Leaf Area Prediction of Pennywort Plants Grown in a Plant Factory Using Image Processing and an Artificial Neural Network
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Factory Preparation and Operation
2.2. Pennywort Seedling Preparation and Transplantation
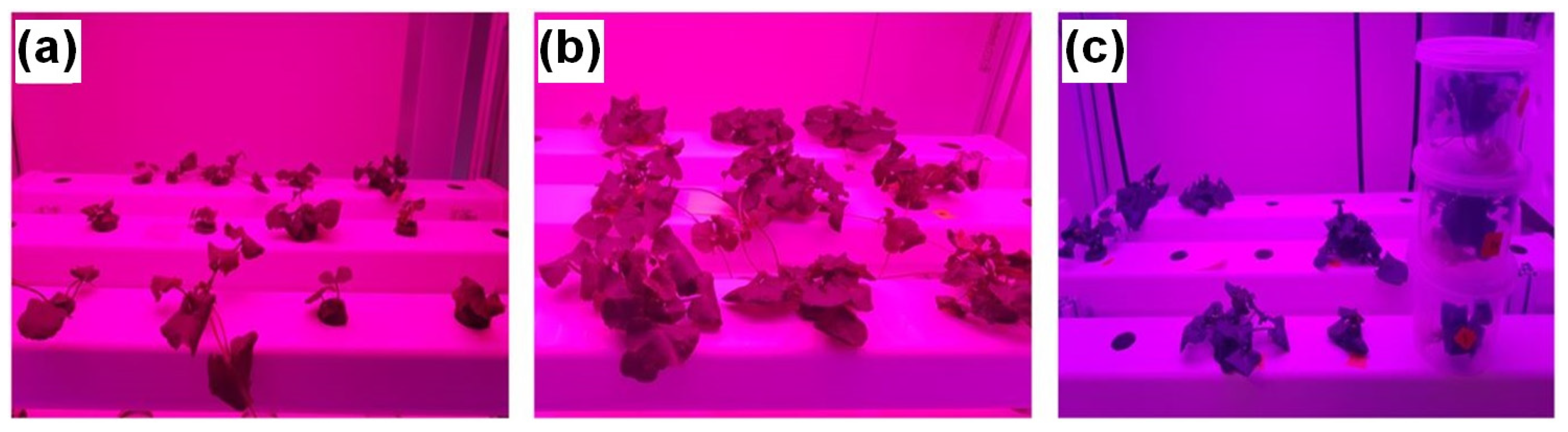
2.3. Experimental Design and Sample Collection
2.4. Leaf Image Acquisition
2.5. Leaf Area (LA) Estimation from Images
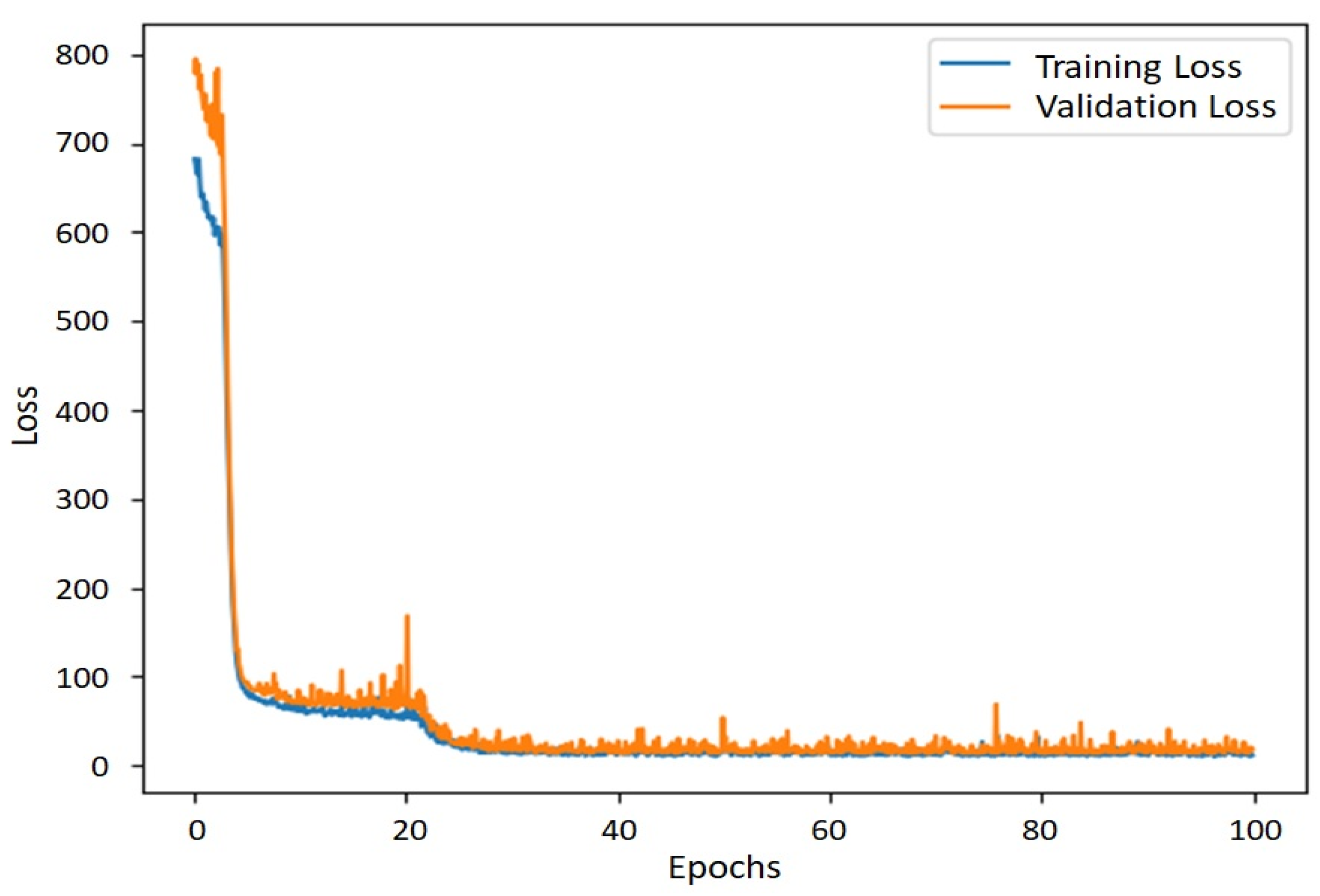
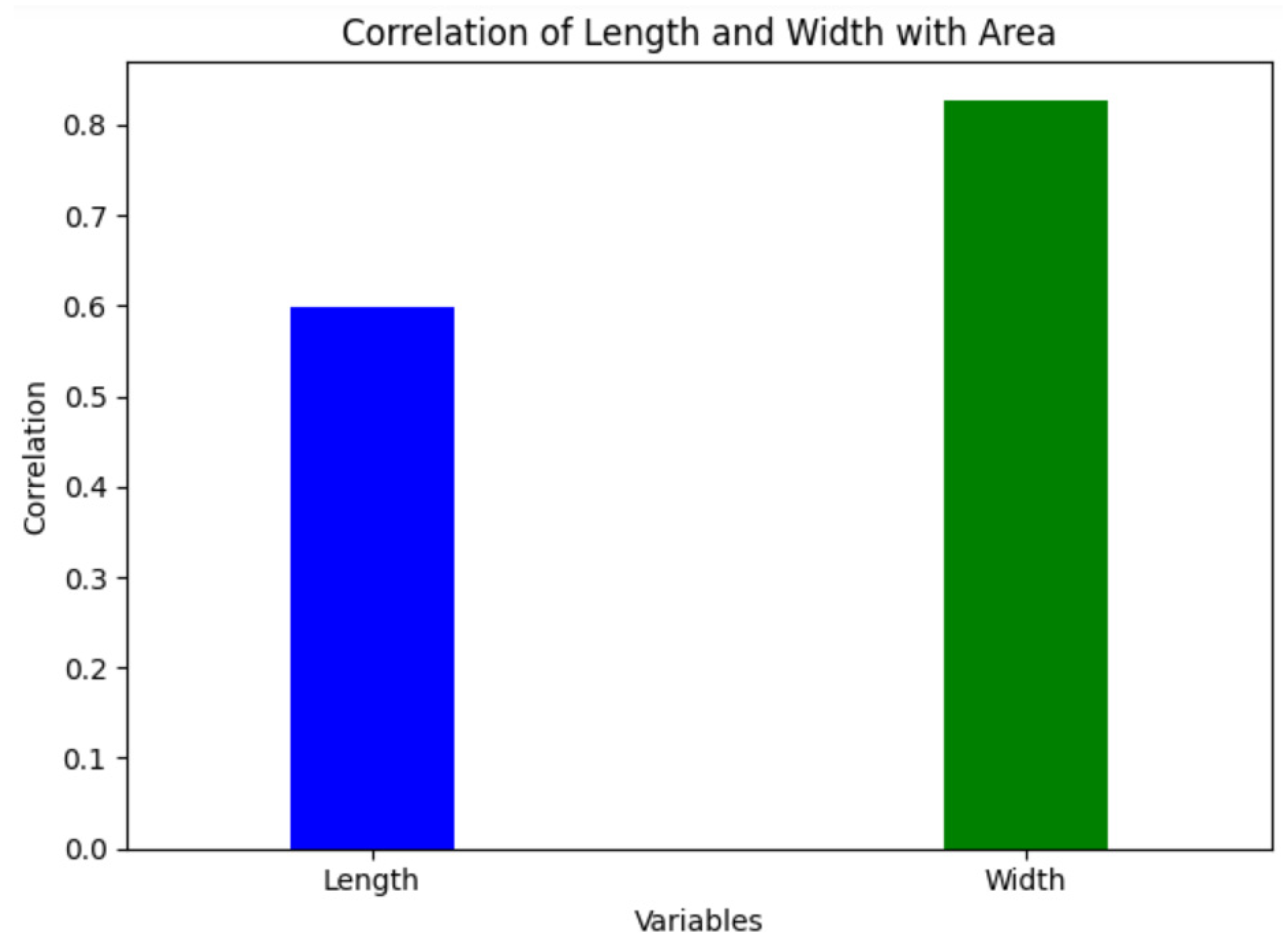
2.6. ANN Model
- 1.
- The model was trained with both L and W to predict LA from the actual measured data.
- 2.
- The model was trained with both L and W to predict LA from the image-extracted data.
- 3.
- The model was trained with both L and W to predict LA from both actual and image-extracted data.
3. Results and Discussion
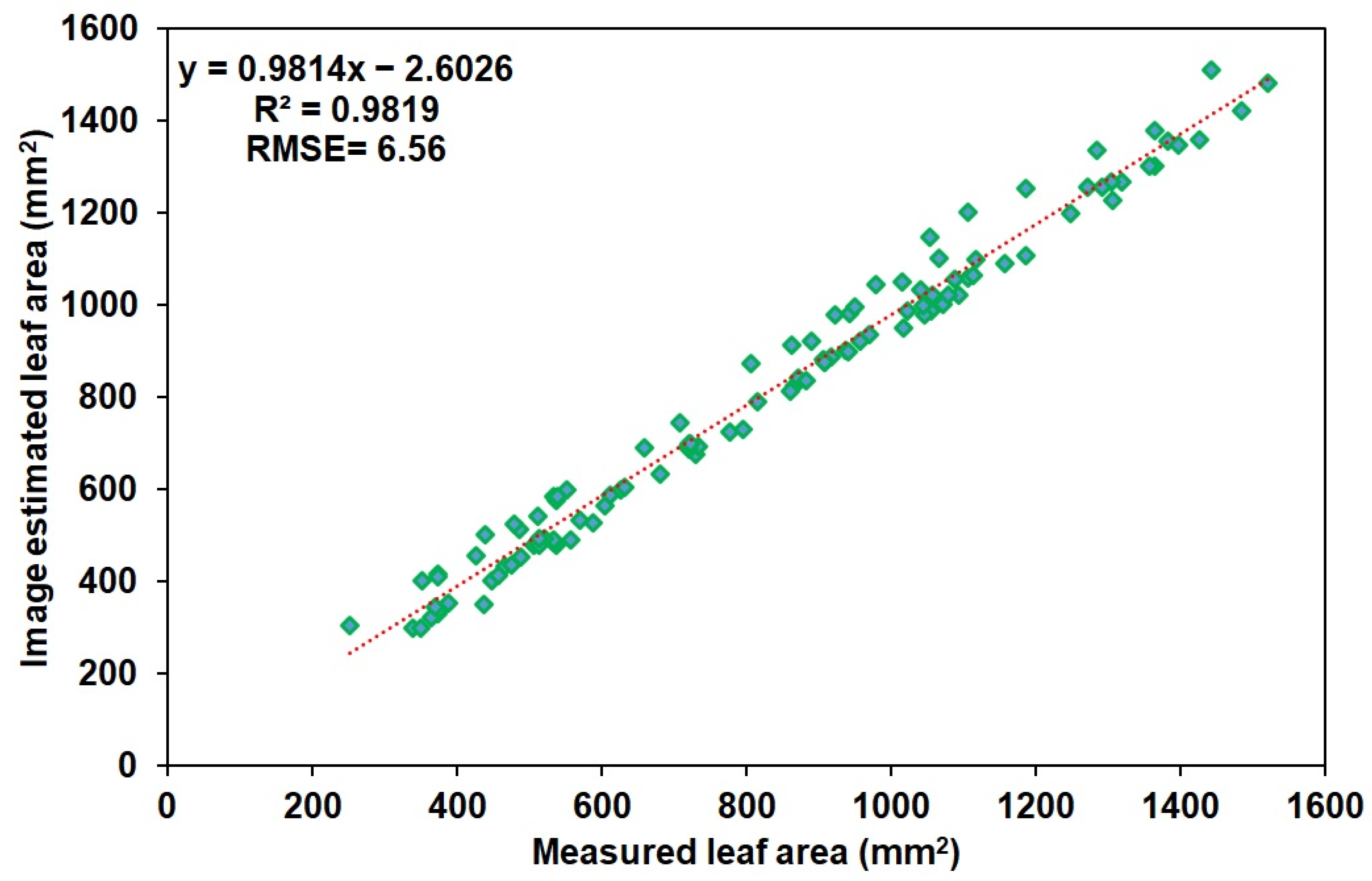
3.1. Leaf Area Estimation Using Image Processing
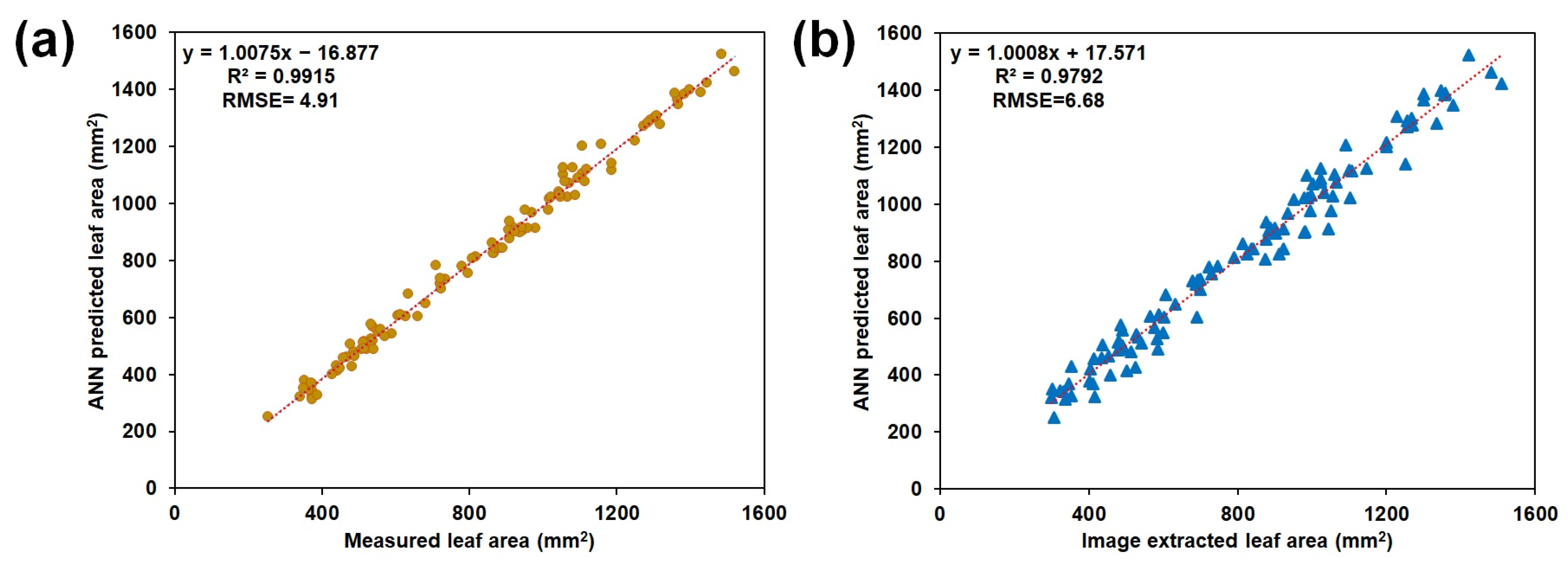
3.2. Leaf Area Estimation Using ANN Model
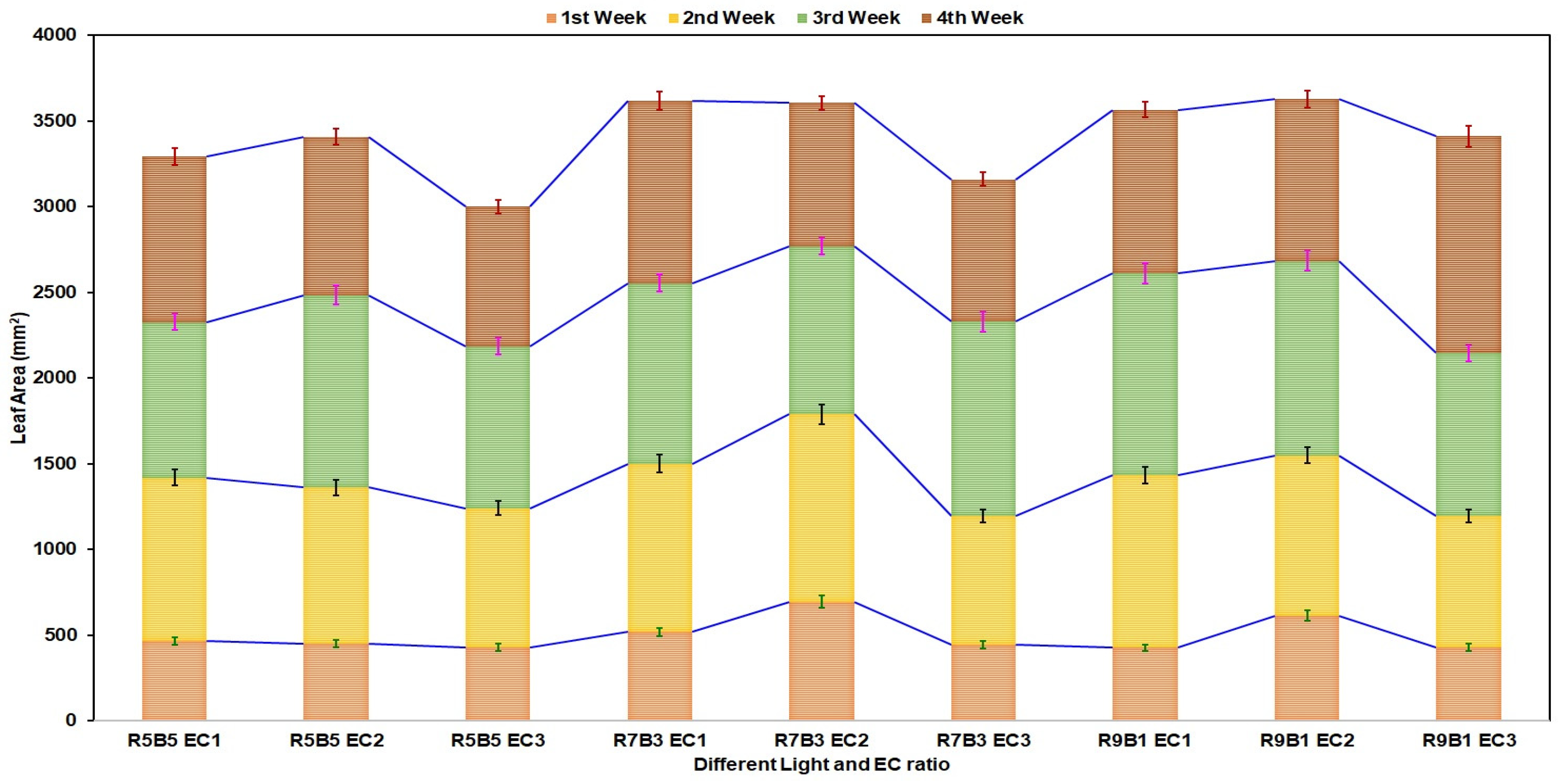
3.3. Effect of Light and Nutrients on Leaf Area
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arora, D.; Kumar, M.; Dubey, S.D. Centella asiatica—A Review of It’s Medicinal Uses and Pharmacological Effects. J. Nat. Remedies 2002, 2, 143–149. [Google Scholar]
- Seevaratnam, V.; Banumathi, P.; Premalatha, M.R.; Sundaram, S.P.; Arumugam, T. Functional properties of Centella asiatica (L.): A review. Int. J. Pharm. Pharm. Sci. 2012, 4, 8–14. [Google Scholar]
- Das, A.J. Review on Nutritional, Medicinal and Pharmacological Properties of Centella asiatica (Indian penny-wort). J. Biol. Act. Prod. Nat. 2011, 1, 216–228. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Use of Asiatic Pennywort Centella asiatica Aqueous Extract as a Bath Treatment to Control Columnaris in Nile Tilapia. J. Aquat. Anim. Health 2010, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gautam, A.; Sharma, A.; Batra, A. Centella asiatica (L.): A plant with immense medicinal potential but threat-ened. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 9–17. [Google Scholar]
- Yasurin, P.; Sriariyanun, M.; Phusantisampan, T. Review: The Bioavailability Activity of Centella asiatica. Appl. Sci. Eng. Prog. 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Subathra, M.; Shila, S.; Devi, M.A.; Panneerselvam, C. Emerging role of Centella asiatica in improving age-related neurological antioxidant status. Exp. Gerontol. 2005, 40, 707–715. [Google Scholar] [CrossRef]
- Jensen, M.H. Controlled Environment Agriculture in Deserts, Tropics and Temperate Regions—A World Review. Acta Hortic. 2002, 578, 19–25. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.H.; Chung, S.O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G. Chapter 4—Plant Factory as a Resource-Efficient Closed Plant Production System. In Plant Factory; Kozai, T., Niu, G., Takagaki, M., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 69–90. [Google Scholar] [CrossRef]
- Kozai, T.; Niu, G.; Masabni, J.G. Plant Factory Basics, Applications and Advances; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Merrill, B.F.; Lu, N.; Yamaguchi, T.; Takagaki, M.; Maruo, T.; Kozai, T.; Yamori, W. Next evolution of agriculture: A review of innovations in plant factories. Handb. Photosynth. 2018, 3, 723–740. [Google Scholar]
- Chowdhury, M.; Jang, B.E.; Kabir, M.S.N.; Kim, Y.J.; Na, K.D.; Park, S.B.; Chung, S.O. Factors affecting the accuracy and precision of ion-selective electrodes for hydroponic nutrient supply systems. Acta Hortic. 2020, 1296, 997–1004. [Google Scholar] [CrossRef]
- Jones, J.B., Jr. Hydroponics: A Practical Guide for the Soilless Grower; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Khan, F.A. A review on hydroponic greenhouse cultivation for sustainable agriculture. Int. J. Agric. Environ. Food Sci. 2018, 2, 59–66. [Google Scholar] [CrossRef]
- Cho, W.J.; Kim, H.J.; Jung, D.H.; Kim, D.W.; Ahn, T.I.; Son, J.E. On-site ion monitoring system for precision hydroponic nutrient management. Comput. Electron. Agric. 2018, 146, 51–58. [Google Scholar] [CrossRef]
- Chowdhury, M.; Islam, M.N.; Reza, M.N.; Ali, M.; Rasool, K.; Kiraga, S.; Lee, D.H.; Chung, S.O. Sensor-based nutrient recirculation for aeroponic lettuce cultivation. J. Biosyst. Eng. 2021, 46, 81–92. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kabir, M.S.N.; Kim, H.T.; Chung, S.O. Method of pump, pipe, and tank selection for aeroponic nutrient management systems based on crop requirements. J. Agric. Eng. 2020, 51, 119–128. [Google Scholar] [CrossRef]
- Jung, D.H.; Kim, H.J.; Choi, G.L.; Ahn, T.I.; Son, J.E.; Sudduth, K.A. Automated lettuce nutrient solution management using an array of ion-selective electrodes. Trans. ASABE 2015, 58, 1309–1319. [Google Scholar]
- Basso, B.; Cammarano, D.; Carfagna, E. Review of crop yield forecasting methods and early warning systems. In Proceedings of the First Meeting of the Scientific Advisory Committee of the Global Strategy to Improve Agricultural and Rural Statistics, FAO Headquarters, Rome, Italy, 18–19 July 2013; p. 19. [Google Scholar]
- Hachicha, M.; Kallel, A.; Louati, M. Prediction of plant growth based on statistical methods and remote sensing data. J. Appl. Remote Sens. 2021, 15, 042410. [Google Scholar] [CrossRef]
- Horie, T.; Yajima, M.; Nakagawa, H. Yield forecasting. Agric. Syst. 1992, 40, 211–236. [Google Scholar] [CrossRef]
- Rizkiana, A.; Nugroho, A.P.; Irfan, M.A.; Sutiarso, L.; Okayasu, T. Crop growth prediction model at vegetative phase to support the precision agriculture application in plant factory. AIP Conf. Proc. 2019, 2202, 020104. [Google Scholar] [CrossRef]
- Islam, S.; Reza, M.N.; Chowdhury, M.; Islam, M.N.; Ali, M.; Kiraga, S.; Chung, S.O. Image processing algorithm to estimate ice-plant leaf area from RGB images under different light conditions. IOP Conf. Ser. Earth Environ. Sci. 2021, 924, 012013. [Google Scholar] [CrossRef]
- Chaudhary, P.; Godara, S.; Cheeran, A.N.; Chaudhari, A.K. Fast and accurate method for leaf area measurement. Int. J. Comput. Appl. 2012, 49, 22–25. [Google Scholar] [CrossRef]
- Easlon, H.M.; Bloom, A.J. Easy Leaf Area: Automated digital image analysis for rapid and accurate measurement of leaf area. Appl. Plant Sci. 2014, 2, 1400033. [Google Scholar] [CrossRef] [PubMed]
- Tech, A.R.B.; da Silva, A.L.C.; Meira, L.A.; de Oliveira, M.E.; Pereira, L.E.T. Methods of image acquisition and software development for leaf area measurements in pastures. Comput. Electron. Agric. 2018, 153, 278–284. [Google Scholar] [CrossRef]
- Hajjdiab, H.; Obaid, A. A vision-based approach for nondestructive leaf area estimation. In Proceedings of the 2010 The 2nd Conference on Environmental Science and Information Application Technology, Wuhan, China, 17–18 July 2010; pp. 53–56. [Google Scholar] [CrossRef]
- Lü, C.; Ren, H.; Zhang, Y.; Shen, Y. Leaf area measurement based on image processing. In Proceedings of the 2010 International Conference on Measuring Technology and Mechatronics Automation, Changsha, China, 13–14 March 2010; Volume 2, pp. 580–582. [Google Scholar] [CrossRef]
- Patil, S.B.; Bodhe, S.K. Betel leaf area measurement using image processing. Int. J. Comput. Sci. Eng. 2011, 3, 2656–2660. [Google Scholar]
- Prasad, A.; Prakash, O.; Mehrotra, S.; Khan, F.; Mathur, A.K.; Mathur, A. Artificial neural network-based model for the prediction of optimal growth and culture conditions for maximum biomass accumulation in multiple shoot cultures of Centella asiatica. Protoplasma 2017, 254, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Nagano, S.; Moriyuki, S.; Wakamori, K.; Mineno, H.; Fukuda, H. Leaf-movement-based growth prediction model using optical flow analysis and machine learning in plant factory. Front. Plant Sci. 2019, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Van Klompenburg, T.; Kassahun, A.; Catal, C. Crop yield prediction using machine learning: A systematic literature review. Comput. Electron. Agric. 2020, 177, 105709. [Google Scholar] [CrossRef]
- Khan, T.; Sherazi, H.H.R.; Ali, M.; Letchmunan, S.; Butt, U.M. Deep Learning-Based Growth Prediction System: Use Case of China Agriculture. Agronomy 2021, 11, 1551. [Google Scholar] [CrossRef]
- Yue, Y.; Li, J.H.; Fan, L.F.; Zhang, L.L.; Zhao, P.F.; Zhou, Q.; Wang, N.; Wang, Z.Y.; Huang, L.; Dong, X.H. Prediction of maize growth stages based on deep learning. Comput. Electron. Agric. 2020, 172, 105351. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of Light Quality on Growth and Phytonutrient Accumulation of Herbs under Controlled Environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Lanoue, J.; St. Louis, S.; Little, C.; Hao, X. Continuous lighting can improve yield and reduce energy costs while increasing or maintaining nutritional contents of microgreens. Front. Plant Sci. 2022, 13, 983222. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, P.; Wang, J.; Fu, W. Growth, photosynthesis, and nutrient uptake at different light intensities and temperatures in lettuce. HortScience 2019, 54, 1925–1933. [Google Scholar] [CrossRef]
- Daud, Z.M.; Ismail, M.F.; Hakiman, M. Effects of LED Red and Blue Spectra Irradiance Levels and Nutrient Solution EC on the Growth, Yield, and Phenolic Content of Lemon Basil (Ocimum citriodurum Vis.). Horticulturae 2023, 9, 416. [Google Scholar] [CrossRef]
- Chowdhury, M.; Gulandaz, M.A.; Islam, S.; Reza, M.N.; Ali, M.; Islam, M.N.; Park, S.U.; Chung, S.O. Lighting conditions affect the growth and glucosinolate contents of Chinese kale leaves grown in an aeroponic plant factory. Hortic. Environ. Biotechnol. 2023, 64, 97–113. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zheng, D.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Effects of supplementary blue and UV-A LED lights on morphology and phytochemicals of Brassicaceae baby-leaves. Molecules 2020, 25, 5678. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, Z.; Jiang, X.; Ahammed, G.J.; Zhou, Y. Light regulation of horticultural crop nutrient uptake and utilization. Hortic. Plant J. 2021, 7, 367–379. [Google Scholar] [CrossRef]
- Kolatkar, V.; Chhatre, U.; Jawalekar, V. Effect of red, blue and uv light on constituents of Centella asiatica L. urban grown under controlled environment. Psychiatry 2004, 238, 242. [Google Scholar]
- Li, K.; Chen, J.; Wei, Q.; Li, Q.; Lei, N. Effects of transgenerational plasticity on morphological and physiological properties of stoloniferous herb Centella asiatica subjected to high/low light. Front. Plant Sci. 2018, 9, 1640. [Google Scholar] [CrossRef]
- Nawae, W.; Yoocha, T.; Narong, N.; Paemanee, A.; Ketngamkum, Y.; Romyanon, K.; Toojinda, T.; Tangphatsornruang, S.; Pootakham, W. Transcriptome sequencing revealed the influence of blue light on the expression levels of light-stress response genes in Centella asiatica. PLoS ONE 2021, 16, e0260468. [Google Scholar] [CrossRef]
- Priyanka, S.P.; Sujatha, S.; Smitha, G.R.; Suryanarayana, M.A.; Kalaivanan, D. Biomass accumulation, bioactive compounds and nutrient uptake in Centella asiatica (L.) in relation to organic nutrition in open-field and shade. Ind. Crops Prod. 2022, 176, 114352. [Google Scholar] [CrossRef]
- Farshana, K.L.; Deepa, P. In vitro salt tolerance induced secondary metabolites production in Abrus precatorius L. Curr. Bot. 2021, 12, 115–120. [Google Scholar]
- Ibrahim, M.H.; Shibli, N.I.; Izad, A.A.; Zain, N.A.M. Growth, chlorophyll fluorescence, leaf gas exchange and phytochemicals of Centella asiatica exposed to salinity stress. Annu. Res. Rev. Biol. 2018, 1–13. [Google Scholar] [CrossRef]
- Müller, V.; Lankes, C.; Zimmermann, B.F.; Noga, G.; Hunsche, M. Centelloside accumulation in leaves of Centella asiatica is determined by resource partitioning between primary and secondary metabolism while influenced by supply levels of either nitrogen, phosphorus or potassium. J. Plant Physiol. 2013, 170, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Sathish, D.K. Ameliorative effect by calcium on NaCI salinity stress related to proline metabolism in the callus of Centella asiatica L. J. Plant Biochem. Biotechnol. 2005, 14, 205–207. [Google Scholar] [CrossRef]
- Pipatsitee, P.; Praseartkul, P.; Theerawitaya, C.; Taota, K.; Tisarum, R.; Singh, H.P.; Cha-um, S. Exogenous NaCl salt elicitor improves centelloside content and physio-morphological adaptations in indian pennywort (Centella asiatica). J. Plant Biochem. Biotechnol. 2021, 31, 777–787. [Google Scholar] [CrossRef]
- Chung, S.O.; Kang, S.W.; Bae, K.S.; Ryu, M.J.; Kim, Y.J. The potential of remote monitoring and control of protected crop production environment using mobile phone under 3G and Wi-Fi communication conditions. Eng. Agric. Environ. Food 2015, 8, 251–256. [Google Scholar] [CrossRef]
- Mohapatra, P.; Ray, A.; Sandeep, I.S.; Nayak, S.; Mohanty, S. Tissue-Culture-Mediated Biotechnological Inter-vention in Centella asiatica: A Potential Antidiabetic Plant. In Biotechnology of Anti-diabetic Medicinal Plants; Springer: Berlin/Heidelberg, Germany, 2021; pp. 89–116. [Google Scholar]
- Solet, J.M.; Simón-Ramiasa, A.; Cosson, L.; Guignard, J.L. Centella asiatica (L.) Urban, (Pennywort): Cell Culture, Production of Terpenoids, and Biotransformation Capacity. In Medicinal and Aromatic Plants X; Springer: Berlin/Heidelberg, Germany, 1998; pp. 81–96. [Google Scholar]
- He, J.; Reddy, G.V.P.; Liu, M.; Shi, P. A general formula for calculating surface area of the similarly shaped leaves: Evidence from six Magnoliaceae species. Glob. Ecol. Conserv. 2020, 23, e01129. [Google Scholar] [CrossRef]
- Kanan, C.; Cottrell, G.W. Color-to-Grayscale: Does the Method Matter in Image Recognition? PLoS ONE 2012, 7, e29740. [Google Scholar] [CrossRef]
- Reza, M.N.; Na, I.S.; Lee, K.H. Automatic Counting of Rice Plant Numbers after Transplanting Using Low Altitude UAV Images. Int. J. Contents 2017, 13, 1–8. [Google Scholar] [CrossRef]
- Guerra-Rosas, E.; Álvarez-Borrego, J. Methodology for diagnosing of skin cancer on images of dermatologic spots by spectral analysis. Biomed. Opt. Express 2015, 6, 3876–3891. [Google Scholar] [CrossRef]
- Worden, K.; Tsialiamanis, G.; Cross, E.J.; Rogers, T.J. Artificial neural networks. In Machine Learning in Modeling and Simulation: Methods and Applications; Springer International Publishing: Cham, Switzerland, 2023; pp. 85–119. [Google Scholar]
- Yang, X.; Yan, J.; Wang, W.; Li, S.; Hu, B.; Lin, J. Brain-inspired models for visual object recognition: An overview. Artif. Intell. Rev. 2022, 55, 5263–5311. [Google Scholar] [CrossRef]
- Halagundegowda, G.R.; Singh, A. Multilayer Perceptron Method of Artificial Neural Network for Classification of Farmers Based on Adoption of Drought Coping Mechanisms. Int. J. Pure Appl. Biosci. 2018, 6, 1408–1414. [Google Scholar]
- Smith, G.N. Probability and statistics in civil engineering. In Collins Professional and Technical Books; Nichols Publishing Company: Asbury, IA, USA, 1986; p. 244. [Google Scholar]
- Kumar, M.K.; Kumar, R.S.; Sankar, V.; Sakthivel, T.; Karunakaran, G.; Tripathi, P.C. Non-destructive estimation of leaf area of durian (Durio zibethinus)—An artificial neural network approach. Sci. Hortic. 2017, 219, 319–325. [Google Scholar] [CrossRef]
- Asriani, E. Application of artificial neural network with backpropagation algorithm for estimating leaf area. IOP Conf. Ser. Earth Environ. Sci. 2020, 599, 012046. [Google Scholar] [CrossRef]
- Da Silva Ribeiro, J.E.; dos Santos Coêlho, E.; de Oliveira, A.K.S.; da Silva, A.G.C.; Lopes, W.D.A.R.; de Almeida Oliveira, P.H.; da Silveira, L.M. Artificial neural network approach for predicting the sesame (Sesamum indicum L.) leaf area: A non-destructive and accurate method. Heliyon 2023, 9, e17834. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, H.; Sajadi, S.J.; Jafarzadeh, M.R.; Rezaei, M.; Ghaffari, S.; Bakhtiari, S. Image processing and prediction of leaf area in cereals: A comparison of artificial neural networks, an adaptive neuro-fuzzy inference system, and regression methods. Crop Sci. 2021, 61, 1013–1029. [Google Scholar] [CrossRef]
- Ercanlı, İ.; Günlü, A.; Şenyurt, M.; Keleş, S. Artificial neural network models predicting the leaf area index: A case study in pure even-aged Crimean pine forests from Turkey. For. Ecosyst. 2018, 5, 29. [Google Scholar] [CrossRef]
- Nair, I.; Mazumdar, P.; Singh, P.; Rengasamy, N.; Harikrishna, J.A. Increasing the ratio of blue to red light improves growth and phytochemical content in Hydrocotyle bonariensis. Russ. J. Plant Physiol. 2021, 68, 337–346. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef]
- Gao, Q.; Liao, Q.; Li, Q.; Yang, Q.; Wang, F.; Li, J. Effects of LED Red and Blue Light Component on Growth and Photosynthetic Characteristics of Coriander in Plant Factory. Horticulturae 2022, 8, 1165. [Google Scholar] [CrossRef]
- Muneer, S.; Kim, E.J.; Park, J.S.; Lee, J.H. Influence of green, red and blue light emitting diodes on multiprotein complex proteins and photosynthetic activity under different light intensities in lettuce leaves (Lactuca sativa L.). Int. J. Mol. Sci. 2014, 15, 4657–4670. [Google Scholar] [CrossRef] [PubMed]
- Naznin, M.T.; Lefsrud, M.; Azad, M.O.K.; Park, C.H. Effect of Different Combinations of Red and Blue LED Light on Growth Characteristics and Pigment Content of In Vitro Tomato Plantlets. Agriculture 2019, 9, 196. [Google Scholar] [CrossRef]


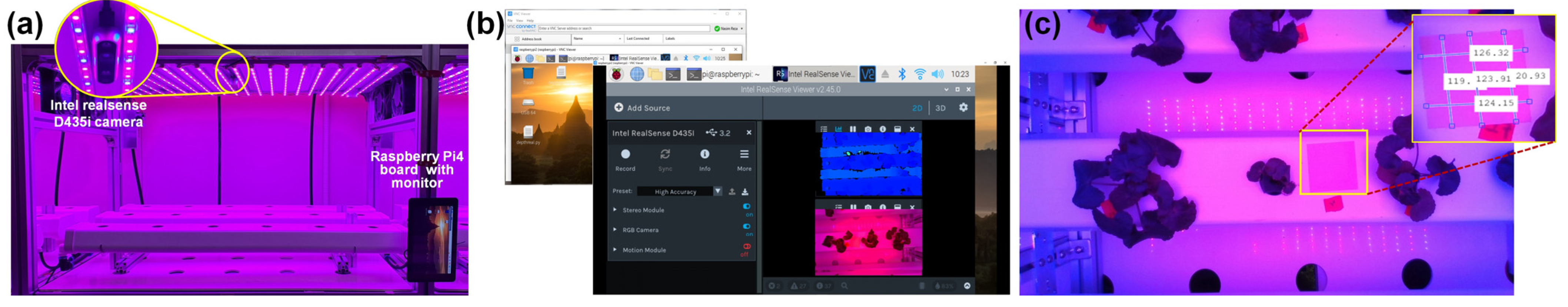
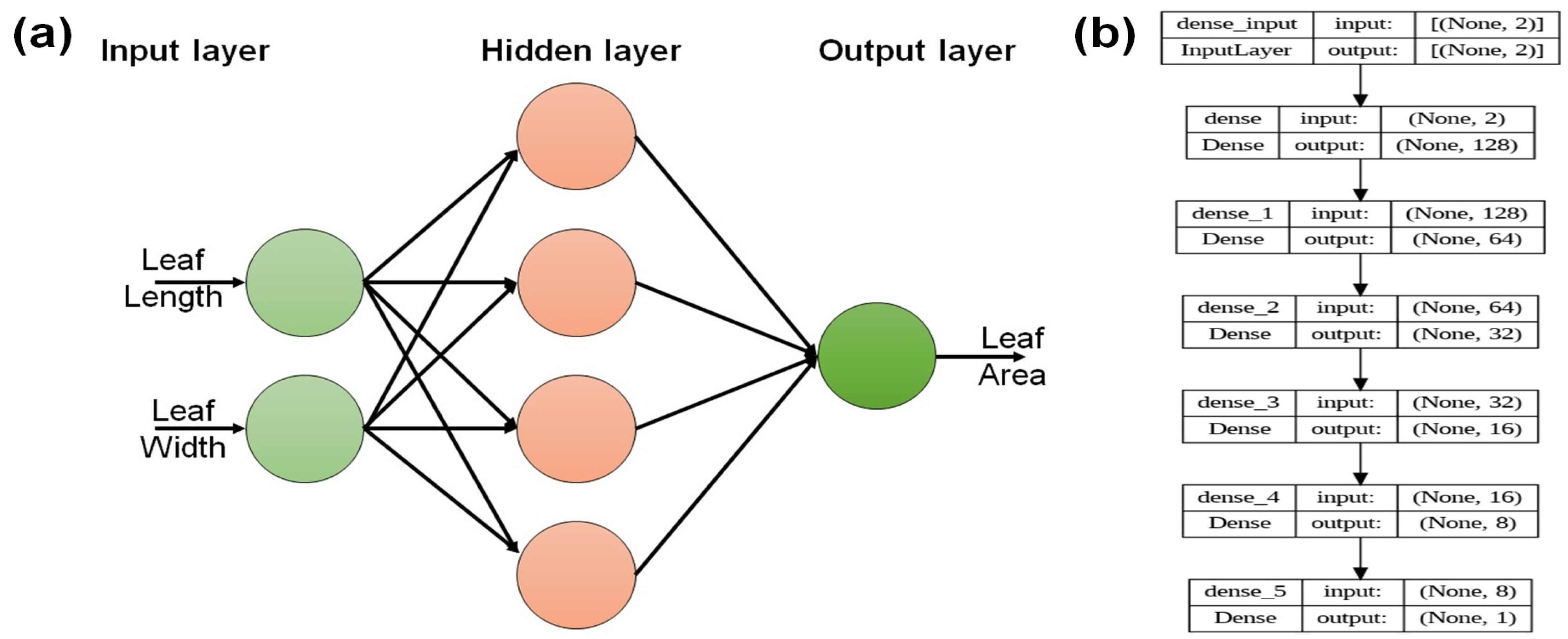
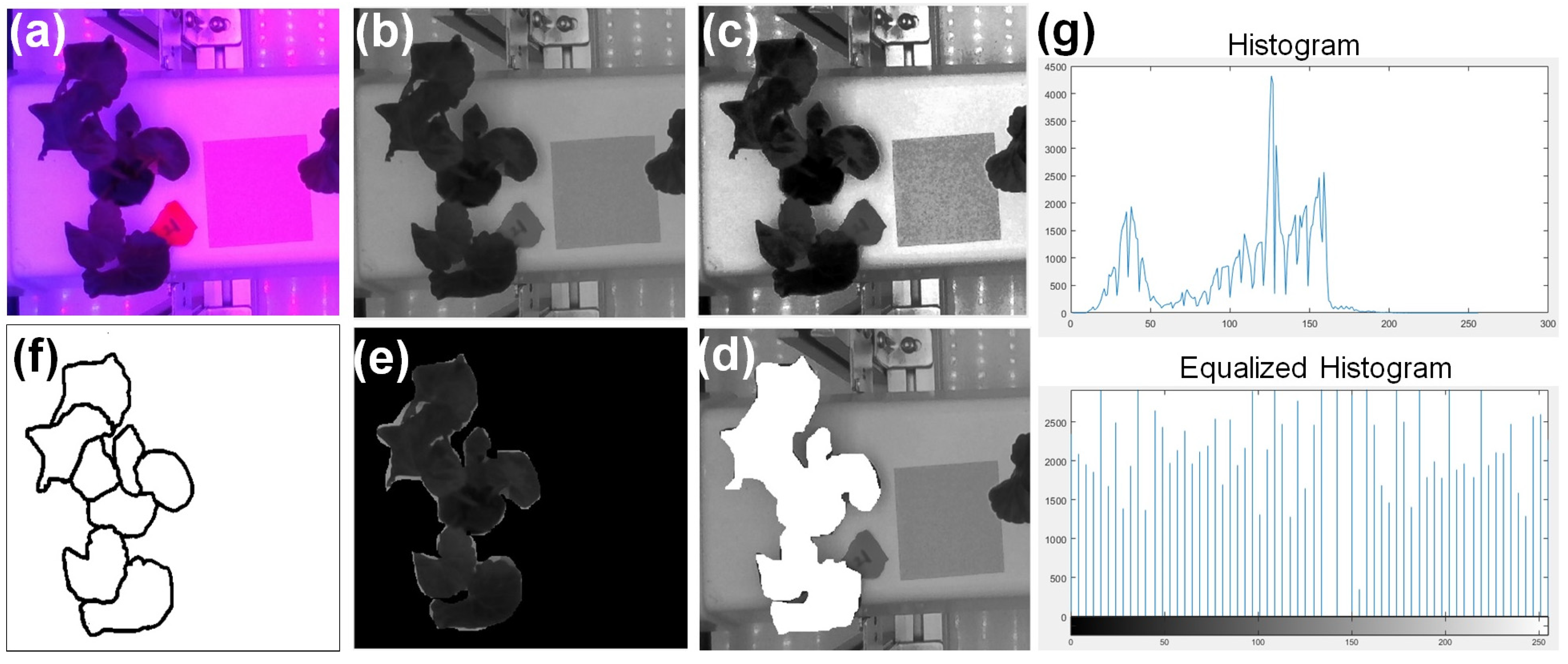
| Parameter | Set Value | Sensor Used | Specification |
|---|---|---|---|
| Temperature (°C) | 25 ± 1 | ETH-01DV, ECONARAE, Seoul, Republic of Korea | Temp. meas. range: −40~80 °C Humi. meas. range: 0–100% Temp. accuracy: ±0.5 Hum. accuracy: ±2% Compatible: 3.0~5 V |
| Humidity (%) | 65 ± 5 | ||
| CO2 (ppm) | 600 ± 100 | SH-300-DS, SOHA TECH Co., Ltd., Seoul, Republic of Korea | Measurement: 0 to 2000 ppm Accuracy: ± 70 ppm Compatible: 4.5~5.25 V |
| LED type (R:B) | 90:10, 70:30, 50:50 | - | - |
| Light intensity (µmol m−2s−1) | 150 ± 10 | GY-30, ROHM Co., Ltd., Kyoto, Japan | Range: 1~65,535 lx Accuracy: ±20% Compatible: 3.3 and 5 V |
| Photoperiod (h) | 18/6 | MaxiRex 5QT, Legrand, Republic of Korea | - |
| Cultivation system | DFT and NFT | - | - |
| EC (dS m−1) | 1, 2, 3 ± 0.3 | EC-BTA, Vernier, OR, USA | Range: 2–2000 dS m−1 Temperature range: 0~80 °C |
| pH | 6.50 ± 0.5 | pH-BTA, Vernier, OR, USA | Range: 0~14 Temperature range: 5–80 °C |
| Parameters | Specifications | Parameters | Specifications |
|---|---|---|---|
| Sensor Name | RealSense D435i | Name | Raspberry Pi 4B board |
| Company | Intel | Company | Raspberry Pi |
| Sensor | Global Shutter | CPU | Quad-core Cortex-A72, 64-bit, 1.8 GHz |
| Resolution | 2.0 MP | RAM | 8 GB LPDDR4-3200 |
| Frame Resolution | 1280 × 720 pixel | Connection | Standard 40-pin GPIO header |
| Frame Rate | 30 fps | Operating system | Linux based |
| Control | Automatic | Power supply | 5 V DC |
| Connection | USB-C 3.1 | Operating temperature | 0° to 50 °C |
| Manufacturer | Raspberry Pi Foundation, UK |
| Parameters | Value |
|---|---|
| Number of neurons in the input layer | 2 |
| Number of hidden layers | 1 |
| Number of hidden layers | 4 |
| Number of output layers | 1 |
| Learning rate | 0.01 |
| Maximum number epoch | 100 |
| Loss function | Mean absolute error (MAE) |
| Activation function | Rectified linear unit (ReLu) |
| Parameter | Max | Min | Mean and Standard Deviation |
|---|---|---|---|
| Leaf length (L), mm | 35.71 | 10.0 | 5.4 ± 0.12 |
| Leaf width (D), mm | 60.45 | 18.0 | 11.43 ± 0.26 |
| Leaf area (LA), mm2 | 1591.74 | 141.3 | 297.197 ± 6.82 |
| Values | Training Data | Testing Data |
|---|---|---|
| RMSE | 3.26 | 4.53 |
| MAPE | 6.33 | 6.59 |
| MAE | 1.33 | 1.59 |
| R2 | 0.97 | 0.96 |
| Leaf Area | Mean | p-Value |
|---|---|---|
| Measured | ||
| Image processing | −116.84 | 0.019 |
| ANN | −94.14 | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, M.N.; Chowdhury, M.; Islam, S.; Kabir, M.S.N.; Park, S.U.; Lee, G.-J.; Cho, J.; Chung, S.-O. Leaf Area Prediction of Pennywort Plants Grown in a Plant Factory Using Image Processing and an Artificial Neural Network. Horticulturae 2023, 9, 1346. https://doi.org/10.3390/horticulturae9121346
Reza MN, Chowdhury M, Islam S, Kabir MSN, Park SU, Lee G-J, Cho J, Chung S-O. Leaf Area Prediction of Pennywort Plants Grown in a Plant Factory Using Image Processing and an Artificial Neural Network. Horticulturae. 2023; 9(12):1346. https://doi.org/10.3390/horticulturae9121346
Chicago/Turabian StyleReza, Md Nasim, Milon Chowdhury, Sumaiya Islam, Md Shaha Nur Kabir, Sang Un Park, Geung-Joo Lee, Jongki Cho, and Sun-Ok Chung. 2023. "Leaf Area Prediction of Pennywort Plants Grown in a Plant Factory Using Image Processing and an Artificial Neural Network" Horticulturae 9, no. 12: 1346. https://doi.org/10.3390/horticulturae9121346
APA StyleReza, M. N., Chowdhury, M., Islam, S., Kabir, M. S. N., Park, S. U., Lee, G.-J., Cho, J., & Chung, S.-O. (2023). Leaf Area Prediction of Pennywort Plants Grown in a Plant Factory Using Image Processing and an Artificial Neural Network. Horticulturae, 9(12), 1346. https://doi.org/10.3390/horticulturae9121346














