Abstract
Water availability, light, management practices, and harvest time impacts on Coffea arabica L. yield and bean quality remain uncertain. It was hypothesized that the soil water and light availability could impact berry distribution, yield, and bean chemical attributes within the plant canopy. Therefore, it was aimed to study berry yield, berry distribution, and bean chemical traits along the canopy strata of four coffee genotypes (Iapar 59, Catuaí 99 and two Ethiopian wild accessions, ‘E083’ and ‘E027’), cultivated with (IRR) and without irrigation (NI) in the two initial harvest years. The maximum height of berry occurrence was lower in NI than in IRR plants in both harvest years. In the 2nd harvest year, higher leaf-to-fruit ratio was found under NI than under IRR for all genotypes, except for Catuaí 99, while the most regular berry distribution among canopy strata was obtained in IRR ‘E083’, the highest bean yield in IRR ‘E083’ and Iapar 59, and the highest percent of useful bean biomass in NI Catuaí 99. The reduced lipid content under IRR was more important in the 1st (all genotypes) than in the 2nd harvest year (Iapar 59 and ‘E027’). As a novelty, chemical bean composition was additionally impacted by light availability along the canopy strata. Proteins declined from bottom (shaded) to upper (highly light exposed) strata, regardless of genotype and harvest year. Similar stratification was observed in caffeine in the 2nd year. Although some traits were somewhat changed among strata, no substantial quality changes occurred, thus allowing that harvest might include the entire plant and not only some specific strata. Iapar 59 and ‘E083’ showed chemical composition usually associated with high bean quality, with the highest lipid, sucrose, and soluble sugar contents, and the lowest caffeine, chlorogenic acids, and phenolic components among four genotypes, but Iapar 59 plants were less affected in their yield under NI. Based on additional responses from space occupation and yield only under IRR, the wild accession ‘E083’ must be considered in future breeding programs as promising material for intensive input conditions. High bean quality and the less variated yield under lower soil water availability qualified the Iapar 59 as the most prominent among the four genotypes.
Keywords:
biomass; caffeine; chlorogenic acids; Ethiopian accessions; irrigation; leaf-to-fruit ratio; lipids; proteins; sucrose 1. Introduction
Among the 130 species from the Coffea genus [1], C. arabica L. and C. canephora Pierre ex Froehner commercially dominate the coffee trade [2]. Coffee plays a relevant role for the subsistence of nearly 25 million coffee-farming families from about 80 developing countries of Asia, Africa, and Latin America [3]. C. arabica contributes today to about 60% of the world’s coffee consumption [4], and C. canephora to the rest.
Coffee trees build their architecture following the Roux model, considering a continuous growth and dimorphism of branches—orthotropic (1st order) and plagiotropic [5]. In C. arabica, the plagiotropic axes develop from the 2nd to the 5th orders [6]. Coffee metamer (basic architectural segment in tree construction) is characterized by internode, two leaves, and two serial buds (up to 5–6 buds formed in each leaf axil). Buds may differ in either inflorescence (up to 4 flowers from each bud in C. arabica), or more plagiotropic branches [7]. Therefore, the maximum possible flower/fruit number produced by one metamer is 40. All plagiotropic orders bear fruits, especially in late production years, but the 5th order axes are very rare [6,8].
The alternate vegetative and reproductive phases, six in total [9], occur in the coffee plant over a two-year cycle, with the coexistence of two distinct phenophases within the meta-population of axes and metamers in a same moment of the biennial cycle [6]. When the number of daylight hours begins to decrease, such photoperiod changes can induce the differentiation of the reproductive buds [10]. These buds grow and enter in dormancy, matching with the dry season in most growing regions [7]. After the first rainfalls, the break of bud dormancy initiates anthesis, or floral opening. In non-equatorial regions, encompassing most Brazilian coffee production areas, blossom occurs at different periods (from August to November) in two or more unsynchronized flushes [11], with the latter promoting the simultaneous presence of mature and immature berries at harvest [6,12]. Expression of coffee florigen suggests a continuum of floral induction that allows different starting points for floral activation, explaining developmental asynchrony and prolonged anthesis events in coffee [13]. After fertilization, fruits develop along ca. 6–9 months, with successive divisions and elongation of the perisperm and endosperm tissues [14].
In the mature coffee fruit (drupe, also called coffee cherry or berry), the exocarp is red or yellow. The berry consists of pericarp and seeds or beans (usually two in C. arabica). To obtain the commercial coffee beans, the pericarp outer skin, pulp, pectic adhesive layer, and parchment (usually together with bean silverskin) are removed, through either dry or wet processing [15]. The remaining part of the coffee beans (processed beans) are then roasted using dry heat at temperatures usually between 200 and 240 °C, with constant stirring to ensure even heat distribution [16].
To obtain a good fruit development, a certain leaf area expressed in leaf-to-fruit ratio is required [17]. Consequently, plant investment in leaf area development must increase with increased leaf area index, to sustain the growth and maintenance of berries. This requirement increases with plant density [6], considering that leaf photosynthesis decreases in the lower canopy layers with greater self-shading [18].
Arabica coffee beans contain up to 15% nitrogenous compounds (10–11% proteins, 0.9–1.3% caffeine, 0.6–2.0% trigonelline, 0.5% of free amino acids), 15–18.5% lipids, 50–60% carbohydrates (6–9% sucrose, 0.1% reducing sugars, 33–44% polysaccharides, 3% lignin, 2% pectin), 3–4.2% minerals, and 4.1–7.9% chlorogenic acids [19]. Under different environmental conditions, the same coffee species, or even genotype, can produce coffee beans with a wide chemical composition [16,20], flavors, and aromas or sensory attributes [21,22]. The estimation is that chemical bean attributes and coffee cup quality are affected by 40% pre-harvest, 40% post-harvest, and 20% export handling [23]. In fact, plant performance, bean yield, and quality can be altered by: (1) genotype and its geographical origin [16,24], (2) environmental conditions, such as soil, topography, altitude and climate [2,25], shade density under agroforestry [24,26,27], and (3) cultural management practices, such as planting density [28], mineral fertilization [29], irrigation [30,31], pruning [32], or even “CO2 fertilization” [18,31,33]. The chemical composition is less impacted by biennial cycle [34], or year of production [22], but the year impact can be significant in some experiments [28].
To ensure large, high-quality seed yields, abundant water availability is crucial during the period of rapid berry expansion [35]. In drought-impacted regions, coffee yield increases with irrigation [36,37], but contradictory results were obtained about the impacts of this management practice on coffee bean and/or cup coffee quality. Partial root zone drying and normal deficit irrigation seemed to preserve cup coffee quality [36], although only minor changes of bean quality were found under irrigation [38]. The 5-caffeilquinic acid (5-CQA), the main chlorogenic acid (CGA) isomer found in coffee seeds, and the lipid content do not vary with irrigation, while coffee bean sucrose and caffeine contents are found to increase in non-irrigated coffee plants grown in warmer regions [38]. Other reports pointed to greater caffeine and CGA contents in beans of irrigated than in non-irrigated plants [30]. Additionally, low shading (ca. 30% irradiance reduction) can enhance dry bean yield, and total sugar and CGA contents in coffee beans [37]. Some evidence suggests that increased altitude (with a more prolonged maturation due to lower temperatures), as well as shade, might improve the sensory attributes of coffee [39]. Contradictory reports about environment and management practice impacts on coffee quality that might result from distinct C. arabica cultivars used in the studies. They intrinsically respond differently to shade/altitude, from yield to quality aspects [26,27], further interacting with management conditions that strongly influence the outcome, or with adverse thermal/light and water availabilities that can greatly reduce the potential bean yield and quality [30,40]. This can interact with harvest year, for example, protein and caffeine contents increase and lipids decrease in inferior plant layers (self-shading) only in latter harvest years [28].
One of the research gaps is related to shifts of coffee yield and quality (increase, decrease, or non-linear of principal primary and secondary metabolites) and their dependency on water deficit and/or light conditions in different genotypes. It was hypothesized that, depending on genotypes, the additional soil water availability and light availability along the canopy strata, could impact on berry yield and bean chemical attributes, from the initial harvest years. To examine this hypothesis, the variations in chemical traits of coffee beans and productivity were evaluated along the canopy strata of plants cultivated under natural rainfall or with supplementary irrigation, in four cropped genotypes (Iapar 59, Catuaí 99, ‘E027’ and ‘E083’) of different genetic origins, in two subsequent harvest years.
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
Seedlings of Coffea arabica L. from the approximately 100 Ethiopian wild accessions and the two test cultivars (Iapar 59 and Catuaí 99) were established in 2009 in nursery. They were planted in 2010 in the experimental fields of IAPAR, Londrina (23°18′ S and 51°17′ W, altitude 620 m.a.s.l.), Paraná state, Brazil. Coffee rows were oriented east–west, with 2.5 m distance among them, and 0.5 m between plants in the row (planting density of 8000 plants ha−1), with four repetitions (plants) of each genotype in each of two water regimes. Plants were randomly distributed in the experimental field. Two Ethiopian wild accessions (‘E027’ and ‘E083’) were chosen because of their outstanding architectural characters shown in spring 2011. The ‘E027’ presented visually large and long leaf blades, branched structure, and very few flowers, whilst the ‘E083’ had smaller elongated leaves, a high number of flowers, and quick vegetative space occupation by higher order branching. Iapar 59 is originated from the cross between the cultivar Villa Sarchi CIFC 971/10 and hybrid of Timor CIFC 832/2, representing C. canephora introgression by spontaneous specific cross with C. arabica [41], while Catuaí 99 is a highly productive C. arabica cultivar characterized by high cup quality [42].
The soil was dusky-red dystrophic latosol, characterized by 790.02 g clay, 160.31 g silt, and 49.67 g sand per kg of soil particle-size in 0 to 0.20 m depth layer [43]. Climate is subtropical, Köpen–Geiger climate type Cfa, with average annual precipitation of about 1585 mm, ranging from 55 mm in the driest month (August) to 245 mm in wettest one (January). The limiting factors for C. arabica growth in Cfa climate are defined by low autumn and winter temperatures [44], such that almost all plants from this experiment died after the strong frost occurred after harvest in 2013, precluding the experimental continuation. The data of 2012–2013 daily rains were obtained from the IAPAR local meteorological station.
Coffee plants were cultivated under rainfed (not irrigated—NI) and with additional irrigation (irrigated—IRR) water regimes. For the latter, drip irrigation was implemented, being triggered based on a soil water balance method, aiming at supplying the difference of rain and soil storage [45]. The irrigation intensity was 3.5 L h−1 by dripper, placed near to the trunk of each coffee plant. Fertilization NPK (20:5:15) was added at 1000 kg ha−1 year−1, split in four times during the most demanding phenophases of the culture.
Light irradiance, measured as photosynthetic photon flux density (PPFD), was obtained from stational sensors distributed along the plant canopy strata, starting from 20 cm from the soil (average height of coffee trunks), and positioned at every subsequent 40 cm of the plant height (60 cm, 100 cm). They were installed in one representative plant of each genotype and each water regime. PPFD was measured before the harvests, during one representative cloudy day, in June of 2012 and 2013. Stational photodiode sensors (Hamamatsu G1118, Japan) used in the experiment were calibrated with sensors (LI-COR 190R, Lincoln, NE, USA). For data acquisition and storage, a datalogger (CR21X, Campbell Scientific, Logan, UT, USA) and a multiplexer (AM416, Campbell Scientific, Logan, UT, USA) were used, allowing the simultaneous data collection of up to 32 irradiance sensors. This included Hamamatsu sensors positioned at the base of each existing stratum along the plant canopy strata of representative plants, while one LI-COR 190R was installed in the middle of the field, as a PPFD reference, at 2 m height (thus, above the plant top). Data were collected every 60 s and were presented as mean values for each 15 min interval.
2.2. Plant Coding, Computational Processing, Berry Harvests, and Yield
Coffee plant coding was performed in the two harvesting periods, in June of 2012 and 2013. Harvest was effectuated in two or three passages, two weeks apart, always collecting only red (cherry) berries under adequate state of maturation. For berry collection, plant canopy was divided in four strata (S): S1: 20–60 cm (or <60 cm); S2: 61–100 cm; S3: 101–140 cm; S4 > 140 cm (Figure 1).
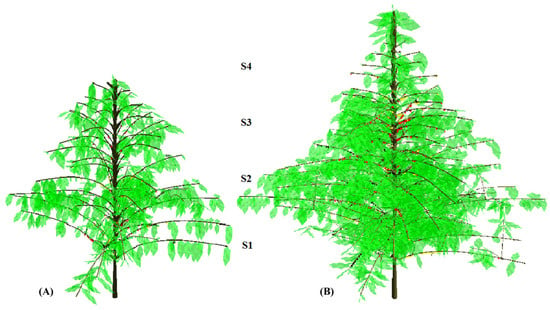
Figure 1.
Snapshots of 3D reconstruction of one ‘E083’ irrigated plant in the (A) 1st (2012) and (B) 2nd (2013) harvest year, illustrating the strata along plant canopy: S1: 20–60 cm (or <60 cm); S2: 61–100 cm; S3: 101–140 cm; S4 > 140 cm.
Topological and geometric codification of coffee trees was performed in three botanical scales—metamers, branches, and plants [8] in multiscale tree graphs [46]. The orthotropic axes were always described at metamer scale, collecting a maximum number of variables, including length of each metamer, leaf position, size and elevation, orientation, and total length of all plagiotropic branches inserted in the orthotropic axis. Four representative 2nd order plagiotropic axes were sampled (one for each cardinal point) in each stratum above the trunk [42]. The sampled plagiotropic axes were described in detail following the same logic as orthotropic axes. In addition, the 3rd to 5th order plagiotropic lateral branches that belonged to the sampled and decomposed 2nd order branches were also described in detail. All other 2nd order plagiotropic axes were described according to their positions along the orthotropic axis, considering elevation and orientation cardinal points, total live length of the axes, mortality/vivacity of 2nd order plagiotropic axes terminal apex, supported by total number of berries [47]. The plants were reconstructed using the VPlants modeling platform [48]. The reconstructions followed the specific proposed modules use, such as AmostraCafe3D, VirtualCafe3D, and Cafe3D and procedures [6,47]. Reconstructed plants (Figure 1) permitted the calculation of leaf and branch area. In this paper, only the information about the total berry number, leaf/branch area per plant, together with berry position relative to the trunk distance, or over the strata were estimated from the virtual 3D plants (Figure 1). This information was used to calculate the ratio of average leaf-to-fruit and branch-to-fruit ratios (cm2 berry−1) of plants in the two harvest years.
The berry yield of coffee cherries (fresh berry mass, FM) was measured separately for each plant and each plant stratum. The collected berries were dried in the sun at a concrete yard until 12.5% of moisture (dry berry mass, DM). Afterwards, the pulp and parchment were removed, and only beans without visual defects (processed bean mass, BM) were selected for further analysis. The initial berry moisture was calculated from the ratio of DM to FM. The dry and processed bean mass performances were calculated as percent of BM or DM to FM, respectively.
2.3. Chemical Attributes of the Coffee Beans
The processed beans were stored in a dry local on paper bags. The coffee beans from each genotype, stratum, water availability, and harvest year were frozen with liquid nitrogen at −196 °C for chemical analyses, ground in a laboratory disk mill (Perten 3600, Kungens Kurva, Sweden) to a particle size of 0.5 mm, packed in plastic bottles, and kept at −18 °C until analysis. The determination of protein (PRO), caffeine (CAF), lipids (LIP), sucrose (SUC), total soluble sugars (TS), total chlorogenic acids (CGA), and total phenolic components (PC) in coffee beans was performed using a near infrared spectroscopy (NIR, SYSTEM 6500 spectrophotometer, Foss-Perstorp employing ISIscan software, Foss, Silver Spring, MD, USA). The NIR reflectance spectra were collected at 2 nm intervals from 1100 to 2500 nm using a rectangular cell containing 6 g of ground coffee beans, and data were saved as the average of 32 scans. The two replicates of the NIR spectra were collected for each coffee sample. The blank spectrum was used from the ceramic plate supplied with the instrument. The ISIscan software package was used to control the recorder, collect the spectra, import, and analyze the data. The concentration of each compound was calculated using the prediction models for coffee beans, developed by Scholz et al. [49].
2.4. Statistical Analyses
The ‘R’ software [50] was used for all statistical analyses. The experimental design was completely randomized, with plant or stratum as statistical units, and four repetitions. Data were subjected to the two-way analysis of variance (ANOVA), after testing the hypothesis of variance homogeneity. ANOVA considered a mixed linear model (‘nlme’ package) and maximum likelihood to test the significance of differences between two water regimes (IRR and NI), and four genotypes (‘E083’, ‘E027’, Iapar 59, and Catuaí 99). If no significant interaction was found, the model reduction was applied and fitted. The significance of local strata conditions (three in 2012 and four in 2013) was estimated for each genotype, not including the water regime as a 3rd factor, to simplify the model and explanations. In comparison among the averages estimated by the ANOVA models, the Tukey HSD test with the significance of 0.05 was used, supported by ‘lsmeans’, and ‘multcompView’ packages. In the 1st harvest year (2012), the ‘E027’ showed extremely low frequency or even lack of berries in plants/strata, which was insufficient for any kind of further chemical analyses.
To analyze the radial and vertical distribution of berries in coffee trees, the resulting 3D reconstructions were explored, using exact berry positions in the Cartesian coordinate system (x, y, z). The cumulative empirical distribution weighted by the inverse of the total number of berries per plant (function ‘Ecdf’, ‘Hmisc’ package) were estimated. The weighted Kolmogorov–Smirnov two-sample test was used to compare water regime levels for each genotype (function ‘ks_test’, ‘Ecume’ package) separately for each harvest year. Finally, the measured and calculated fruit architectural, yield, and chemical parameters along the plant canopy strata were correlated separately for each harvest year, and graphically presented using the ‘Hmisc’ and ‘corrplot’.
3. Results
3.1. Environmental Conditions—Rainfall and Light Distribution along the Plant Canopy Strata
The experimental fields were localized in Northwest of Paraná state, Brazil (Figure 2A). During the experimental period, one not usual summer drought occurred in the beginning of 2012, in the phenophase of leaf area and berry expansion (Figure 2B), the critical phase for coffee yield and quality. The winter of 2012 was very dry, preceding the blossom. The early autumn dry period in 2013 matched with the phenophases of berry maturation [9], and the last dry period (winter of 2013) was used for berry harvest. The experiment finished due to strong frost that killed almost all plants in the experimental area, with exception of four IRR ‘E083’ plants.
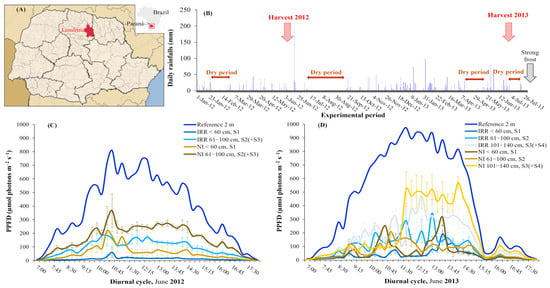
Figure 2.
Experimental site localization and the environmental factors during experiment. (A) Londrina localization is tagged in red at Northwest of Paraná state, Brazil; (B) Daily rainfalls during the experimental period, periods of 2012 and 2013 harvests, dry periods, and the strong frost that ended the experiment are tagged. Photosynthetic photon flux density (PPFD, μmol photons m−2 s−1), values (means ± standard errors, n = 4), registered along the plant canopy strata and in the reference (2 m above soil) in (C) June 2012, and (D) June 2013.
Irradiance strongly and progressively decreased from the top to the bottom layers of plant canopy (Figure 2C,D), even in the 1st harvest—2012 (Figure 2C), with quite small plants. In fact, PPFD values registered at plant base (<60 cm) showed that a large part was intercepted by leaves, since from the ~700 μmol photons m−2 s−1 measured at 2 m height at noon (cloudy sky), only ~10 μmol photons m−2 s−1 and 55 μmol photons m−2 s−1 reached S1 in IRR or NI plants, respectively. Most irradiance was intercepted by S2 and S3, as inferred from the difference among PPFD of reference and at the bottom of S2(+S3), in IRR and NI plants in 2012. In this year, the IRR plants intercepted more than NI plants, as shown by the PPFD at S2(+S3), thus pointing to a greater leaf area of IRR than NI plants.
A more complex light distribution along canopy strata occurred in the 2nd harvest year (2013, Figure 2D), due to an additional leaf stratum (S4, Figure 1B) as compared with 2012 (Figure 1A), which increased light interception (Figure 2D). The S3 + S4 strata would have lower leaf area to intercept the incident light in NI than in IRR plants, as inferred from the higher light measured at the bottom of S3 in NI plants, when compared to their IRR counterparts (Figure 2D), in line with the 3D reconstruction (Figure 1B).
3.2. Plant Scale—Berry Production and Distribution, Leaf-to Fruit, and Branch-to Fruit Dependency on Water Regime and Genotype
Total berry number per plant in 2012 greatly differed among the four genotypes, between ca. 530 (‘E083’ = Iapar 59) and ca. 50 berries (‘E027’), but with no significant impact of water regime, despite a common tendency to somewhat lower values in NI plants of all genotypes except ‘E027’ (Figure 3A). In the 2nd harvest year, the ‘E027’ also showed the lowest berry number per plant under IRR, whilst the other three genotypes showed similar values, close to 3000 (Figure 3B). Contrasting with the 1st harvest year, significant reductions of berry number per plant were observed under NI in all genotypes except in Catuaí 99, as compared with their respective IRR plants.
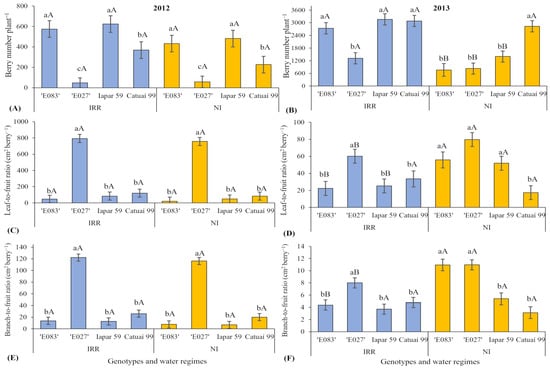
Figure 3.
Reproductive and vegetative investments at plant scale: (A) Berry number in 2012 and (B) 2013; Leaf-to-fruit ratio (cm2 berry−1) in (C) 2012 and (D) 2013; Branch-to-fruit ratio (cm2 berry−1) in (E) 2012 and (F) 2013) of four genotypes of Coffea arabica cultivated under two water regimes (irrigated-IRR; not irrigated-NI). Mean values ± standard errors (n = 4) are shown. Lower-case letters compare genotype effects within each water regime; upper-case letters compare water regime effects within each genotype, always separately for each harvest year.
In 2012, the greatest leaf-to-fruit ratio was found in ‘E027’ (Figure 3C), and with quite low values in the other three genotypes, but without impact of soil water conditions in all genotypes. In 2013, this parameter still presented maximal values in ‘E027’, but in all genotypes presented much lower values than in 2012. Additionally, the response to soil water availability was different in 2013, when greater leaf-to-fruit ratio values were found under NI than under IRR for all genotypes, except Catuaí 99 (Figure 3D, p-value of Genotype × Water regime = 0.0330).
In the 1st harvest year, the branch-to-fruit ratio (Figure 3E) showed a similar pattern to the leaf-to-fruit ratio, with the highest values found in ‘E027’, quite low values in the other three genotypes, and without impact of soil water conditions in all genotypes (Figure 3E). In the 2nd harvest year, ‘E027’ showed higher values of branch-to-fruit ratio than the other three genotypes under IRR (Figure 3F). At the same time under NI, two wild Ethiopian accessions showed higher values of this parameter than two cultivars (significant interaction of Genotype × Water regime, p-value = 0.0012). In 2013, the branch-to-fruit ratio had higher values under NI than under IRR in two Ethiopian accessions, but without soil water regime impact in two cultivars.
In both harvest years, the distribution of berries along the height of the plant canopy differed between the two water regimes, in all four studied genotypes (Figure 4A). Higher density of berries in NI than in IRR was observed in lower plant strata. The berry distribution reached lower height in NI than in IRR plants of all genotypes, in both harvest years. In 2013, the IRR ‘E083’ showed a regular berry distribution along the canopy height that tended to linearity, attaining 200 cm of height, the highest among four genotypes.
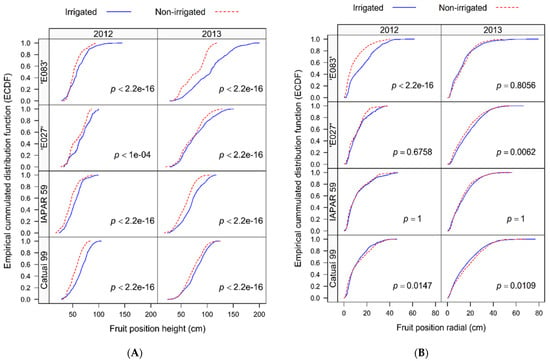
Figure 4.
Empirical cumulative distribution function (ECDF) for berries along the (A) height of the canopy and (B) radial plant profile analyzed for four genotypes of Coffea arabica cultivated under two water regimes (irrigated and not irrigated). The p-values are shown (n = 4), comparing water regimes, separately for each genotype and harvest year (2012 and 2013).
Additionally, the radial berry distribution (horizontal distribution over x- and y-axes) was also assessed, showing slightly delayed berry accumulation over the plagiotropic branches in NI compared to IRR in Catuaí 99 plants, in both harvest years (Figure 4B). The opposite, i.e., the delayed berry accumulation over the plagiotropic branches in IRR than in NI plants was calculated for ‘E083 in the 1st harvest year and for ‘E027’ the 2nd harvest year. The radial berry occupation zone referent to trunk attained the maximum from ca. 40 cm (‘E027’) to 60 cm (‘E083’) in the 1st harvest year. In the 2nd harvest year, the radial zone of berry occupation attained the maximum from ca. 60 cm (Iapar 59) to 80 cm (‘E083’ and Catuaí 99).
3.3. Components of the Berry and Bean Yields at Plant Scale Dependent on Water Regime, Genotype, and Local Light Availability
In the 1st harvest year (2012) the yield components (fresh dry mass (FM), dry mass (DM), processed bean mass (BM)), and mass performances were positively impacted by irrigation in the three analyzed genotypes (Table 1). In this year, greater initial berry moisture (indicator of delayed fruit maturation), and lower processed bean mass performance were found in Catuaí 99 than in ‘E083’, while Iapar 59 showed intermediary values.

Table 1.
Components of the berry and bean yields: fresh berry mass (FM), dry berry mass (DM), and processed bean mass (BM) (g plant−1), initial berry moisture (%), DM and BM performances (%) of two harvests (2012 and 2013) analyzed in four genotypes of Coffea arabica cultivated under two water regimes (irrigated—IRR; not irrigated—NI). Estimated means and p-values are shown (n = 4), where “-“ indicates that the model reduction and fitting were applied. When significant, p-value is marked in bold. Lower-case letters compare genotype effects within each water regime; upper-case letters compare water regime effects within each genotype, always separately for each harvest year.
In the 2nd harvest year (2013), important increments in berry FM, DM, and bean BM were obtained due to supplementary irrigation, as compared to NI values (Table 1). Those increments were higher for two Ethiopia accessions than for two cultivars, although ‘E083’ and Iapar 59 did not differ as regards their absolute values under IRR conditions. The mass performances were positively impacted by irrigation for ‘E027’ and Iapar 59, while the opposite pattern was found for ‘E083’ and Catuaí 99 in the 2nd harvest year. Among the four genotypes in 2013, the DM and BM performances showed maximum values in Catuaí 99, followed by ‘E083’, both of which with greater values than Iapar 59 and ‘E027’, with the latter showing the smallest values. In 2013, berries of Catuaí 99 had the lowest initial berry moisture among the four studied genotypes, meaning the most advanced maturity of Catuaí 99.
The variation in yield parameters was additionally studied among the canopy strata of studied genotypes, irrespective of water regime. In 2012, no significant differences in FM, DM, BM, or initial berry moisture were observed among three canopy strata in three studied genotypes, despite the variation in estimated average values (Table 2). The DM and BM performances showed lower values in Catuaí 99 than in other genotypes, in all canopy strata, in 2012.

Table 2.
Components of the berry and bean yields: fresh berry mass (FM), dry berry mass (DM), and processed bean mass (BM) (g stratum−1 initial berry moisture (%), DM and BM performances (%) of two harvests (2012 and 2013) analyzed in four genotypes of Coffea arabica along the canopy strata (S1: 20–60 cm; S2: 61–100 cm; S3: 101–140 cm; S4: > 40 cm). Estimated means and p-values are shown (n = 2–4), where “-“ indicates that the model reduction and fitting were applied. When significant, p-value is marked in bold. Lower-case letters compare genotype effects in each stratum, whilst the upper-case letters compare stratum effects in each genotype, always separately for each harvest year.
Notably, in 2013, the highest FM, DM, and BM values were obtained in Iapar 59, and the lowest in ‘E027’ consistently in all canopy strata (Table 2). The highest and lowest values of DM and BM were found in S3 and S1, respectively, for all genotypes, showing greater berry production in the stratum that received high PPFD. In 2013, the initial berry moisture did not differ among strata, but was the lowest in Catuaí 99, opposite to BM performance that was the highest in this genotype. The DM performance showed the interaction of stratification with genotype: it was stable among the strata in ‘E083’ and Iapar 59; had the highest values in S2 and the lowest in S1 (two self-shaded strata) in ‘E027’; and had the highest values in the well-lighted S4 in Catuaí 99. Among the four genotypes, Catuaí 99 showed the lowest DM performance in the S1, intermediate in S2, but the highest in S3 and S4, which was related to low initial berry moisture in this genotype. This genotype also showed the highest BM performance in all strata among the four genotypes, suggesting its lowest losses in bean processing.
3.4. Chemical Attributes of the Coffee Dependency on Water Regime, Genotype, and Irradiance Availability
High water availability (IRR) significantly altered only PRO and LIP contents, which were reduced although not in great extent (between 5 and 6%) in the 1st harvest year in all genotypes (Table 3). Furthermore, these compounds were mostly unresponsive to water conditions in the 2nd year, with a marginal (but significant) decline of LIP content only in ‘E027’ and Iapar 59 under IRR. On the other hand, the PRO, LIP, CAF, and CGA varied among studied genotypes in the 1st harvest year, and in all studied components in the 2nd harvest year in both water conditions. The highest PRO and CAF contents were found in Catuaí 99 and Iapar 59 in the 1st harvest year, and in Catuaí 99 in the 2nd year, when this genotype also showed the greatest contents of CGA and PC in both water regime. By contrast, Catuaí 99 showed the lowest values of LIP, SUC, and TS in the 2nd harvest year, with the highest contents of those compounds being found in ‘E083’ and Iapar 59 under both water regimes.

Table 3.
Chemical contents (% of dry matter): PRO (proteins), LIP (lipids), CAF (caffeine), SUC (sucrose), TS (total soluble sugars), CGA (total chlorogenic acids), PC (phenolic compounds) of two harvests (2012 and 2013) analyzed in four genotypes of Coffea arabica cultivated under two water regimes (irrigated—IRR; not irrigated—NI). Estimated means and p-values are shown (n = 4), where “-“ indicates that the model reduction and fitting were applied. When significant, p-value is marked in bold. Lower-case letters compare genotype effects within each water regime, whilst the upper-case letters compare water regime effects within each genotype, always separately for each harvest year.
Additionally, the variation in chemical composition of coffee beans was analyzed along the canopy strata in studied genotypes (Figure 5). In the 1st harvest year, only PRO content showed a stratification, being the lowest in the upper S3 in all three analyzed genotypes, as compared to their respective S1 and S2 (Figure 5A). In the 2nd harvest year, additionally to PRO, bean CAF content of all four genotypes also varied among the canopy strata, with greater values found in S1 in all genotypes, and usually gradually decreasing at higher (S3 or S4) strata, that is, with greater irradiance (Figure 5B).
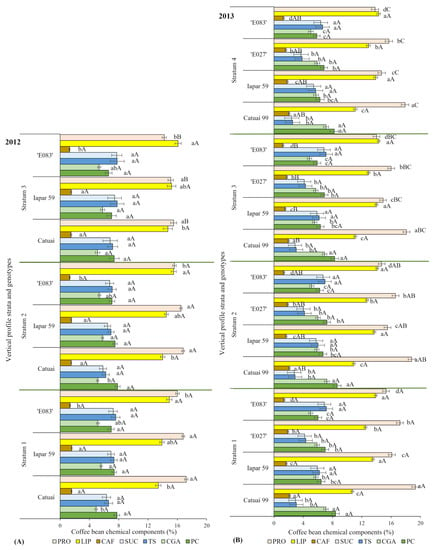
Figure 5.
Chemical contents (% of dry matter) of coffee grains: PRO (proteins), LIP (lipids), CAF (caffeine), SUC (sucrose), TS (total soluble sugars), CGA (total chlorogenic acids), PC (phenolic compounds) of two harvests, (A) 2012 and (B) 2013, analyzed in four genotypes of Coffea arabica among the plant canopy strata (S1: 20–60 cm; S2: 61–100 cm; S3: 101–140 cm; S4: >140 cm). Estimated means ± standard error (n = 2–4) are shown. Lower-case letters compare genotype effects in each stratum, whilst the upper-case letters compare stratum effects in each genotype.
3.5. Correlations among the Berry Distribution per Strata, Yield, and Chemical Attributes
To synthesize the previous results, a correlation analysis was performed (Figure 6) considering berry distribution along the strata (sequential growing numbers for ‘Stratum’ in correlations), yield, and chemical coffee bean characteristics. In 2012, a decline of berry number was observed from S1 to S3 (Figure 6A), as a general response not observed when genotype and soil water availability impacts were analyzed (Table 2). Positive correlation of FM, DM, and BM was observed with increased canopy strata in 2013 (Figure 6B). Increased stratum height was associated with a decline of protein content in both harvest years (Figure 6A,B), decreased content in CAF in 2013 (Figure 6B), and increased in LIP in 2012 (Figure 6A). The FM over the plant canopy strata was positively associated to DM, BM in both years (Figure 6), and to increased initial berry moisture in 2012 (Figure 6A). The last suggested that in the first harvest year, the higher strata produced berries that that were induced lately (from latter flowering), gaining the red color rapidly, but still retaining more moisture than lower strata.
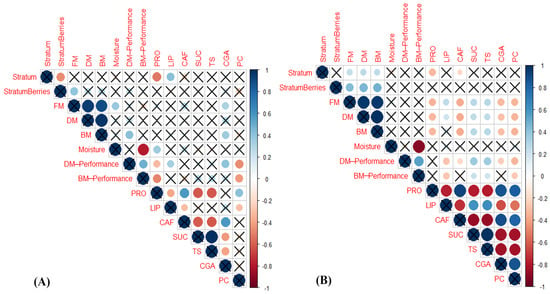
Figure 6.
Graphical presentation of coefficients (values corresponding to circle size and color intensities) and p-values < 0.05 (not crossed circles) for general correlations among berry distribution along plant canopy strata (Stratum (S1–S4), StratumBerries (number of berries stratum−1)), yield [FM and DM (fresh and dry berry mass stratum−1 respectively), BM (processed bean mass stratum−1), initial berry moisture, DM and BM performances, and chemical parameters (PRO (proteins), LIP (lipids), CAF (caffeine), SUC (sucrose), TS (total soluble sugars), CGA (total chlorogenic acids), PC (phenolic compounds)) of four Coffea arabica genotypes in (A) 2012, and (B) 2013.
Interestingly, greater FM and DM of berries per stratum was associated with greater LIP, SUC, and TS bean contents, and lower PRO, CAF, CGA, and PC values in 2013 (Figure 6B). The initial berry moisture was negatively correlated to BM performance in both harvest years (Figure 6), and positively to PRO grain content in 2012 (Figure 6A). The DM and BM performances were negatively associated with LIP, CAF, CGA and PC contents and positively to sugar contents in 2012 (Figure 6B).
The general patterns in bean chemical composition in both harvest years were negative correlations of PRO to LIP, SUC, and TS contents, and positive to CAF, TC, and PC, coherent with the opposite situation observed in LIP (negative correlations to CAF, TC, and PC, and positive to SUC and TS contents), especially in 2013 (Figure 6B). TS and SUC were negatively correlated to CGA and PC compounds.
4. Discussion
Coffee bean quality results from complex interactions between the relation of genetics and environment, which influences the presence/content of more than 1000 bean components [21]. The ongoing climate changes and the predicted future conditions demand evidence-based insights as regards coffee yield and quality, to guarantee the sustainability and promote resilience of the coffee sector, from field to cup [39], namely through plant breeding and adequate crop management. Here, we provided some novel data regarding yield and bean quality traits in the two initial harvest years, associated with water management, genotype, and microenvironment irradiance from the bottom (shaded) to the top (highly lighted) strata of plant canopy.
Additional irrigation significantly increased the berry number plant−1 in the 2nd harvest year (except Catuaí 99) (Figure 3) and on FM, DM, and BM in both harvest years (Table 1). This denoted a positive impact of irrigation on plant yield potential, in line with the previously reported gains in accumulation of biomass per fruit in C. canephora cv. Conilon [51]. In 2013, IRR plants showed strong increases of FM, DM, and BM in all genotypes, with ‘E083’ having the strongest (up to ca. 500%) and Catuaí 99 moderate yield improvements (ca. 50%). Conversely, the extent of the yield reduction (especially of BM) under NI conditions in the 2nd harvest year, was lower in Iapar 59 and Catuaí 99 than in the two Ethiopian wild accessions (Table 1). The studied coffee plants under NI experienced some dry periods (Figure 2B). The molecular mechanisms of drought-resistance in C. arabica genotypes involve ABA signaling, together with predominance of protective genes expression, associated with antioxidant activities, including genes involved in water deprivation and desiccation [52]. That could have been the case in Catuaí 99 and Iapar 59 plants under NI, as judged by their elevated berry/bean yielding (Figure 3, Table 1). Such lower sensitivity to lower water availability in Iapar 59 than in other genotypes could be related to its C. canephora introgression genetics [41].
The initial berry moisture is about 55–65%, which after drying falls to ca. 12% [53]. Our results of initial berry moisture (54–67%) were close to the usual values [53], although they additionally differed among the genotypes in the 2nd harvest year, the lowest being in Catuaí 99, indicating its advanced fruit maturation [54], as compared to the other three genotypes (Table 1 and Table 2). As the exocarp is more attached in immature berries, which will induce more breaks and losses during the dry processing than in mature berries [15], consequently diminishing the bean mass. The bean mass performance was 12.1–20.6% in 2012 and 14.9–24.3% in 2013. In 2012, this trait was higher under IRR than under NI in all genotypes, but values differed among the genotypes, the highest in irrigated being ‘E083’ and the lowest in not irrigated Catuaí 99 (Table 1). Those trends were strongly modified in 2013, showing the greatest values in NI Catuaí 99, followed by ‘E083’, and with the lowest values in ‘E027’. This suggested that Catuaí 99 (and ‘E083’) plants, with increased yield in the 2nd compared to the 1st harvest year, especially under IRR, could have higher flowering and maturation uniformity and earlier maturation, which impacted on higher yield performances than other studied genotypes. The higher flowering uniformity is normally expected from IRR than from NI coffees [55], but the Catuaí 99 and ‘E083’ responses in the 2nd year suggested their advanced maturation, and lower bean defect contents under lower water availability, even if a much greater yield was obtained under IRR conditions.
The leaf-to-fruit and branch-to-fruit ratios differed among the genotypes in both harvest years, always being the highest in ‘E027’ (Figure 3C–F), which resulted mainly from the lowest berry yield among these genotypes, and a concomitant elevated investment in vegetative structures (Table 1 and Table 2). Leaf-to-fruit ratio is an expression of source–sink ratios considering growing sinks and maintenance of sources [56]. Previous reports estimated that ca. 20 cm2 of leaf area is needed to support each coffee berry development [17]. Still, this value can be somewhat smaller in heavily bearing coffee trees [57], in line with our smallest values (ca. 17 cm2 berry−1). Leaf-to-fruit ratios below the 20 cm2 berry−1 were registered in NI plants of ‘E083’ in the 1st harvest year, while in the 2nd year, those values were higher than 20 cm2 berry−1, and greater in NI than IRR plants (except in Catuaí 99). These high values of leaf-to-fruit ratio, although being positive for bean development, also suggest that the berry production was relatively more impacted by low soil water availability (Table 1) than the vegetative growth [40]. The elevated branch-to-fruit ratios under NI in ‘E027’ and ‘E083’ in 2013 showed relatively higher structural than reproductive investments of Ethiopian accessions than of test cultivars, indicated a higher sensitivity of former to lower water availability.
Greater concentration of some secondary metabolites of coffee beans is usually associated with increased sensory attributes (e.g., trigonelline), whereas an increase of others (e.g., feruloylquinic acids) is often associated with a decline in sensory attributes, although with a high degree of uncertainty resulting from their specific thresholds, impacts, and cross interaction [21,22,39]. In fact, some compounds, such as chlorogenic acids, are associated to both positive and negative impacts in cup quality, depending on their absolute values and their interactions with other chemical compounds in each specific coffee [2,58]. More than 30 chlorogenic acid isomers are detected in coffee beans, as bioactive compounds with antioxidant activity against free radicals and metal ions [59]. They accumulate in the beans as the berries mature, and greatly contribute to the final acidity, aroma, flavor, bitterness, and astringency of the coffee beverage [2]. The presence/content of primary metabolites (proteins, lipids, and carbohydrates) are also related to cup quality [22,60]. In both harvest years, the highest PRO and CAF and the lowest LIP contents, together with the highest CGA and PC contents in the 2nd harvest year were registered in Catuaí 99. In addition, the highest LIP, SUC, and TS content was found ‘E083’, irrespective of harvest year or water regime (Table 3). On the other hand, the most elevated CGA and PC content were found in Iapar 59 in the 1st harvest year, showing strong genetic impact on bean chemical quality. Phenolic compounds are secondary metabolites that can be involved in leaves during the plant adaptation to environmental stress conditions, among them the chlorogenic acids that are the main components of the phenolic fraction of coffee beans [61]. Chlorogenic acid impacts on the beverage are more dependent on roasting level than on coffee species [58], but we found here that CGA can be genotype dependent.
Iapar 59 has more genetic variation, more uniform maturation, and earlier yielding than Catuaí 99 [62]. In our experiment, the berries were collected when they were visually mature in all plants, excluding maturity as chemical composition factor. Later berry harvest performed in the same year can increase LIP and reducing sugar and decrease CAF contents [63], but maturity states of red berries do not show differences in organic acids, free fatty acids, lipids, total chlorogenic acids, proteins, alkaloids, or sucrose [54]. Catuaí 99 is seen as a highly productive cultivar, of high bean quality for the final coffee cup [42], but here it showed elevated PRO, CAF, TCA, and PC contents, generally associated with lower cup quality [22]. ‘E083’, usually together with Iapar 59, seemed to present the best chemical components composition, with the highest LIP, SUC, and TS and the lowest CAF, CGA, and PC contents among genotypes, irrespective of harvest year or water regime (Table 3).
An interesting finding was that irrigation promoted a decline on LIP and PRO bean contents of all genotypes harvested in 1st harvest year, and on LIP of Iapar 59 and ‘E027’ in the 2nd harvest year (Table 3). This can be an interesting issue since, lipids of C. arabica beans are responsible for flavor carriers, texture, and mouthfeel in the beverage [19,64,65,66]. Additionally, sugars are also greatly present and intended components of coffee beans, with SUC being the most represented TS irrespective of the environmental conditions [2], in line with the absence of changes in SUC and TS between water conditions (Table 3). Shade is reported to either reduce sucrose content [67,68], or increase reducing sugars in coffee beans [69], contrary to our findings that, in turn, agreed with absence of effect of light level on the content of sucrose, glucose, fructose, arabinose, and total soluble sugars [2]. In this regard, the greater values LIP, SUC, and TS could have a potential positive impact in cup quality [70], thus pointing to a greater potential bean quality in Iapar 59 and ‘E083’, despite the relevant sensitivity to low water availability in the latter, as reflected in the greatest yield decline from IRR to NI conditions.
The lower levels of CAF, CGA, PC in ‘E083’ in both harvest years, irrespective of water availability and in Iapar 59 in the 2nd harvest year (Table 3) could have positive implications on coffee quality [22], although with a certain degree of uncertainty. It is usually accepted that CAF and CGA contribute to coffee bitterness, and their significant rise under higher temperature conditions can result in poor bean quality [67,69]. Chlorogenic acids are greatly represented by monocaffeoylquinic acids (CQAs), among them by 5-CQA. The increase of CQAs level has an inverse association with cup quality, particularly concerning 5-CQA [21,71]. 5-CQA constitutes the major substrate for polyphenol oxidase, producing ortho-quinones that in turn will cause darkening of the beans and a worse coffee quality [72]. The 5-CQA rise is inconsistent with first grade coffee beans [73], as it is also characterized as low acidic, with a small amount of bitterness [58]. Furthermore, the presence of feruloylquinic acids and diCQAs isomers (also CGA) can have detrimental impact on the sensory properties of espresso coffee beverage, associated to greater bitterness, together with a metallic taste and astringency [74]. However, the impacts on cup quality of increased presence of chlorogenic acids and phenolic compounds would strongly depend on the specific compounds in each category and, mostly, with the interaction with other compounds [58]. Low soil water availability can promote the increase of CGA in the bean [75], which was not observed in our experiment regardless of genotype.
Altogether, lower water availability resulted in important yield decline but the direct impact on bean quality reported by others [30] was not likely to occur in our experimental conditions and genotypes. In fact, in 2013, with exception of LIP content in Iapar 59 and ‘E027’, no significant changes were depicted among the studied compounds, irrespective of genotype (Table 3).
The ‘E083’ presented the most expansive vertical and horizontal berry spatial occupations under irrigation (Figure 4). Despite Iapar 59 had less expansive space occupation than ‘E083’, the berry distribution tended to occupy the whole canopy strata, continuing with berry production in self-shaded strata, and producing the most in both ‘E083’ and Iapar 59 (Table 2, Figure 3). Judging by the produced fresh berry mass related to berry number, ‘E083’ produced the berries of smaller size than Iapar 59. In the 2nd harvest year, the highest biomass was produced in the 3rd stratum, and the lowest in the 1st and 4th strata, irrespective of genotype (Table 2). Among the studied chemical components considering both harvest years, only the PRO varied between the canopy strata, showing a pattern of increase from the top to the bottom in all genotypes, particularly in 2013 (Figure 5 and Figure 6). Under high irradiance, the common stress responses in green leaves are usually associated with efficient dissipation of excess of excitation energy, and antioxidant mechanisms, an increased abundance of proteins associated with the photosynthetic apparatus, together with relevant dynamics of the lipid profile of cell membranes [76]. As regards the bean, LIP fraction did not vary among the coffee canopy strata, which were submitted to contrasting irradiances (Figure 5 and Figure 6), thus showing a different response than that found in leaves. In the 2nd harvest year, CAF showed differences between S1 and S3, with lower values in the latter. In fact, under greater self-shading (S1), the beans tended to have greater CAF content and lower production, whereas moderately lighted S3 (Figure 2D) showed the opposite yield (Table 2) and CAF content (Figure 5 and Figure 6), confirming earlier findings [18].
Recently, various review studies tried to synthetize results about coffee responses to stresses associated with coffee quality and chemical bean composition, but many data are still contradictory [2,23,39,59]. Yield and chemical traits differed more regarding the strata in the 2nd than in the 1st harvest year, indicating that those interannual variations were apparently more related to overall increased variation in microenvironment light conditions among canopy strata due to tree growth, than to soil water availability. This rationale is associated to one dry period in the 1st harvest year that occurred in extremely dry summer, during leaf and berry expansion, the crucial phenophase for fruit development and quality, while in the 2nd harvest year one dry period occurred during berry maturation, when its impact was less detrimental. The greater was the grain biomass per strata, greater were the LIP, SUC, and TS, and lower the PRO, CAF, CGA, and PC bean contents in the 2nd harvest year (Figure 6), suggesting strong light/temperature impacts on berry content, and/or costs of investments of the primary and secondary metabolism products. The expensive investments in the bean in biosynthesis of secondary metabolites that play role in protection and acclimation to stress conditions in the leaves (PC, CGA, CAF) including the synchronization with primary metabolism (PRO, TS, and LIP fractions) [77], might indicate that will be prevalent under climate challenging conditions, which will continue to be a focus of world agriculture interest.
5. Conclusions
Our hypothesis was that, depending on genotype, additional soil water availability on one side, and light availability along the canopy strata on the other, could impact coffee berry yield and bean chemical traits, studied in the two initial harvest years. Additional irrigation strongly increased the berry number and yield, modifying the berry distribution, but revealed to influence only some chemical bean attributes (reducing LIP and PRO contents). On the other hand, the genotype factor impacted practically all observed variables. The microenvironment light variation impacted on berry distribution and only on PRO and CAF bean contents among canopy strata. All metrics were variable between the two the harvest years, namely, berry yield and distribution, leaf-to-fruit ratio, and chemical composition of the bean. Yield and chemical traits differed more regarding the strata in the 2nd than in the 1st harvest year, indicating that those interannual variations were apparently more related to overall increased variation in microenvironment light conditions among canopy strata due to tree growth, than to soil water availability.
Iapar 59 plants were the less influenced by lower water availability among the four genotypes, showing the lowest yield reduction from irrigated to not irrigated plants at the 2nd harvest year. CGA was shown as genotype dependent, being the highest in Iapar 59 and Catuaí 99, irrespective of water regime of strata. Among the studied chemical components, PRO presented the greatest variation among the canopy strata, with a clear trend to decline from the shaded (bottom) to the well-lighted (upper) strata, in all genotypes and both harvest years. Similar stratification (now between S1 and S3) was observed for CAF, but only in the 2nd harvest year. In this harvest year, the most regular berry distribution along the plant canopy strata was obtained in irrigated ‘E083’, the highest bean yield in irrigated ‘E083’ and Iapar 59, and the highest DM and BM performances in not irrigated Catuaí 99. Iapar 59 and the Ethiopian wild accession ‘E083’ showed chemical composition associated with high cup quality, with the highest LIP, SUC, and TS and the lowest CAF, CGA, and PC contents among studied genotypes. Overall, these genotypes showed intrinsic differences on yield potential, which was impacted by water availability and self-shading.
Although some chemical bean traits had varied among strata (PRO), no substantial quality changes occurred. These suggest that the entire plant could be harvested, not only some specific strata, without an important deleterious impact on quality. High bean quality and the less reduced yield under reduced soil water availability qualified Iapar 59 as the most prominent among the four genotypes. Based on berry vertical and radial space occupation, yield, bean quality, the Ethiopian wild accession ‘E083’ could also be an interesting genetic material for the future breeding programs, although to be used with irrigation input, together with high fertilization condition, as applied in this experiment.
Author Contributions
Conceptualization, M.R.; methodology, M.R. and M.B.d.S.S.; software, F.T.M.; validation, M.R. and J.C.R.; formal analysis, R.A.A.P. and M.R.; investigation, M.R. and M.B.d.S.S.; resources, M.R.; data curation, R.A.A.P. and M.R.; writing—original draft preparation, M.R.; writing—review and editing, J.C.R.; visualization, M.R. and J.C.R.; supervision, M.R.; project administration, M.R.; funding acquisition, M.R. and M.B.d.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research work was carried out with the support of Consórcio Pesquisa Café (Brazil) projects (02.09.20.008.00.00 and 02.13.02.042.00.00). The authors acknowledge the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil, for Invited Researcher fellowships awarded to M.R. (350509/2020–4) and the Fundação para a Ciência e a Tecnologia I.P., Portugal, awarded to J.C.R., through the units UID/04129/2020 (CEF), and UIDP/04035/2020 (GeoBioTec).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. The authors can provide the experimental data for all interested researchers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Davis, A.P.; Rakotonasolo, F. Six new species of coffee (Coffea) from northern Madagascar. Kew. Bull. 2021, 76, 497–511. [Google Scholar] [CrossRef]
- Cassamo, C.T.; Mangueze, A.V.; Leitão, A.E.; Pais, I.P.; Moreira, R.; Campa, C.; Chiulele, R.; Reis, F.O.; Marques, I.; Scotti-Campos, P.; et al. Shade and altitude implications on the physical and chemical attributes of green coffee beans from Gorongosa Mountain, Mozambique. Agronomy 2022, 12, 2540. [Google Scholar] [CrossRef]
- FAO. Available online: https://www.fao.org/markets-and-trade/commodities/coffee/en/ (accessed on 18 October 2022).
- Coffee Industry Statistics. Available online: https://coffeeaffection.com/coffee-industry-statistics/ (accessed on 4 November 2022).
- Hallé, F.; Oldeman, R.A.A.; Tomlinson, P.B. Tropical Trees and Forests—An Architectural Analysis; Springer: Berlin, Germany, 1978; p. 441. [Google Scholar]
- Rakocevic, M.; Matsunaga, F.T.; Baroni, D.F.; Campostrini, E.; Costes, E. Multiscale analyses of growth and berry distributions along four branching orders and vertical profile of Coffea arabica L. cultivated under high-density planting systems. Sci. Hortic. 2021, 281, 109934. [Google Scholar] [CrossRef]
- Ferreira, T.; Shuler, J.; Guimarães, R.; Farah, A. Introduction to coffee plant and genetics. In Coffee: Production, Quality and Chemistry; Royal Society of Chemistry: Cambridge, UK, 2019; pp. 1–25. [Google Scholar] [CrossRef]
- Rakocevic, M.; Androcioli-Filho, A. Morphophysiological characteristics of Coffea arabica L. in different arrangements: Lessons from a 3D virtual plant approach. Coffee Sci. 2010, 5, 54–166. [Google Scholar]
- Camargo, A.P.; Camargo, M.B.P. Definição e esquematização das fases fenológicas do cafeeiro arábica nas condições tropicais do Brasil. Bragantia 2001, 60, 65–68. [Google Scholar] [CrossRef]
- Quiñones, A.J.P.; Builes, V.H.R.; Robledo, A.J.; Sáenz, J.R.R.; Pulgarín, J.A. Effects of daylength and soil humidity on the flowering of coffee Coffea arabica L. in Colombia. Rev. Fac. Nac. Agron. Medellin 2011, 64, 5745–5754. [Google Scholar]
- Rena, A.B.; Barros, R.S. Aspectos críticos no estudo da floraçãao do café. In Efeitos da Irrigaçãao Sobre a Qualidade e Produtividade do Café; Zambolim, L., Ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2004; pp. 149–172. [Google Scholar]
- Rakocevic, M.; Braga, K.S.M.; Batista, E.R.; Maia, A.H.N.; Scholz, M.B.S.; Filizola, H.F. The vegetative growth assists to reproductive responses of Arabic coffee trees in a long-term FACE experiment. Plant Growth Regul. 2020, 91, 305–316. [Google Scholar] [CrossRef]
- Cardon, C.H.; de Oliveira, R.R.; Lesy, V.; Ribeiro, T.H.; Fust, C.; Pereira, L.P.; Colasanti, J.; Chalfun-Junior, A. Expression of coffee florigen CaFT1 reveals a sustained floral induction window associated with asynchronous flowering in tropical perennials. Plant Sci. 2022, 325, 111479. [Google Scholar] [CrossRef]
- Morais, H.; Caramori, P.H.; Koguishi, M.S.; Ribeiro, A.M.A. Detailed phenological scale of the reproductive phase of Coffea arabica. Bragantia 2008, 67, 257–260. [Google Scholar] [CrossRef]
- Kitzberger, C.S.G.; Pot, D.; Marraccini, P.; Pereira, L.F.P.; Scholz, M.B.S. Flavor precursors and sensory attributes of coffee submitted to different post-harvest processing. AIMS Agric. Food 2020, 5, 700–714. [Google Scholar] [CrossRef]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of total phenols and antioxidants in coffee samples of different origins and evaluation of the effect of degree of roasting on their levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef] [PubMed]
- Cannell, M.G. Physiology of the coffee crop. In Coffee—Botany, Biochemistry and Production of Beans and Beverage; Clifford, M.N., Willson, K.C., Eds.; Crom Helm: London, UK, 1985; pp. 108–134. [Google Scholar]
- Rakocevic, M.; Batista, E.R.; Pazianotto, R.A.A.; Scholz, M.B.S.; Souza, G.A.R.; Campostrini, E.; Ramalho, J.C. Leaf gas exchange and bean quality fluctuations over the whole canopy vertical profile of Arabic coffee cultivated under elevated CO2. Funct. Plant Biol. 2021, 48, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Farah, A. Coffee constituents. In Coffee: Emerging Health Effects and Disease Prevention; Chu, Y.-F., Ed.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 21–58. [Google Scholar]
- Brige, F.A.A.; Celestino, S.M.C.; Amabile, R.F.; Fagioli, M.; Delvico, F.M.S.; Montalvão, A.P.L.; Sala, P.I.A.L. Genetic variability in conilon coffee related to grain attributes in an irrigated crop in the Cerrado. Pesqui. Agropecu Bras. 2019, 54, e00358. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Food chemistry correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Durand, N.; Rakocevic, M. From the field to coffee cup: Impact of planting design on chlorogenic acid isomers and other compounds in coffee beans and sensory attributes of coffee beverage. Eur. Food. Res. Technol. 2018, 244, 793–1802. [Google Scholar] [CrossRef]
- Velásquez, S.; Banchón, C. Influence of pre-and post-harvest factors on the organoleptic and physicochemical quality of coffee: A short review. J. Food Sci. Technol. 2022, 15, 1–13. [Google Scholar] [CrossRef]
- Lambot, C.; Herrera, J.C.; Bertrand, B.; Sadeghian, S.; Benavides, P.; Gaitán, A. Cultivating coffee quality—Terroir and agro-ecosystem. In The Craft and Science of Coffee; Folmer, B., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 17–49. [Google Scholar] [CrossRef]
- Ginbo, T. Heterogeneous impacts of climate change on crop yields across altitudes in Ethiopia. Clim. Chang. 2022, 170, 12. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bertrand, B.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Léran, S.; Markussen, B.; et al. Shade effects on yield across different Coffea arabica cultivars-how much is too much? A meta-analysis. Agron. Sustain. Dev. 2022, 42, 55. [Google Scholar] [CrossRef]
- Koutouleas, A.; Sarzynski, T.; Bordeaux, M.; Bosselmann, A.S.; Campa, C.; Etienne, H.; Turreira-García, N.; Rigal, C.; Vaast, P.; Ramalho, J.C.; et al. Shaded-coffee: A nature-based strategy for coffee production under climate change? A Review. Front. Sustain. Food Syst. 2022, 6, 877476. [Google Scholar] [CrossRef]
- Rakocevic, M.; Scholz, M.B.S.; Kitzberger, C.S.G. Berry distributions on coffee trees cultivated under high densities modulate the chemical composition of respective coffee beans during one biannual cycle. Int. J. Fruit Sci. 2018, 18, 117–137. [Google Scholar] [CrossRef]
- Reis, A.R.; Favarin, J.L.; Gallo, L.A.; Moraes, M.F.; Tezotto, T.; Lavres, J.J. Influence of nitrogen fertilization on nickel accumulation and chemical composition of coffee plants during fruit development. J. Plant Nutr. 2011, 34, 1853–1866. [Google Scholar] [CrossRef]
- Vinecky, F.; Davrieux, F.; Mera, A.C.; Alves, G.S.; Lavagnini, G.; Leroy, T.; Bonnot, F.; Rocha, O.C.; Bartholo, G.F.; Guerra, A.F.; et al. Controlled irrigation and nitrogen, phosphorous and potassium fertilization affect the biochemical composition and quality of Arabica coffee beans. J. Agric. Sci. 2017, 155, 902–918. [Google Scholar] [CrossRef]
- Marcheafave, G.G.; Tormena, C.D.; Terrile, A.E.; Salamanca-Neto, C.A.; Sartori, E.R.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Pauli, E.D. Ecometabolic mixture design-fingerprints from exploratory multi-block data analysis in Coffea arabica beans from climate changes: Elevated carbon dioxide and reduced soil water availability. Food. Chem. 2021, 362, 129716. [Google Scholar] [CrossRef] [PubMed]
- Gokavi, N.; Mote, K.; Jayakumar, M.; Raghuramulu, Y.; Surendran, U. The effect of modified pruning and planting systems on growth, yield, labour use efficiency and economics of Arabica coffee. Sci. Hortic. 2021, 276, 109764. [Google Scholar] [CrossRef]
- Ramalho, J.C.; Pais, I.P.; Leitão, A.E.; Guerra, M.; Reboredo, F.H.; Máguas, C.M.; Carvalho, M.L.; Scotti-Campos, P.; Ribeiro-Barros, A.I.; Lidon, F.J.; et al. Can elevated air [CO2] conditions mitigate the predicted warming impact on the quality of coffee bean? Front. Plant Sci. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.B.; Nogueira, F.D.; Guimarães, P.T.G.; Chagas, S.J.R.; Costa, L. Sources and doses of potassium on the yield and quality of green coffee. Pesq. Agropec. Bras. 1999, 34, 335–345. [Google Scholar] [CrossRef]
- Carr, M. The water relations and irrigation requirements of coffee. Exp. Agric. 2001, 37, 1–36. [Google Scholar] [CrossRef]
- Tesfaye, S.G.; Ismail, M.R.; Kausar, H.; Marziah, M.; Ramlan, M.F. Plant water relations, crop yield and quality in coffee (Coffea arabica L.) as influenced by partial root zone drying and deficit irrigation. AJCS 2013, 7, 1361–1368. [Google Scholar] [CrossRef]
- Liu, X.; Qi, Y.; Li, F.; Yang, Q.; Yu, L. Impacts of regulated deficit irrigation on yield, quality and water use efficiency of Arabica coffee under different shading levels in dry and hot regions of southwest China. Agric. Water Manag. 2018, 204, 292–300. [Google Scholar] [CrossRef]
- Silva, E.A.; Mazzafera, P.; Brunini, O.; Sakai, E.; Arruda, F.B.; Mattoso, L.H.C.; Carvalho, C.R.L.; Pires, R.C.M. The influence of water management and environmental conditions on the chemical composition and beverage quality of coffee beans. Braz. J. Plant Physiol. 2005, 17, 229–238. [Google Scholar] [CrossRef]
- Ahmed, S.; Brinkley, S.; Smith, E.; Sela, A.; Theisen, M.; Thibodeau, C.; Warne, T.; Anderson, E.; Van Dusen, N.; Giuliano, P.; et al. Climate change and coffee quality: Systematic review on the effects of environmental and management variation on secondary metabolites and sensory attributes of Coffea arabica and Coffea canephora. Front. Plant Sci. 2021, 12, 708013. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.; Ramalho, J.C. Impacts of drought and temperature stress on coffee physiology and production: A review. Braz. J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Anthony, F.; Quiros, O.; Topart, P.; Bertrand, B.; Lashermes, P. Detection by simple sequence repeat markers of introgression from Coffea canephora in Coffea arabica cultivars. Plant Breed. 2002, 121, 542–544. [Google Scholar] [CrossRef]
- Pérez-Molina, J.P.; de Toledo Picoli, E.A.; Oliveira, L.A.; Silva, B.T.; de Souza, G.A.; dos Santos Rufino, J.L.; Pereira, A.A.; de Freitas Ribeiro, M.; Malvicini, G.L.; Turello, L.; et al. Treasured exceptions: Association of morphoanatomical leaf traits with cup quality of Coffea arabica L. cv. “Catuaí”. Food Res. Int. 2021, 141, 110118. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.L.; Cortez, G.L.; Zaro, G.C.; Zorzenoni, T.O.; Melo, T.R.; Figueiredo, A.; Aquino, G.S.; Medina, C.D.; Ralisch, R.; Caramori, P.H.; et al. Soil morphostructural characterization and coffee root distribution under agroforestry system with Hevea Brasiliensis. Sci. Agric. 2021, 78, e20190150. [Google Scholar] [CrossRef]
- Meireles, E.J.; Camargo, M.B.; Pezzopane, J.R.; Thomaziello, R.A.; Fahl, J.I.; Bardin, L.; Santos, J.C.; Japiassú, L.B.; Garcia, A.W.; Miguel, A.E.; et al. Fenologia do Cafeeiro: Condições Agrometeorológicas e Balanço Hídrico do ano Agrícola 2004–2005; Embrapa Informação Tecnológica: Brasíla, Brazil, 2009; p. 128. [Google Scholar]
- Cesanelli, A.; Guarracino, L. Numerical modeling of actual evapotranspiration of a coffee crop. Sci. Agric. 2011, 68, 395–399. [Google Scholar] [CrossRef]
- Godin, C.; Caraglio, Y. A multiscale model of plant topological structures. J. Theor. Biol. 1998, 191, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, F.T.; Tosti, J.B.; Androcioli-Filho, A.; Brancher, J.D.; Costes, E.; Rakocevic, M. Strategies to reconstruct 3D Coffea arabica L. plant structure. SpringerPlus 2016, 5, 2075. [Google Scholar] [CrossRef]
- Pradal, C.; Boudon, F.; Nouguier, C.; Chopard, J.; Godin, C. PlantGL: A Python-based geometric library for 3D plant modelling at different scales. Graph. Models 2009, 71, 1–21. [Google Scholar] [CrossRef]
- Scholz, M.B.S.; Kitzberger, C.S.G.; Pereira, L.F.P.; Davrieux, F.; Pot, D.; Charmetant, P.; Leroy, T. Application of near infrared spectroscopy for green coffee biochemical phenotyping. J. Near Infrared Spectrosc. 2014, 22, 411–421. Available online: https://opg.optica.org/jnirs/abstract.cfm?URI=jnirs-22-6-411 (accessed on 15 December 2022). [CrossRef]
- R Core Team. Available online: https://wwwr-projectorg/ (accessed on 18 December 2022).
- Covre, A.M.; Oliveira, M.G.; Martins, L.D.; Bonomo, R.; Rodrigues, W.N.; Tomaz, M.A.; Duarte, H.; de Sá Paye, H.; Partelli, F.L. How is the fruit development of Coffea canephora trees modulated by the water supply? An analysis of growth curves for irrigated and rainfed systems. Semin. Ciênc. Agrár. Londrina 2022, 43, 2359–2374. [Google Scholar] [CrossRef]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; DaMatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the impact of drought in Coffea genotypes: Transcriptomic analysis supports a common high resilience to moderate water deficit but a genotype dependent sensitivity to severe water deficit. Agronomy 2021, 11, 2255. [Google Scholar] [CrossRef]
- Ghosh, P.; Venkatachalapathy, N. Processing and drying of coffee—A review. Int. J. Eng. Res. Technol. 2014, 3, 784–794. [Google Scholar]
- Pérez, V.O.; Pérez, L.G.M.; Fernandez-Alduenda, M.R.; Barreto, C.I.A.; Agudelo, C.P.G.; Restrepo, E.C.M. Chemical composition and sensory quality of coffee fruits at different stages of maturity. Agronomy 2023, 13, 341. [Google Scholar] [CrossRef]
- Soares, A.R. Irrigação Fertirrigação Fisiologia e Produção do Cafeeiro Adulto na Região da Zona da Mata de Minas Gerais. Master’s Thesis, Universidade Federal de Viçosa, Viçosa, Brazil, 2001; p. 84. [Google Scholar]
- Baïram, E.; leMorvan, C.; Delaire, M.; Buck-Sorlin, G. Fruit and leaf response to different source–sink ratios in apple, at the scale of the fruit-bearing branch. Front. Plant Sci. 2019, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Cunha, R.L.; Antunes, W.C.; Martins, S.C.V.; Araújo, W.L.; Fernie, A.; Moraes, G.A.B.K. In field-grown coffee trees source-sink manipulation alters photosynthetic rates, independently of carbon metabolism, via alterations in stomatal function. New Phytol. 2008, 178, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Yeager, S.E.; Batali, M.E.; Guinard, J.-X.; Ristenpart, W.D. Acids in coffee: A review of sensory measurements and meta-analysis of chemical composition. Crit. Rev. Food Sci. Nutr. 2021, 23, 1–27. [Google Scholar] [CrossRef]
- González, A.R.; Hernández, C.Y.F.; Rios, O.G.; Quiroz, M.L.S.; Amaro, R.M.G.; Estrada, Z.J.H.; Duarte, P.R. Coffee chlorogenic acids incorporation for bioactivity enhancement of foods: A review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Vaast, P.; Bertrand, B.; Perriot, J.-J.; Guyot, B.; Génard, M. Fruit thinning and shade improve bean characteristics and beverage quality of coffee (Coffea arabica L.) under optimal conditions. J. Sci. Food Agric. 2006, 86, 197–204. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Silveira, S.R.; Ruas, P.M.; Ruas, C.F.; Sera, T.; Carvalho, V.P.; Coelho, A.S.G. Assessment of genetic variability within and among coffee progenies and cultivars using RAPD markers. Genet. Mol. Biol. 2003, 26, 329–336. [Google Scholar] [CrossRef]
- Silva, F.B.; Tormena, C.D.; Pauli, E.D.; de Almeida, A.G.; Berg, A.B.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S.; Marcheafave, G.G. Time dependent berry maturation for planting density levels in Coffea arabica L. beans: Mixture design-fingerprinting using near-infrared transmittance spectroscopy. J. Food Compos. Anal. 2021, 97, 103795. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Chemistry of Coffee: Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Cambridge, MA, USA, 2013; pp. 1–28. [Google Scholar] [CrossRef]
- Toci, A.T.; Neto, V.J.; Torres, A.G.; Farah, A. Changes in triacylglycerols and free fatty acids composition during storage of roasted coffee. Food Sci. Technol. 2013, 50, 581–590. [Google Scholar] [CrossRef]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; Kochko, A.; Dussert, S. Influence of environmental factors, wet processing, and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Worku, M.; Meulenaer, B.; Duchateau, L.; Boeckx, P. Effect of altitude on biochemical composition and quality of green arabica coffee beans can be affected by shade and postharvest processing method. Food Res. Int. 2018, 105, 278–285. [Google Scholar] [CrossRef]
- Santos, C.A.F.; Leitão, A.E.; Pais, I.P.; Lidon, F.C.; Ramalho, J.C. Perspectives on the potential impacts of climate changes on coffee plant and bean quality. Emir. J. Food Agric. 2015, 27, 152–163. [Google Scholar] [CrossRef]
- Barbosa, M.S.G.; Scholz, M.B.S.; Kitzberger, C.S.G.; Benassi, M.T. Correlation between the composition of green Arabica coffee beans and the sensory quality of coffee brews. Food Chem. 2019, 292, 275–280. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Borém, F.M.; Cirillo, M.A.; Malta, M.R.; Alvarenga, A.A.; Alves, H.M.R. Coffee quality and its interactions with environmental factors in Minas Gerais. Brazil. J. Agric. Sci. 2012, 4, 181–190. [Google Scholar] [CrossRef]
- Mazzafera, P.; Robinson, S.P. Characterization of polyphenol oxidase in coffee. Phytochemistry 2000, 55, 285–296. [Google Scholar] [CrossRef]
- Mintesnot, A.; Dechassa, N. Effect of altitude, shade, and processing methods on the quality and biochemical composition of green coffee beans in Ethiopia. East Afr. J. Sci. 2018, 12, 87–100. [Google Scholar]
- Upadhyay, R.; Rao, L.J.M. An outlook on chlorogenic acids-occurrence, chemistry, technology, and biological activities. Crit. Rev. Food Sci. Nutr. 2013, 53, 968–984. [Google Scholar] [CrossRef] [PubMed]
- Marcheafave, G.G.; Pauli, E.D.; Tormena, C.D.; Ortiz, M.C.; de Almeida, A.G.; Rakocevic, M.; Bruns, R.E.; Scarminio, I.S. Factorial design fingerprint discrimination of Coffea arabica beans under elevated carbon dioxide and limited water conditions. Talanta 2020, 209, 120591. [Google Scholar] [CrossRef] [PubMed]
- Semedo, J.N.; Rodrigues, A.P.; Lidon, F.C.; Pais, I.P.; Marques, I.; Gouveia, D.; Armengaud, J.; Silva, M.J.; Martins, S.; Semedo, M.C.; et al. Intrinsic non-stomatal resilience to drought of the photosynthetic apparatus in Coffea spp. is strengthened by elevated air [CO2]. Tree Physiol. 2021, 41, 708–727. [Google Scholar] [CrossRef] [PubMed]
- Geromel, C.; Ferreira, L.P.; Davrieux, F.; Guyot, B.; Ribeyre, F.; dos Santos Scholz, M.B.; Pereira, L.F.; Vaast, P.; Pot, D.; Leroy, T.; et al. Effects of shade on the development and sugar metabolism of coffee (Coffea arabica L.) fruits. Plant Physiol. Biochem. 2008, 46, 569–579. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).