Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site, Treatment and Design
2.2. Plant and Planting Materials
2.3. Synthesis Method of Zinc Oxide Nanoparticles (ZnO-NPs)
2.4. Agronomic Practices
2.5. Data Collection of Growth, Yield and Yield Parameters
2.6. Data Collecton of Photosynthetic Parameters
2.7. Determination of Quality Parameters
2.8. Plant Samples Analysis
Determination of N, P, K, S, Mg, B, Fe, and Zn Content in Leaves and Fruits
2.9. Soil Sample Analysis
2.10. Calculation of Nutrient Uptake
2.11. Calculation of Apparent Nutrient Recovery Efficiency (ANR)
2.12. Relative Data in Percentage
2.13. Statistical Analysis
3. Results and Discussion
3.1. Growth Parameters of Tomato
3.2. Physiological Characters
3.3. Yield and Yield-Contributing Characters
3.4. Quality Parameters
3.5. Nutrient Contents in Leaves
3.6. Nutrient Contents in Fruits
3.7. Effect of Nutrient Uptake by Leaves
3.8. Effect of Nutrient Uptake by Fruits
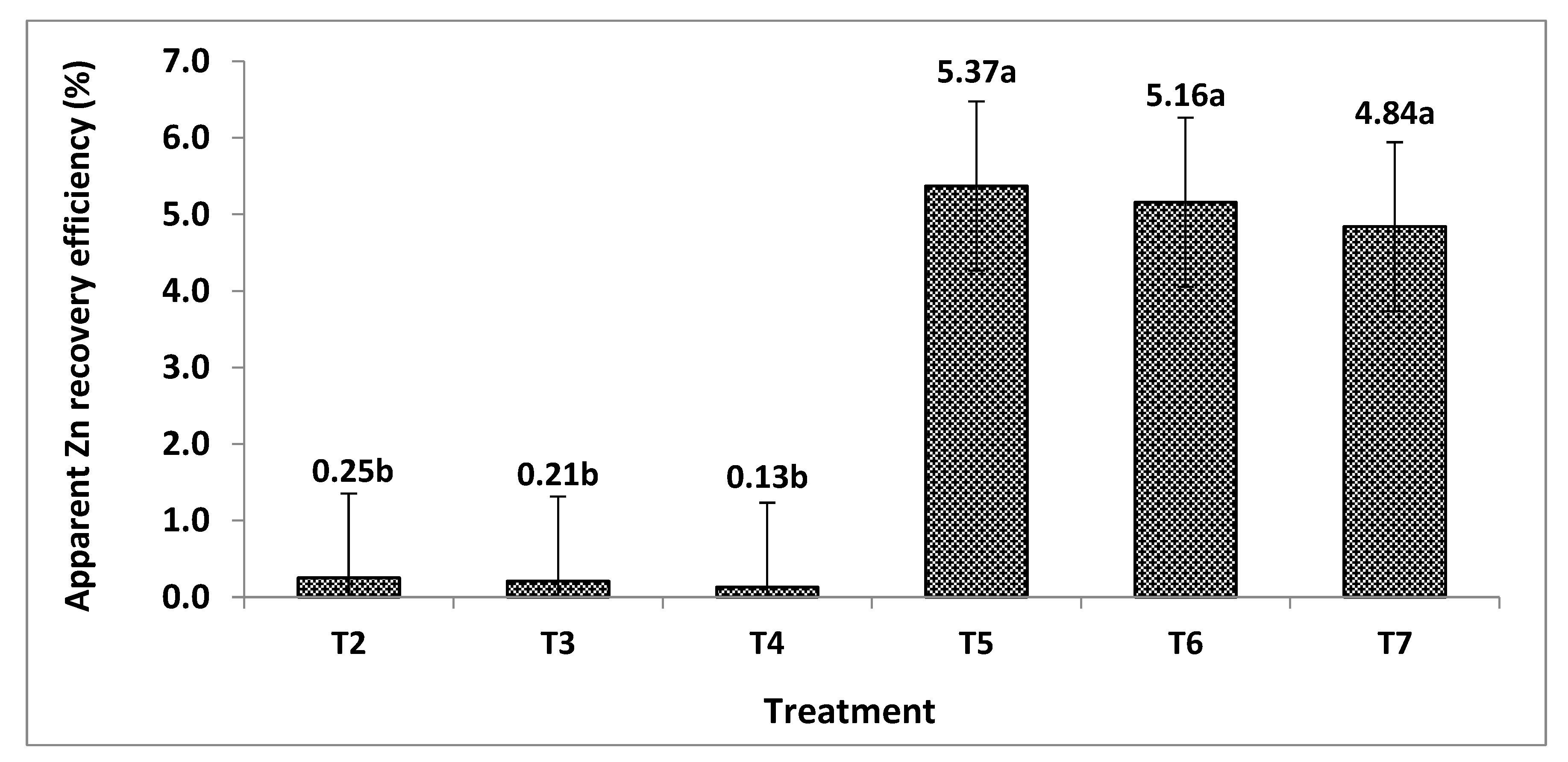
3.9. Apparent Zinc Recovery Efficiency of Tomato
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Srividya, S.; Reddy, S.S.; Sudhavani, V.; Reddy, R. Effect of post-harvest chemicals on fruit physiology and shelf Life of tomato under ambient conditions. Int. J. Agric. Food Sci. Technol. 2014, 5, 99–104. [Google Scholar]
- Ali, M.R.; Mehrajb, H.; Jamal Uddin, A.F.M. Effects of foliar application of zinc and boron on growth and yield of summer tomato. J. Biosci. Agric. Res. 2015, 6, 512–517. [Google Scholar] [CrossRef]
- Sultana, S.; Naser, H.M.; Akhter, S.; Begum, R.A. Effectiveness of soil and foliar applications of zinc and boron on the yield of tomato. Bangladesh J. Agric. Res. 2016, 41, 411–418. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Khanm, H.; Vaishnavi, B.A.; Shankar, A.G. Raise of Nano-Fertilizer Era: Effect of nano scale zinc oxide particles on the germination, growth and yield of tomato (Solanum lycopersicum). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1861–1871. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R.; Lakzian, A. Effects of coated and non-coated ZnO nano particles on cucumber seedlings grown in gel chamber. Arch. Agron. Soil Sci. 2017, 63, 1108–1120. [Google Scholar] [CrossRef]
- Zhang, T.; Sun, H.; Lv, Z.; Cui, L.; Mao, H.; Kopittke, P.M. Using synchrotron-based approaches to examine the foliar application of ZnSO4 and ZnO nanoparticles for field grown winter wheat. J. Agric. Food Chem. 2018, 66, 2572–2579. [Google Scholar] [CrossRef]
- Munir, T.; Rizwan, M.; Kashif, M.; Shahzad, A.; Ali, S.; Amin, N.; Zahid, R.; Alam, M.F.E.; Imran, M. Effect of zinc oxide nanoparticles on the growth and Zn uptake in wheat (Triticum aestivum L.) by seed priming method. Dig. J. Nanomater. Biostructures 2018, 13, 315–323. [Google Scholar]
- Shamshuddin, J.; Rabileh, M.A.; Fauziah, C.I. Can the Acidic Ultisols in Peninsular Malaysia Be Alleviated by Biochar Treatment for Corn Cultivation? Malays. J. Soil Sci. 2020, 24, 1–10. [Google Scholar]
- Mohan, A.C.; Renjanadevi, B. Preparation of Zinc Oxide Nanoparticles and its Characterization Using Scanning Electron Microscopy (SEM) and X-Ray Diffraction(XRD). Procedia Technol. 2016, 24, 761–766. [Google Scholar] [CrossRef]
- Cox, D. Water quality: PH and alkalinity. University of Massachusetts Extension. Dep. Plant Soil Sci. 1995, 50–51. [Google Scholar]
- Nirupama, P.; Gol, N.B.; Rao, T.V.R. Effect of postharvest treatments on physicochemical characteristics and storage life of tomato (Lycopersicon esculentum Mill.) fruits during storage. Am.-Eurasian J. Agric. Environ. Sci. 2010, 9, 470–479. [Google Scholar]
- Kumah, P.; Olympio, N.S.; Tayviah, C.S. Sensitivity of three tomatoes (Lycopersicon esculentum) cultivars-Akoma, Pectomech, and power to chilling injury. Agric. Biol. J. N. Am. 2011, 2, 799–805. [Google Scholar] [CrossRef]
- Mohammadi-Aylar, S.; Jamaati-e-Somarin, S.; Azimi, J. Effect of stage of ripening on mechanical damage in tomato fruits. Am. Eur. J. Agric. Environ. Sci. 2010, 9, 297–302. [Google Scholar]
- Ding, P.; Mashah, N.C. Growth, maturation, and ripening of underutilized Carissa congesta fruit. Fruits 2016, 71, 171–176. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. A simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Influence of ripening stages on antioxidant properties of papaya fruit (Carica papaya L.). AIP Conf. Proc. 2013, 1571, 696–701. [Google Scholar]
- Musa, K.H.; Abdullah, A.; Jusoh, K.; Subramaniam, V. Antioxidant activity of pink-flesh guava (Psidium guajava L.): Effect of extraction techniques and solvents. Food Anal. Methods 2011, 4, 100–107. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Page, A.L.; Miller, R.H.; Keeney, D.R. Methods of Soil Analysis. Part 2, Chemical and Microbial Properties; (Agronomy Series); American Society of Agronomy, Inc.: Madison, WI, USA; Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, p. 2. [Google Scholar]
- Sharma, N.K.; Singh, R.J.; Kumar, K. Dry matter accumulation and nutrient uptake by wheat under poplar based agroforestry system. Agronomy 2012, 2012, 359673. [Google Scholar]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Ashraf, M.; Waheed, A. Screening of local/exotic accessions of lentil (Lens culinaris Medic.) for salt tolerance at two growth stages. Plant Soil 1990, 128, 167–176. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Wang, R.; Zhang, P.; Ju, Q.; Xu, J. Physiological, Transcriptomic and Metabolomic Analyses Reveal Zinc Oxide Nanoparticles Modulate Plant Growth in Tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Faizan, M.; Hayat, S. Effect of foliar spray of ZnO-NPs on the physiological parameters and antioxidant systems of Lycopersicon esculentum. Pol. J. Nat. Sci. 2019, 34, 87–105. [Google Scholar]
- Wang, X.P.; Li, Q.Q.; Pei, Z.M.; Wang, S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant 2018, 62, 801–808. [Google Scholar] [CrossRef]
- Basavarajeswari, C.P.; Hosamni, R.M.; Ajjappalavara, P.S.; Naik, B.H.; Smitha, R.P.; Ukkund, K.C. Effect of foliar application of micronutrients on growth, yield components of Tomato (Lycopersicon esculentum Mill). Karnataka J. Agric. Sci. 2008, 21, 428–430. [Google Scholar]
- Isah, A.S.; Amans, E.B.; Odion, E.C.; Yusuf, A.A. Growth rate and yield of two tomato varieties (Lycopersicon esculentum Mill) under green manure and NPK fertilizer Rate Samaru Northern Guinea Savanna. Int. J. Agron. 2014, 2014, 932759. [Google Scholar] [CrossRef]
- Olaniyi, J.O.; Akanbi, W.B.; Adejumo, T.A.; Akande, O.G. Growth, fruit yield and nutritional quality of tomato varieties. Afr. J. Food Sci. 2010, 4, 398–402. [Google Scholar]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.A.M.; Soliman, A.M.; Dougdoug, N.K.E. Ameliarating the adverse effects of tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef] [PubMed]
- Dixit, A.; Sharma, D.; Sharma, T.K.; Bairwa, P.L. Effect of foliar application of some macro and micronutrients on growth and yield of tomato (Solanum lycopersicum L.) cv. ArkaRakshak. Int. J. Curr. Microbiol. Appl. Sci. 2018, 6, 197–203. [Google Scholar]
- Alloway, B.J. Fundamental aspects of zinc in soils and plants. Zinc Soils Crop Nutr. 2008, 2, 30–52. [Google Scholar]
- Quddus, M.A.; Siddiky, M.A.; Hussain, M.J.; Rahman, M.A.; Ali, R.; Masud, M.A.T. Magnesium influences growth, yield, nutrient uptake, and fruit quality of tomato. Int. J. Veg. Sci. 2022, 28, 441–464. [Google Scholar] [CrossRef]
- Razzaque, M.A.; Haque, M.M.; Rahman, M.M.; Bazzaz, M.M.; Khan, M.S.A. Screening of mungbean (Vigna radiata L. wilczek) genotypes under nutrient stress in soil. Bangladesh J. Agric. Res. 2016, 41, 377–386. [Google Scholar] [CrossRef]
- Harris, K.D.; Mathuma, V. Effect of foliar application of boron and zinc on growth and yield of tomato (Lycopersicon esculentum MILL.). Asian J. Pharm. Sci. Technol. 2015, 5, 74–78. [Google Scholar]
- Kumar, U.J.; Bahadur, V.; Prasad, V.M.; Mishra, S.; Shukla, P.K. Effect of different concentrations of iron oxide and zinc oxide nanoparticles on growth and yield of strawberry (Fragaria x ananassa Duch) cv. chandler. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2440–2445. [Google Scholar] [CrossRef]
- Prasad, T.N.V.K.V.; Sudhakar, P.; Sreenivasulu, P.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.S.; Sajanlal, P.R.; Pradeep, T. Effect of nanoscale Zinc Oxide Nanoparticles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Ullah, M.Z.; Hassan, L.; Shahid, S.B.; Patwary, A.K. Variability and inter relationship studies in tomato (Solanum lycopersicum L.). J. Bangladesh Agril. Univ. 2015, 13, 65–69. [Google Scholar] [CrossRef]
- Almendros, P.; Gonzalez, D.; Fernandez, M.D.; García-Gomez, C.; Obrador, A. 2022. Both Zn biofortification and nutrient distribution pattern in cherry tomato plants are influenced by the application of ZnO nanofertilizer. Heliyon 2022, 8, 09130. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, G.; Vijayalatha, K.R.; Anitha, T. Heavy metals and its impact in vegetable crops. Int. J. Chem. Stud. 2019, 7, 1612–1621. [Google Scholar]
- Du, W.; Yang, J.; Peng, Q.; Liang, X.; Mao, H. Comparison study of zinc nanoparticles and zinc sulphate on wheat growth: From toxicity and zinc biofortification. Chemosphere 2019, 227, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Karim, M.R.; Oliver, M.M.H.; Urmi, T.A.; Hossain, M.A.; Haque, M.M. Impacts of trace element addition on lentil (Lens culinaris L.). Agronomy 2018, 8, 100. [Google Scholar] [CrossRef]
- Gutierrez-Miceli, F.A.; Oliva-Llavan, M.A.; Lujan-Hidalgo, M.C.; Velazquez-Gamboa, M.C.; Gonzalez-Mendoza, D.G.; Sanchez-Roque, Y. Zinc oxide phytonanoparticles’ effects of yield and mineral contents in fruits of tomato (Solanum lycopersicum L. cv. cherry) under field conditions. Sci. World J. 2021, 2021, 5561930. [Google Scholar] [CrossRef]
- Subedi, P.; Walsh, K. Non-invasive techniques for measurement of fresh fruit firmness. Postharvest Biol. Technol. 2009, 51, 297–304. [Google Scholar] [CrossRef]
- Ayuso-Yuste, M.C.; González-Cebrino, F.; Lozano-Ruiz, M.; Fernández-León, A.M.; Bernalte-García, M.J. Influence of Ripening Stage on Quality Parameters of Five Traditional Tomato Varieties Grown under Organic Conditions. Horticulturae 2022, 8, 313. [Google Scholar] [CrossRef]
- Siva Prasad, P.N.; Subbarayappa, C.T.; Sathish, A.; Ramamurthy, V. Impact of zinc fertilization on tomato (Solanum lycopersicum L.) yield, zinc use efficiency, growth and quality parameters in Eastern Dry Zone (EDZ) soils of Karnataka, India. Int. J. Plant Soil Sci. 2021, 33, 20–38. [Google Scholar] [CrossRef]
- Raliya, R.; Nair, R.; Chavalmane, S.; Wangab, W.N.; Biswas, P. Mechanistic evaluation of translocation and physiological impact of titanium dioxide and zinc oxide nanoparticles on the tomato (Solanum lycopersicum L.) plant. Metallomics 2015, 7, 1584–1594. [Google Scholar] [CrossRef]
- Helyes, L.; Pék, Z.; Lugasi, A. Function of the variety technological traits and growing conditions on fruit components of tomato (Lycopersicon lycopersicum L. karsten). Acta Aliment. 2008, 37, 427–436. [Google Scholar] [CrossRef]
- Trivedi, N.; Singh, D.; Bahadur, V.; Prasad, V.M.; Collis, J.P. Effect of foliar application of zinc and boron on yield and fruit quality of guava (Psidium gujava L.). Hort Flora Res. Spectr. 2012, 1, 281–283. [Google Scholar]
- Gurmani, A.R.; Din, J.U.; Khan, S.U.; Andaleep, R.; Waseem, K.; Khan, A.; Ullah, H. Soil Application of Zinc Improves Growth and Yield of Tomato. Int. J. Agric. Biol. 2012, 14, 91–96. [Google Scholar]
- Singh, A.K.; Meena, M.K.; Bharati, R.C.; Gade, R.M. Effect of sulphur and zinc management on yield, nutrient uptake, changes in soil fertility and economics in rice (Oryza sativa)–lentil (Lens culinaris) cropping system. Indian J. Agric. Sci. 2013, 83, 344–348. [Google Scholar]
- Agrawal, B.; Shrivastava, A.; Harmukh, N. Effect of irrigation methods and micronutrients on nutrient uptake of tomato F1 hybrid avinash-2. Int. J. Curr. Trends Sci. Technol. 2010, 1, 20–26. [Google Scholar]
- Divyashree, K.S.; Prakash, S.S.; Yogananda, S.B.; Munawery, A. Effect of micronutrients mixture application on growth, yield and nutrient uptake of mungbean in Southern Dry Zone (Zone 6) of Karnataka. Int. J. Pure Appl. Biosci. 2018, 6, 56–62. [Google Scholar]
- Quddus, M.A.; Anwar, M.B.; Naser, H.M.; Siddiky, M.A.; Hussain, M.J.; Aktar, S.; Mondol, A.T.M.A.I.; Islam, M.A.; Amin, M.R. Impact of Zinc, Boron and Molybdenum Addition in Soil on Mungbean Productivity, Nutrient Uptake and Economics. J. Agric. Sci. 2020, 12, 115–129. [Google Scholar] [CrossRef]
- Ibiang, Y.B.; Innami, H.; Sakamoto, K. Effect of excess zinc and arbuscular mycorrhizal fungus on bioproduction and trace element nutrition of tomato (Solanum lycopersicum L. cv. Micro-Tom). Soil Sci. Plant Nutr. 2018, 3, 342–351. [Google Scholar] [CrossRef]
- Haydon, M.J.; Kawachi, M.; Wirtz, M.; Hillmer, S.; Hell, R.; Kramer, U. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell 2012, 24, 724–737. [Google Scholar] [CrossRef]
- Briat, J.; Rouached, H.; Tissot, N.; Gaymard, F.; Dubos, C. Integration of P, S, Fe and Zn nutrition signals in Arabidopsis thaliana: Potential involvement of phosphate starvation response 1 (PHR1). Front. Plant Sci. 2015, 6, 290. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. The interaction of zinc with other elements in plants: A review. Intl. J. Agri. Crop Sci. 2012, 4, 1881–1884. [Google Scholar]
- Imtiaz, M.; Alloway, B.J.; Shah, K.H.; Siddiqui, S.H.; Memon, M.Y.; Aslam, M.; Khan, P. Zinc nutrition of wheat: Growth and zinc uptake. Asian J. Plant Sci. 2003, 2, 152–155. [Google Scholar] [CrossRef][Green Version]
- Kaya, C.; Higgs, D. Response of tomato (Lycopersicon esculentum L.) cultivars to foliar application of zinc when grown in sand culture at low zinc. Sci. Hortic. 2002, 93, 53–64. [Google Scholar] [CrossRef]
- Grzebisz, W.; Wrońska, M.; Diatta, J.B.; Dullin, P. Effect of zinc foliar application at early stages of maize growth on patterns of nutrients and dry matter accumulation by the canopy. Part I. Zinc uptake patterns and its redistribution among maize organs. J. Elem. 2008, 13, 17–28. [Google Scholar]
- Saravaiya, S.N.; Wakchaure, S.S.; Jadhav, P.B.; Tekale, G.S.; Patil, N.B.; Dekhane, S.S.; Patel, D.J. Influence of foliar application of micronutrients on tomato (Lycopersicon esculentum mill.) cv. gujarat tomato 2. Int. J. Dev. Res. 2014, 4, 1539–1542. [Google Scholar]
- Elia, A.; Conversa, G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012, 40, 64–74. [Google Scholar] [CrossRef]
| Properties and Unit | Value |
|---|---|
| % Sand | 46.35 |
| % Silt | 21.48 |
| % Clay | 32.17 |
| pH | 6.52 ± 0.409 |
| Total C (%) | 2.44 ± 0.179 |
| Total N (%) | 0.18 ± 0.022 |
| Total S (%) | 0.05 ± 0.011 |
| Available P (mg//kg) | 9.39 ± 0.61 |
| Potassium (mg/kg) | 140.25 ± 10.091 |
| Calcium (mg/kg) | 775 ± 58.248 |
| Magnesium (mg/kg) | 85.35 ± 6.284 |
| Boron (mg/kg) | 2.4 ± 0.189 |
| Zinc (mg/kg) | 10.7 ± 0.718 |
| Treatment | Plant Height (cm) | No. of Primary Branch/Plant | Leaf Area (cm2) |
|---|---|---|---|
| V1 = MT1 | 123 a ± 2.31 | 13.1 a ± 0.75 | 18.1 a ± 0.93 |
| V2 = MT3 | 125 a ± 2.13 | 13.7 a ± 0.76 | 19.1 a ± 0.98 |
| Level of significance | ns | ns | ns |
| MSD value | 5.09 | 1.16 | 1.01 |
| CV (%) | 8.42 | 14.5 | 8.36 |
| T1 | 114 c ± 2.85 | 8.50 d ± 0.46 | 13.1 d ± 0.41 |
| T2 | 124 abc ± 2.84 | 14.0 bc ± 0.71 | 19.1 c ± 0.50 |
| T3 | 121 abc ± 3.63 | 11.0 cd ± 0.60 | 14.8 d ± 0.47 |
| T4 | 117 bc ± 3.02 | 9.38 d ± 0.60 | 12.7 d ± 0.33 |
| T5 | 127 abc ± 3.95 | 16.0 ab ± 0.76 | 22.1 b ± 0.66 |
| T6 | 135 a ± 4.28 | 18.5 a ± 0.71 | 25.4 a ± 0.66 |
| T7 | 131 ab ± 3.73 | 16.5 ab ± 0.65 | 23.1 ab ± 0.76 |
| Level of significance | ** | ** | ** |
| MSD value | 16.37 | 3.05 | 2.43 |
| CV (%) | 8.42 | 14.57 | 8.36 |
| Interaction (V*T) significance | ns | ns | ns |
| Treatment | Chlorophyll Content in Leaf (SPAD) | Photosynthetic Rate (µmol/m2/s) | Stomatal Conductance (mol/m2/s) | Transpiration Rate (mmol/m2/s) |
|---|---|---|---|---|
| V1 = MT1 | 40.8 a ± 1.33 | 23.6 b ± 0.91 | 0.83 a ± 0.05 | 12.0 b ± 0.51 |
| V2 = MT3 | 41.8 a ± 1.33 | 24.5 a ± 0.96 | 0.87 a ± 0.04 | 12.4 a ± 0.52 |
| Level of significance | ns | * | ns | * |
| MSD value | 1.30 | 0.86 | 0.08 | 0.33 |
| CV (%) | 8.41 | 9.38 | 10.4 | 11.2 |
| T1 | 34.5 e ± 0.97 | 19.1 e ± 0.64 | 0.54 d ± 0.02 | 8.96 d ± 0.37 |
| T2 | 40.6 cd ± 1.01 | 23.8 cd ± 0.72 | 0.90 bc ± 0.03 | 13.1 bc ± 0.48 |
| T3 | 37.8 de ± 1.02 | 21.3 de ± 0.77 | 0.80 c ± 0.03 | 11.4 c ± 0.30 |
| T4 | 33.0 e ± 0.95 | 18.4 e ± 0.59 | 0.55 d ± 0.02 | 9.08 d ± 0.32 |
| T5 | 44.6 bc ± 1.15 | 27.1 bc ± 0.75 | 1.00 ab ± 0.03 | 13.1 bc ± 0.49 |
| T6 | 51.6 a ± 1.31 | 30.8 a ± 0.97 | 1.13 a ± 0.04 | 16.1 a ± 0.63 |
| T7 | 47.0 ab ± 1.24 | 28.1 ab ± 0.95 | 1.06 a ± 0.04 | 13.9 b ± 0.44 |
| Level of significance | ** | ** | ** | ** |
| MSD value | 5.43 | 3.53 | 0.14 | 2.15 |
| CV (%) | 8.41 | 9.38 | 10.41 | 11.26 |
| Interaction (V*T) significance | ns | ns | ns | ns |
| Treatment | Fruit Length (cm) | Fruit Diameter (cm) | Individual Fruit Weight (g) | No. of Fruits/Plant | Yield/Plant (kg) | Fruit Yield (t/ha) |
|---|---|---|---|---|---|---|
| V1 = MT1 | 4.05 a ± 0.12 | 3.59 a ± 0.09 | 38.1 a ± 1.66 | 33.4 a ± 1.06 | 1.31 a ± 0.09 | 26.1 b ± 1.74 |
| V2 = MT3 | 4.12 a ± 0.12 | 3.65 a ± 0.09 | 38.6 a ± 1.63 | 33.8 a ± 1.03 | 1.34 a ± 0.08 | 26.7 a ± 1.72 |
| Level of significance | ns | ns | ns | ns | ns | * |
| MSD value | 0.38 | 0.21 | 0.67 | 1.86 | 0.08 | 0.40 |
| CV (%) | 9.18 | 9.21 | 9.92 | 10.54 | 16.44 | 10.07 |
| T1 | 3.10 d ± 0.09 | 3.02 d ± 0.08 | 24.7 e ± 0.68 | 24.9 c ± 0.79 | 0.62 e ± 0.03 | 12.3 g ± 0.63 |
| T2 | 4.43 ab ± 0.14 | 3.87 ab ± 0.13 | 43.5 ab ± 1.42 | 36.2 a ± 1.16 | 1.58 ab ± 0.08 | 31.6 c ± 1.50 |
| T3 | 3.90 bc ± 0.12 | 3.45 bcd ± 0.09 | 35.8 cd ± 1.48 | 33.2 ab ± 1.31 | 1.19 cd ± 0.06 | 23.8 e ± 1.28 |
| T4 | 3.60 cd ± 0.12 | 3.23 cd ± 0.15 | 30.4 de ± 0.92 | 29.6 bc ± 1.13 | 0.90 de ± 0.05 | 18.0 f ± 0.93 |
| T5 | 4.23 ab ± 0.11 | 3.73 abc ± 0.11 | 40.4 bc ± 1.15 | 35.7 a ± 1.18 | 1.45 bc ± 0.07 | 28.9 d ± 1.46 |
| T6 | 4.73 a ± 0.14 | 4.13 a ± 0.12 | 48.1 a ± 1.59 | 38.4 a ± 1.40 | 1.85 a ± 0.10 | 36.9 a ± 1.77 |
| T7 | 4.63 a ± 0.15 | 3.93 ab ± 0.12 | 45.6 ab ± 1.33 | 37.0 a ± 1.24 | 1.69 ab ± 0.08 | 33.7 b ± 1.77 |
| Level of significance | ** | ** | ** | ** | ** | ** |
| MSD value | 0.59 | 0.52 | 5.94 | 5.52 | 0.34 | 2.09 |
| CV (%) | 9.18 | 9.21 | 9.92 | 10.54 | 16.44 | 10.07 |
| Interaction (V*T) significance | ns | ns | ns | ns | ns | ns |
| Treatment | TSS (0Brix) | Firmness (N) | Titratable Acidity (%) |
|---|---|---|---|
| V1 = MT1 | 8.16 a ± 0.20 | 14.7 a ± 0.41 | 0.63 a ± 0.03 |
| V2 = MT3 | 8.23 a ± 0.19 | 14.8 a ± 0.40 | 0.65 a ± 0.03 |
| Level of significance | ns | ns | ns |
| MSD value | 0.83 | 1.39 | 0.03 |
| CV (%) | 9.99 | 7.89 | 7.59 |
| T1 | 7.05 c ± 0.20 | 11.7 e ± 0.34 | 0.82 a ± 0.02 |
| T2 | 8.50 ab ± 0.39 | 15.6 abc ± 0.46 | 0.57 cd ± 0.02 |
| T3 | 8.00 abc ± 0.27 | 14.2 cd ± 0.37 | 0.70 b ± 0.02 |
| T4 | 7.55 bc ± 0.25 | 13.0 de ± 0.34 | 0.78 a ± 0.02 |
| T5 | 8.38 ab ± 0.28 | 15.3 bc ± 0.43 | 0.62 c ± 0.02 |
| T6 | 9.05 a ± 0.32 | 17.3 a ± 0.43 | 0.48 e ± 0.02 |
| T7 | 8.85 a ± 0.30 | 16.3 ab ± 0.43 | 0.50 de ± 0.02 |
| Level of significance | ** | ** | ** |
| MSD value | 1.28 | 1.82 | 0.08 |
| CV (%) | 9.99 | 7.89 | 7.59 |
| Interaction (V*T) significance | ns | ns | ns |
| Treatment | Ascorbic Acid (mg/100 g) | Lycopene Content (µg/100 g) | TPC (mg/g) | DPPH (%) |
|---|---|---|---|---|
| V1 = MT1 | 18.1 a ± 0.69 | 206 a ± 8.81 | 0.38 a ± 0.02 | 48.6 a ± 1.68 |
| V2 = MT3 | 18.3 a ± 0.69 | 212 a ± 8.59 | 0.39 a ± 0.02 | 49.2 a ± 1.66 |
| Level of significance | ns | ns | ns | ns |
| MSD value | 1.81 | 11.62 | 0.06 | 2.63 |
| CV (%) | 9.23 | 10.49 | 13.24 | 10.02 |
| T1 | 12.0 e ± 0.32 | 136 c ± 5.72 | 0.28 e ± 0.01 | 34.5 d ± 0.80 |
| T2 | 20.0 abc ± 0.60 | 239 a ± 8.25 | 0.42 abc ± 0.02 | 53.0 ab ± 1.60 |
| T3 | 18.2 c ± 0.57 | 192 b ± 6.60 | 0.37 cd ± 0.02 | 47.9 bc ± 1.57 |
| T4 | 15.0 d ± 0.43 | 171 b ± 6.22 | 0.32 de ± 0.01 | 41.8 cd ± 1.29 |
| T5 | 19.3 bc ± 0.58 | 232 a ± 8.71 | 0.40 bc ± 0.02 | 51.2 ab ± 1.73 |
| T6 | 22.1 a ± 0.65 | 246 a ± 9.43 | 0.48 a ± 0.02 | 58.4 a ± 2.05 |
| T7 | 20.9 ab ± 0.61 | 248 a ± 9.02 | 0.45 ab ± 0.02 | 55.5 ab ± 1.46 |
| Level of significance | ** | ** | ** | ** |
| MSD value | 2.63 | 34.32 | 0.08 | 7.65 |
| CV (%) | 9.23 | 10.49 | 13.24 | 10.02 |
| Interaction (V*T) significance | ns | ns | ns | ns |
| Treatment | Nitrogen (N) Content (g/kg) | Phosphorus (P) Content (g/kg) | Potassium (K) Content (g/kg) | Sulfur (S) Content (g/kg) | Magnesium (Mg) Content (g/kg) |
|---|---|---|---|---|---|
| V1 = MT1 | 23.2 a ± 0.89 | 10.6 a ± 0.39 | 20.8 a ± 0.93 | 10.4 b ± 0.39 | 5.35 b ± 0.22 |
| V2 = MT3 | 23.6 a ± 0.89 | 10.9 a ± 0.39 | 21.6 a ± 0.90 | 10.7 a ± 0.40 | 5.56 a ± 0.21 |
| Level of significance | ns | ns | ns | * | * |
| MSD value | 1.65 | 0.56 | 0.88 | 0.16 | 0.18 |
| CV (%) | 7.97 | 8.54 | 7.84 | 8.24 | 7.17 |
| T1 | 14.3 d ± 0.38 | 6.85 c ± 0.17 | 12.4 e ± 0.31 | 6.87 f ± 0.27 | 3.53 d ± 0.10 |
| T2 | 25.3 b ± 0.67 | 10.3 b ± 0.27 | 27.4 a ± 0.68 | 12.6 a ± 0.50 | 7.02 a ± 0.18 |
| T3 | 29.1 a ± 0.80 | 12.4 a ± 0.35 | 22.7 bc ± 0.61 | 11.3 c ± 0.46 | 6.09 b ± 0.16 |
| T4 | 26.2 ab ± 0.68 | 11.9 a ± 0.32 | 19.7 d ± 0.71 | 10.2 e ± 0.41 | 4.93 c ± 0.16 |
| T5 | 22.3 c ± 0.56 | 10.1 b ± 0.27 | 25. ab ± 0.74 | 10.5 de ± 0.42 | 6.23 b ± 0.16 |
| T6 | 24.2 bc ± 0.68 | 11.4 ab ± 0.58 | 22.4 c ± 0.56 | 11.7 b ± 0.47 | 5.29 c ± 0.15 |
| T7 | 22.2 c ± 0.64 | 12.4 a ± 0.38 | 18.8 d ± 0.48 | 10.8 d ± 0.47 | 5.10 c ± 0.14 |
| Level of significance | ** | ** | ** | ** | ** |
| MSD value | 2.91 | 1.44 | 2.60 | 0.37 | 0.61 |
| CV (%) | 7.97 | 8.54 | 7.84 | 8.24 | 7.17 |
| Interaction (V*T) significance | ns | ns | ns | ns | ns |
| Treatment | Boron Content (mg/kg) | Iron Content (mg/kg) | Zinc Content (mg/kg) |
|---|---|---|---|
| V1 = MT1 | 51.4 a ± 2.02 | 81.3 a ± 2.61 | 488 a ± 39.28 |
| V2 = MT3 | 52.3 a ± 2.02 | 83.3 a ± 2.97 | 497 a ± 39.93 |
| Level of significance | ns | ns | ns |
| MSD value | 3.29 | 6.38 | 31.91 |
| CV (%) | 7.97 | 7.64 | 8.02 |
| T1 | 36.7 d ± 1.05 | 112 a ± 2.84 | 21.2 d ± 0.57 |
| T2 | 58.7 b ± 1.50 | 87.8 b ± 2.51 | 547 bc ± 15.83 |
| T3 | 66 a ± 1.77 | 77.9 cd ± 1.94 | 573 b ± 14.11 |
| T4 | 61.9 ab ± 1.69 | 68.9 d ± 1.68 | 700 a ± 16.12 |
| T5 | 43.2 cd ± 0.99 | 83.1 bc ± 2.23 | 498 c ± 11.81 |
| T6 | 49 c ± 1.24 | 76.8 cd ± 2.14 | 506 c ± 11.95 |
| T7 | 47.5 c ± 1.16 | 71.5 d ± 1.88 | 604 b ± 16.18 |
| Level of significance | ** | ** | ** |
| MSD value | 6.45 | 9.85 | 61.77 |
| CV (%) | 7.97 | 7.64 | 8.02 |
| Interaction (V*T) significance | ns | ns | ns |
| Treatment | Nitrogen Content (g/kg) | Phosphorus Content (g/kg) | Potassium Content (g/kg) | Sulfur Content (g/kg) | Magnesium Content (g/kg) |
|---|---|---|---|---|---|
| V1 = MT1 | 21.8 a ± 0.78 | 9.54 a ± 0.44 | 17.9 a ± 0.76 | 9.17 b ± 0.39 | 2.13 a ± 0.05 |
| V2 = MT3 | 22.2 a ± 0.78 | 10. a ± 0.46 | 18.7 a ± 0.81 | 9.44 a ± 0.39 | 2.18 a ± 0.06 |
| Level of significance | ns | ns | ns | * | ns |
| MSD value | 1.37 | 0.53 | 0.96 | 0.16 | 0.08 |
| CV (%) | 8.66 | 7.87 | 8.43 | 8.96 | 7.58 |
| T1 | 14.2 e ± 0.43 | 5.00 e ± 0.15 | 10.9 f ± 0.27 | 5.35 d ± 0.22 | 1.60 b ± 0.04 |
| T2 | 20.0 d ± 0.54 | 10.1 cd ± 0.39 | 23.7 a ± 0.67 | 11.2 a ± 0.45 | 2.39 a ± 0.06 |
| T3 | 22.8 bcd ± 0.59 | 12.1 a ± 0.34 | 19.5 bc ± 0.58 | 10.1 b ± 0.39 | 2.28 a ± 0.06 |
| T4 | 22.0 cd ± 0.59 | 11.6 ab ± 0.35 | 16.9 de ± 0.47 | 9.15 c ± 0.37 | 2.15 a ± 0.05 |
| T5 | 24.0 abc ± 0.70 | 8.90 d ± 0.23 | 21.6 ab ± 0.68 | 9.33 c ± 0.38 | 2.20 a ± 0.06 |
| T6 | 25.3 ab ± 0.79 | 9.95 cd ± 0.31 | 19.1 cd ± 0.52 | 10.4 b ± 0.42 | 2.34 a ± 0.06 |
| T7 | 25.8 a ± 0.69 | 10.9 bc ± 0.30 | 16.2 e ± 0.44 | 9.55 c ± 0.43 | 2.17 a ± 0.06 |
| Level of significance | ** | ** | ** | ** | ** |
| MSD value | 2.98 | 1.20 | 2.41 | 0.43 | 0.26 |
| CV (%) | 8.66 | 7.87 | 8.43 | * | 7.58 |
| Interaction (V*T) significance | ns | ns | ns | ns | ns |
| Treatment | Boron Content (mg/kg) | Iron Content (mg/kg) | Zinc Content (mg/kg) |
|---|---|---|---|
| V1 = MT1 | 24.3 a ± 1.26 | 78.9 a ± 2.23 | 35.4 a ± 1.60 |
| V2 = MT3 | 24.9 a ± 1.27 | 78.8 a ± 2.33 | 36.6 a ± 1.67 |
| Level of significance | ns | ns | ns |
| MSD value | 0.84 | 4.40 | 1.53 |
| CV (%) | 8.75 | 8.53 | 7.05 |
| T1 | 11.8 f ± 0.34 | 99.1 a ± 2.51 | 19.5 d ± 0.47 |
| T2 | 30.1ab ± 0.86 | 81.6 bc ± 2.20 | 29.1 c ± 0.71 |
| T3 | 32.3 a ± 0.93 | 76.5 bcd ± 1.93 | 37.1 b ± 0.84 |
| T4 | 28.1 bc ± 0.88 | 71.5 cd ± 2.03 | 40.7 b ± 1.15 |
| T5 | 21.3 e ± 0.58 | 86.2 b ± 2.45 | 40.1b ± 0.97 |
| T6 | 23.4 de ± 0.66 | 68.1 d ± 2.06 | 40.9 b ± 1.00 |
| T7 | 25.4 cd ± 0.69 | 69.2 d ± 2.33 | 45.1 a ± 1.10 |
| Level of significance | ** | ** | ** |
| MSD value | 3.37 | 10.50 | 3.97 |
| CV (%) | 8.75 | 8.53 | 7.05 |
| Interaction (V*T) significance | ns | ns | ns |
| Treatment | Nitrogen Uptake (g/Plant) | Phosphorus Uptake (g/Plant) | Potassium Uptake (g/Plant) | Sulfur Uptake (g/Plant) | Magnesium Uptake (g/Plant) | Boron Uptake (mg/Plant) | Zinc Uptake (mg/Plant) |
|---|---|---|---|---|---|---|---|
| V1 = MT1 | 2.44 b ± 0.14 | 1.12 b ± 0.07 | 2.21 b ± 0.15 | 1.10 b ± 0.07 | 0.56 b ± 0.04 | 5.33 b ± 0.29 | 53.0 a ± 4.51 |
| V2 = MT3 | 2.62 a ± 0.15 | 1.22 a ± 0.08 | 2.42 a ± 0.16 | 1.20 a ± 0.08 | 0.62 a ± 0.04 | 5.72 a ± 0.32 | 57.2 a ± 4.90 |
| Level of significance | * | * | * | * | * | * | ns |
| MSD value | 0.31 | 0.04 | 0.03 | 0.03 | 0.02 | 0.15 | 17.55 |
| CV (%) | 14.9 | 15.8 | 13.6 | 7.74 | 15.4 | 14.1 | 14.08 |
| T1 | 0.91 c ± 0.03 | 0.43 d ± 0.02 | 0.78 e ± 0.03 | 0.43 d ± 0.02 | 0.22 d ± 0.01 | 2.32 d ± 0.07 | 1.34 c ± 0.05 |
| T2 | 2.97 ab ± 0.15 | 1.21 bc ± 0.06 | 3.20 a ± 0.13 | 1.47 a ± 0.08 | 0.82 a ± 0.04 | 6.87 a ± 0.30 | 64.1 b ± 3.97 |
| T3 | 2.85 ab ± 0.14 | 1.21 bc ± 0.06 | 2.22 cd ± 0.09 | 1.11 b ± 0.06 | 0.60 b ± 0.03 | 6.46 ab ± 0.30 | 56.0 b ± 3.06 |
| T4 | 2.39 b ± 0.12 | 1.09 c ± 0.05 | 1.80 d ± 0.11 | 0.93 c ± 0.05 | 0.45 c ± 0.02 | 5.64 bc ± 0.26 | 63.8 b ± 3.60 |
| T5 | 2.55 b ± 0.12 | 1.16 c ± 0.06 | 2.86 ab ± 0.12 | 1.20 b ± 0.06 | 0.71 ab ± 0.04 | 4.93 c ± 0.21 | 56.9 b ± 3.04 |
| T6 | 3.16 a ± 0.13 | 1.48 ab ± 0.10 | 2.91 ab ± 0.11 | 1.53 a ± 0.08 | 0.69 ab ± 0.03 | 6.39 ab ± 0.27 | 65.9 ab ± 2.95 |
| T7 | 2.86 ab ± 0.15 | 1.59 a ± 0.08 | 2.43 bc ± 0.12 | 1.40 a ± 0.08 | 0.66 b ± 0.03 | 6.12 abc ± 0.27 | 77.8 a ± 4.53 |
| Level of significance | ** | ** | ** | ** | ** | ** | ** |
| MSD value | 0.59 | 0.29 | 0.49 | 0.14 | 0.14 | 1.21 | 12.12 |
| CV (%) | 14.9 | 15.8 | 13.6 | 7.74 | 15.4 | 14.1 | 14.08 |
| Interaction (V*T) significance | ns | ns | ns | ns | ns | ns | ns |
| Treatment | Nitrogen Uptake (g/Plant) | Phosphorus Uptake (g/Plant) | Potassium Uptake (g/Plant) | Sulfur Uptake (g/Plant) | Magnesium Uptake (g/Plant) | Boron Uptake (mg/Plant) | Zinc Uptake (mg/Plant) |
|---|---|---|---|---|---|---|---|
| V1 = MT1 | 2.52 b ± 0.20 | 1.09 b ± 0.08 | 2.07 b ± 0.17 | 1.06 b ± 0.09 | 0.24 b ± 0.02 | 2.80 b ± 0.22 | 4.11 b ± 0.34 |
| V2 = MT3 | 2.58 a ± 0.22 | 1.16 a ± 0.09 | 2.15 a ± 0.17 | 1.10 a ± 0.09 | 0.25 a ± 0.09 | 2.87 a ± 0.22 | 4.29 a ± 0.39 |
| Level of significance | * | * | * | * | * | * | * |
| MSD value | 0.03 | 0.02 | 0.04 | 0.02 | 0.01 | 0.03 | 0.03 |
| CV (%) | 9.11 | 8.50 | 9.96 | 10.6 | 14.5 | 9.62 | 9.33 |
| T1 | 0.61 f ± 0.03 | 0.21 f ± 0.01 | 0.46 e ± 0.03 | 0.23 e ± 0.02 | 0.07 d ± 0.01 | 0.50 e ± 0.03 | 0.83 f ± 0.04 |
| T2 | 2.48 d ± 0.09 | 1.25 c ± 0.05 | 2.94 a ± 0.10 | 1.39 b ± 0.07 | 0.30 ab ± 0.02 | 3.72 a ± 0.14 | 3.60 de ± 0.13 |
| T3 | 2.42 d ± 0.10 | 1.29 c ± 0.05 | 2.07 c ± 0.08 | 1.07 c ± 0.06 | 0.24 b ± 0.01 | 3.42 b ± 0.13 | 3.93 d ± 0.15 |
| T4 | 1.79 e ± 0.07 | 0.94 e ± 0.04 | 1.38 d ± 0.05 | 0.74 d ± 0.04 | 0.18 c ± 0.01 | 2.29 d ± 0.09 | 3.32 e ± 0.16 |
| T5 | 2.92 c ± 0.12 | 1.08 d ± 0.04 | 2.63 b ± 0.10 | 1.13 c ± 0.06 | 0.27 b ± 0.02 | 2.59 c ± 0.11 | 4.86 c ± 0.20 |
| T6 | 3.95 a ± 0.20 | 1.46 b ± 0.06 | 2.81 a ± 0.10 | 1.53 a ± 0.08 | 0.34 a ± 0.02 | 3.44 b ± 0.12 | 5.99 b ± 0.22 |
| T7 | 3.72 b ± 0.13 | 1.66 a ± 0.09 | 2.49 b ± 0.14 | 1.46 ab ± 0.11 | 0.33 a ± 0.02 | 3.89 a ± 0.20 | 6.88 a ± 0.35 |
| Level of significance | ** | ** | ** | ** | ** | ** | ** |
| MSD value | 0.20 | 0.10 | 0.16 | 0.13 | 0.06 | 0.20 | 0.35 |
| CV (%) | 9.11 | 8.50 | 9.96 | 10.6 | 14.5 | 9.62 | 9.33 |
| Interaction (V*T) significance | ns | ns | ns | ns | ns | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, R.; Uddin, M.K.; Quddus, M.A.; Samad, M.Y.A.; Hossain, M.A.M.; Haque, A.N.A. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae 2023, 9, 162. https://doi.org/10.3390/horticulturae9020162
Ahmed R, Uddin MK, Quddus MA, Samad MYA, Hossain MAM, Haque ANA. Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae. 2023; 9(2):162. https://doi.org/10.3390/horticulturae9020162
Chicago/Turabian StyleAhmed, Razu, Md. Kamal Uddin, Md. Abdul Quddus, Mohd Yusoff Abd Samad, M. A. Motalib Hossain, and Ahmad Numery Ashfaqul Haque. 2023. "Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato" Horticulturae 9, no. 2: 162. https://doi.org/10.3390/horticulturae9020162
APA StyleAhmed, R., Uddin, M. K., Quddus, M. A., Samad, M. Y. A., Hossain, M. A. M., & Haque, A. N. A. (2023). Impact of Foliar Application of Zinc and Zinc Oxide Nanoparticles on Growth, Yield, Nutrient Uptake and Quality of Tomato. Horticulturae, 9(2), 162. https://doi.org/10.3390/horticulturae9020162








