Morphogenesis of Stamens and Petaloid Stamens in Lilium hybrid ‘Red Twin’ under Different Temperatures and the Expression Characteristics of Two AGAMOUS-like Genes Linked to These Processes
Abstract
1. Introduction
2. Materials and Methods
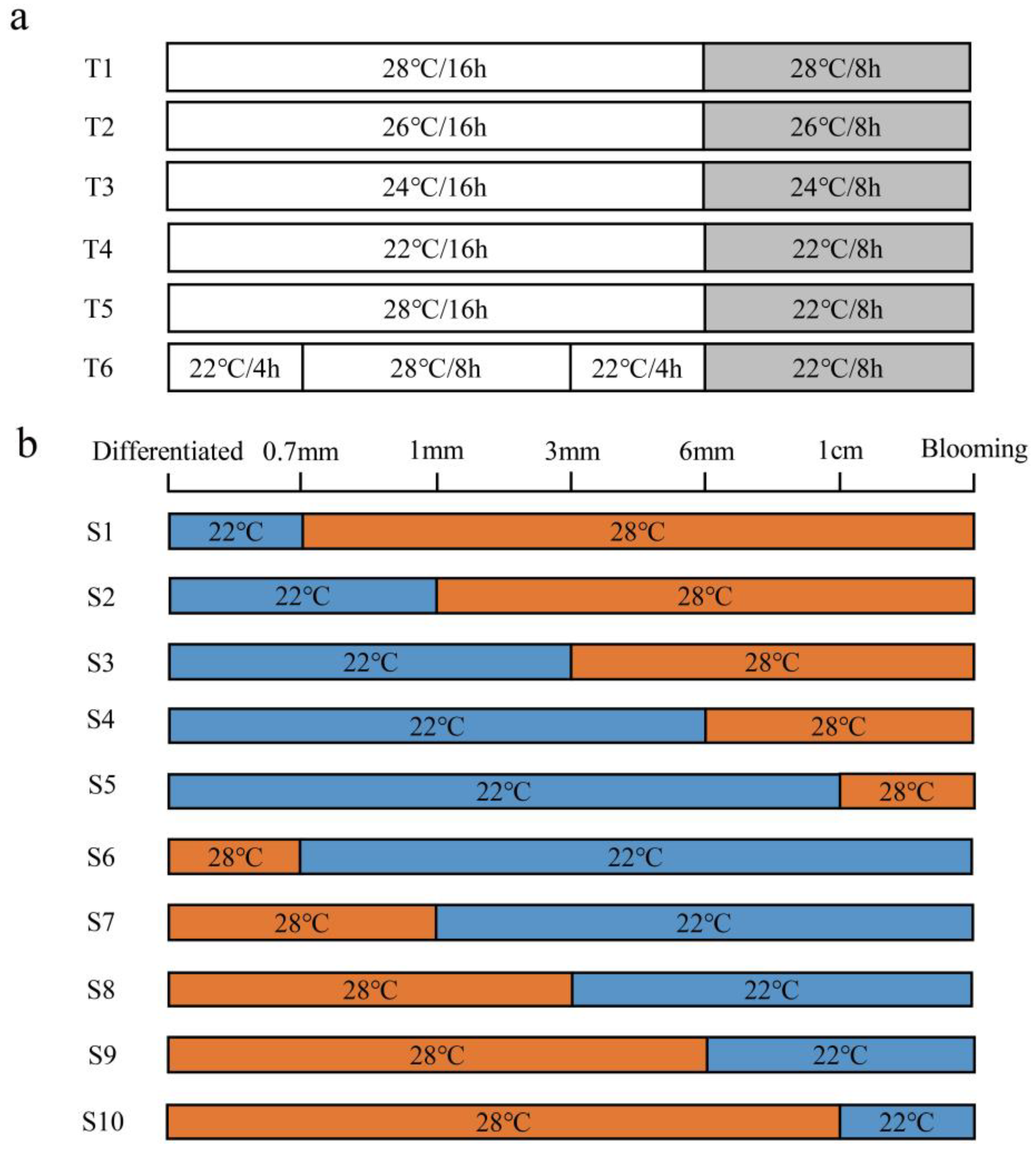
2.1. Plant Material and Temperature Treatment
2.2. Morphological Observation and Statistics of Petaloid Value
2.3. RNA Extraction and cDNA Synthesis
2.4. Isolation of the AGAMOUS-like Genes in ‘Red Twin’ and Sequence Analysis
2.5. Quantitative Real-Time PCR Analysis
3. Results
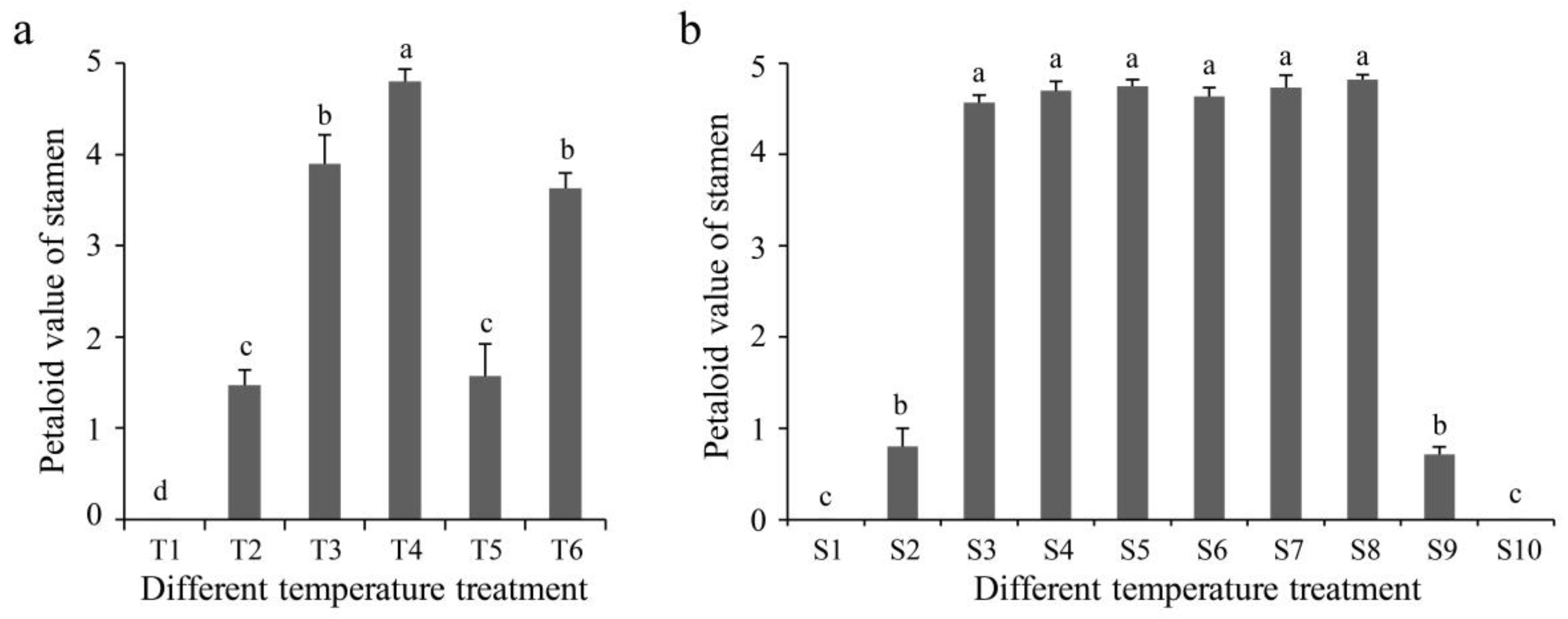
3.1. Petaloidy of Stamens under Different Temperatures
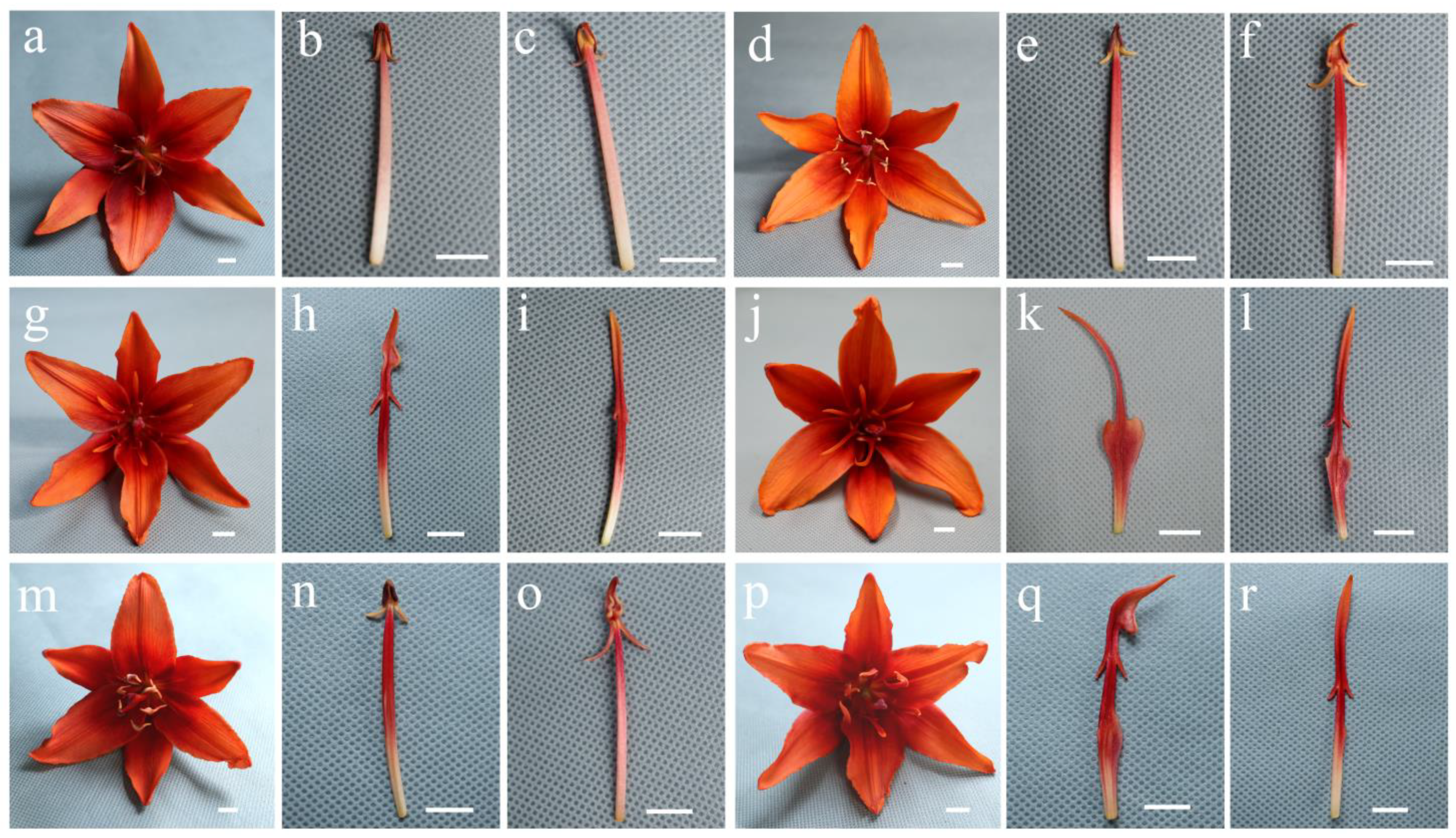
3.2. Morphological Courses of Stamens and Petaloid Stamens
3.3. Identification of Critical Developmental Periods of Stamens and Petaloid Stamens
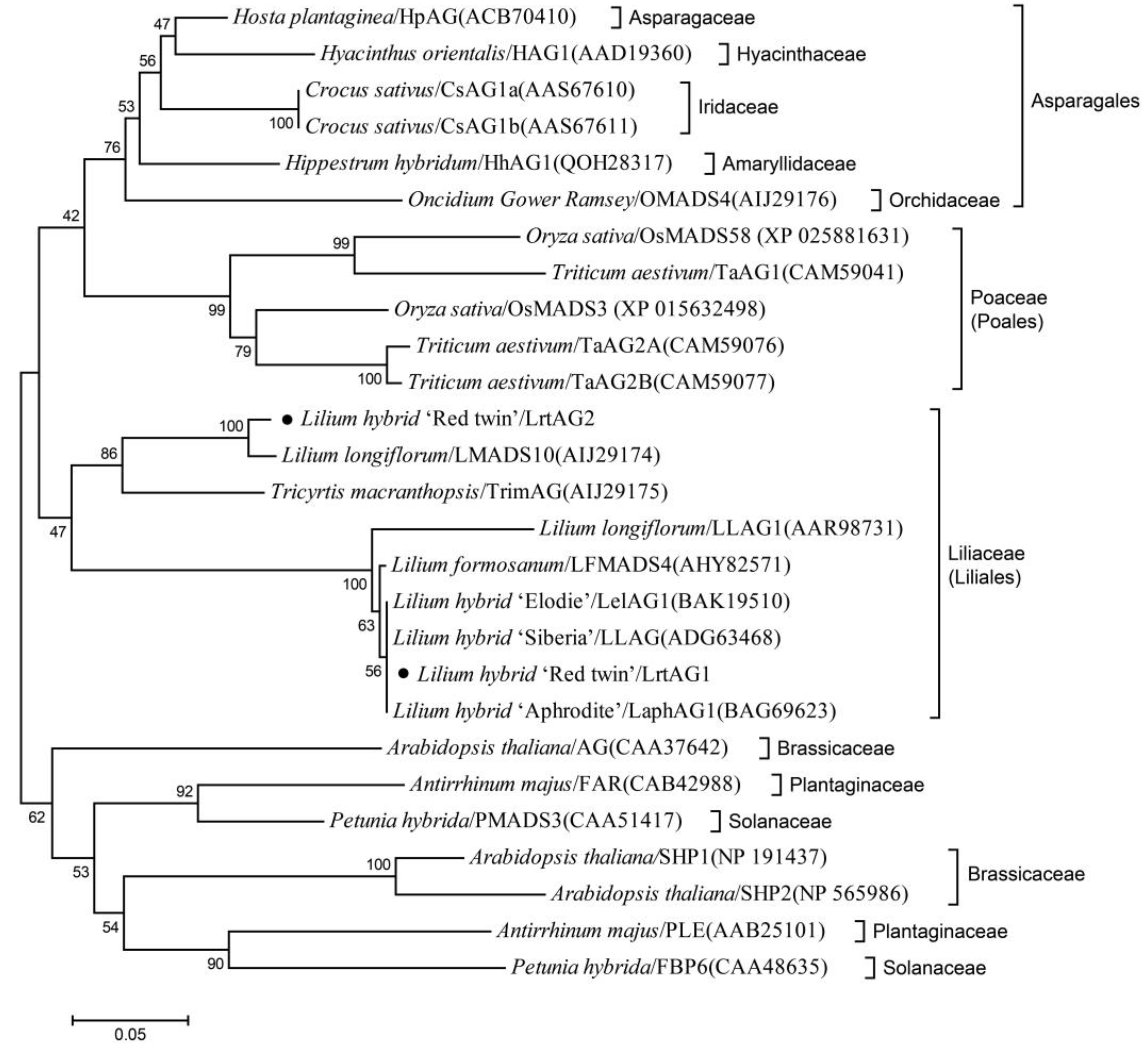
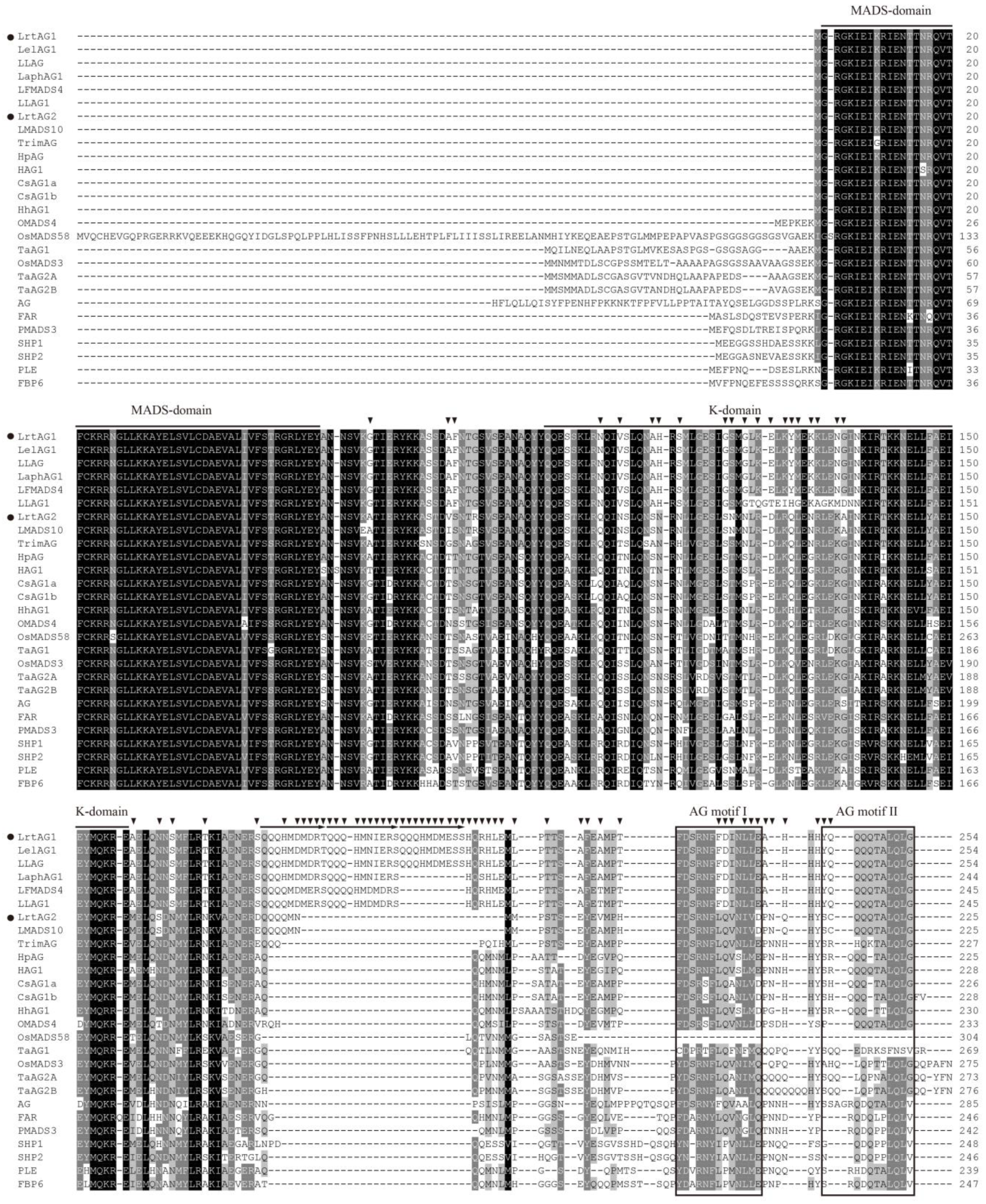
3.4. Isolation and Sequence Analysis of Two AGAMOUS-like Genes from ‘Red Twin’
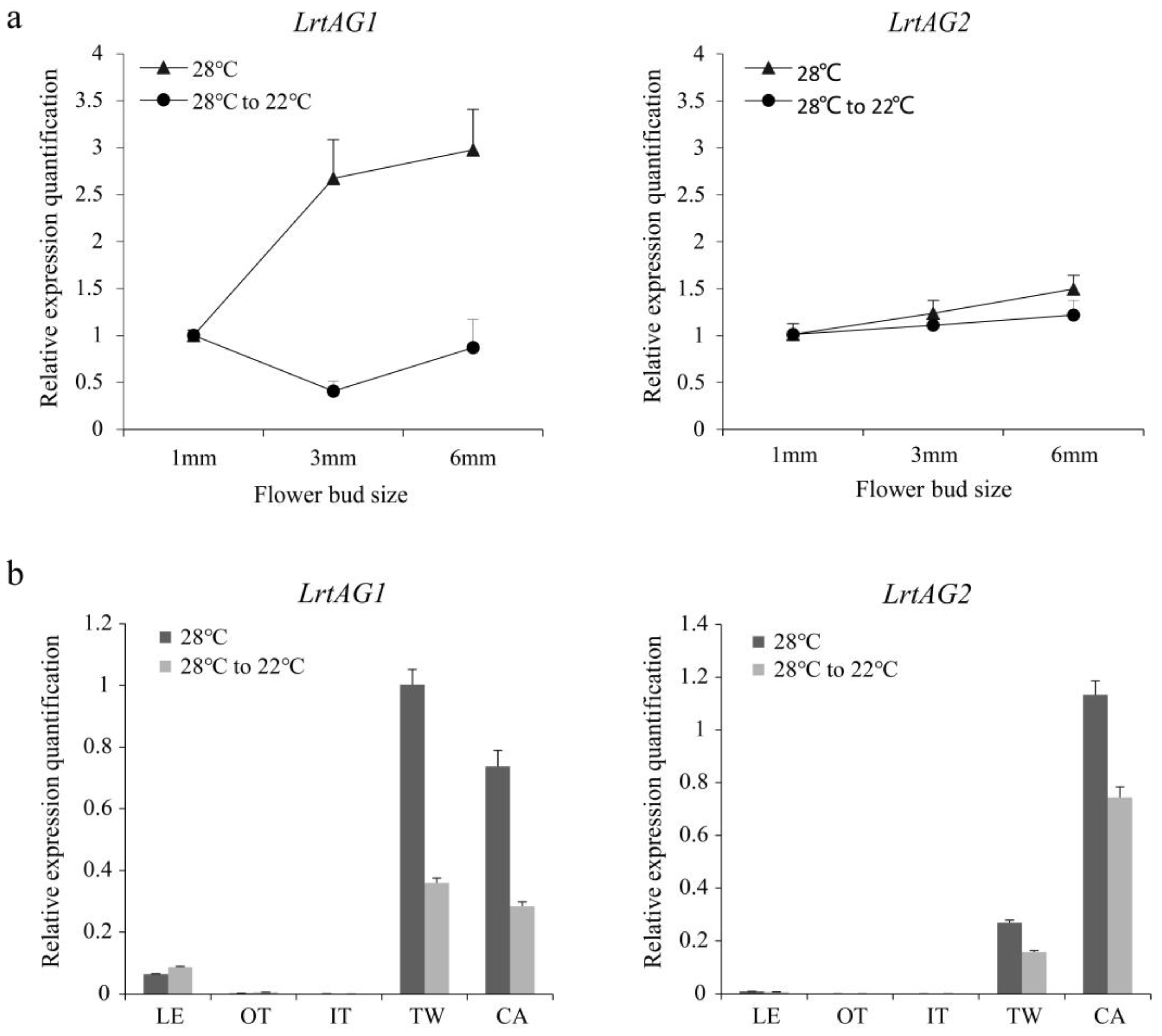
3.5. Expression Analysis of LrtAG1 and LrtAG2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Coen, E.S.; Meyerowitz, E.M. The War of the Whorls: Genetic Interactions Controlling Flower Development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Causier, B.; Schwarz-Sommer, Z.; Davies, B. Floral organ identity: 20 years of ABCs. Semin. Cell Dev. Biol. 2010, 21, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Ma, H. Ectopic Expression of the Floral Homeotic Gene AGAMOUS in Transgenic Arabidopsis Plants Alters Floral Organ Identity. Cell 1992, 71, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, Y.; Ma, H. Determination of Arabidopsis Floral Meristem Identity by AGAMOUS. Plant Cell 1997, 9, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R. Flowering and Determinacy in Arabidopsis. J. Exp. Bot. 2007, 58, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Drews, G.N.; Bowman, J.L.; Meyerowitz, E.M. Negative Regulation of the Arabidopsis Homeotic Gene AGAMOUS by the APETALA2 Product. Cell 1991, 65, 991–1002. [Google Scholar] [CrossRef]
- Prunet, N.; Morel, P.; Negrutiu, I.; Trehin, C. Time to Stop: Flower Meristem Termination. Plant Physiol. 2009, 150, 1764–1772. [Google Scholar] [CrossRef]
- Galimba, K.D.; Tolkin, T.R.; Sullivan, A.M.; Melzer, R.; Theißen, G.; Stilio, V.S.D. Loss of Deeply Conserved C-Class Floral Homeotic Gene Function and C- and E-Class Protein Interaction in a Double-Flowered Ranunculid Mutant. Proc. Natl. Acad. Sci. USA 2012, 109, E2267–E2275. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.; Liu, D.; Li, F.; Lu, H. Exon Skipping of AGAMOUS Homolog PrseAG in Developing Double Flowers of Prunus lannesiana (Rosaceae). Plant Cell Rep. 2013, 32, 227–237. [Google Scholar] [CrossRef]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M.L. Genetics and Genomics of Flower Initiation and Development in Roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef]
- Ma, N.; Chen, W.; Fan, T.; Tian, Y.; Zhang, S.; Zeng, D.; Li, Y. Low Temperature-Induced DNA Hypermethylation Attenuates Expression of RhAG, an AGAMOUS Homolog, and Increases Petal Number in Rose (Rosa hybrida). BMC Plant Biol. 2015, 15, 237. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.H.; Ushijima, K.; Murata, A.; Yoshida, K.; Tanabe, M.; Tanigawa, T.; Kubo, Y.; Nakano, R. Double Flower Formation Induced by Silencing of C-Class MADS-Box Genes and Its Variation among Petunia cultivars. Sci. Hortic. 2014, 178, 1–7. [Google Scholar] [CrossRef]
- Sun, Y.; Fan, Z.; Li, X.; Liu, Z.; Li, J.; Yin, H. Distinct Double Flower Varieties in Camellia japonica Exhibit Both Expansion and Contraction of C-Class Gene Expression. BMC Plant Biol. 2014, 14, 288. [Google Scholar] [CrossRef]
- Mizunoe, Y.; Ozaki, Y. Effects of Growth Temperature and Culture Season on Morphogenesis of Petaloid-Stamen in Double-Flowered Cyclamen. Hortic. J. 2015, 84(3), 269–276. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Koishi, K. Molecular Characterization of a Double-Flower Mutation in Matthiola Incana. Plant Sci. 2018, 268, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Yoshioka, S.; Aida, R.; Ohtsubo, N. Production of Petaloid Phenotype in the Reproductive Organs of Compound Flowerheads by the Co-Suppression of Class-C Genes in Hexaploid Chrysanthemum morifolium. Planta 2021, 253, 100. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Yang, L.; Xu, J.; Zhang, Y. Expression Level of B- and C-Class MADS-Box Genes Is Associated with the Petaloidy of Stamens in Cultivated Amaryllis (Hippeastrum hybridum). J. Hortic. Sci. Biotechnol. 2022, 97, 211–223. [Google Scholar] [CrossRef]
- Akita, Y.; Horikawa, Y.; Kanno, A. Comparative Analysis of Floral MADS-Box Genes between Wild-Type and a Putative Homeotic Mutant in Lily. J. Hortic. Sci. Biotechnol. 2008, 83, 453–461. [Google Scholar] [CrossRef]
- Akita, Y.; Nakada, M.; Kanno, A. Effect of the Expression Level of an AGAMOUS-like Gene on the Petaloidy of Stamens in the Double-Flowered Lily, ‘Elodie’. Sci. Hortic. 2011, 128, 48–53. [Google Scholar] [CrossRef]
- Wang, W.-B.; He, X.; Li, X.; Wang, W.; Wang, W.-B.; He, X.; Li, X.; Wang, W. Transcriptome Profiling during Double-Flower Development Provides Insight into Stamen Petaloid in Cultivated Lilium. Ornam. Plant Res. 2022, 2, 1–11. [Google Scholar] [CrossRef]
- Eisa, H.M.; Wallace, D.H. Factors Influencing Petaloidy Expression in the Carrot, Daucus carota L.1. J. Am. Soc. Hortic. Sci. 1969, 94, 647–649. [Google Scholar] [CrossRef]
- Sawhney, V.K. The Role of Temperature and Its Relationship with Gibberellic Acid in the Development of Floral Organs of Tomato (Lycopersicon esculentum). Can. J. Bot. 1983, 61, 1258–1265. [Google Scholar] [CrossRef]
- Garrod, J.F.; Harris, G.P. Studies on the Glasshouse Carnation: Effects of Temperature and Growth Substances on Petal Number. Ann. Bot. 1974, 38, 1025–1031. [Google Scholar] [CrossRef]
- Yamane, K.; Sumida, K.; Terui, Y.; Kojima, N.; Burana, C.; Kurokura, T. Temperature-Dependent Flower Malformation in Carnations (Dianthus caryophyllus L.). Hort. J. 2018, 87, 406–412. [Google Scholar] [CrossRef]
- Chmelnitsky, I.; Colauzzi, M.; Algom, R.; Zieslin, N. Effects of Temperature on Phyllody Expression and Cytokinin Content in Floral Organs of Rose Flowers. Plant Growth Regul. 2001, 35, 207–214. [Google Scholar] [CrossRef]
- Polowick, P.L.; Sawhney, V.K. Temperature Effects on Male Fertility and Flower and Fruit Development in Capsicum annuum L. Sci. Hortic. 1985, 25, 117–127. [Google Scholar] [CrossRef]
- Ohnishi, S.; Miyoshi, T.; Shirai, S. Low Temperature Stress at Different Flower Developmental Stages Affects Pollen Development, Pollination, and Pod Set in Soybean. Environ. Exp. Bot. 2010, 69, 56–62. [Google Scholar] [CrossRef]
- Sato, T.; Miyoshi, K. Restoration of Intact Anthers in a Thermosensitive, Antherless, Male-Sterile Cultivar of Asiatic Hybrid Lily in Response to High Temperature. J. Hortic. Sci. Biotechnol. 2007, 82, 791–797. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular Evolutionary Genetics Analysis Using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Nicholas, K.B. GeneDoc: Analysis and visualization of genetic variation. Embnew. News 1997, 4, 14. [Google Scholar]
- Eisa, H.M.; Wallace, D.H. Morphological and Anatomical Aspects of Petaloidy in the Carrot (Daucus carota L.). J. Am. Soc. Hortic. Sci. 1969, 94, 545–548. [Google Scholar] [CrossRef]
- Smyth, D.R.; Bowman, J.L.; Meyerowitz, E.M. Early Flower Development in Arabidopsis. Plant Cell 1990, 2, 755–767. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ng, K.-H.; Lim, T.-S.; Yu, H.; Meyerowitz, E.M. The Homeotic Protein AGAMOUS Controls Late Stamen Development by Regulating a Jasmonate Biosynthetic Gene in Arabidopsis. Plant Cell 2007, 19, 3516–3529. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Franks, R.G.; Levin, J.Z.; Liu, Z. Repression of AGAMOUS by BELLRINGER in Floral and Inflorescence Meristems. Plant Cell 2004, 16, 1478–1489. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genes Directing Flower Development in Arabidopsis. Plant Cell 1989, 1, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Bowman, J.L.; Smyth, D.R.; Meyerowitz, E.M. Genetic Interactions among Floral Homeotic Genes of Arabidopsis. Development 1991, 112, 1–20. [Google Scholar] [CrossRef]
- Liao, W.-Y.; Lin, L.-F.; Lin, M.-D.; Hsieh, S.-C.; Li, A.; Tsay, Y.-S.; Chou, M.-L.; Liao, W.-Y.; Lin, L.-F.; Lin, M.-D.; et al. Overexpression of Lilium Formosanum MADS-Box (LFMADS) Causing Floral Defects while Promoting Flowering in Arabidopsis thaliana, Whereas Only Affecting Floral Transition Time in Nicotiana tabacum. Int. J. Mol. Sci. 2018, 19, 2217. [Google Scholar] [CrossRef]
- Benedito, V.A.; Visser, P.B.; van Tuyl, J.M.; Angenent, G.C.; de Vries, S.C.; Krens, F.A. Ectopic Expression of LLAG1, an AGAMOUS Homologue from Lily (Lilium longiflorum Thunb.) Causes Floral Homeotic Modifications in Arabidopsis. J. Exp. Bot. 2004, 55, 1391–1399. [Google Scholar] [CrossRef]
- Hsu, H.-F.; Hsieh, W.-P.; Chen, M.-K.; Chang, Y.-Y.; Yang, C.-H. C/D Class MADS Box Genes from Two Monocots, Orchid (Oncidium gower Ramsey) and Lily (Lilium longiflorum), Exhibit Different Effects on Floral Transition and Formation in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1029–1045. [Google Scholar] [CrossRef]
- Singh, S.; Sawhney, V.K.; Pearce, D.W. Temperature Effects on Endogenous Indole-3-Acetic Acid Levels in Leaves and Stamens of the Normal and Male Sterile ‘stamenless-2’ Mutant of Tomato (Lycopersicon esculentum Mill.). Plant Cell Environ. 1992, 15, 373–377. [Google Scholar] [CrossRef]
- Singh, S.; Sawhney, V.K. Abscisic Acid in a Male Sterile Tomato Mutant and Its Regulation by Low Temperature. J. Exp. Bot. 1998, 49, 199–203. [Google Scholar] [CrossRef]
- Chen, M.; Nie, G.; Yang, L.; Zhang, Y.; Cai, Y. Homeotic Transformation from Stamen to Petal in Lilium Is Associated with MADS-Box Genes and Hormone Signal Transduction. Plant Growth Regul. 2021, 95, 49–64. [Google Scholar] [CrossRef]

| Gene | Oligo Name | Sequence (5’-3’) | Remarks |
|---|---|---|---|
| LrtAG1 | 5GSP1 | GTTGCGCCCTTTACTTGCCA | 5’-RACE |
| 5GSP2 | GAGCTCATAGGCCTTCTTGAGCAGGCCA | 5’-RACE | |
| 5nested-GSP | CTGCCTGTTGGTGGTGTTCTCGATCCTC | 5’-RACE | |
| 3GSP | CACATGGACATGGACCGAACGCAGCAGC | 3’-RACE | |
| 3nested-GSP | GCGACACTTGGAGATGCTGCCCACCAC | 3’-RACE | |
| RT-F | CCATGCCTACATTCGATTCG | qRT-PCR | |
| RT-R | GGAGATTGTTCAGAGTCTTCATAA | qRT-PCR | |
| LrtAG2 | 5GSP1 | TCGCTTTGCAATTCCATCTC | 5’-RACE |
| 5GSP2 | TCATGTTGCTGAGGGACTCGCCCAACAG | 5’-RACE | |
| 5nested-GSP | GGCGGCCGCGAGTGGAGAAGACGATGAG | 5’-RACE | |
| 3GSP | AGATCTGTCTCGGAAGCCAATGCACAGT | 3’-RACE | |
| 3nested-GSP | AGGAATCTGTTGGGCGAGTCCCTCAGCA | 3’-RACE | |
| RT-F | GCCACACTTCGATTCACGG | qRT-PCR | |
| RT-R | GATTCAGCATAAAACCACATTG | qRT-PCR | |
| 18S | RT-F | CGTTTCGGGCATGATTTGTGG | qRT-PCR |
| RT-R | TCGCATTTCGCTACGTTCTTC | qRT-PCR |
| Petaloid Value | Characterization | Related Pictures |
|---|---|---|
| 0 | Four pollen sacs, more pollen grains, a filiform filament | Figure 2b,c |
| 1 | Two pollen sacs, less pollen grains, a filiform filament | Figure 2e,n |
| 2 | Abnormal pollen sacs, pollen-free, a filiform filament | Figure 2f,h,o |
| 3 | Abnormal pollen sacs, pollen-free, a petaloid filament | Figure 2q |
| 4 | No pollen sacs, a filiform filament | Figure 2i,r |
| 5 | No pollen sacs, a petaloid filament | Figure 2k,l |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, Z.; Chen, M.; Yang, L.; Zhang, Y. Morphogenesis of Stamens and Petaloid Stamens in Lilium hybrid ‘Red Twin’ under Different Temperatures and the Expression Characteristics of Two AGAMOUS-like Genes Linked to These Processes. Horticulturae 2022, 8, 1184. https://doi.org/10.3390/horticulturae8121184
Li X, Wang Z, Chen M, Yang L, Zhang Y. Morphogenesis of Stamens and Petaloid Stamens in Lilium hybrid ‘Red Twin’ under Different Temperatures and the Expression Characteristics of Two AGAMOUS-like Genes Linked to These Processes. Horticulturae. 2022; 8(12):1184. https://doi.org/10.3390/horticulturae8121184
Chicago/Turabian StyleLi, Xin, Zhen Wang, Minmin Chen, Liuyan Yang, and Yongchun Zhang. 2022. "Morphogenesis of Stamens and Petaloid Stamens in Lilium hybrid ‘Red Twin’ under Different Temperatures and the Expression Characteristics of Two AGAMOUS-like Genes Linked to These Processes" Horticulturae 8, no. 12: 1184. https://doi.org/10.3390/horticulturae8121184
APA StyleLi, X., Wang, Z., Chen, M., Yang, L., & Zhang, Y. (2022). Morphogenesis of Stamens and Petaloid Stamens in Lilium hybrid ‘Red Twin’ under Different Temperatures and the Expression Characteristics of Two AGAMOUS-like Genes Linked to These Processes. Horticulturae, 8(12), 1184. https://doi.org/10.3390/horticulturae8121184






