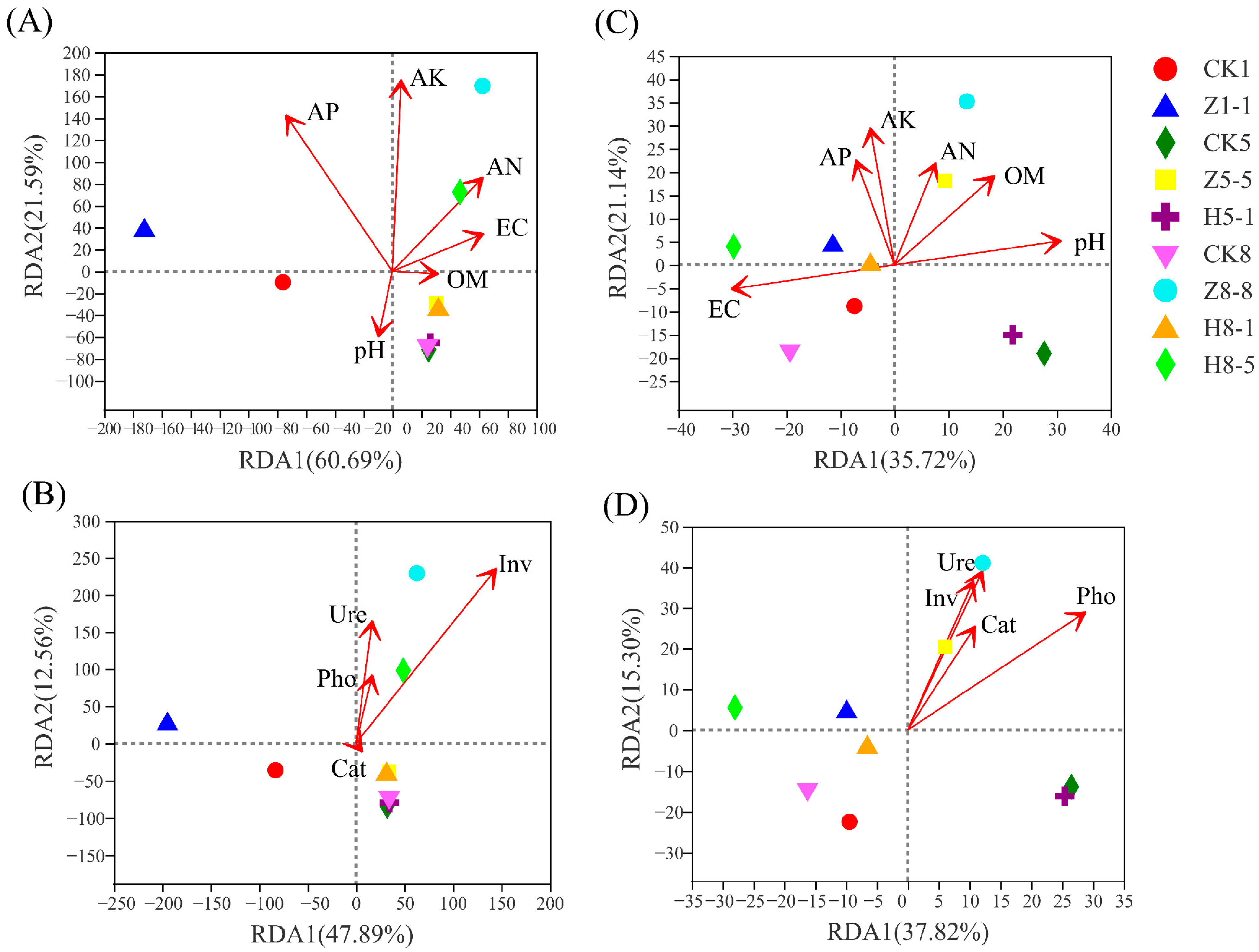
3.5.5. Correlation between Soil Microbial Community and Soil Environmental Factors
4. Discussion
4.1. Herbaceous Peony Growth and Development
4.2. Effects of Different Growing and Replanting Years on Soil Environment
4.3. Effects of Different Growing and Replanting Years on Soil Microbial Adiversity
4.4. Effects of Different Growing and Replanting Years on Soil Fungal Community Composition
4.5. Effects of Different Growing and Replanting Years on Soil Bacterial Community Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, X.-N. Herbaceous Peoniesies; China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Yang, Y. The Japanese Grow Peony Like This—A Visit to the Peony Industry in Japan (I). Available online: https://mp.weixin.qq.com/s/HC0kABcyzumiqbJIOxoD3Q (accessed on 15 December 2020).
- Sun, X. Appreciation of Chinese Peony Varieties; China Forestry Publishing House: Beijing, China, 2019. [Google Scholar]
- Xie, A.-Q.; Sun, L.-M.; Zhang, D.-L.; Li, Y.; Liu, Z.-M.; Li, X.; Sun, X. Changes in the root system of the herbaceous peony and soil properties under different years of continuous planting and replanting. Hortic. Plant J. 2021; in press. [Google Scholar] [CrossRef]
- Grunewaldt-Stöcker, G.; Mahnkopp, F.; Popp, C.; Maiss, E.; Winkelmann, T. Diagnosis of apple replant disease (ARD): Microscopic evidence of early symptoms in fine roots of different apple rootstock genotypes. Sci. Hortic. 2019, 243, 583–594. [Google Scholar] [CrossRef]
- Shi, G.Y.; Sun, H.Q.; Calderon-Urrea, A.; Jia, X.X.; Yang, H.Y.; Su, G.L. Soil Fungal Diversity Loss and Appearance of Specific Fungal Pathogenic Communities Associated With the Consecutive Replant Problem (CRP) in Lily. Front. Microbiol. 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Westphal, A.; Browne, G.T.; Schneider, S. Evidence for biological nature of the grape replant problem in California. Plant Soil 2002, 242, 197–203. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.C.; Chen, L.S. Impact of monoculture of poplar on rhizosphere microbial communities over time. Pedosphere 2020, 30, 487–495. [Google Scholar] [CrossRef]
- Wei, X.; Wang, X.; Cao, P.; Gao, Z.; Chen, A.; Jianping, H. Microbial Community Changes in the Rhizosphere Soil of Healthy and Rusty Panax ginseng and Discovery of Pivotal Fungal Genera Associated with Rusty Roots. BioMed Res. Int. 2020, 2020, 8018525. [Google Scholar] [CrossRef]
- Larkin, R.P. Relative effects of biological amendments and crop rotations on soil microbial communities and soilborne diseases of potato. Soil Biol. Biochem. 2008, 40, 1341–1351. [Google Scholar] [CrossRef]
- Wu, L.K.; Wu, H.M.; Chen, J.; Wang, J.Y.; Lin, W.X. Microbial community structure and its temporal changes in Rehmannia glutinosa rhizospheric soils monocultured for different years. Eur. J. Soil Biol. 2016, 72, 1–5. [Google Scholar] [CrossRef]
- Hua, C.-p.; Xie, Z.-k.; Wu, Z.; Zhang, Y.-b.; Guo, Z.-h.; Qiu, Y.; Wang, L.; Wang, Y.-j. The Physiological and Biochemical Effects of Phthalic Acids and the Changes of Rhizosphere Fungi Diversity under Continuous Cropping of Lanzhou Lily (Lilium davidii var. unicolor). HortScience 2019, 54, 253–261. [Google Scholar] [CrossRef]
- Zhou, X.G.; Liu, J.; Wu, F.Z. Soil microbial communities in cucumber monoculture and rotation systems and their feedback effects on cucumber seedling growth. Plant Soil 2017, 415, 507–520. [Google Scholar] [CrossRef]
- Liu, Q.W.; Wang, S.X.; Li, K.; Qiao, J.; Guo, Y.S.; Liu, Z.D.; Guo, X.W. Responses of soil bacterial and fungal communities to the long-term monoculture of grapevine. Appl. Microbiol. Biotechnol. 2021, 105, 7035–7050. [Google Scholar] [CrossRef]
- Wu, L.K.; Wang, J.Y.; Huang, W.M.; Wu, H.M.; Chen, J.; Yang, Y.Q.; Zhang, Z.Y.; Lin, W.X. Plant-microbe rhizosphere interactions mediated by Rehmannia glutinosa root exudates under consecutive monoculture. Sci. Rep. 2015, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, R.-M.; Chen, D.; Ye, Q.; Zhao, H.-Y.; Piao, R.-Z. Correlation of bacterial community with soil physicochemical properties and enzyme activities in rhizosphere soil under different cultivation years of ginseng (Panax ginseng C. A. Mey.). J. Plant Nutr. Fertil. 2022, 28, 313–324. [Google Scholar]
- Xia, X.-Y. Effects of Continuous Cropping on Bacteria Community and Diversity in Tobacco Rhizospheric Soil. Master’s Thesis, Shandong Agricultural University, Taian, China, 2018. [Google Scholar]
- Niu, L.-J. Cut Flower Production Technology of Herb Peony in Openland and Greenhouse. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2010. [Google Scholar]
- Cang, J.; Zhao, H.-J. Experimental Course of Plant Physiology; Higher Education Press: Beijing, China, 2013. [Google Scholar]
- Bao, S.-D. Soil Agrochemical Analysis; China Agriculture Press: Beijing, China, 2000. [Google Scholar]
- Guan, S.-Y. Enzyme and Its Research Method; Agriculture Press: Beijing, China, 1986. [Google Scholar]
- Wang, R.; Zhang, H.C.; Sun, L.G.; Qi, G.F.; Chen, S.; Zhao, X.Y. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci. Rep. 2017, 7, 10. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Chandra, J.; Retuerto, M.; Sikaroodi, M.; Brown, R.E.; Jurevic, R.; Salata, R.A.; Lederman, M.M.; Gillevet, P.M.; Ghannoum, M.A. Oral Mycobiome Analysis of HIV-Infected Patients: Identification of Pichia as an Antagonist of Opportunistic Fungi. PLoS Pathog. 2014, 10, 17. [Google Scholar] [CrossRef]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- STACKEBRANDT, E.; GOEBEL, B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994, 44, 846–849. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Meng, Q.-W.; Gao, H.-Y. Plant Physiology; China Agriculture Press: Beijing, China, 2017. [Google Scholar]
- Dai, H.-g.; Dong, W.-K.; Chai, S.-J.; Ma, H.-L. The Growth and Physiological Characteristics of Astragalus cicer L. Seedlings under Simulated Drought Stress. Chin. J. Grassl. 2021, 43, 63–75. (In Chinese) [Google Scholar] [CrossRef]
- Ma, H.-P.; Peng, Z.-F. Causes and countermeasures of continuous cropping obstacle of Peony. Chin. Hortic. Abstr. 2011, 27, 122–123. (In Chinese) [Google Scholar]
- Sun, H.-Q. Continuous Cropping on Physiological Characteristics in Lanzhou Lily and Soil Environmen Effect. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2017. [Google Scholar]
- Du, Q.; Zhao, Y.; Zhou, D.-Y.; Wang, X.-G.; Jiang, C.-J.; Wang, J.; Zhao, X.-H.; Yu, H.-Q. Response of root growth and structure of different potassium sensitive maize cultivars (lines) to low potassium stress after flowering stage. J. Plant Nutr. Fertil. 2021, 27, 301–311. (In Chinese) [Google Scholar]
- Sun, Z.-G. Soil Enzyme Activity and Microbial Diversity in Rhizosphere of Continuous Watermelon Cropping. Resrarch Soil Water Conserv. 2015, 22, 46–51. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, W.-M.; Qiu, H.-Z.; Liu, X.; Zhang, J.-L.; Wang, D. Effects of continuous cropping on morphology characteristics and absorptive capacity of potato root. Agric. Res. Arid. Areas 2014, 32, 34–37. (In Chinese) [Google Scholar]
- Huang, X.-D.; Xue, D. Changes in Microbial Biomass, Activity, Functional Diversity, and Enzyme Activity in Tree Peony (Paeonia suffruticosa) Garden Soils. HortScience 2014, 49, 1408–1413. [Google Scholar] [CrossRef]
- Xue, D.; Huang, X.D. Changes in soil microbial community structure with planting years and cultivars of tree peony (Paeonia suffruticosa). World J. Microbiol. Biotechnol. 2014, 30, 389–397. [Google Scholar] [CrossRef]
- Yao, Q.; Xu, Y.-X.; Liu, X.-F.; Liu, J.-J.; Huang, X.-Y.; Yang, W.-G.; Yang, Z.; Lan, L.; Zhou, J.-M.; Wang, G.-H. Dynamics of soil properties and fungal community structure in continuous-cropped alfalfa fields in Northeast China. PeerJ 2019, 7, e7127. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, W.-T.; Xu, S.-Z.; Fan, H.; Chen, X.-S.; Shen, X.; Yin, C.-M.; Mao, Z.-Q. An emerging chemical fumigant: Two-sided effects of dazomet on soil microbial environment and plant response. Environ. Sci. Pollut. Res. 2022, 29, 3022–3036. [Google Scholar] [CrossRef]
- Zydlik, Z.; Zydlik, P. Effect of a preparation containing humic acids on selected physico-chemical and biological properties of replanted soil. J. Elem. 2020, 25, 993–1004. [Google Scholar] [CrossRef]
- Gao, J.-X.; Gao, Y.; Niu, Y.-Q.; Wu, X.-M.; Xie, H. Effect of Decomposition of Different Crop Straws on Growth and Rhizosphere Environment of Continuous Cropping Pepper. Acta Agric. Boreali-Occident. Sin. 2021, 30, 1220–1226. (In Chinese) [Google Scholar] [CrossRef]
- Xu, S.-Q.; Zhang, R.-Z.; Dong, B.; Zhang, M. Effect of tillage practices on structural properties and content of organic carbon in tilth soil. Chin. J. Eco-Agric. 2009, 17, 203–208. (In Chinese) [Google Scholar] [CrossRef]
- Liu, Y.-L. Effects of Continuous Cropping Years on the Growth of Codonopsis pilosula, Soil Physical and Chemical Properties and Enzyme Activities. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2021. [Google Scholar]
- Shao, X.-X.; Yang, W.-Y.; Wu, M. Seasonal Dynamics of Soil Labile Organic Carbon and Enzyme Activities in Relation to Vegetation Types in Hangzhou Bay Tidal Flat Wetland. PLoS ONE 2015, 10, e0142677. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Liu, W.-H.; Qi, J.; Yao, T.; Li, J.-H.; Li, C.-N. Rhizosphere soil nutrition and bacterial community diversity of Elymus sibiricus in different planting years. Agric. Res. Arid. Areas 2020, 38, 8–14. (In Chinese) [Google Scholar] [CrossRef]
- She, S.Y.; Niu, J.J.; Zhang, C.; Xiao, Y.H.; Chen, W.; Dai, L.J.; Liu, X.D.; Yin, H.Q. Significant relationship between soil bacterial community structure and incidence of bacterial wilt disease under continuous cropping system. Arch. Microbiol. 2017, 199, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.H.; Yi, X.L.; Liu, S.S.; Zhou, C.; Wang, A.Y. Effect of transgenic cotton continuous cropping on soil bacterial community. Ann. Microbiol. 2020, 70, 10. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chang, S.-L.; Li, X.; Zhang, Y.-T. Soil fungal diversity and its community structure in Tianshan Forest. Acta Ecol. Sin. 2021, 41, 124–134. (In Chinese) [Google Scholar] [CrossRef]
- Guo, H.; Tang, W.-P. Enzyme activity and microbial community diversity in rhizosphere and non-rhizosphere of Larix principis-rupprechtii. Ecol. Environ. Sci. 2020, 29, 2163–2170. (In Chinese) [Google Scholar] [CrossRef]
- Dong, L.L.; Xu, J.; Li, Y.; Fang, H.L.; Niu, W.H.; Li, X.W.; Zhang, Y.J.; Ding, W.L.; Chen, S.L. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biol. Biochem. 2018, 125, 64–74. [Google Scholar] [CrossRef]
- Radl, V.; Winkler, J.B.; Kublik, S.; Yang, L.H.; Winkelmann, T.; Vestergaard, G.; Schroder, P.; Schloter, M. Reduced microbial potential for the degradation of phenolic compounds in the rhizosphere of apple plantlets grown in soils affected by replant disease. Environ. Microbiome 2019, 14, 12. [Google Scholar] [CrossRef]
- Yuan, X.F.; Song, T.J.; Yang, J.S.; Huang, X.G.; Shi, J.Y. Changes of microbial community in the rhizosphere soil of Atractylodes macrocephala when encountering replant disease. S. Afr. J. Bot. 2019, 127, 129–135. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; van Veen, J.A.; Kowalchuk, G.A. Soil characteristics more strongly influence soil bacterial communities than land-use type. Fems Microbiol. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.W.; Asao, S.; Calderon, F.; Wolk, B.; Wallenstein, M.D. Plant nitrogen uptake drives rhizosphere bacterial community assembly during plant growth. Soil Biol. Biochem. 2015, 85, 170–182. [Google Scholar] [CrossRef]
- Li, M.; Wang, G.X.; Kang, X.M.; Hu, H.L.; Wang, Y.; Zhang, X.R.; Sun, X.L.; Zhang, H.; Hu, Z.Y.; Xi, B.D. Long-term fertilization alters microbial community but fails to reclaim soil organic carbon stocks in a land-use changed soil of the Tibetan Plateau. Land Degrad. Dev. 2020, 31, 531–542. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Qi, Y.-K.; Jiao, J.; Li, C.-Z. Diversity of reed (Phragmites australis) rhizosphere soil microbial community in hexi Corridor sandy land. J. Desert Res. 2021, 41, 1–9. (In Chinese) [Google Scholar]
- Su, L.; Zhu, H.; Niu, Y.C.; Guo, Y.X.; Du, X.P.; Guo, J.G.; Zhang, L.; Qin, C. Phylogeny and taxonomic revision of Kernia and Acaulium. Sci. Rep. 2020, 10, 11. [Google Scholar] [CrossRef]
- Wang, G.S.; Yin, C.M.; Pan, F.B.; Wang, X.B.; Xiang, L.; Wang, Y.F.; Wang, J.Z.; Tian, C.P.; Chen, J.; Mao, Z.Q. Analysis of the Fungal Community in Apple Replanted Soil Around Bohai Gulf. Hortic. Plant J. 2018, 4, 175–181. [Google Scholar] [CrossRef]
- Ferrigo, D.; Mondin, M.; Ladurner, E.; Fiorentini, F.; Causin, R.; Raiola, A. Effect of seed biopriming with Trichoderma harzianum strain INAT11 on Fusarium ear rot and Gibberella ear rot diseases. Biol. Control 2020, 147, 9. [Google Scholar] [CrossRef]
- Jiang, N.-W.; Liang, C.-F.; Zhang, Y.; Jiang, Z.-L.; Dong, J.-Q.; Wu, J.-S.; Fu, W.-J. Microbial composition and diversity in soil of torreya grandis cv. Merrillii Relative to rifferent cultivation years after land use conversion. Environ. Sci. 2022, 43, 530–539. (In Chinese) [Google Scholar] [CrossRef]
- Linkies, A.; Jacob, S.; Zink, P.; Maschemer, M.; Maier, W.; Koch, E. Characterization of cultural traits and fungicidal activity of strains belonging to the fungal genus Chaetomium. J. Appl. Microbiol. 2021, 131, 375–391. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Xu, R.-R.; Ji, H.-L.; Chang, Z.-L. Isolation, screening and identification of an endophytic fungus and the detection of its antifungal effects. J. Plant Prot. 2015, 42, 806–812. (In Chinese) [Google Scholar] [CrossRef]
- Cai, Y.-L. Diversity of Yeast and Resource Assessment of Peach Orchard in Xinjiang. Master’s Thesis, Shihezi University, Shihezi, China, 2018. (In Chinese). [Google Scholar]
- Nong, Q.; Zhang, W.-L.; Xie, L.; Liao, S.-T.; Qin, L.-P. Optimization of Protoplast Preparation and Regeneration Conditions of Schizothecium sp. L-14. Southwest China J. Agric. Sci. 2019, 32, 1302–1306. (In Chinese) [Google Scholar] [CrossRef]
- Hua, L.; Wang, D.-G.; Huang, T.; Gao, J.; Chen, P. Three quarantine pathogenic fungi harming wheat in the United States. Plant Quarantne 2010, 24, 30–33. (In Chinese) [Google Scholar] [CrossRef]
- Li, J.R.; Chen, X.Z.; Li, S.M.; Zuo, Z.M.; Zhan, R.T.; He, R. Variations of rhizospheric soil microbial communities in response to continuousAndrographis paniculatacropping practices. Bot. Stud. 2020, 61, 13. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Khafipour, E.; Krause, D.O.; Entz, M.H.; de Kievit, T.R.; Fernando, W.G.D. Pyrosequencing Reveals the Influence of Organic and Conventional Farming Systems on Bacterial Communities. PLoS ONE 2012, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- Bharti, N.; Barnawal, D.; Maji, D.; Kalra, A. Halotolerant PGPRs Prevent Major Shifts in Indigenous Microbial Community Structure Under Salinity Stress. Microb. Ecol. 2015, 70, 196–208. [Google Scholar] [CrossRef]
- Zhang, Y.-G.; Zhao, J.; Zhang, Y.-J.; Wu, T.; Wu, X.-B.; Zheng, Y. Bacterial diversity in rhizosphere soil of the medicinal tree peony(Paeonia suffruticosa)revealed by amplified ribosomal DNA restriction analys. Acta Ecol. Sin. 2016, 36, 5564–5574. (In Chinese) [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, G.-C.; Ding, H.; Qin, F.-F.; Zhang, Z.-M.; Dai, L.-X. Effects of soil types on bacterial community diversity on the rhizosphere soil of arachis hypogaea and yield. Biotechnol. Bull. 2022, 38, 221. (In Chinese) [Google Scholar] [CrossRef]
- Ahemad, M.; Kibret, M. Mechanisms and applications of plant growth promoting rhizobacteria: Current perspective. J. King Saud Univ. Sci. 2014, 26, 1–20. [Google Scholar] [CrossRef]
- Qiao, T.-M.; Li, S.-J.; Han, S.; Zhu, T.-H.; Wang, S.-S. The phosphate-solubilizing ability and growth promoting effects of Pseudomonas vancouverensis strain PAN4 on walnut. J. S. China Agric. Univ. 2015, 36, 117–124. (In Chinese) [Google Scholar] [CrossRef]
- Rfaki, A.; Nassiri, L.; Ibijbijen, J. Isolation and Characterization of Phosphate Solubilizing Bacteria from the Rhizosphere of Faba Bean (Vicia faba L.) in Meknes Region, Morocco. Br. Microbiol. Res. J. 2015, 6, 247–254. [Google Scholar] [CrossRef]
| Sample | Flowering Rate (%) | Plant Height (cm) | Stem Diameter (cm) | Leaf Area (cm2) | |
|---|---|---|---|---|---|
| 1-year plantation | Z1-1 | 43.33% ± 37.34% ab | 56.20 ± 9.17 b | 6.28 ± 0.97 bc | 10.19 ± 4.11 b |
| 5-year plantation | Z5-5 | 80.05% ± 20.89% a | 70.33 ± 12.76 a | 7.92 ± 0.40 a | 21.35 ± 2.16 a |
| H5-1 | 42.22% ± 36.72% ab | 51.90 ± 1.64 b | 6.15 ± 0.16 bc | 7.12 ± 2.38 b | |
| 8-year plantation | Z8-8 | 55.96% ± 5.27% ab | 53.00 ± 3.00 b | 7.00 ± 0.42 ab | 19.21 ± 8.82 a |
| H8-1 | 34.92% ± 7.27% ab | 45.13 ± 4.83 bc | 5.59 ± 0.09 c | 7.25 ± 0.68 b | |
| H8-5 | 19.44% ± 17.35% b | 35.73 ± 5.39 c | 5.04 ± 0.74 c | 10.16 ± 3.42 b | |
| Sample | pH | EC (μm·cm−1) | AN (mg·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | OM (g·kg−1) | |
|---|---|---|---|---|---|---|---|
| 1-year plantation | CK1 | 7.55 ± 0.04 b | 119.27 ± 1.29 d | 58.33 ± 2.02 e | 59.76 ± 1.55 d | 112.70 ± 11.60 bc | 11.46 ± 2.96 c |
| Z1-1 | 7.58 ± 0.04 b | 77.70 ± 0.36 f | 86.10 ± 1.85 c | 108.88 ± 1.25 b | 121.12 ± 2.08 b | 19.40 ± 3.58 b | |
| 5-year plantation | CK5 | 7.68 ± 0.09 a | 65.53 ± 0.42 g | 79.57 ± 17.60 c | 29.62 ± 1.65 f | 103.07 ± 5.51 de | 18.44 ± 2.20 b |
| Z5-5 | 7.73 ± 0.04 a | 66.17 ± 0.25 g | 98.70 ± 2.52 b | 53.73 ± 0.66 d | 113.90 ± 5.51 bc | 30.36 ± 3.93 a | |
| H5-1 | 7.54 ± 0.02 b | 120.73 ± 0.93 d | 73.27 ± 2.46 cd | 29.14 ± 4.63 f | 100.66 ± 3.61 e | 17.95 ± 1.33 b | |
| 8-year plantation | CK8 | 7.50 ± 0.02 b | 136.4 ± 1.92 c | 108.50 ± 7.00 b | 86.07 ± 2.29 c | 122.32 ± 3.61 b | 15.91 ± 4.82 bc |
| Z8-8 | 7.57 ± 0.08 b | 89.50 ± 0.36 e | 123.43 ± 4.66 a | 124.77 ± 5.94 a | 147.59 ± 6.25 a | 19.67 ± 1.31 b | |
| H8-1 | 7.49 ± 0.04 bc | 150.70 ± 0.30 b | 76.30 ± 5.60 cd | 39.35 ± 8.13 e | 111.49 ± 3.61 bcd | 15.32 ± 1.47 bc | |
| H8-5 | 7.40 ± 0.07 c | 239.33 ± 0.58 a | 66.27 ± 4.28 de | 33.73 ± 1.94 ef | 109.09 ± 5.51 de | 14.08 ± 2.75 bc | |
| Sample | Fungi | Bacteria | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Shannon | Ace | Chao1 | Coverage | Shannon | Ace | Chao1 | Coverage | ||
| 1-year plantation | CK1 | 3.55 ± 0.05 c | 399.35 ± 0.87 g | 413.06 ± 2.98 g | 0.99 ± 0.00 a | 6.82 ± 0.04 ab | 3253.04 ± 0.03 h | 3192.15 ± 0.14 h | 0.97 ± 0.00 a |
| Z1-1 | 2.59 ± 0.40 e | 479.73 ± 0.29 f | 495.02 ± 2.85 f | 0.99 ± 0.00 a | 6.92 ± 0.04 a | 3522.64 ± 0.34 a | 3481.84 ± 0.06 b | 0.97 ± 0.00 a | |
| 1-year plantation | CK5 | 4.63 ± 0.08 a | 646.47 ± 1.76 b | 651.02 ± 0.02 b | 0.99 ± 0.00 a | 6.63 ± 0.2 c | 3295.89 ± 2.09 f | 3259.98 ± 0.64 g | 0.97 ± 0.00 a |
| Z5-5 | 4.46 ± 0.11 a | 646.44 ± 0.06 b | 650.37 ± 1.15 b | 0.99 ± 0.00 a | 6.73 ± 0.06 bc | 3348.13 ± 0.14 e | 3337.28 ± 0.19 d | 0.97 ± 0.00 a | |
| H5-1 | 4.51 ± 0.00 a | 608.21 ± 4.96 d | 619.33 ± 1.00 c | 0.99 ± 0.00 a | 6.76 ± 0.14 abc | 3449.20 ± 0.09 c | 3491.00 ± 0.01 a | 0.97 ± 0.00 a | |
| 1-year plantation | CK8 | 4.54 ± 0.06 a | 586.58 ± 0.35 e | 594.03 ± 0.13 e | 0.99 ± 0.00 a | 6.81 ± 0.03 abc | 3456.24 ± 0.03 b | 3433.39 ± 0.00 c | 0.97 ± 0.00 a |
| Z8-8 | 3.12 ± 0.02 d | 618.15 ± 0.16 c | 616.26 ± 0.75 d | 0.99 ± 0.00 a | 6.64 ± 0.01 bc | 3290.89 ± 0.00 g | 3279.62 ± 0.08 f | 0.97 ± 0.00 a | |
| H8-1 | 4.38 ± 0.01 a | 677.35 ± 0.04 a | 699.86 ± 0.87 a | 0.99 ± 0.00 a | 6.79 ± 0.12 abc | 3372.85 ± 0.09 d | 3304.96 ± 0.06 e | 0.97 ± 0.00 a | |
| H8-5 | 3.83 ± 0.01 b | 588.53 ± 0.09 e | 593.72 ± 0.06 e | 0.99 ± 0.00 a | 6.68 ± 0.03 bc | 3106.45 ± 0.00 i | 3082.50 ± 0.17 i | 0.97 ± 0.00 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Liu, Z.; Yang, L.; Yang, X.; Shi, Y.; Li, X.; Dong, L.; Zheng, C.; Zhang, D.; Sun, X. Analysis of Changes in Herbaceous Peony Growth and Soil Microbial Diversity in Different Growing and Replanting Years Based on High-Throughput Sequencing. Horticulturae 2023, 9, 220. https://doi.org/10.3390/horticulturae9020220
Li Y, Liu Z, Yang L, Yang X, Shi Y, Li X, Dong L, Zheng C, Zhang D, Sun X. Analysis of Changes in Herbaceous Peony Growth and Soil Microbial Diversity in Different Growing and Replanting Years Based on High-Throughput Sequencing. Horticulturae. 2023; 9(2):220. https://doi.org/10.3390/horticulturae9020220
Chicago/Turabian StyleLi, Yang, Zemiao Liu, Lijin Yang, Xiao Yang, Yajie Shi, Xue Li, Lingling Dong, Chengshu Zheng, Dongliang Zhang, and Xia Sun. 2023. "Analysis of Changes in Herbaceous Peony Growth and Soil Microbial Diversity in Different Growing and Replanting Years Based on High-Throughput Sequencing" Horticulturae 9, no. 2: 220. https://doi.org/10.3390/horticulturae9020220
APA StyleLi, Y., Liu, Z., Yang, L., Yang, X., Shi, Y., Li, X., Dong, L., Zheng, C., Zhang, D., & Sun, X. (2023). Analysis of Changes in Herbaceous Peony Growth and Soil Microbial Diversity in Different Growing and Replanting Years Based on High-Throughput Sequencing. Horticulturae, 9(2), 220. https://doi.org/10.3390/horticulturae9020220





