Abstract
Invasive tomato leaf miner, Phthorimaea absoluta causes serious damage and yield loss in tomato production in open-field and protected cultivation. Use of chemical pesticides is uneconomical and adversely affects humans and the environment. Host-plant resistance is an effective, economical and eco-friendly alternative to chemical pesticides. In this study, four wild tomato accessions from the World Vegetable Center along with one susceptible check were evaluated for their antixenosis and antibiosis effects on P. absoluta. The accessions VI037241 (Solanum galapagense) and VI037240 (S. cheesmaniae) were highly resistant, leading to 85% larval mortality under no-choice conditions. Choice assay also showed less oviposition preference and reduced pupal weight. Both VI037241 and VI037240 showed the highest resistance under field conditions. The accessions of S. habrochaites (LA1777) and S. habrochaites var. glabratum (VI030462) demonstrated moderate resistance against P. absoluta. Wild accessions recorded significantly less eggs and leaf damage in field trials compared to the susceptible genotype, S. lycopersicum (CL5915). Trichome density, type and higher production of acylsugar contributed to the insect resistance. Acylsugar production in wild accessions was less during the rainy season but significantly higher than in susceptible genotype. These findings can be useful to develop P. absoluta-resistant tomato varieties in tropics.
1. Introduction
Tomato, Solanum lycopersicum L., is the second most important vegetable crop in India, annually grown on 779,000 ha with an annual production of 19.38 mt [1], with Bihar, Karnataka, Uttar Pradesh, Odisha, Andhra Pradesh, Maharashtra, Madhya Pradesh, and West Bengal being the major tomato producing states. However, most tomato cultivars are susceptible to a wide range of arthropod pests, which can cause significant losses, including the invasive tomato leaf miner, Phthorimaea absoluta (Meyrick) (Lepidoptera: Gelechiidae), which threaten the tomato industry in India.
Phthorimaea absoluta is one of the major pests of tomatoes in South America. It was introduced to Europe via Spain in 2006, and later spread into Africa and Asia [2,3], with heavy damage to tomato crops. Phthorimaea absoluta was first reported in India (Maharashtra) in 2014 [3], and subsequently spread to other parts of India including Karnataka, Tamil Nadu, Andhra Pradesh, Telangana, Gujarat, Chhattisgarh, Madhya Pradesh, Uttarakhand, Punjab, and Meghalaya [3]. After the introduction of this pest in India, it started causing havoc, and it has been reported from 13 district of Maharashtra state out of 18 surveyed districts [3], with more than 50% damage. Besides tomato, P. absoluta also feeds on other Solanaceous plants including potato (Solanum tuberosum), eggplant (Solanum melongena), as well as other host plants, such as Jimson weed (Datura stramonium), broad bean (Vicia faba) and alfalfa (Medicago sativa) of the Fabaceae family and other members of the Cucurbitaceae, Euphorbiaceae, Asteraceae, Amaranthaceae, Cleomaceae and Verbenaceae families [4,5]. Larvae of P. absoluta can damage both the leaves and fruits of tomatoes, causing up to 90% yield losses and severely affecting fruit quality under greenhouse and field conditions [6]. Recently, tomato production losses of USD 59.3 and 8.5 million were reported in Kenya and Zambia, respectively, due to the P. absoluta attack [7].
The spread of P. absoluta is alarming as it can feed and attack several solanaceous host plants. The pest has developed resistance to several insecticides at field level [8]. After the first report of developing resistance against organophosphates and pyrethroids in Chile [8], a few more studies were conducted to find the resistance level in P. absoluta against various groups of insecticides. A few years ago, field-evolved resistance has been reported in various parts of Brazil, where after successful control for 6 long years, P. absoluta had developed resistance against the diamide group of insecticides under field conditions [9]. A study conducted in India reported that the severity of P. absoluta resulted in the crop losses ranging from 20–90 per cent, and the main reasons for the outbreak of P. absoluta were due to lack of knowledge on IPM among the farmers, delay in implementing management strategies, over reliance on insecticide sprays, and usage of spurious and un-recommended insecticides [10]. In a recent study conducted in the tomato field of Hyderabad and Tamil Nadu, India, the results indicated a variable level of susceptibility of different field population of P. absoluta against frequently used insecticides, viz., flubendiamide 39.35% SC, cyantraniliprole 10.25% SC and indoxacarb 14.5% SC. The biochemical assays of the resistant population have indicated a significant increase in the detoxifying enzymes, especially cytochrome P450, monooxygenase and esterase level of P. absoluta [11]. Therefore, controlling P. absoluta with chemical means is often futile, and hence, managing them in an eco-friendly manner helps us to achieve sustainable management. Therefore, finding resistant sources, which are adapted to tropical conditions, is essential to combat this invasive pest species.
Plants offer various defensive strategies to cope with insect herbivory [6,12]. Morphological parameters such as trichomes are very important plant resistance traits. Besides conferring physical protection against biotic stress factors, trichomes also produce antibiotic and antixenotic chemical compounds that may confer resistance against insect pest attacks. Three main groups of trichome-produced allelochemicals associated with herbivore resistance [12,13] are sesquiterpenes, methyl ketones, and acylsugars. Resistant sources to insect pests have been found in wild tomato species such as Solanum habrochaites var. hirsutum, S. pennellii, S. cheesmaniae, S. galapagense, S. peruvianum, etc. [14,15,16,17]. Therefore, studying the stability of the resistance traits under tropical and subtropical conditions and also incorporating those into breeding lines are needed to develop insect-resistant tomato cultivars.
Acylsugars synthesized in the glandular trichomes of the Solanaceae family are implicated in protection against abiotic and biotic stresses, and is very important in pest resistance [4,18,19]. Acylsugars are composed of either sucrose or glucose, esterified with varying numbers of acyl chains of differing lengths. However, acylsugar and its chemotypes can vary based on the weather parameters and geographical area [20]. Trichome metabolite analysis revealed the presence of at least 34 structurally diverse acyl sucroses and two acyl glucoses. Distinct phenotypic classes that varied based on the presence of glucose or sucrose, the numbers and lengths of acyl chains, and the relative total amounts of acylsugars were discovered. The presence or absence of an acetyl chain on the acyl sucrose hexose ring caused clustering of the accessions into two main groups [21], which are very important in pest resistance [18,19]. The World Vegetable Center (WorldVeg) had identified accessions of S. galapagense, S. habrochaites and S. cheesmaniae that are resistant to whitefly (Bemisia tabaci), spider mite (Tetranychus urticae), and P. absoluta [15,16].
Hence, the objectives of the current study were to investigate resistance levels, trichome density and acylsugar levels of selected accessions grown under different seasons (summer, rainy and winter) and under open-field conditions compared to controlled condition. In addition, our study also had an objective to find the categories of resistance, with a special focus on the expression of antixenosis and antibiosis against invasive P. absoluta among selected wild tomato accessions.
2. Materials and Methods
2.1. Plant Materials
Seeds of wild tomato accessions, VI037241 (S. galapagense), VI037240 (S. cheesmaniae), LA1777 (S. habrochaites), and VI030462 (S. habrochaites var. glabratum), which were previously reported as insect resistant [16,17], along with a susceptible accession CL5915 (S. lycopersicum) used as a check were supplied by WorldVeg. Field trials to evaluate the resistance of the wild tomato accessions and perform a susceptibility check against P. absoluta were conducted in three different seasons, i.e., summer, autumn (kharif) and winter (rabi) during 2017–2018 and 2018–2019 under field conditions at M/s I & B Seeds Pvt. Ltd., Uttarahalli, Bengaluru, Karnataka (Latitude 12.9041844 N, Longitude 77.5071723 E) (Table S1). The seeds were treated with gibberellic acid @ 250 ppm overnight to break the dormancy and placed in Petri plates for germination. The germinated seeds were planted in small jiffy pots. Seedlings were grown in a greenhouse under ambient temperature (28–35 °C, 16/8 h day/night). Seedlings were raised in a polyhouse and later used in field screening experiments as well as in lab bioassays. One-month-old seedlings were transplanted to the field in single-row plots (row length = 7.20 m) with entries arranged in a randomized complete block design (RCBD), with four replications for each entry (Figure S1). Spacing between and within rows were 90 cm and 60 cm, respectively. Plant spacing was 20 and 15 cm between and within accessions, respectively. The minimum number of plants per plot was 12, and data were collected from 5 inner plants per plot/replication. The number of eggs and larvae and the percent foliage and fruit damage were recorded on fortnightly basis till 15th week after transplanting of the crop.
2.2. Sampling for Insects and Damage
Non-destructive sampling was performed on the 3rd, 6th, 9th, 12th and 15th week after transplanting. At each sampling date, five tomato plants were randomly selected in each plot. From each selected plant, twenty leaflets per canopy were randomly chosen and the numbers of the eggs, larvae, and percent leaf damage were recorded. A rating scale utilized from Ayalew (2011) for Plutella xylostella damage on brassicas [22,23] was used for scoring the P. absoluta leaf damage (Table 1).

Table 1.
Rating scale adapted for assessing P. absoluta foliage damage on tomato genotypes.
2.3. Trichome Type and Density Analysis
Sampling was performed by collecting 2nd, 5th, and 7th leaf from the apex of a branch at three canopy levels by plucking with forceps and used for observing the trichome types and density, with four replications for each genotype. Trichome (both glandular and non-glandular) types were identified by using a Hitachi TM 3030 plus Scanning Electron Microscope (SEM) (Hitachi High-Technologies Corporation, Tokyo, Japan) with low vacuum analysis [24] at Indian Institute of Horticultural Research (IIHR), Bengaluru, Karnataka. After identifying the different types of trichomes occurring on each accession, the total number of trichomes present on the abaxial and adaxial surfaces was recorded under a stereo-microscope using leaves at different phenological stages of the crop. Densities were determined from the interior middle section of the abaxial surface of the second leaf from the apex of six randomly selected plants per accession by using a 0–3 visual scale, adapted from Simmons and Gurr (2005) [23] where 3 = abundant (5 per mm2); 2 = sparse (1–5 per mm2); 1 = very sparse (1 per mm2); and 0 = absent. The mean trichome density was computed and later correlated with infestation levels. Additionally, trichome types and density were further correlated with acylsugar levels produced by each of the evaluated genotypes.
Since glandular trichomes has been previously reported to impart insect resistance [15,25,26], detailed investigation was made on the type and density of glandular trichomes using SEM. Three tomato leaflets were chosen from each accession and type of glandular trichomes was studied by using the SEM at the Laboratory of IIHR, Bengaluru. Samples were washed in 0.1 M phosphate buffer (pH 7) and post-fixed in 2% Osmium tetra-oxide (OsO4) (pH 7). The samples were then taken through an alcohol dehydration series (15%, 25%, 40%, 50%, 70%, and 95% EtOH). SEM samples were critical point dried, sputter-coated to 20 nm with gold/palladium and mounted on aluminum stubs for observation under a Phillips SEM at 10–15 kV [27,28]. The average trichome/mm2 was recorded and then correlated with percent larval mortality data.
2.4. Acylsugar Analysis
Acylsugar was assessed at different phenological stages of the crop in different seasons. Leaf samples from three different canopy levels were collected fortnightly starting from two weeks after transplanting with the help of forceps and dried in the biological oxygen demand (BOD) incubator chamber, at 27 °C. Such sampling of leaves was continued till 15th week after transplanting. Acylsugar level was quantified by using the modified protocol [29]. After drying the leaf samples for 4–5 days, the extraction was carried out by using methanol as the solvent. The extracted leaves were kept inside a dryer and the dry leaf weight was measured after 2 days. The leaf extract was further taken and mixed with other reagents. The sample with reagents were kept inside a shaker for 3 h, and then observation was taken with a spectrophotometer. The observed reading was further used to quantify the total acylsugar present in a particular leaf sample. Acylsugar was correlated with trichome type, trichome density, and insect foliage damage.
2.5. Rearing of P. absoluta and Maintenance of the Colonies
Initially, fifty adult moths of P. absoluta were collected from the polyhouse of M/s I&B Seeds Company (Latitude 12.9041844 N, Longitude 77.5071723 E) and 20 adults were collected from farmers’ fields in and around Magadi taluk of Ramanagara district, Kadur taluk of Chikkamagaluru district (Latitude 12.9577° N, Longitude 77.2261° E) and Department of Horticulture, UAS, GKVK, Bengaluru, Karnataka (Latitude 13.0781° N, Longitude 77.5792° E) in August, 2017. Adults were fed ad libitum with a 10% (w/v) sugar solution dispensed on cotton wool placed inside the cages. Adult male and female P. absoluta moths were reared in cages of 65 cm × 45 cm × 45 cm size of plexi-glass and kept with potted tomato plants of the variety “Rishika” (from Clause Vegetable Seeds, Hyderabad). Fresh seedlings were used as oviposition substrate and kept changed regularly by replacing with fresh leaves of same genotypes. The plants were then kept in similar cages, where the eggs hatched. To obtain adults of the same age, prepupal stage of the larvae were placed in cages of 20 cm × 15 cm × 15 cm size and provided with fresh tomato leaves daily until pupation. Newly formed pupae were placed in a cold chamber, at 10 °C, until all the larvae became pupae, which were then sexed based on sex-specific external morphology [13,30]. Thirty pairs of pupae were selected, and male and female pupae were separated. Each pair was placed in a climatic chamber (the average emergence time for males is 7.8 ± 0.28 days, while that of females is 8.7 ± 0.22 days, at 25 °C) and kept undisturbed for 3–4 days.
After adult emergence, the males and females of the same age were released into a mating cage of 90 cm × 45 cm × 45 cm size for 12 h, and then insect pairs were separated into individual cages of 50 cm × 40 cm × 40 cm. Males and females were identified based on different morphological features, including color (i.e., the male is darker), and abdomen width (i.e., males have a narrower abdomen than females). The mated female adults were placed in the same vial and provided with water and honey before using them for choice assays. A part of the mated females was used for maintaining the insect colony. They were allowed to lay the eggs. After hatching from the eggs, the neonate larvae were transferred to new cages with healthy tomato plants as dietary substrate for multiplication, and the remaining larvae were used for laboratory no-choice bioassays.
2.6. No Choice Bioassay (Larval Feeding)
All five tomato accessions (treatments) were planted in plastic pots and kept in a polyhouse, and the leaves of each accession were collected and used for the no-choice bioassays under laboratory conditions (25 ± 1 °C, R.H. of 65 ± 5% and a photoperiod of 16L: 8D h). Furthermore, twenty early second-instar larvae were released on the leaves of each accession to assess the effect of different accessions on their development. Percent mortality, days taken for pupation, pupal weight and percent adult emergence were recorded in the no-choice test. The assay was replicated 6 times.
2.7. Choice Bioassay (Oviposition Preference)
To assess the preference for oviposition, P. absoluta adults were released in the cages, and the effects of these genotypes on oviposition were studied under controlled conditions (25 ± 1 °C, R.H. of 65 ± 5% and a photoperiod of 16L: 8D h). The branches of each accession along with few leaves were picked up for choice assays and the branches of all different accessions were kept in equidistance manner inside a cage and exposed to ten pairs of P. absoluta adults. The number of eggs laid on the leaf surface (both abaxial and adaxial) was counted to assess the total egg load.
The experiment was replicated four times. Egg hatchability was recorded and converted into percent egg hatching. The first evaluation of oviposition was performed at 3 days after exposure to the moth, by counting the number of eggs present on the leaf using a binocular stereoscopic microscope with 20 to 80× magnification. The evaluation was repeated once in every two days, till 10th day of exposure. Numbers of eggs and larvae were counted, besides scoring the overall plant damage due to P. absoluta larvae. Proportion of eggs was calculated to find out the most preferred tomato accession (the susceptible accession) for oviposition by P. absoluta female moths.
The proportion of eggs laid was calculated by using the following formula:
Proportion of egg laid = [(Number of eggs laid per plant of each accession in a choice cage)/(Sum of eggs laid in all different accessions within a choice cage)] × 100
2.8. Data Analysis
Data on field observation on egg and larval load and foliage damage caused by P. absoluta was analyzed using a combined analysis approach of several experiments [31,32]. Preliminary analysis of variance (ANOVA) was completed for each individual analysis (each accession in each season); experimental errors were examined for heterogeneity. The data were then analyzed using ANOVA with the Proc GLM MIXED of SAS, version 9.1 (SAS Institute, Cary, NC, USA). Each variety/season/damage in terms of egg load, larval load and foliage damage was considered as a particular environment for the combined analysis. Years were considered as random effects, while treatments were considered as fixed effects. When significant treatment differences were identified, means were separated by Tukey’s HSD test (differences were considered significant at α = 0.05). Data on egg and larval counts were square-root transformed (sqrt + 0.5), and percent foliage damage was Arcsine transformed [ASIN (SQRT (I2)] before analysis.
Pearson’s correlation coefficient was calculated to assess the relationship between moth population density, number of larvae and leaf trichome density by using SPSS 13.0 (2004). Host-plant resistance can be governed by multiple factors under open-field condition. Hence, tobit regression was performed using total glandular trichomes and acylsugar content of leaves as independent factors and the larval mortality as a dependent factor by using Stata 13.0 software (StataCorp, 2013. Stata Statistical Software: Release 13. College Station, TX, USA) [33]. To generate the tobit model, we listed the outcome variable followed by the predictors, and then specified the lower limit and the upper limit of the outcome variable. The lower limit of dependent variable (LL) was specified as 10, and the upper limit (UL) was 85. The number of observations in the dataset was 150 for which all the response and predictor variables were non-missing.
3. Results
3.1. Field Trials
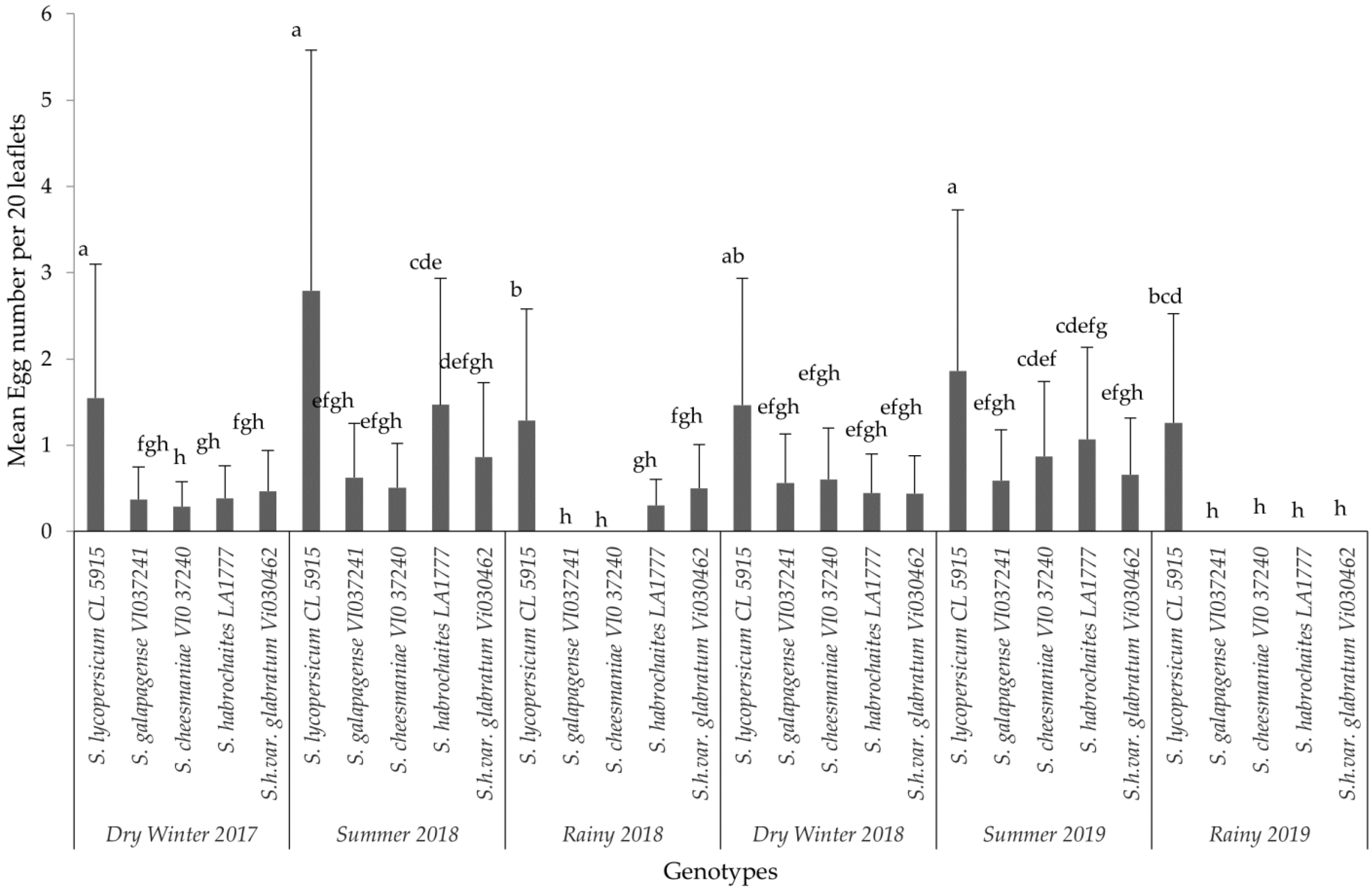
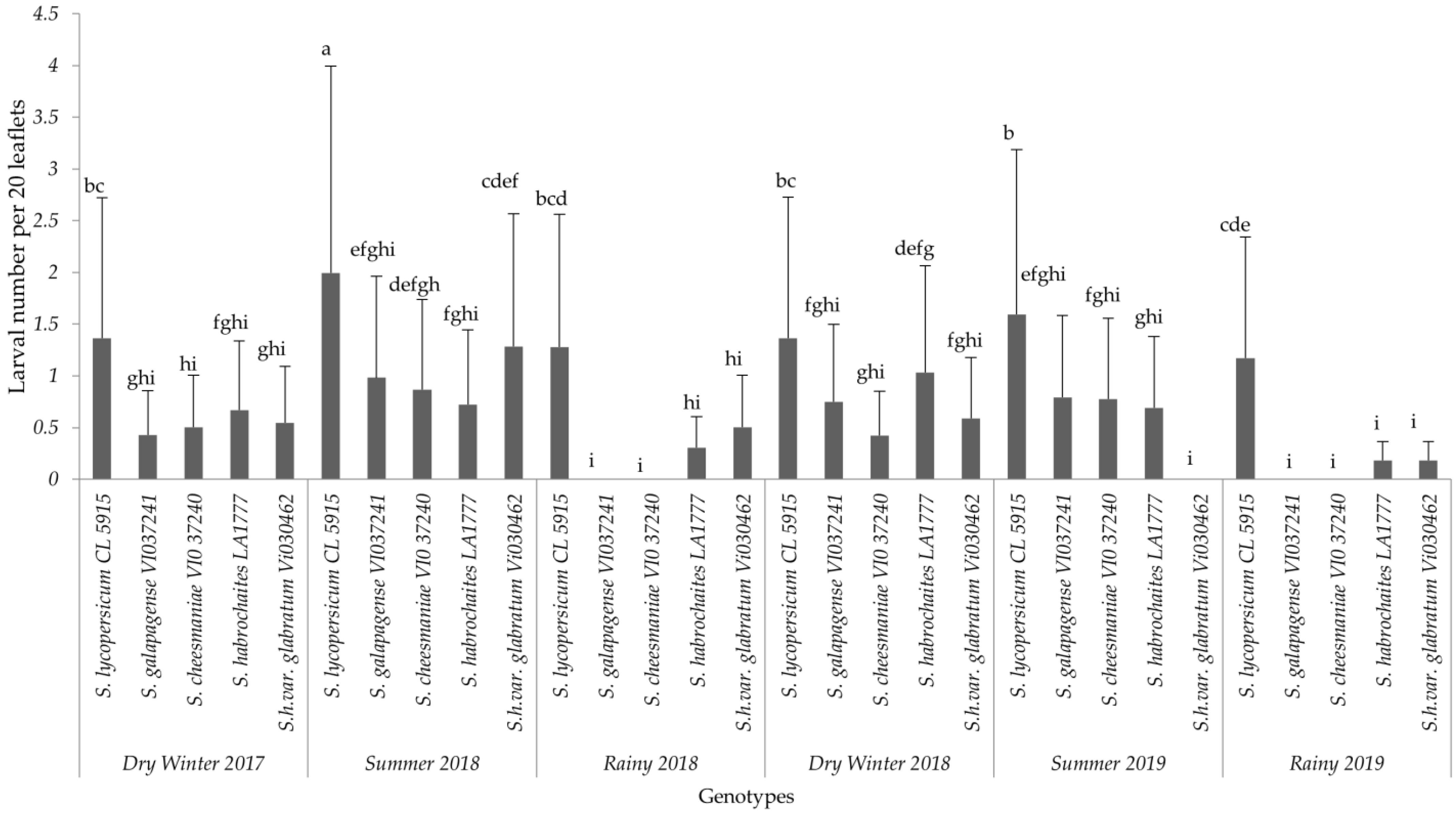
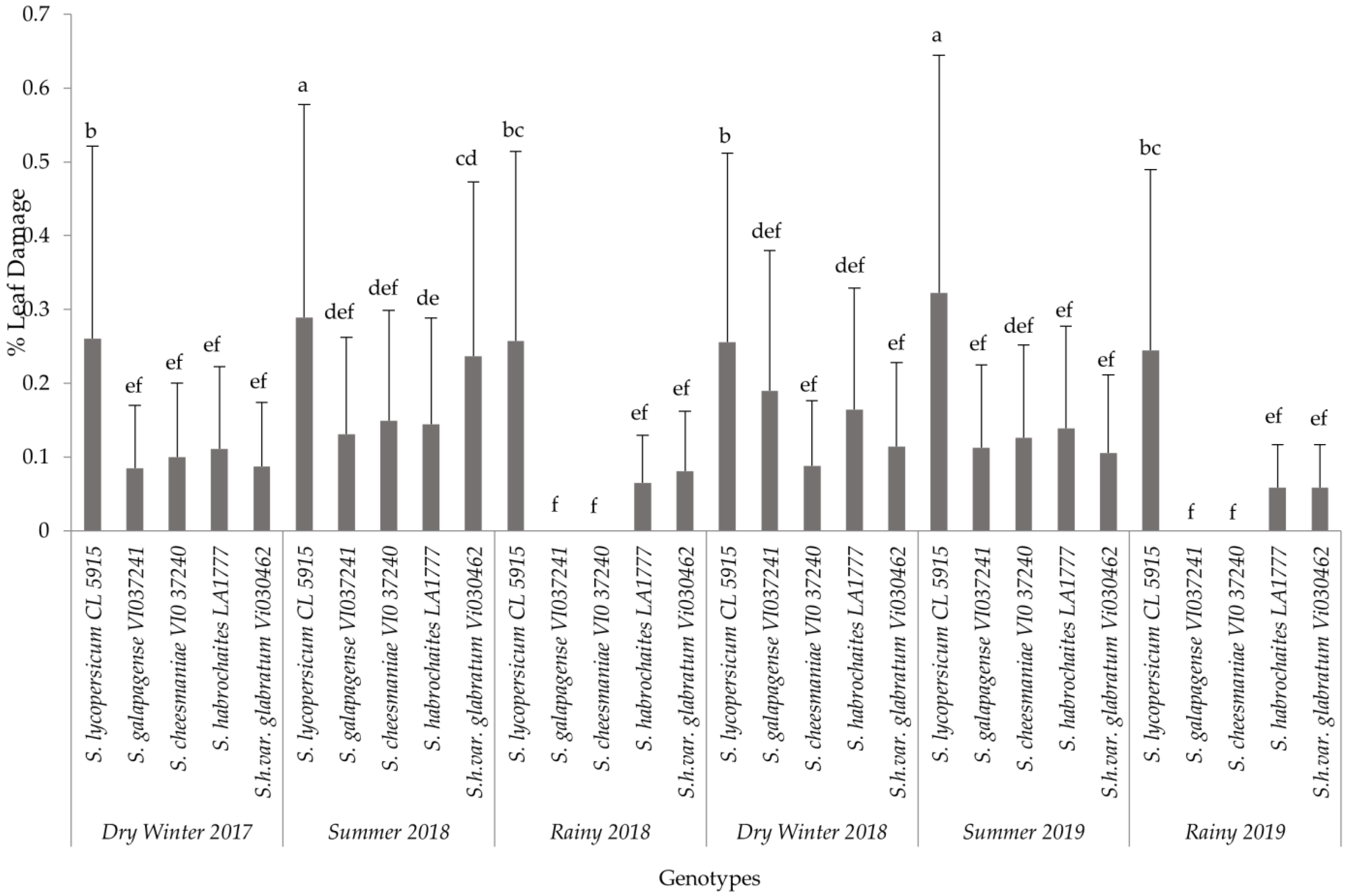
The analysis for each variable showed significant differences among the interaction effects of treatments and seasons. Based on the combined analyses for each evaluated variable, only interaction effects are presented in this paper (Figure 1, Figure 2 and Figure 3). The results of non-significant differences among tomato accessions for adult survival, emerged larvae, and non-significant correlated parameters were not presented in a graph or a table. For all six field trials, significant differences were observed among the genotypes (p ≤ 0.0001) in terms of egg load, larval number and leaf damage. More specifically, the susceptible variety recorded the highest mean larval number and percent foliage damage due to P. absoluta infestation. In contrast, the wild Solanum species, S. galapagense (VI037241) and S. cheesmaniae (VI037240) accessions recorded lower P. absoluta egg load (Figure 1), larval population (Figure 2) and foliage damage (Figure 3), followed by S. habrochaites (LA1777) and S. habrochaites var. glabratum (VIO30462) accessions when compared to susceptible genotype S. lycopersicum (CL5915/CH45) (Figure 2). In addition, significant differences were observed for egg load among the canopy levels (p ≤ 0.0001). Furthermore, egg load was significantly higher in upper and middle canopy leaves, whereas no or very few egg load was observed in lower canopy leaves in wild tomato accessions especially in S. galapagense (VI037241) and S. cheesmaniae (VI037240). However, it did not differ significantly at different weeks after transplanting.
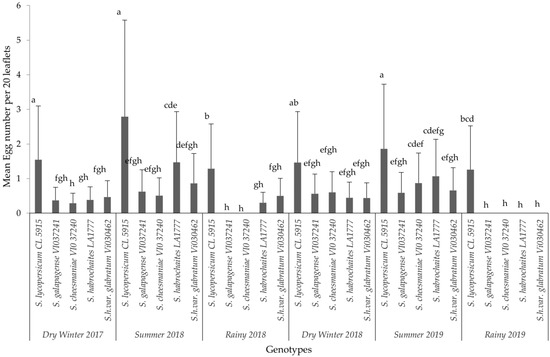
Figure 1.
Mean (±SD) number of P. absoluta eggs on tomato genotypes in different years and seasons. Significant differences are presented for the interaction of Seasons X Genotypes. Genotypes with the same letter(s) did not differ statistically across seasons based on Tukey’s HSD (p = 0.05).
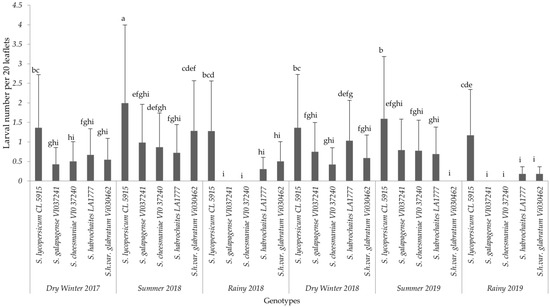
Figure 2.
Mean (±SD) number of P. absoluta larvae on tomato genotypes in different seasons. Significant differences are presented for the interaction of Seasons X Genotypes. Genotypes with the same letter(s) did not differ statistically across seasons based on Tukey’s HSD (p = 0.05).
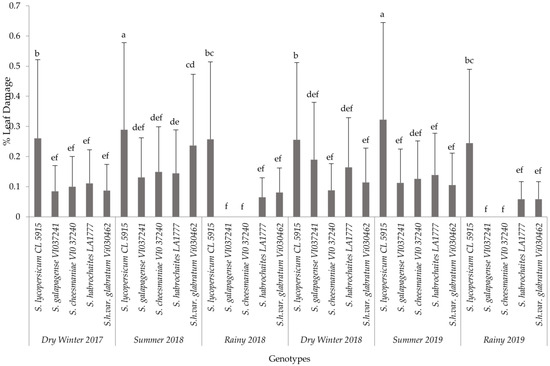
Figure 3.
Mean (±SD) of P. absoluta foliage damage (%) on tomato genotypes in different seasons. Significant differences are presented for the interaction of Seasons X Genotypes. Genotypes with the same letter(s) did not differ statistically across seasons based on Tukey’s HSD (p = 0.05).
In general, during the wet season trials, the P. absoluta incidence was low, but egg load, larval population and foliage damage were still significantly lower on wild tomato accessions than on the susceptible genotype (p < 0.05). A similar trend in foliage damage level was observed during the wet seasons of different years (Table 2). P. absoluta infestation in the summer season significantly differed with the damage in the winter and rainy seasons (Table 2). Larvae, which were observed on leaves of wild accessions, were not able to grow to later instars, and they were confined into smaller mining blotches. To confirm the results of field screening, lab bioassays and a morphometric analysis of trichomes were conducted using choice and no-choice assays under controlled conditions for all the genotypes.

Table 2.
Analyses for resistance reaction of tomato genotypes to P. absoluta oviposition, larval number and foliage damage in different seasons under field conditions during 2017–2019.
3.2. Choice Assay
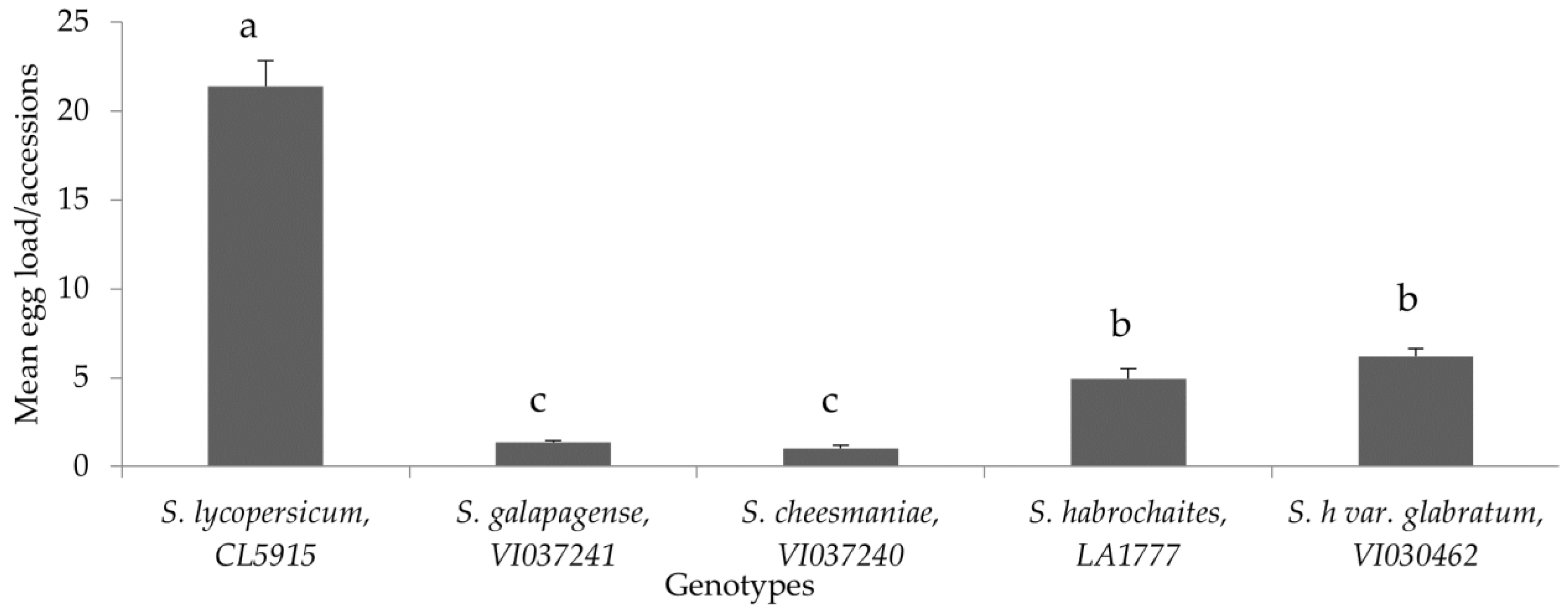
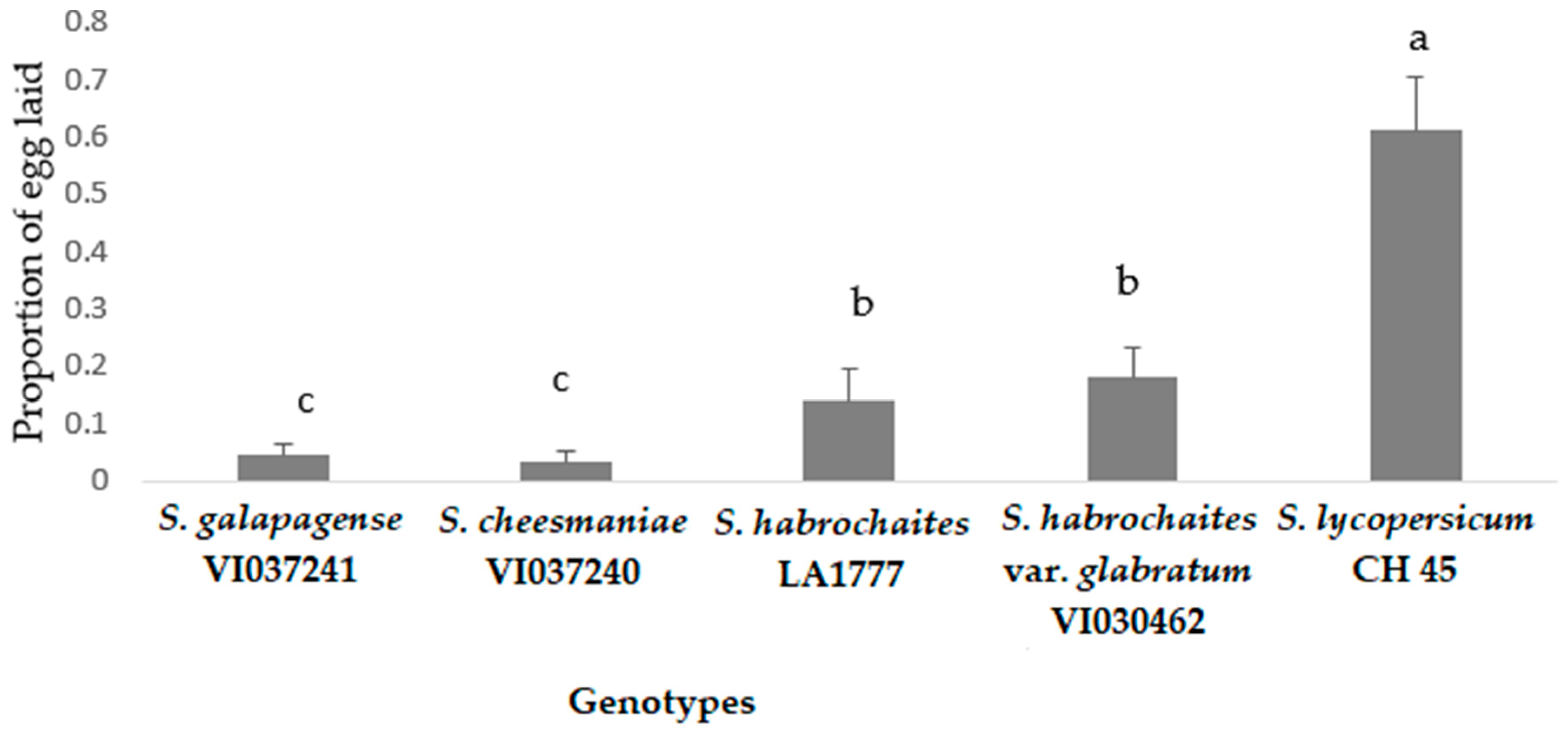
Oviposition preference of P. absoluta on different tomato genotypes under choice conditions showed that all the wild accessions recorded significantly lower egg load compared to the susceptible control, S. lycopersicum (CL5915) (F = 27.127, p < 0.005, df = 24) (Figure 4). In addition, egg load was consistently lower in S. cheesmaniae (VI037240), followed by S. galapagense (VI037241), S. habrochaites (LA1777) and S. habrochaites var. glabratum (VI030462), whereas S. lycopersicum (CL5915) recorded the highest number of eggs (Figure 4). The high proportion of eggs laid on the susceptible genotype suggested a significantly overwhelming preference of female moths for susceptible genotype compared to the wild Solanum genotypes (F = 70.47, p < 0.005, df = 24) (Figure 5).
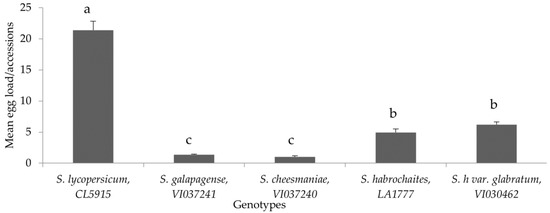
Figure 4.
Mean (±SE) number of P. absoluta eggs on tomato genotypes in choice bioassay under laboratory conditions. Genotypes with the same letter did not differ statistically based on Tukey’s HSD (p = 0.05).
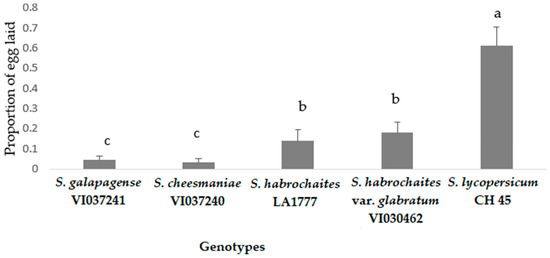
Figure 5.
Proportion of eggs laid (mean ± SE) by P. absoluta female moths on tomato genotypes in choice bioassay under laboratory conditions. Genotypes with the same letter did not differ statistically based on Tukey’s HSD (p = 0.05).
3.3. No-Choice Assay
Larval mortality of P. absoluta varied significantly among genotypes (p ≤ 0.0001) (Table 3). Most of the larvae died in late second instar or in third instar stage in S. cheesmaniae (VI037240) (83.60%), S. galapagense (VI037241) (82.60%), whereas larval mortality observed for S. habrochaites var. glabratum (VI030462) and S. habrochaites (LA1777) accessions was 46.00% and 40.60%, respectively, compared to 15% of larval mortality recorded on the susceptible genotype S. lycopersicum (CL5915). In addition, larvae fed on wild Solanum accessions and reaching the pupal stage were significantly underweight (p ≤ 0.0001) compared to the susceptible accession, sometimes reaching only half of the weight observed on pupa growing on susceptible genotype S. lycopersicum (CL5915). Similarly, pupal duration was significantly shorter (11 days) (p ≤ 0.0001) and the adult emergence percentage was significantly higher (76%) (p ≤ 0.0001) on susceptible genotype S. lycopersicum (CL5915) (Table 3).

Table 3.
Phthorimaea absoluta mean larval mortality percentage, pupal duration, pupal wight and adult emergence percentage in wild tomato accessions compared to the tomato check CL5915 evaluated in no-choice feeding bioassay under laboratory conditions.
3.4. Morphological Bases of Resistance
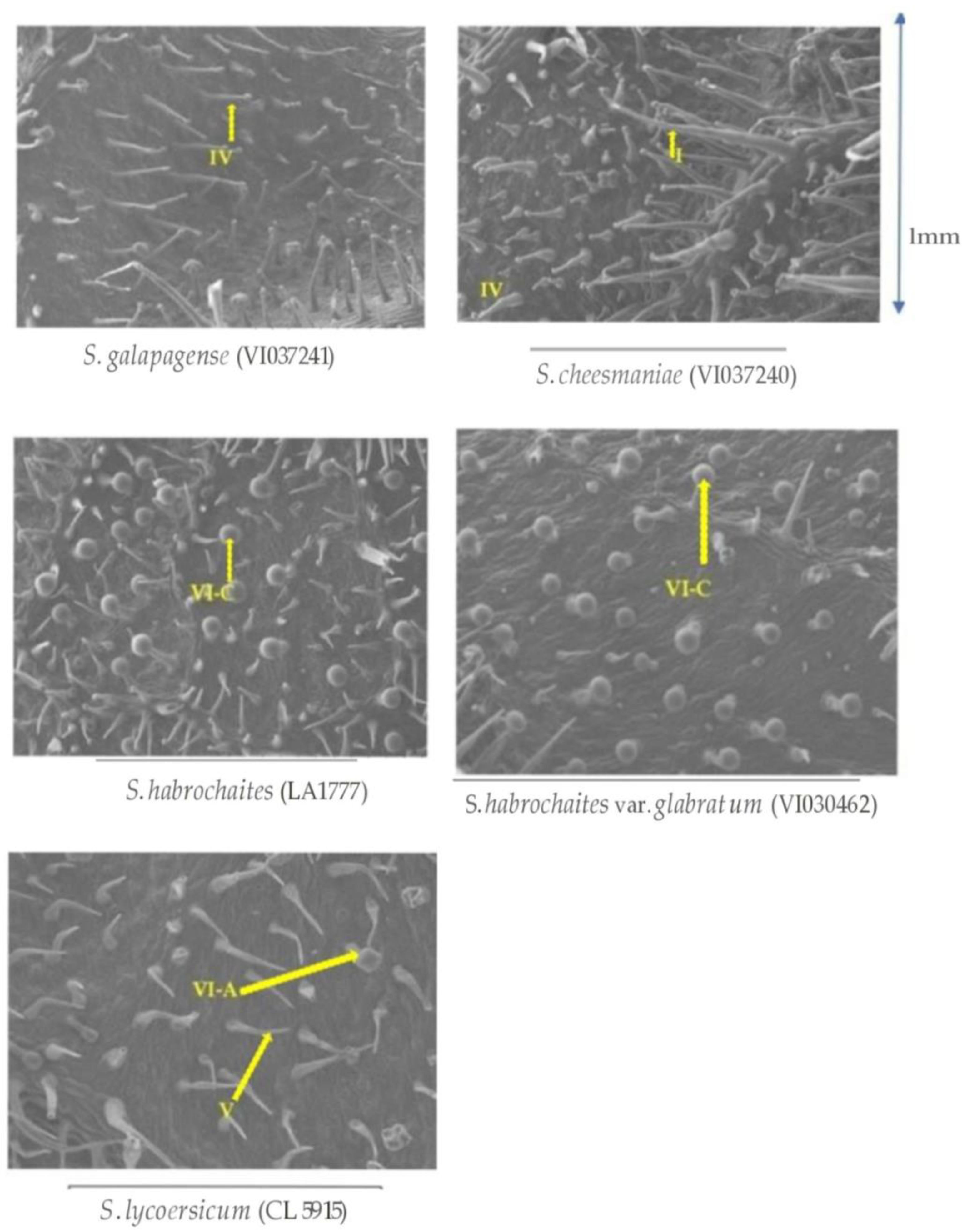
Density of glandular trichomes was significantly higher on wild tomato accessions compared to the total glandular trichomes in S. lycopersicum (CL5915) based on morphometric analysis of the trichomes. Furthermore, there was combination of different glandular types of trichomes (type IV, VI, I and VII) in resistant accessions, whereas only one type of glandular trichomes (type VI) with a low density was observed in the susceptible accession (Figure 6).
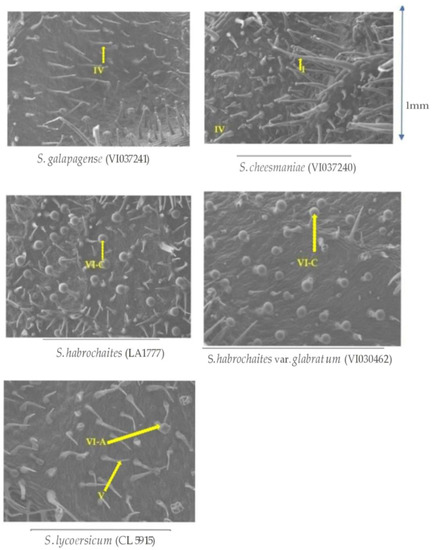
Figure 6.
Scanning electron micrographs of trichome morphology and density on abaxial leaf surfaces of five selected Solanum accessions/check evaluated in this study. The arrow head in VI-A indicates type VI trichomes in S. lycopersicum with looped shape, and VI-C indicates type VI trichomes in S. habrochaites accessions with spherical shape.
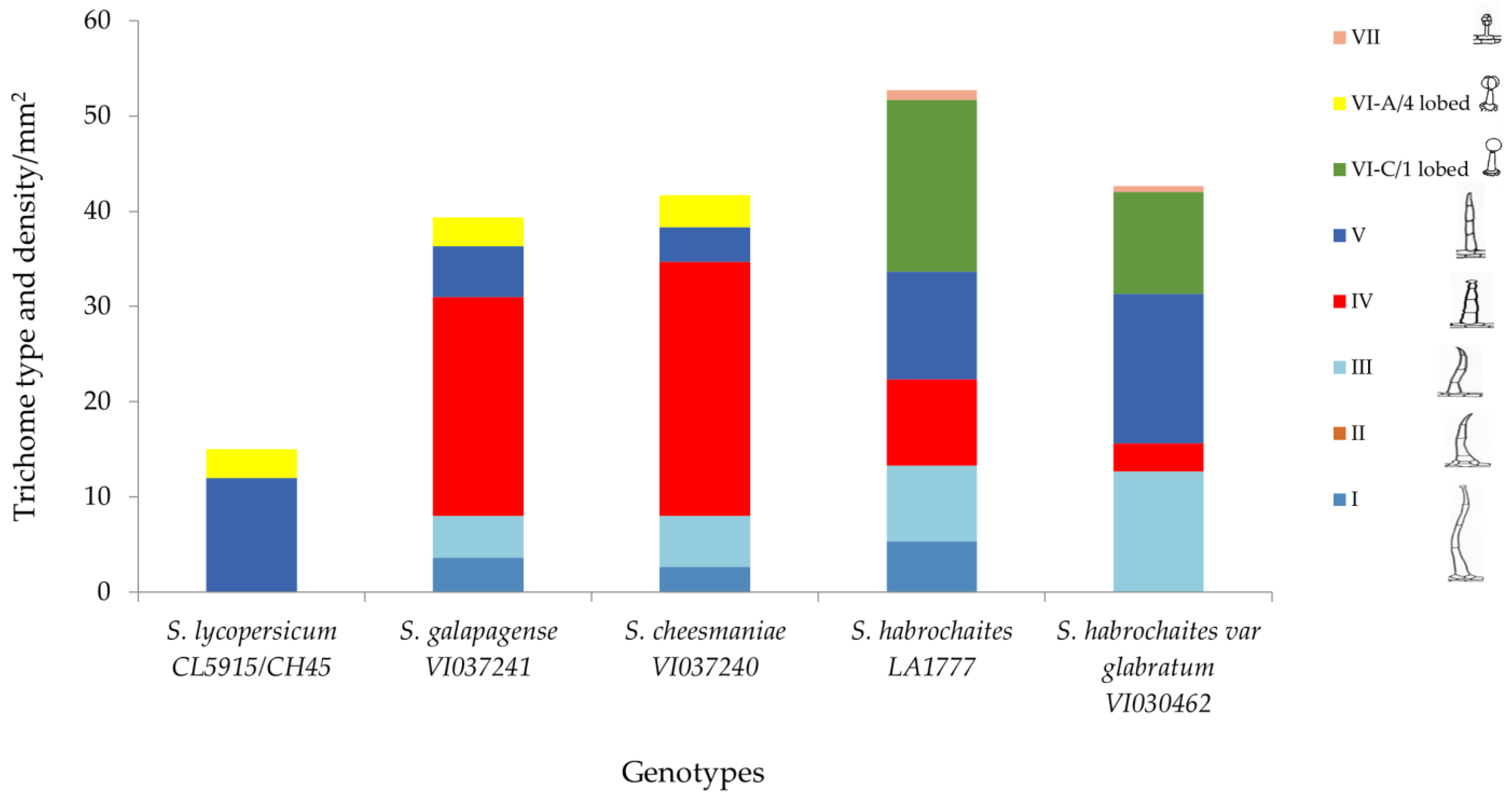
Higher glandular trichome density and especially presence of type I and type IV trichomes in wild tomato accessions conferred resistance against P. absoluta in the present investigation. Scanning electron microscopic results revealed that S. galapagense (VI037241) and S. cheesmaniae (VI037240) possessed about 20–32 type IV trichomes per mm2 of leaf area (Figure 7). The density of glandular trichomes was very high in all the four wild tomato accessions, but very few or none were present in the susceptible genotype.
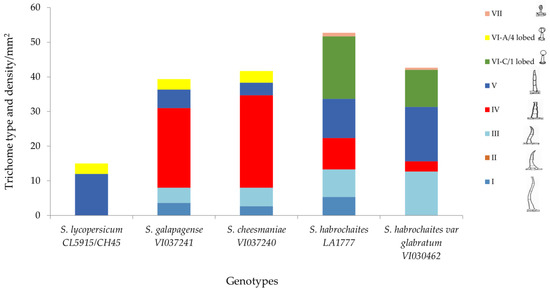
Figure 7.
Trichome type and mean trichome density/mm2 on abaxial leaf surfaces of five selected Solanum accessions/check evaluated in this study.
Figure 7 shows the combination of trichome types present in each accession when observed under microscope. The finding showed that type IV glandular trichome is more abundant in S. galapagense (VI037241) and S. cheesmaniae (VI037240), followed by S. habrochaites (LA1777) and S. habrochaites var. glabratum (VI030462) accessions, but it is absent in S. lycopersicum (CL5915). The density of glandular trichomes was higher in all the wild Solanum accessions, whereas nonglandular type V trichome density was comparatively higher in the susceptible genotype. In addition, type VII glandular trichomes were found only in S. habrochaites (LA1777) and S. habrochaites var. glabratum (VI030462) accessions but absent in other accessions.
3.5. Acylsugar Content
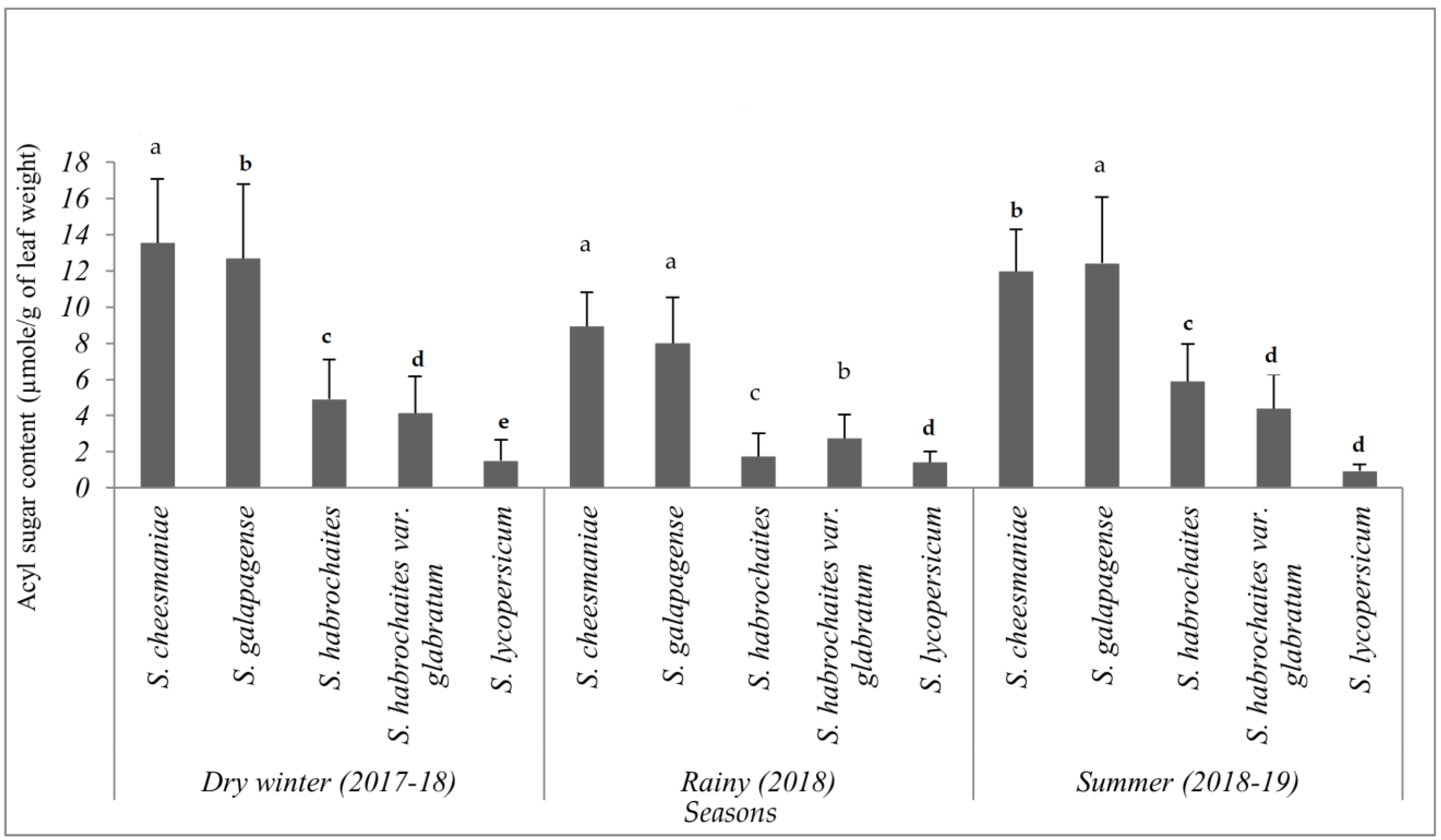
Although acylsugar content was drastically reduced during wet seasons at different phenological stages, the total acylsugar was found to be significantly higher in S. galapagense (VI037241) and S. cheesmaniae (VI037240) accessions, followed by S. habrochaites var. glabratum (VI030462) and S. habrochaites (LA1777) accessions, compared to the susceptible S. lycopersicum (CL5915) (F = 317.19, p < 0.001, df = 4) (Figure 8). Similarly, S. galapagense (VI037241) and S. cheesmaniae (VI037240) accessions recorded significantly higher acylsugars in the winter (F = 335.86, p < 0.001, df = 4) and summer (F = 308.84, p < 0.001, df = 4) seasons as well (Figure 8).
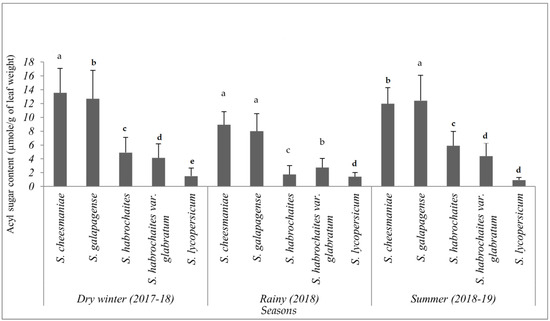
Figure 8.
Total acylsugars levels (µmol acylsugar/g dry leaf tissue) (mean ± SE) measured in four selected wild tomato (Solanum spp.) accessions and tomato check (CL5915). Genotypes with the same letter in a particular season did not differ statistically, according to least significant difference (LSD) test at p < 0.05.
3.6. Correlation between P. absoluta Damage and Morphological or Biochemical Traits
Total number of non-glandular trichomes was found to be positively correlated (r = 0.73) with the foliage damage in field conditions but statistically non-significant (p > 0.05), whereas total glandular trichomes showed highly significant negative correlation with the foliage damage (r = −0.92) (Table 4). Trichome metabolite acylsugar showed significant negative correlation with foliage damage (r = −0.89). Acylsugar content showed significant negative correlation with larval mines, oviposition preference, and egg load under field conditions. The results showed that the number of leaf mines (r = −0.71 **), percent fruit damage (r = −0.60 **), and egg counts under field conditions were significantly negatively correlated with acylsugar content (r = −0.65 **). Similarly, egg counts under laboratory assays showed significant negative correlation with acylsugar content (r = −0.69 **).

Table 4.
Linear correlation between trichome types and P. absoluta damage; acylsugar and damage and oviposition of P. absoluta.
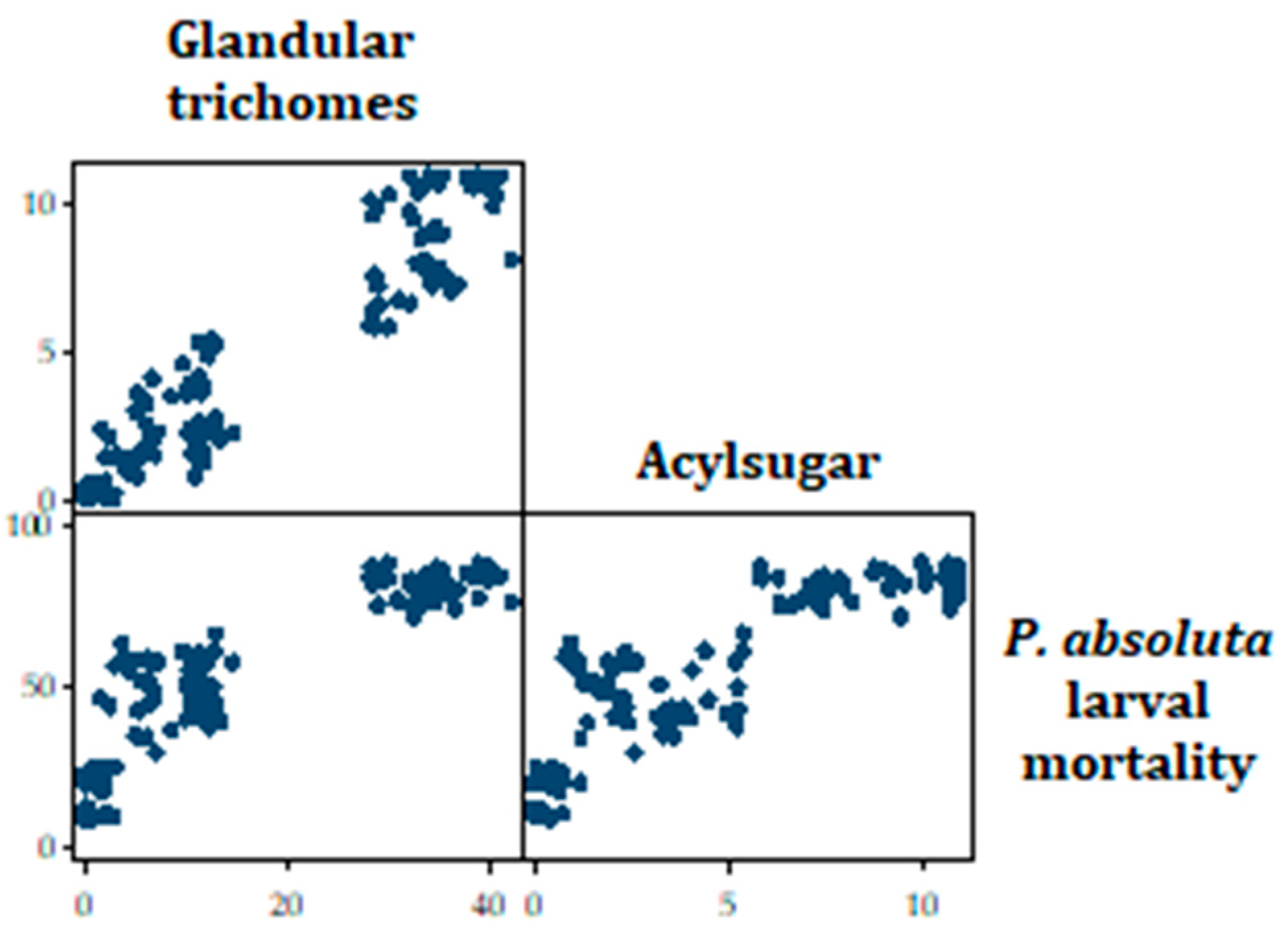
Tobit regression analysis indicated that the t test statistic for acylsugar is 2.07 with an associated p-value of 0.04. Hence, it can be concluded that the regression coefficient for acylsugar has been found to be statistically different from zero, given glandular trichomes is in the model (Table 5). Similarly, the t test statistic for glandular trichomes is 6.45 with an associated p-value of <0.001. Hence, it can be concluded that the regression coefficient for glandular trichomes has been found to be statistically different from zero, given acylsugar is in the model. The results also indicated that the larval mortality is expected to increase by 1.60 points, if the acylsugar content in a tomato accession increases by one point, while holding all other variables in the model constant (Table 5). Similarly, the larval mortality is expected to increase by 1.30 points, if the glandular trichomes in a tomato accession increase by one point, while holding all other variables in the model constant. Thus, the higher a tomato accession’s acylsugar or glandular trichomes, the higher the predicted larval mortality (Figure 9).

Table 5.
Relationship between acylsugar and glandular trichomes on P. absoluta larval mortality. Single asterisk (*) indicates significance at p ≤ 0.05; double asterisk (**) indicates significance at p ≤ 0.01.
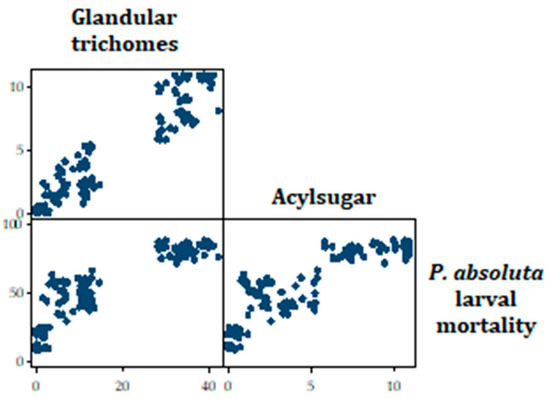
Figure 9.
Scatterplot matrix showing the relationship between total glandular trichomes and P. absoluta larval mortality, as well as acylsugar and P. absoluta larval mortality.
4. Discussion
The invasive leaf miner (P. absoluta) is a serious pest of tomato (S. lycopersicum) in the tropics and subtropics presently. The continental invasion of P. absoluta represents a considerable biosecurity threat that affects most livelihoods dependent on agricultural sustenance. The rapid spread increases the risk of crop failure, if not controlled in an efficient manner. The climatic conditions in the newly invaded regions are also favorable for the survival and reproduction of P. absoluta. Therefore, there is a high likelihood of the long-time survival of this destructive pest as some of the most trusted strategies, such as chemical control measures fail to manage this insect. Previous WorldVeg studies found glandular trichome-based resistance to whitefly and spider mite in certain wild tomato accessions of S. cheesmaniae, S. galapagense and S. pimpinellifolium [17,22]. Host-plant resistance can be an important tool to manage such invasive species. P. absoluta uses semiochemicals produced by glandular trichomes to select tomato host plants for oviposition and/or feeding [29,34]; P. absoluta and other lepidopteran larvae also depend on taste to select host plants for feeding [31]. Therefore, any morphological or biochemical parameter of the plants, which could resist the feeding or host selection can be an effective step to reduce the initial level of attack. Hence, the present study aimed to evaluate five wild tomato accessions for resistance to P. absoluta in choice and no-choice feeding bioassays as well as their field performance against P. absoluta damage.
Low oviposition rate and reduced larval survival in all the four wild tomato accessions indicated that those accessions were able to reduce damage caused by P. absoluta. Moreover, our results showed that S. cheesmaniae and S. galapagense were the best-performing genotypes, and found to be the most resistant accessions. Few earlier studies have reported about a few moderately resistant tomato cultivars against the P. absoluta attack [35,36], although the majority were found to be highly susceptible to P. absoluta infestation. In this investigation, fortnightly observations were mainly taken to record the pest load, its survival and damage intensity on selected tomato accessions. It has been observed that the mean number of eggs on leaves at different canopy levels significantly differed among the tomato genotypes. S. galapagense (VI037241) and S. cheesmaniae (VI037240) received a significantly lower egg load, which was observed only in upper and middle canopy leaves, whereas no egg load was observed in lower canopy leaves in these two wild accessions. The laboratory experiments also confirmed that S. galapagense (VI037241), S. cheesmaniae (VI037240) and S. habrochaites (LA1777) genotypes recorded low egg numbers. Thus, wild tomato accessions belonging to S. galapagense and S. cheesmaniae were confirmed to be the least preferred genotypes by P. absoluta for oviposition.
In general, three mechanisms, namely, antixenosis, antibiosis and tolerance account for plant resistance to insect attack [36]. Choice and no-choice bioassays were conducted to assess the antixenosis (choice assay) and antibiosis (no-choice) resistance mechanisms in this study [18]. Tested wild tomato accessions were found to possess prominent biophysical and biochemical properties to confer resistance against P. absoluta. Resistant accessions possessed different types of glandular trichomes, especially type IV. Glandular trichomes in wild tomatoes confer resistance by releasing toxic exudates, mainly acylsugar [37,38], which shows resistance against herbivory. In our findings, the mean acylsugar content was almost 8- to 12-fold higher in S. galapagense (VI037241) and S. cheesmaniae (VI037240) than susceptible genotype, S. lycopersicum (CL5915). Acylsugar concentration in leaves of different tomato genotypes was shown to have a highly positive correlation with larval mortality. The no-choice bioassay also confirmed the antibiosis effect, since the larvae feeding on resistant tomato accessions were unable to complete their life cycle due to mortality in immature stages. No precocious development was observed but slow, and less feeding by the larvae was noticed under laboratory conditions in the wild accessions, especially on S. galapagense and S. cheesmaniae accessions.
Phthorimaea absoluta also chooses tomato host plants for oviposition and/or feeding using semiochemicals produced by glandular trichomes [3,22,37,39]. Earlier studies also indicated that resistance is positively associated with trichome density, and it is well known fact that the diversity and concentrations of host-plant secondary metabolites may impart varied level of resistance [40,41,42]. Indeed, tomato plants possessing glandular trichomes that produce volatile and non-volatile secondary metabolites, e.g., acylsugars, terpenoids, phenylpropanoids, flavonoids and phenolic compounds, can repel, deter, and cause antibiosis effect on insects’ biology [25,34]. It was reported that accession of S. galapagense is resistant to a broad range of pest, and that resistance is associated with the cooccurrence of high densities of type IV glandular trichomes and acyl-sugars [26,34]. Vosman et al. [43] also identified broad spectrum resistance towards insects in close relatives of the cultivated tomato. Type IV trichomes in the species from the Lycopersicon group of Solanum section Lycopersicon showed resistance to a range of insect pests, and accessions of S. galapagense were found to be highly resistant [43,44,45]. This observation was also consistent with our investigation. The metabolite content of S. galapagense was very similar with S. cheesmaniae wild accessions, which also contain a high density of type IV trichomes in this study, although other accessions of these two species showed large differences in the levels of both acylsugars and several O-methylated forms of the flavanol myricetin [43].
Besides the secondary metabolites produced by glandular trichomes, there is a close relationship between trichome (both glandular and non-glandular) density and insect resistance. In our findings, resistant wild tomato accessions possessed significantly higher glandular trichome density than the susceptible genotype. These results are in line with Darbain et al. (2016) [46], who reported that glandular trichome length and density showed significant negative correlation with P. absoluta infestation, and the length of normal trichomes also showed significant negative correlation with the pest infestation. They suggested that the density of glandular trichomes might play an important role in tomato cultivars’ susceptibilities. The present findings are similar with those of the studies in terms of trichome density imparting resistance against herbivores [47,48,49,50]. Some studies in cotton revealed that the insect pest resistance is associated with various morphological traits including trichomes [30,35,36,48], which make the plants less attractive to pests, thus reducing their survival or growth or interactions. Trichomes were reported to impart resistance against the sucking as well as chewing insect pests of cotton [51,52]. Hooked trichomes on leaves of field bean cultivars effectively captured nymph and adult leafhoppers. The mortality was highly correlated with trichome density [18]. For many Lepidopterans, there is an apparent trade off: moths prefer pubescent plant surfaces for oviposition, but early instar larvae may suffer higher mortality. In our studies, the wild tomato accessions with high trichome density showed very high resistance against P. absoluta due to less preference for oviposition and by showing antibiosis effect in larval stages. Thus, these studies indicate the major role of trichomes in imparting resistance against insect pests.
The selected wild tomato accessions viz., S. galapagense (VI037241), S. cheesmaniae (VI037240) and S. habrochaites (LA1777) were also found to significantly reduce egg load by spider mites in an earlier study [53]. Similarly, earlier studies reported higher larval mortality, when the P. absoluta larvae were fed on wild tomato accessions [22,54]. Similar reports with wild tomato accessions against T. urticae and B. tabaci were documented by Rakha et al. [15,25,53]. Hence, it is obvious from the current study that the wild tomato accessions can reduce the egg load and increase the larval mortality, leading to a substantial reduction in newly emerged adults of P. absoluta. Thus, an overall reduction in P. absoluta populations is likely to be expected when resistant tomato genotypes are becoming available. Incorporation of these resistance traits into cultivated tomato varieties can definitely help with the gradual reduction in the pest population under field conditions. Therefore, the development of tomato cultivars resistant to pests is an important component in integrated pest management (IPM) packages.
The use of resistant cultivars should contribute to integrated P. absoluta management in both greenhouse and field tomato production by farmers. The use of resistant varieties would reduce pesticide applications and associated input costs [55,56]. The highly resistant S. galapagense (VI037241) and S. cheesmaniae (VI037240) accessions identified in this study were able to significantly reduce numbers of eggs, larvae and newly emerged adults, which may reduce P. absoluta populations and fruit damage. Overall findings of the present investigation throw light on the morphological and biochemical parameters of wild tomato accessions, which has a major role in conferring resistance against P. absoluta damage either by deterring or repelling them from egg laying or causing deleterious impact on their lifecycle.
It is reasonable to assume that indirect selection for acylsugar contents for breeding studies can be more effective towards increasing insect resistance in tomato than direct selection for arthropod resistance per se. The antixenosis and antibiosis effect on egg laying responses found in our experiment support this assertion. Therefore, the findings can be useful for developing P. absoluta-resistant varieties through breeding techniques quite rapidly. The breeding programs can have targeted lines with high acylsugars and other secondary metabolites, e.g., zingiberene for host resistance against pests in tomato production [57,58,59,60]. Therefore, the use of resistant varieties may also offer a cost effective and sustainable approach to the fight against the P. absoluta menace in India and beyond.
5. Conclusions
Aggressive invasive insect pests can adapt well when they are introduced into a new area and cause considerable crop loss. The results reported in this study demonstrate that the morphological and biochemical parameters of different wild tomato accessions influence the P. absoluta attack. Damage to tomatoes were high in the summer season, and the maximum and most evident examples of damage were on the leaves during vegetative stage. It is important to emphasize that heavy foliage damage leads to heavy fruit damage once the crop attains its fruiting stage. The presence of P. absoluta is observed throughout the year if the host is available and mostly depending on phenological stages. In this investigation, the damage recorded in the field was more visible during the second cropping cycle, but under greenhouse conditions, specifically, the leaves were totally infested before fruit setting. The presence of dense glandular trichomes, especially type IV, contributes to high resistance against this pest attack. Trichome-derived biochemicals, especially the total acylsugar content, are significantly negatively correlated with foliage damage. Hence, to manage the invasive P. absoluta, this information can be utilized to know when to begin monitoring and take effective control measures. Based on our findings, we hypothesize that wild tomato accessions having a high density of glandular trichomes, and a high content of acyl-sugars can be explored by plant breeders in developing P. absoluta-resistant lines.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/horticulturae9020143/s1, Table S1: Location and details of experimental plots for field screening; Figure S1: Field layout of the tomato accessions for screening against P. absoluta resistance.
Author Contributions
Conceptualization, P.G., R.S. and P.S.-C.; methodology, P.G., R.S., P.S.-C., P.H., K.S.J. and M.G.P.; software, P.G. and P.S.-C.; validation, P.G., P.S.-C. and R.S.; formal analysis, P.G., P.S.-C. and R.S.; investigation, P.G., M.G.P., S.V. and R.S.; resources, P.H., M.R. and S.V.; data curation, P.S.-C. and R.S.; writing—original draft preparation, P.G., R.S., P.S.-C., P.H. and K.S.J.; writing—review and editing, P.G., R.S. and P.S.-C.; visualization, P.G., R.S., P.S.-C. and P.H.; supervision, P.G., R.S. and P.S.-C.; project administration, R.S. and M.G.P.; funding acquisition, M.G.P., R.S. and P.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Federal Ministry for Economic Cooperation and Development (GIZ Project number 16.7860.6-001.00), Germany.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available for a certain period of time and later can be accessed from https://worldveg.tind.io/ accessed on 1 February 2023.
Acknowledgments
This work was also supported by core donors to the World Vegetable Center: Taiwan, UK aid from the UK government, United States Agency for International Development (USAID), Australian Centre for International Agricultural Research (ACIAR), Germany, Thailand, Philippines, Korea, and Japan, besides M/s I and B Seeds Pvt. Ltd., Uttarahalli, Bengaluru and Indian Institute of Horticultural Research, Bengaluru, Karnataka, India.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chandrashekar, K.; Shashank, P.R. Invasive Pest Alert. 2014. Available online: http://www.iari.res.in/files/Latest-News/INVASIVE_PEST_ALERT-05022015.pdf (accessed on 25 March 2015).
- Biondi, A.; Guedes, R.; Wan, F.; Desneux, N. Ecology, worldwide spread, and management of the invasive South American tomato pinworm, Tuta absoluta: Past, present, and future. Annu. Rev. Entomol. 2018, 63, 239–258. [Google Scholar] [CrossRef] [PubMed]
- Shashank, P.R.; Suroshe, S.; Singh, P.K.; Chandrashekar, K.; Nebapure, S.; Meshram, N.M. Report of invasive tomato leaf miner, Tuta absoluta (Lepidoptera: Gelechiidae) from northern India. Indian J. Agric. Sci. 2016, 86, 1635–1636. [Google Scholar]
- Lucini, T.; Marcos, V.F.; Cristhiane, R.; Juliano, T.V.R.; Joao Ronaldo, F.D. Acylsugar and the role of trichomes in tomato genotypes resistance to Tetranychus urticae. Arthropod-Plant Interact. 2015, 9, 45–53. [Google Scholar] [CrossRef]
- Mohamed, E.S.I.; Mahmoud, M.E.E.; Elhaj, M.A.M.; Mohamed, S.A.; Ekesi, S. Host plants record for tomato leaf miner Tuta absoluta (Meyrick) in Sudan. EPPO Bull. 2015, 45, 108–111. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- CABI. Tomato Leafminer (Tuta absoluta): Impacts and Coping Strategies for Africa. Evidence Note. 2019. Available online: https://www.cabi.org/isc/search/ (accessed on 19 May 2019).
- Solomon, J.D. Characters for determining sex in elm span worm pupae. J. Econ. Entomol. 1962, 55, 269–270. [Google Scholar] [CrossRef]
- Simmons, A.T.; Gurr, G.M. Trichomes of Lycopersicon species and their hybrids: Effects on pests and natural enemies. Agric. For. Entomol. 2005, 7, 265–276. [Google Scholar] [CrossRef]
- Prasannakumar, N.R.; Jyothi, N.; Kumar, G.R.; Saroja, S.; Sridhar, V. Studies on outbreak of tomato pinworm, Tuta absoluta (Meyrick) in South India and its differential susceptibility to insecticides. Pest Manag. Hort. Ecosys. 2020, 26, 97–103. [Google Scholar] [CrossRef]
- Prasannakumar, N.R.; Jyothi, N.; Saroja, S.; Ram Kumar, G. Relative toxicity and insecticide resistance of different field population of tomato leaf miner, Tuta absoluta (Meyrick). Int. J. Trop. Insect Sci. 2021, 41, 1397–1405. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Galdino, T.V.S.; Picanço, M.C.; Ferreira, D.O.; Silva, G.A.R.; Souza, T.C.; Silva, G.A. Is the performance of a specialist herbivore affected by female choices and the adaptability of the offspring? PLoS ONE 2015, 10, e0143389. [Google Scholar] [CrossRef]
- Gill, M.A. Insect resistance in tomato (Solanum spp.). Cultiv. Trop. 2015, 36, 100–110. [Google Scholar]
- Rakha, M.; Hanson, P.; Ramasamy, S. Identification of resistance to Bemisia tabaci (Genn.) in closely related wild relatives of cultivated tomato based on trichome type analysis and choice and no-choice assays. Genet. Resour. Crop Evol. 2017, 64, 247–264. [Google Scholar] [CrossRef]
- Rakha, M.; Zekeya, N.; Sevgan, S.; Musembi, M.; Srinivasan, R.; Hanson, P. Screening recently identified whitefly/spider mite-resistant wild tomato accessions for resistance to Tuta absoluta. Plant Breed. 2017, 136, 562–568. [Google Scholar] [CrossRef]
- Salama, H.S.A.; Ismail, I.A.; Fouda, M.; Ebadah, I.; Shehata, I. Some ecological and behavioral aspects of the tomato leaf miner Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Ecol. Balk. 2015, 7, 35–44. [Google Scholar]
- Baldin, E.L.; Eneduzzi, R.A. Characterization of antibiosis and antixenosis to the whitefly silverleaf Bemisia tabaci B biotype (Homoptera: Aleyrodidae) in several squash varieties. J. Pest Sci. 2010, 83, 221–227. [Google Scholar] [CrossRef]
- Buta, G.J.; Lusby, W.R.; Neal, J.W.J.; Waters, R.M.; Pittarelli, G.W. Sucrose esters from Nicotiana gossei active against the greenhouse whitefly Trialeuroides vaporarium. Phytochemistry 1993, 32, 859–864. [Google Scholar] [CrossRef]
- Leckie, B.M.; Dejong, D.M.; Mutschler, M.A. Quantitative trait loci increasing acylsugars in tomato breeding lines and their impacts on silver leaf whiteflies. Mol. Breed. 2012, 30, 1621–1634. [Google Scholar] [CrossRef]
- Kim, J.; Kang, K.; Gonzales, V.E.; Shi, F.; Daniel, J.A.; Barry, C.S.; Last, R.L. Striking natural diversity in glandular trichome acylsugar composition is shaped by variation at the acyltransferase2 locus in the wild tomato Solanum habrochaites. Plant Physiol. 2012, 160, 1854–1870. [Google Scholar] [CrossRef]
- Ayalew, G. Effect of the insect growth regulator novaluron on diamondback moths, Plutella xylostella (Lepidoptera: Plutellidae), and its indigenous parasitoids. Crop Prot. 2011, 30, 1087–1090. [Google Scholar] [CrossRef]
- Carter, C.D.; Gianfagna, T.J.; Sacalis, J.N. Sesquiterpenes in glandular trichomes of a wild tomato species and toxicity to the Colorado potato beetle. J. Agric. Food Chem. 1989, 37, 1425–1428. [Google Scholar] [CrossRef]
- Luckwill, L.C. The Genus Lycopersicon: An Historical, Biological, and Taxonomic Survey of the Wild and Cultivated Tomatoes; Aberdeen University: Aberdeen, UK, 1943; p. 44. [Google Scholar]
- Rakha, M.; Bouba, N.; Ramasamy, S.; Regnard, J.; Hanson, P. Evaluation of wild tomato accessions (Solanum spp.) for resistance to two-spotted spider mite (Tetranychus urticae Koch) based on trichome type and acyl sugar content. Genet. Resour. Crop Evol. 2015, 64, 1011–1022. [Google Scholar] [CrossRef]
- Tobin, J. Estimation of relationships for limited dependent variables. Econometrica 1958, 26, 24. [Google Scholar] [CrossRef]
- Karnowsky, M.J. A formaldehyde–glutaraldehyde fixative of high osmolarity for use in electron microscopy. J. Cell Biol. 1965, 27, 137A. [Google Scholar]
- Eric, A.P.; Ward, M.T. Hooked trichomes: A physical plant barrier to a major agricultural pest. Science 1976, 193, 482–484. [Google Scholar]
- Setter, T.L.; Flannigan, B.A.; Melkonian, J. Loss of kernel set due to water deficit and shade in maize: Carbohydrate supplies, abscisic acid, and cytokinins. Crop Sci. 2001, 41, 1530–1540. [Google Scholar] [CrossRef]
- Silva, J.E.; Assis, C.P.; Rebeiro, M.S.; Herbert, A.S. Field-Evolved Resistance and Cross-Resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) Populations to Diamide Insecticides. J. Econ. Entomol. 2016, 109, 2190–2195. [Google Scholar] [CrossRef]
- Peterson, R.G. Agriculture Field Experiments: Design and Analysis; Marcel Dekker: New York, NY, USA, 1994. [Google Scholar]
- Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 1 November 2019).
- McDonald, J.F.; Moffitt, R.A. The uses of tobit analysis. Rev. Econ. Stat. 1980, 62, 318. [Google Scholar] [CrossRef]
- Kant, M.R.; Ament, K.; Sabelis, M.W.; Harin, M.A.; Schuurink, R.C. Differential timing of spider mite-induced direct and indirect defences in tomato plants. Plant Physiol. 2004, 135, 483–495. [Google Scholar] [CrossRef]
- Gharekhani, G.H.; Salek-Ebrahimi, H. Evaluating the damage of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on some cultivars of tomato under greenhouse condition. Arch. Phytopathol. Pflanzenschutz 2014, 47, 429–436. [Google Scholar] [CrossRef]
- Peter, A.J.; Shanower, T.G.; Romeis, J. The role of plant trichomes in insect-resistance: A selective review. Phytophaga 1995, 7, 41–64. [Google Scholar]
- Levin, D.A. The role of trichomes in plant defense. Q. Rev. Biol. 1973, 48, 3–15. [Google Scholar] [CrossRef]
- De Oliveira, C.M.; De Rade, V.C.J.; Maluf, W.R.; Neiva, I.P.; Maciel, G.M. Resistance of tomato strains to the moth Tuta absoluta imparted by allelochemicals and trichome density. Ciênc. Agrotec. 2012, 36, 45–52. [Google Scholar] [CrossRef]
- Torres, J.B.; Faria, C.A.; Evangelista, W.S.; Pratissoli, D. Within-plant distribution of the leaf miner Tuta absoluta (Meyrick) immatures in processing tomatoes, with notes on plant phenology. Int. J. Pest Manag. 2001, 47, 173–178. [Google Scholar] [CrossRef]
- Watson, J.S. Recent progress in breeding for insect resistance in three types of cotton. In Cotton Breeding, 2nd ed.; Singh, P., Ed.; Kalyani Publishers: New Delhi, India, 2004; pp. 136–146. [Google Scholar]
- Sohrabi, F.; Nooryazdan, H.R.; Gharati, B.; Saeidi, Z. Plant resistance to the moth Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) in tomato cultivars. Neotrop. Entomol. 2016, 46, 203–209. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Da Silva, D.J.H.; Leite, G.L.D.; Jham, G.N.; Picanço, M. Resistance of 57 greenhouse-grown accessions of Lycopersicon esculentum and three cultivars to Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae). Sci. Hortic. 2009, 119, 182–187. [Google Scholar] [CrossRef]
- Vosman, B.; Wendy, P.C.; Westende, V.; Henken, B.; Henriëtte, D.L.M.; Eekelenric, V.; Vosroel, C.H.D.; Voorrips, E. Broad spectrum insect resistance and metabolites in close relatives of the cultivated tomato. Euphytica 2018, 214, 46. [Google Scholar] [CrossRef]
- Lucatti, A.F.; Heusden, V.A.W.; Vos, R.C.; Visser, R.G.; Vosman, B. Differences in insect resistance between tomato species endemic to the Galapagos Islands. BMC Evol. Biol. 2013, 13, 175. [Google Scholar] [CrossRef]
- Fischer, E.R.; Hansen, B.T.; Nair, V.; Hoyt, F.H.; Dorward, D.W. Scanning Electron Microscopy. Curr. Protoc. Microbiol. 2012, 25, 2B. [Google Scholar] [CrossRef]
- Darbain, S.; Emam, A.K.; Helmi, A.; El-Badawy, S.S.; Moussa, S. Susceptibility of certain tomato cultivars to infestation with Tuta absoluta (meyrick) (Lepidoptera: Gelechiidae) in relation to leaflet trichomes. Egypt J. Agric. Res. 2016, 94, 829–839. [Google Scholar]
- Jayaraj, S.; Uthamasamy, S.; Parameswaran, S. Host plant resistance to insects with reference to biochemical parameters. In Dynamics of Insect-Plant Interaction—Recent Advances and Future Trends; Ananthakrishnan, T.N., Raman, A., Eds.; Oxford and IBH Publishing Company: New Delhi, India, 1988; pp. 29–43. [Google Scholar]
- Jenkins, J.N. State of the art in host plant resistance in cotton. In Cotton Breeding, 2nd ed.; Singh, P., Ed.; Kalyani Publishers: New Delhi, India, 1992; Volume 2, pp. 627–633. [Google Scholar]
- Moore, J.K.; Dixon, P.M. Analysis of combined experiments revisited. Agron. J. 2015, 107, 763–777. [Google Scholar] [CrossRef]
- Kamel, S.A. Relationship between leaf hairiness and resistance to cotton leaf worm. Emp. Cotton Grow. Rev. 1965, 42, 41–48. [Google Scholar]
- Giustolin, T.A.; Vendramim, J.D.; Alves, S.B.; Vieira, S.A.; Pereira, R.M. Susceptibility of Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) reared on two species of Lycopersicon to Bacillus thuringiensis var. kurstaki. J. Appl. Entomol. 2001, 125, 551–556. [Google Scholar] [CrossRef]
- Mehta, R.C. Survival and egg production of the cotton spotted bollworm, Earias fabia Stoll. (Lepidoptera: Noctuidae) in relation to plant infestation. Appl. Entomol. Zool. 1971, 6, 206–209. [Google Scholar] [CrossRef]
- Cruz, P.L.; Baldin, E.L.L.; de Castro, J.P.M. Characterization of antibiosis to the silverleaf whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in cowpea entries. J. Pest Sci. 2015, 87, 639–645. [Google Scholar] [CrossRef]
- Salazar, E.R.; Araya, J.E. Tomato moth, Tuta absoluta (Meyrick) response to insecticides in Arica, Chile. Agric. Tec. 2001, 61, 429–435. [Google Scholar]
- Desneux, N.; Wajnberg, E.; Wyckhuys, K.A.; Burgio, G.; Arpaia, S.; Narváez-Vasquez, C.A.; González-Cabrera, J.; Catalán Ruescas, D.; Tabone, E.; Frandon, J.; et al. Biological invasion of European tomato crops by Tuta absoluta: Ecology, geographic expansion and prospects for biological control. J. Pest Sci. 2010, 83, 197–215. [Google Scholar] [CrossRef]
- Fernandes, M.E.F.; Fernandes, F.L.; Silva, D.J.H.; Picanço, M.; Jhamc, G.N.; Carneiro, P.; Queiroz, R.B. Trichomes and hydrocarbons associated with the tomato plant antixenosis to the leafminer. An. Acad. Bras. Ciências. 2012, 84, 201–210. [Google Scholar] [CrossRef]
- Borisade, O.A.; Kolawole, A.O.; Adebo, G.M.; Uwaidem, Y.I. The tomato leafminer (Tuta absoluta) (Lepidoptera: Gelechiidae) attack in Nigeria: Effect of climate change on over-sighted pest or agro bioterrorism? J. Agric. Ext. Rural Dev. 2017, 9, 163–171. [Google Scholar] [CrossRef]
- Han, P.; Bayram, Y.; Shaltiel-Harpaz, L.; Sohrabi, F.; Saji, A.; Esenali, U.T.; Jalilov, A.; Ali, A.; Shashank, P.R.; Ismoilov, K. Tuta absoluta continues to disperse in Asia: Damage, ongoing management, and future challenges. J. Pest Sci. 2019, 92, 1317–1327. [Google Scholar] [CrossRef]
- Proffit, M.; Birgersson, G.; Bengtsson, M.; Reis, R.; Witzgall, P.; Lima, E. Attraction and oviposition of Tuta absoluta females in response to tomato leaf volatiles. J. Chem. Ecol. 2011, 37, 565–574. [Google Scholar] [CrossRef]
- Channarayappa, C.; Shivashankar, G.; Muniyappa, V.; Frist, R. Resistance of Lycopersicon species to Bemisia tabaci a tomato leaf curl virus vector. Can. J. Bot. 1992, 11, 2184–2192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).