Abstract
Onions, essential in various salads and cooked dishes, are sought after by producers for high yields, while consumers value their quality, particularly the presence of antioxidant compounds. This study investigates the impact of production methods and biostimulants on the biomass yield and quality of onions. The two-year experiment was conducted in Vojvodina, Serbia, and compared directly seeded (DS) and from-set (FS) onions with four biostimulant treatments: control (C), seaweed extracts (T1), humic and fulvic acids (T2), and Trichoderma sp. (T3). DS onions yielded significantly more biomass, while FS onions had higher dry matter content. DS onions treated with T1 showed a significant increase in phenols (↑ 5.30%), while T2 and T3 led to declines (↓ 8.66% and ↓ 7.55%, respectively). All biostimulants reduced phenol content in FS onions. T1 and T2 significantly increased the flavonoid concentration in DS onions, with no significant changes in FS onions. T1 enhanced antioxidant properties in DS onions and reduced them in FS onions. Additionally, T2 and T3 decreased antioxidant activity in both DS and FS onions, as evidenced by DPPH, FRAP, and ABTS tests. These findings guide onion production, advocating for the fresh consumption of DS onions with higher biomass and industrial processing suitability for FS onions, emphasizing the potential of bio-based products.
1. Introduction
The onion (Allium cepa) is an essential ingredient in many raw or cooked dishes. The annual per capita consumption of onions worldwide amounts to approximately 11.4 kg [1]. Compared to other vegetables, onions contain a high level of minerals, vitamins, and bioactive compounds, such as chlorophyll, phenols, flavonoids, and various other compounds with antioxidant properties [2,3,4,5]. Notably, among the most prevalent individual phenolic compounds in onions are vanillic acid, ferulic acid, peonidin, and isoeugenol [2].
Regarding onions intended for fresh consumption, directly seeded (DS) is often preferred as a production method due to its cost-effectiveness. DS onion also results in higher yields, allowing for a more significant number of plants per unit area. On the other hand, onions intended for processing are typically produced from sets (FS) due to their higher dry matter content.
From the perspective of onion producers (farmers), achieving the highest possible yield of first-class bulbs is essential. Conversely, from the standpoint of onion consumers, it is desirable for the produce to be of the highest quality and to contain a significant amount of bioactive compounds [2,3,5]. With these considerations in mind, there is a need to enhance onion production to enable farmers to achieve optimal yields while ensuring that consumers can enjoy high-quality onions, whether fresh or processed, daily.
The average onion yield worldwide stands at 18.4 t/ha, while in Serbia, it is 8.9 t/ha [6]. Low yields in our country are often the result of unfavorable ecological conditions and the inadequate implementation of agronomic practices. To address these challenges and enhance onion yields, one approach involves the utilization of various biostimulants. By influencing various physiological processes, biostimulants can provide numerous benefits to the growth and development of plants [7,8,9]. They are particularly valuable when plants face stress, as they enhance tolerance to different types of stress that can occur in open-field production [10,11]. However, under stress conditions, plants may reduce yields but increase the synthesis of secondary metabolites, such as various phenolic compounds (e.g., phenolic acids, flavonoids, and catechins) with potent antioxidant properties, as part of their adaptation to challenging environments [12].
In recent years, scientists from various parts of the world have been working to uncover the effects of biostimulants on the yield and quality of vegetables. In a study involving peppers (Capsicum annuum) and tomatoes (Solanum lycopersicum), a biostimulant derived from seaweed extracts (SWE) significantly increased biomass [13]. However, in research conducted by Francke et al. [14], biostimulants with SWE did not significantly affect shallots (Allium cepa Aggregatum group). Concerning quality, in previous experiments with beans (Phaseolus vulgaris) and carrots (Daucus carota) SWE-based biostimulants significantly increased the content of antioxidant compounds such as flavonoids and anthocyanins [15,16]. In the case of lettuce (Lactuca sativa), there was an increase in phenol content in the presence of arbuscular mycorrhiza (Glomus mossae) [17]. In a study on tomato, a yeast-based biostimulant, reduced phenol content compared to untreated plants [18].
Furthermore, humic and fulvic acid-based biostimulants are commonly utilized in vegetable production [19]. Research involving potatoes (Solanum tuberosum) and lettuce revealed that humic compounds had a noteworthy impact on increasing yields in treated plants [20,21]. Additionally, microorganism-based biostimulants, such as those derived from Trichoderma sp. (e.g., T. virens strain GV41 or T. harzianum strain T22), are widely used as they can colonize the root systems of most cultivated plants [22,23]. In the context of onion production, the application of Trichoderma sp. increased protein content, whereas in tomato, it reduced phenol content [24,25].
Despite the significance of onions in people’s daily diets, there has been a notable scarcity of research exploring the impact of production methods and the application of biostimulants on onion yield and quality. Existing studies investigating the effects of biostimulants [3,4,5] have primarily focused on onion production from seedlings, with limited research on onions grown from direct seeding or sets. This research addresses a critical need for practical applicability and aims to enhance onion production for fresh consumption and for processing.
In light of this challenge, the objective of our study was to comprehensively assess the influence of biostimulants on biomass yield and the content of bioactive compounds in onions, under two production methods.
2. Materials and Methods
2.1. Experimental Design and Agroecological Conditions
The field experiments were conducted during 2021 and 2022 on the territory of the Autonomous Province (AP) of Vojvodina, Republic of Serbia. Weather conditions during the experiments are depicted in Figure 1. The field experiment was set up in three replicates with randomized treatments, where the first factor represented the production method: 1. directly seeded (DS) onion, and 2. From-set (FS) onion. The second factor consisted of biostimulants: 1. control without biostimulants (C); 2. Agasi® by Agafert S.R.L. (Bari, Italy), based on SWE (T1); 3. HumiBlack® by DRN Kimya (Antalya, Turkey), based on humic and fulvic acids (T2); and 4. Tifi® by Italpollina S.P.A. (Rivoli, Italy), formulated with Trichoderma sp. (T3).
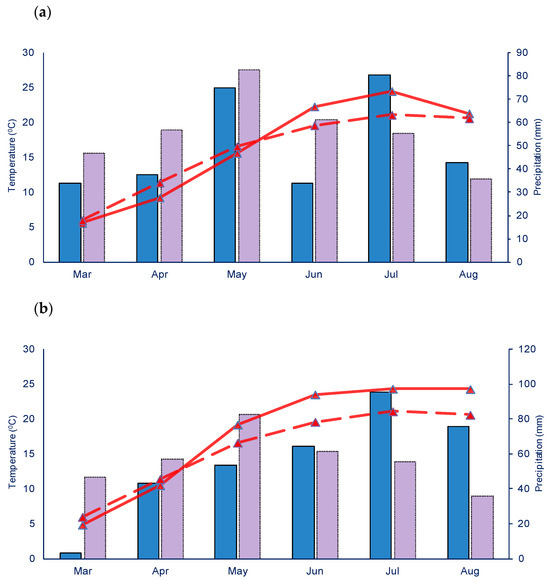
Figure 1.
Weather conditions in the AP, Vojvodina, during onion production in 2021 (a) and 2022 (b). The solid red line represents air temperatures, while the dashed red line shows the multi-year (1990–2020) average temperature. Blue bars represent precipitation, and purple bars indicate the multi-year (1990–2020) average precipitation.
The highest average temperatures in 2021 and 2022 were recorded in July, reaching 24.5 °C and 24.3 °C, respectively, which exceeded the multi-year average (1990–2020) by 3.4 °C and 3.2 °C. In terms of precipitation, the most significant rainfall in 2021 and 2022 also occurred in July, at 80.6 and 95.4 mm, respectively. This marked an increase of 25.3 and 40.1 mm compared to the multi-year average (1990–2020). The monitoring of meteorological parameters was conducted using the automated meteorological station Vantage Pro2TM by Davis Instruments (Hayward, CA, USA) [26] located near the field experiments.
2.2. Agrotechnical Practices
The applied agronomic practices have been described in detail by Vojnović et al. [27]. In summary, during the autumn, 64 kg/ha of nitrogen (N), phosphorus (P), and potassium (K) were applied using mineral fertilizers. Subsequently, plowing was carried out to a depth of 27 cm. In early spring, harrowing was performed. Seven days before sowing, nitrogen fertilizer was applied at 86 kg/ha, followed by soil preparation for sowing and planting.
The hybrid Elenka F1 by Cora Seeds® (Cesena, Italy) was used for the DS. This hybrid has bronze-colored dry outer scales, and its bulbs are suitable for long-term storage. The Stuttgarter Riesen® cultivar (imported from The Netherlands) with golden-colored dry outer scales was used for the FS onion.
The quality indicators of the seeds used in the experiment in 2021 and 2022 are presented in Table 1. Germination energy was assessed after 6 days, and total germination after 12 days, following the guidelines of the regulation on the quality of agricultural plant seeds [28]. The used seeds were treated with a fungicide based on fludioxonil by the seed producer.

Table 1.
Seed quality indicators of seeds used in the experiment with directly seeded onions.
The quality indicators of onion sets in 2021 and 2022 are given in Table 2. The classification of sets was conducted following Ilin [29], wherein sets belonging to the first class are divided into five classes according to diameter. A randomly selected sample of 1 kg of bulbs was used to classify onion sets. This classification is of utmost importance, as practical observations have shown that larger-diameter bulbs have the tendency to initiate early flowering and develop flower stalks, which is not the desired outcome in the production of marketable, consumable bulbs.

Table 2.
Sets quality indicators used in the experiment where onion is production from sets.
Irrigation for the onions was carried out using a drip irrigation method. Irrigation timing was chosen according to measurements of soil moisture, following the method described in the study involving potatoes [30]. Soil moisture sensors (SKU-6440 by Davis Instruments, Hayward, CA, USA) were connected to a Vantage Pro2TM meteorological station near the plots for monitoring and control.
Biostimulants were applied through drip irrigation, according to the manufacturer’s recommendations. T1 was applied at a rate of 10 L/ha, T2 at 50 L/ha, and T3 at 3 kg/ha [27]. During the onion’s growth cycle, herbicides based on bromoxynil (Xinca®, 0.6 L/ha) and fluoxypyr (Bonaca®, 0.25 L/ha) were used. Applied fungicides were based on metalaxyl m + mancozeb (Ridomil®, 2.5 kg/ha) and boscalid + pyraclostrobin (Signum®, 0.75 kg/ha), whereas insecticide treatments included formetanate hydrochloride (Dikarzol®, 1 kg/ha) and imidacloprid (Lobo®, 0.12 L/ha).
The biomass of produced onion was measured in the field by harvesting all plants after removing the edge effect. Subsequently, after separating the bulbs from the green leaves, random samples of fifteen bulbs were taken for measuring bioactive compounds, following the method described in the vegetable quality assessment guidelines [31].
2.3. Methods for Measuring Bioactive Compounds
2.3.1. Preparation of Extracts
Sample preparation for analysis is detailed in Vojnović et al. [27]. In brief, 10 g of ground bulbs were placed in a 50 mL Erlenmeyer flask, and approximately 25 mL of 95% methanol was added as the extraction agent. The mixture was shaken for 24 h in the dark. After 24 h, the samples were quantitatively transferred to 50 mL measuring flasks, which were filled to the nominal volume with the extraction agent. The flask contents were filtered into plastic vials and stored in the fridge until analysis. Methanol extracts were utilized in determining the content of total phenols, total flavonoids, and antioxidant status.
2.3.2. Dry Matter Content
The dry matter (DM) content was measured using the thermogravimetric method after drying the samples to a constant mass [31].
2.3.3. Total Phenol Content
The phenol content was measured using the Folin–Ciocalteu method [32] with gallic acid serving as the standard equivalent. The absorbance of the solution, exhibiting a blue coloration as a result of the reaction, was measured at a wavelength of 750 nm with a UV/VIS spectrophotometer (LLG-uniSPEC 2 Spectrophotometer by Lab Logistics Group GmbH from Meckenheim, Germany).
2.3.4. Total Flavonoid Content
The flavonoids were assessed according to Harbone [33], using the colorimetric aluminum chloride assay, with absorbance values measured at 510 nm.
2.3.5. DPPH Assay
We employed a slightly modified version of the DPPH (2,2-diphenyl-1-picrylhydrazyl) free radical scavenging assay to assess the antioxidant activity of methanol extracts from onions. This methodology is primarily detailed in the study conducted by Brand-Williams et al. [34]. The determination of the Trolox equivalent antioxidant capacity using the DPPH assay was conducted using the following equations [35]:
where cekv is the concentration of the Trolox equivalent calculated from the Trolox calibration curve, A is the absorbance from the tested sample, n is the intercept of the calibration curve, k is the slope of the calibration curve, and c is the concentration of the sample solution.
2.3.6. FRAP Assay
The purpose of the Ferric Reducing Antioxidant Power (FRAP) assay was to evaluate the reducing power of onion extracts against positively charged trivalent ferric ions (Fe3+) [36]. The freshly prepared FRAP reagent comprised 10 mM TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) in 40 mM HCl, a 20 mM iron (III)-chloride (FeCl3) aqueous solution, and a 300 mM acetate buffer at pH 3.6. Subsequently, the absorbance values of the reaction mixture at 593 nm were determined spectrophotometrically after a 10 min incubation in the dark at 37 °C [36]. FRAP was calculated the same way as it is described for the DPPH assay in Section 2.3.5.
2.3.7. ABTS Assay
The antioxidant activity assay using 2,2′-azino-bis-(-3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS) was conducted following a modified methodology outlined by Re et al. [37]. The preparation of the ABTS reagent involved the reaction of a 7 mM aqueous ABTS solution with a 2.45 mM aqueous potassium persulfate solution in the dark for 16 h at room temperature. The reaction mixture, obtained by combining 2.9 mL of the ABTS reagent with 0.1 mL of diluted extracts, was incubated for 300 min at room temperature in the dark. After incubation, absorbance values were recorded at a wavelength of 734 nm [37]. ABTS was calculated in the same way as it is described for the DPPH assay in Section 2.3.5.
Depending on the antioxidant test, the results of measuring DPPH (mg Trolox (TC)/100 g DM), FRAP (mg Fe2+/100 g DM), and ABTS (mg TC/100 g DM) are interpreted in a way in which higher test results indicate higher antioxidant activity.
Sample preparation for the analyses was conducted in the Vegetable Science Laboratory at the Faculty of Agriculture Novi Sad, while bioactive compounds were determined in the Laboratory for Quality Analysis of Fruits and Vegetables at the Faculty of Technology, Novi Sad.
2.4. Statistical Data Analysis
To determine the effect of cultivation methods and biostimulants on biomass yield and the quality of onion, the research results were analyzed using a factorial analysis of variance (ANOVA). The significance of mean differences was determined by the LSD test at p < 0.05. Before analyzing the variance, data normality was confirmed using the Kruskal–Wallis test. Due to the extensive dataset, the biomass yield is presented as a two-year average. The yearly effect for bioactive compounds is displayed individually in graphs, while the influence of biostimulants and production methods is presented as a two-year average in tables. The relationship between phenols and antioxidant activity was established through regression analysis. Statistical analyses were performed using Statistica 14 [38] software (TIBCO) and Excel 2007 [39].
3. Results
3.1. Biomass (t/ha)
On average, in 2021 and 2022, production methods and biostimulants significantly influenced the biomass yield of onion (Figure 2).
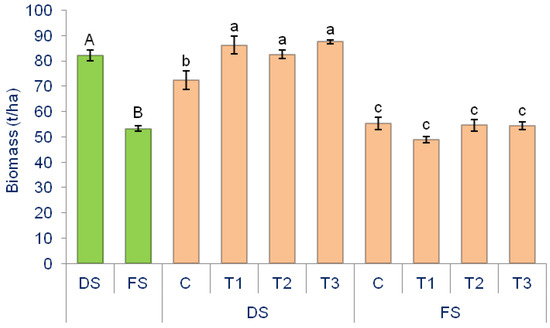
Figure 2.
The effect of production methods (P) and biostimulants (B) on the onion biomass average in 2021 and 2022. Green bars represent the main effect of the P, while orange bars represent the interaction between P and B. Small different letters indicate differences between interactions, whereas capital letters represent the effect of production method, denoting the significance of the differences at p ˂ 0.05 according to the LSD test. The lines on the bars represent the standard error of the mean. DS—Directly seeded onion; FS—from-set onion. C—control (without B); T1—B based on SWE; T2—B based on humic and fulvic acids; T3—B based on Trichoderma sp.
Depending on the production method, a significantly higher biomass yield of onion was observed in DS (82.15 t/ha) compared to FS (53.32 t/ha). In DS treatments, the highest biomass was recorded in DS × T1 (86.16 t/ha), while the lowest was in DS × C (72.43 t/ha), with a difference of 18.95% which is statistically significant. However, there was no difference between DS × T1, DS × T2, and DS × T1. For the FS treatment, the highest yield was in FS × C (55.45 t/ha), while the lowest was in FS × T1 (48.87 t/ha), with a difference of 6.58%. However, there was no significant difference between FS × C, FS × T1, FS × T2, and FS × T3.
3.2. Dry Matter (%)
The cultivation method significantly influenced the dry matter content (Figure 3).
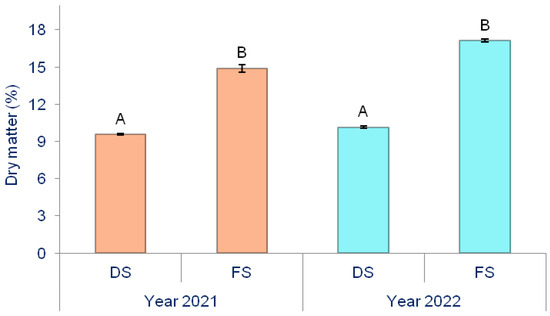
Figure 3.
The effect of production methods on the dry matter content of onion. Different letters indicate the significance of the difference at p < 0.05 according to the LSD test. The lines on the bars represent the standard error of the mean. DS—Directly seeded onion; FS—from-set onion.
During both years of the study (2021 and 2022), onion FS had a significantly higher dry matter content than onion DS. The dry matter content in onion bulbs depended on the production methods and applied biostimulants (Table 3).

Table 3.
The effect of production methods and biostimulants on the average dry matter content in onion in 2021 and 2022.
For the whole experiment, the average dry matter content was 12.94%. When comparing production methods, a significantly higher dry matter level was observed in FS (16.03%) compared to DS (9.86%). Biostimulants significantly increased the dry matter content in the bulbs. On average, for biostimulants, the highest dry matter content was measured in T3 (13.49%), while the lowest was in the control (12.57%), with both being statistically significant.
On plots with DS onion, the highest dry matter content was found in DS × T1 (10.02%) and lowest in DS × C (9.66%), with their significant difference. In the case of FS onion, the highest dry matter content was measured in bulbs treated with FS × T3 (17.06%) and the lowest in FS × C (15.49%), with their difference being significant.
3.3. Phenol Content (mg/100 g DM)
The cultivation method significantly influences the phenolic content in onion bulbs (Figure 4).
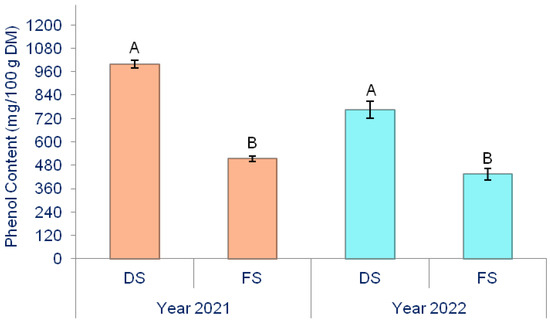
Figure 4.
The effect of production methods on the phenol content in onion. Different letters indicate the significance of the differences at p < 0.05 according to the LSD test. The lines on the bars represent the standard error of the mean. DS—Directly seeded onion; FS—from-set onion.
Over 2021 and 2022, DS onions (999.12 and 764.96 mg/100 g DM) exhibited a statistically significantly higher phenolic content than FS onions (764.96 and 433.99 mg/100 g DM) (Figure 4). The production method and biostimulants significantly influenced the phenolic content in onions (Table 4).

Table 4.
The effect of production methods and biostimulants on the average phenol content in onion in 2021 and 2022.
The average phenolic content in this experiment was 678.13 mg/100 g DM. On average, for the production method, DS onions (882.04 mg/100 g DM) showed significantly higher phenolic content than FS onions (474.23 mg/100 g DM).
On average, for the biostimulants, the highest phenolic content was found in the control (721.58 mg/100 g DM), while the lowest was in T2 (640.58 mg/100 g DM), and their difference of 11.22% is significant.
For DS onion, the highest phenolic content was observed in DS × T1 (954.92 mg/100 g DM), while the lowest was measured in DS × T2 (828.19 mg/100 g DM), and their difference is significant of 15.30%.
In the case of FS onions, the highest phenolic content was recorded in FS × C (536.36 mg/100 g DM), while the lowest was in FS × T1 (441.75 mg/100 g DM), with their difference of 17.63% being significant.
3.4. Flavonoid Content (mg/100 g DM)
The production method significantly influenced the flavonoid content in onion bulbs (Figure 5).
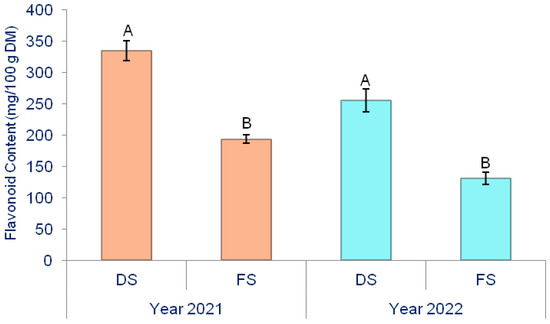
Figure 5.
The effect of production methods on the flavonoid content of onion. Different letters indicate the significance of the difference at p < 0.05 according to the LSD test. The lines on the bars represent the standard error of the mean. DS—Directly seeded onion; FS—from-set onion.
Depending on the year, the flavonoid content was significantly higher in DS onion, as confirmed during the 2021 and 2022 field experiments. On average, for both years, the production method and biostimulants significantly influenced the flavonoid levels in onions (Table 5).

Table 5.
The effect of production methods and biostimulants on the average flavonoid content of onion in 2021 and 2022.
The overall average flavonoid content was 228.56 mg/100 g DM. A significantly higher flavonoid content was observed in DS onions (294.68 mg/100 g DM) compared to FS onions (162.44 mg/100 g DM). Among all the biostimulants, the highest flavonoid content was measured in T1 (259.00 mg/100 g DM), while the lowest was in T3 (201.8 mg/100 g DM), and their difference is significant. In plots growing DS onion, the highest flavonoid content was measured in DS × T1 (355.04 mg/100 g DM), and the lowest was measured in DS × C (262.78 mg/100 g DM), with their difference of 35.10% being statistically significant. FS onions exhibited the highest flavonoid content in FS ×T2 (169.03 mg/100 g DM), while the lowest was recorded in FS ×T3 (157.04 mg/100 g DM), and their difference is significant.
3.5. Antioxidant Indicators
The cultivation method significantly influenced the antioxidant activity in onions (Figure 6).
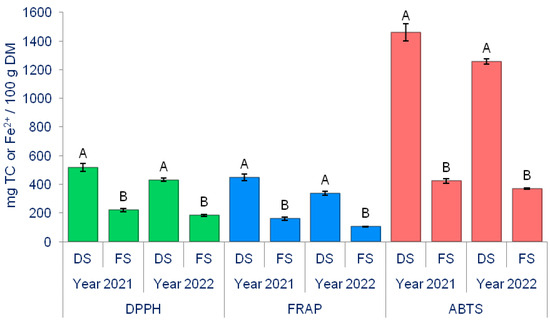
Figure 6.
The effect of production methods on the onion’s antioxidant status (DPPH, FRAP, and ABTS assay). Different letters indicate the significance of the differences at p < 0.05, according to the LSD test. The lines on the bars represent the standard error of the mean. DS—Directly seeded onion; FS—From sets onion.
During both years of the experiment (2021 and 2022), antioxidant activity measurements using the DPPH, FRAP, and ABTS tests revealed that bulbs from DS onion have significantly higher levels of antioxidants than FS onions. The production method and biostimulants significantly affected the antioxidant activity of onions (Table 6).

Table 6.
The effect of production methods and biostimulants on the average antioxidant status (DPPH, FRAP, and ABTS assay) of onion in 2021 and 2022.
For DS onions, the highest antioxidant activity measured by the DPPH, FRAP, and ABTS tests was observed in the DS × T1 treatment, while the lowest was in DS × T3 for the DPPH test and in DS × T1 for the FRAP and ABTS tests. For FS onions, it is evident that the biostimulants significantly reduced the antioxidant activity of the onions. In the case of the DPPH test, the most substantial reduction in antioxidant activity, compared to the control (231.26 mg/100 g DM), was observed in the FS × T2 treatment (179.46 mg/100 g DM), amounting to 22.39%. Similarly, in the FRAP test, the FS × T2 treatment (106.39 mg/100 g DM) reduced the activity by 36.16% compared to the control (166.67 mg/100 g DM). Regarding the ABTS test, the most substantial reduction in antioxidant activity compared to the control (439.89 mg/100 g DM) was induced by the FS×T1 treatment (363.87 mg/100 g DM), representing a 17.28% decrease.
The effect of biostimulants significantly influenced the antioxidant activity of onions (Table 7).

Table 7.
The effect of biostimulants on the average antioxidant status of onion in 2021 and 2022.
All three antioxidant tests revealed the highest activity in onions treated with biostimulant T1. Conversely, the lowest antioxidant content was observed in the plots treated with T2, as confirmed by the DPPH, FRAP, and ABTS tests.
By observing the graphs, a clear positive correlation between phenol content and the antioxidant activity of onions can be noticed. In all three antioxidant tests (DPPH, FRAP, and ABTS), a linear regression curve was obtained concerning phenol content, indicating that an increase in phenol content leads to a corresponding increase in the antioxidant activity of the bulbs (Figure 7).
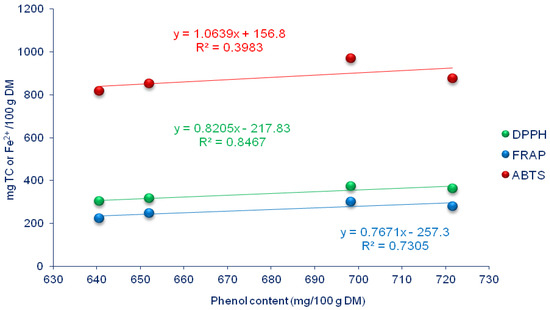
Figure 7.
Relationship between onion’s antioxidant activity (DPPH, FRAP, and ABTS assay) and phenol content.
4. Discussion
The biomass of onion comprises the mass of roots, bulbs, pseudostems, and green leaves. Based on biomass yield, it is possible to obtain a clearer picture of the impact of biostimulants on onion growth, as this eliminates their effect on the allocation of assimilates to the underground or aboveground parts of the plant. Depending on the production method, significantly higher biomass of onion was observed when it was directly seeded (DS) than when it was produced from sets (FS). This can be explained by a higher number of plants per unit area when using DS, as plant density is the most critical factor for yield, with one plant yielding one bulb.
In the case of DS onion, the biostimulant T1 significantly increased biomass yield. Similar effects of biostimulants, based on SWE, were observed by Ali et al. [13] on pepper and tomato. They have also found that those biostimulants affected the expression of genes responsible for synthesis of auxins, gibberellins, and cytokinins. This is in line with the research of Mógor et al. [40], who managed to increase the red beet (Beta vulgaris) yield by using a similar biostimulant.
On the DS × T2 plots, there was a significant increase in biomass yield compared to DS × C. Similarly, an increase in yield on plots where T2 was applied was observed in potatoes and lettuce [20,21]. The biostimulatory effect of T2 can be explained by its ability to enhance the activity of proton pumps on the plasma membrane and tonoplast of plant cells [41]. Also, a significant increase in biomass yield compared to DS × C was observed in plants treated with DS × T3. The positive impact of Trichoderma sp. on yield enhancement has been noted in lettuce and tomatoes [42,43]. According to Fiorentino et al. [44], the stimulatory effect of Trichoderma sp. primarily relies on these fungi’s release of auxins, peptides, and various organic compounds that promote plant productivity.
The absence of any significant impact of biostimulants on biomass in FS onions compared to the control can be attributed to the relatively shorter vegetative period of FS onions. For example, in the case of Trichoderma sp., the shorter vegetative period of FS onion may have limited the development of symbiotic relationships between the onion root and fungi, thus reducing the efficacy of biostimulants. Indeed, FS onions in both years were harvested earlier (18 and 20 days earlier in 2021 and 2022, respectively) compared to DS onions.
The dry matter content is one of the most critical indicators of the quality of onions, influencing their storage duration and processing methods. Our research showed that FS onions had significantly higher dry matter content than onions from DS. This difference in dry matter content in favor of FS onions is one of the reasons why producers often choose to use sets to produce onions intended for industrial processing.
The biostimulant T1 significantly increased the dry matter content compared to the control. Given that 80% of the dry matter of onions is sugars [5], the increase in dry matter due to the T1 treatment can be explained by the potential of SWE to enhance photosynthesis. Indeed, it was found that SWE enhance the expression of genes encoding enzymes involved in carbon fixation during the Calvin cycle, thus contributing to an increase in starch content, as suggested by Jannin et al. [45]. Mzibra et al. [46] observed an increase in the dry matter content in the roots and stems of tomatoes treated with SWE. In the case of humic and fulvic acids, Hafez and Geries [47] reported a significant increase in the dry matter content of onion bulbs through their application, which is consistent with our research findings.
The phenolic content in onions significantly affects the taste and biological value of the bulbs. A significantly higher phenolic content was measured in DS bulbs than FS bulbs, which can also be attributed to genotype characteristics. Under stress, plants synthesize more secondary metabolites, such as phenols [12]. Interestingly, the main effects of all biostimulants significantly reduced the phenolic content compared to the control. This reduction can be attributed to the biostimulants’ capacity to alleviate the impact of abiotic stress resulting from environmental conditions.
The increase in phenolic content in the bulbs treated with DS × T1 and FS × T1 can be explained by the fact that the biostimulant based on SWE enhances the synthesis of the enzyme phenylalanine ammonia-lyase, which catalyzes the conversion of l-phenylalanine into trans-cinnamic acid in the biosynthesis of phenols [48,49]. A significantly increased phenolic content was noted in onions treated with SWE by Abbas et al. [5], as was reported in lettuce by Rasouli et al. [17].
In this study, the application of T2 significantly reduced the phenolic content in onions, while in the case of peppers, there was a significant increase in phenolic content [50,51]. This suggests that the concentration of phenolics is a result of complex interactions of factors that affect plant growth and may also vary with respect to plant species and plant parts (e.g., onion bulb and pepper fruit).
The application of biostimulant T3 significantly reduced the phenolic content in DS and FS onion bulbs. This result aligns with the findings of Vukelić et al. [25], who reported that applying Trichoderma sp. significantly reduced the phenolic content in tomatoes. Furthermore, the same authors emphasize that the effect of Trichoderma sp. fungi varies depending on the cultivars, as demonstrated in the case of tomatoes.
Onions are known for their high flavonoid content compared to other vegetable species. The beneficial effect of flavonoids is mainly attributed to their ability to scavenge free radicals, demonstrating their antioxidant properties [52]. In this study, DS onions had significantly higher flavonoid content than FS onions, as observed during the research conducted in both 2021 and 2022. According to Sharma [53], the flavonoid content in onions is often a characteristics of the cultivar, and this observation is consistent with our research.
On average, bulbs treated with T1 had significantly higher flavonoid content than the control. This aligns with the findings of Jithesh et al. [54], who reported that treatment with SWE in Arabidopsis sp. increased the expression of genes responsible for the enzymes involved in the phenylpropanoid pathway during flavonoid synthesis. Synthesized flavonoids play a crucial role in contributing to the defense against stresses such as pathogens, wounding, and UV light damage [54]. The favorable impact of SWE has been observed in carrots and beans [15,16]. Bulbs treated with T2 had significantly higher flavonoid content than the control. Similarly, treatments with humic acids significantly increased flavonoid content in peppers and yarrow (Achillea millefolium) [51,55]. This can be explained by the fact that humic substances can enhance the synthesis of phenyl and tyrosine ammonia-lyase enzymes, which play a role in flavonoid biosynthesis [56]. According to Schiavon [56], many synthesized compounds of the phenylpropanoid pathway are attributed to co-purified fungal elicitors and/or other signaling molecules. On average, biostimulant T3 reduced the flavonoid content in DS and FS onions. This can be attributed to the limited root surface area where Trichoderma sp. establishes symbiosis [57].
Consuming onions can have numerous health benefits, primarily due to their high antioxidant content [58]. Antioxidants play a crucial role in the human body by neutralizing free radicals that can damage cells. In this study, DS onions had significantly higher levels of antioxidants than those from FS, as indicated by the three antioxidant tests (DPPH, FRAP, and ABTS assay). On average, for both production methods, bulbs treated with T1 showed significantly higher levels of antioxidants than control. This can be related to the increased flavonoid content in the T1 plot, as flavonoids possess potent antioxidant properties, as suggested by Pietta [52]. This finding is also in line with Vojnović et al. [27], who revealed a connection between flavonoids and the antioxidant activity of onions. The observed decline in antioxidant activity, as measured by the DPPH, FRAP, and ABTS tests in FS onions, can be attributed to the overall reduction in phenolic content across all biostimulant-treated bulbs. This decrease is significant because total phenols are known for their potent antioxidant properties, contributing to the overall antioxidant capacity of the plant. The relationship between phenolic content and the results of the antioxidant tests (DPPH, FRAP, and ABTS) is visually demonstrated in Figure 7, highlighting the apparent correlation between phenols and antioxidant performance.
5. Conclusions
The application of biostimulants T1, T2, and T3 significantly increased the biomass of DS onions, while it had no significant impact on FS onions. Furthermore, DS onions consistently displayed higher levels of phenols, flavonoids, and total antioxidants compared to FS onions. Notably, only the application of the T1 biostimulant led to a significant increase in phenol content and antioxidant activity in DS onions, as evidenced by DPPH, FRAP, and ABTS assays. In the case of FS onions, all applied biostimulants resulted in a significant decrease in antioxidant activity. To maximize the benefits of DS onions, we strongly recommend the use of biostimulant T1, while for FS onions, the application of biostimulants is discouraged due to their limited impact on biomass and antioxidant status.
Author Contributions
Conceptualization, Đ.V.; methodology, Đ.V., I.M., A.T.H. and Ž.I.; software, Đ.V.; validation, I.M., A.T.H., D.Ž. and B.A.; formal analysis, Đ.V.; investigation, B.A., A.M. and Z.Š.; resources, Đ.V., V.S. and Ž.I.; data curation, Đ.V.; writing—original draft preparation, Đ.V.; writing—review and editing, Đ.V., I.M., A.T.H., A.M. and Z.Š.; visualization, Đ.V., D.Ž. and B.A.; supervision, V.S. and Ž.I.; funding acquisition, D.Ž., A.M., Z.Š., V.S. and Ž.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We sincerely thank the technical and logistical support to the family farm Đukić from Gospođinci, Hoya V.S. company, from Subotica, and PrincipEco from Belgrade. I would also like to express my appreciation to Ljiljana Nešović (Faculty of Agriculture) and Ljubinka Kuvalja (Faculty of Technology) for their invaluable assistance in conducting the analyses in the laboratory. Furthermore, I would like to thank all the Department of Field and Vegetable Crops (Faculty of Agriculture) employees for their understanding during the sample preparation despite the pungent odor of the onions. The support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (contract No. 451-03-47/2023-01/200117, No. 451-03-47/2023-01/200134, and No. 451-03-1524/2023-04/17) is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Helgi Library. Available online: https://helgilibrary.com/indicators/onion-consumption-per-capita/ (accessed on 10 October 2022).
- Sagar, A.N.; Pareek, S.; Benkeblia, N.; Xiao, J. Onion (Allium cepa L.) bioactives: Chemistry, pharmacotherapeutic functions, and industrial applications. Food Front. 2022, 3, 380–412. [Google Scholar] [CrossRef]
- Ortega-García, G.J.; Montes-Belmont, R.; Rodríguez-Monroy, M.; Ramírez-Trujillo, A.J.; Suárez-Rodríguez, R.; Sepúlveda-Jiménez, G. Effect of Trichoderma asperellum applications and mineral fertilization on growth promotion and the content of phenolic compounds and flavonoids in onions. Sci. Hortic. 2015, 195, 8–16. [Google Scholar] [CrossRef]
- Gemin, L.G.; Mógor, Á.F.; De Oliveira Amatussi, J.; Mógor, G. Microalgae associated to humic acid as a novel biostimulant improving onion growth and yield. Sci. Hortic. 2019, 256, 108560. [Google Scholar] [CrossRef]
- Abbas, M.; Anwar, J.; Zafar-Ul-Hye, M.; Khan, R.I.; Saleem, M.; Rahi, A.A.; Danish, S.; Datta, R. Effect of seaweed extract on productivity and quality attributes of four onion cultivars. Horticulturae 2020, 6, 28. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 7 November 2023).
- Rouphael, Y.; Spíchal, L.S.; Panzarová, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, A.G. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulatory activities of Ascophyllum nodosum extract in tomato and sweet pepper crops in a tropical environment. PLoS ONE 2019, 14, e0216710. [Google Scholar] [CrossRef] [PubMed]
- Francke, A.; Majkowska-Gadomska, J.; Kaliniewicz, Z.; Jadwisieńczak, K. No effect of biostimulants on the growth, yield and nutritional value of shallots grown for bunch harvest. Agronomy 2022, 12, 1156. [Google Scholar] [CrossRef]
- Kocira, S.; Szparaga, A.; Hara, P.; Treder, K.; Findura, P.; Bartoš, P.; Filip, M. Biochemical and economical effect of application biostimulants containing seaweed extracts and amino acids as an element of agroecological management of bean cultivation. Sci. Rep. 2020, 10, 17759. [Google Scholar] [CrossRef] [PubMed]
- Szczepanek, M.; Pobereżny, J.; Wszelaczyńska, E.; Gościnna, K. Effect of biostimulants and storage on discoloration potential of carrot. Agronomy 2020, 10, 1894. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and antioxidant responses of lettuce (Lactuca sativa L.) to arbuscular mycorrhiza inoculation and seaweed extract foliar application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Canellas, P.L.; Olivares, L.F.; Aguiar, O.N.; Jones, L.J.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Akimbekov, N.; Qiao, X.; Digel, I.; Abdieva, G.; Ualieva, P.; Zhubanova, A. The effect of leonardite-derived amendments on soil microbiome structure and potato yield. Agriculture 2020, 10, 147. [Google Scholar] [CrossRef]
- Taha, A.A.; Omar, M.M.; Ghazy, A.M. Effect of humic and fulvic acids on growth and yield of lettuce plant. J. Soil Sci. and Agric. Eng. 2016, 7, 517–522. [Google Scholar] [CrossRef]
- Harman, G.E. Myths and dogmas of biocontrol. Changes in perceptions derived from research on Trichoderma harzianum T-22. Plant Dis. 2007, 84, 377–393. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Metwally, A.R. Arbuscular mycorrhizal fungi and Trichoderma viride cooperative effect on biochemical, mineral content, and protein pattern of onion plants. J. Basic Microbiol. 2020, 60, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Vukelić, D.I.; Prokić, T.L.; Racić, M.G.; Pešić, B.M.; Bojović, M.M.; Sierka, M.E.; Kalaji, M.H.; Panković, M.D. Effects of Trichoderma harzianum on photosynthetic characteristics and fruit quality of tomato plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef] [PubMed]
- Davis Instruments. Available online: https://www.davisinstruments.com/pages/vantage-pro2 (accessed on 1 November 2023).
- Vojnović, Đ.; Maksimović, I.; Tepić Horecki, A.; Karadžić Banjac, M.; Kovačević, S.; Daničić, T.; Podunavac-Kuzmanović, S.; Ilin, Ž. Onion (Allium cepa L.) yield and quality depending on biostimulants and nitrogen fertilization-a chemometric perspective. Processes 2023, 11, 684. [Google Scholar] [CrossRef]
- Regulation on the Quality of Agricultural Plant Seeds. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/slsfrj/drugidrzavniorganiorganizacije/pravilnik/1987/47/1/reg (accessed on 4 November 2023).
- Ilin, Ž. Effect Nitrogen on Yield and Quality of Onion Seed. Master’s Thesis, University of Novi Sad, Faculty of Agriculture, Novi Sad, Serbia, 1990. [Google Scholar]
- Žunić, D.; Sabadoš, V.; Vojnović, Đ.; Maksimović, I.; Ilin, D.; Tepić Horecki, A.; Ilin, Ž. Potato (Solanum tuberosum L.) cultivar yield and quality affected by irrigation and fertilization-from field to chip bag. Horticulturae 2023, 9, 1153. [Google Scholar] [CrossRef]
- Regulation on Food Analysis “Official Gazette SFRY 29/83”. Available online: http://demo.paragraf.rs/demo/combined/Old/t/t2004_09/t09_0137.htm (accessed on 29 October 2023).
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Statistica “TIBCO”. Available online: https://docs.tibco.com/pub/stat/14.0.1/doc/html/UsersGuide/Default.htm#user-guide/experimental-design-a.htm (accessed on 29 October 2023).
- Excel “Microsoft”. Available online: https://learn.microsoft.com/en-us/lifecycle/products/microsoft-office-excel-2007 (accessed on 15 October 2023).
- Mógor, A.F.; de Oliveira Amatussi, J.; Mógor, G.; de Lara, G.B. Bioactivity of cyanobacterial biomass related to amino acids induces growth and metabolic changes on seedlings and yield gains of organic red beet. Am. J. Plant Sci. 2018, 9, 966–978. [Google Scholar] [CrossRef]
- Zandonadi, D.B.; Canellas, L.P.; Façanha, A.R. Indolacetic and humic acids induce lateral root development through a concentrated plasmalemma and tonoplast H+ pumps activation. Planta 2007, 225, 1583–1595. [Google Scholar] [CrossRef] [PubMed]
- Visconti, D.; Fiorentino, N.; Cozzolino, E.; Woo, S.L.; Fagnano, M.; Rouphael, Y. Can Trichoderma-based biostimulants optimize N use efficiency and stimulate growth of leafy vegetables in greenhouse intensive cropping systems? Agronomy 2020, 10, 121. [Google Scholar] [CrossRef]
- Carillo, P.; Woo, L.S.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, M.G.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-pentyl-l-pyrone and plant biopolymer formulations modulate plant metabolism and fruit quality of plum tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Jannin, L.; Arkoun, M.; Etienne, P.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Brassica napus growth is promoted by Ascophyllum nodosum (L.) Le Jol. seaweed extract: Microarray analysis and physiological characterization of N, C, and S Metabolisms. J. Plant Growth Regul. 2013, 32, 31–52. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; El Arroussi, H.; Khouloud, M.; Dhiba, D.; Meftah Kadmiri, I.; Bamouh, A. Polysaccharides extracted from Moroccan seaweed: A promising source of tomato plant growth promoters. J. Appl. Phycol. 2018, 30, 2953–2962. [Google Scholar] [CrossRef]
- Hafez, E.; Geries, L. Effect of nitrogen fertilization and biostimulative compounds on onion productivity. Certec. Agron. Mold. 2018, 1, 75–90. [Google Scholar] [CrossRef][Green Version]
- Ali, O.; Ramsubhag, A.; Ramnarine, S.; Jayaraman, J. Transcriptomic changes induced by applications of a commercial extract of Ascophyllum nodosum on tomato plants. Sci. Rep. 2022, 12, 8042. [Google Scholar] [CrossRef]
- Jun, S.Y.; Sattler, S.A.; Cortez, G.S.; Vermerris, W.; Sattler, S.E.; Kang, C. Biochemical and structural analysis of substrate specificity of a phenylalanine ammonia-lyase. Plant Physiol. 2018, 176, 1452–1468. [Google Scholar] [CrossRef]
- Aminifard, H.M.; Aroiee, H.; Azizi, M.; Nemati, H.; Hawa, Z.; Jaafar, E. Effect of humic acid on antioxidant activities and fruit quality of hot pepper (Capsicum annuum L.). J. Herbs Spices Med. Plants 2021, 18, 360–369. [Google Scholar] [CrossRef]
- Akladious, A.S.; Mohamed, I.H. Ameliorative effects of calcium nitrate and humic acid on the growth, yield component and biochemical attribute of pepper (Capsicum annuum) plants grown under salt stress. Sci. Hortic. 2018, 236, 244–250. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Assefa, A.D.; Ko, E.Y.; Lee, E.T.; Park, S.W. Quantitative analysis of flavonoids, sugars, phenylalanine and tryptophan in onion scales during storage under ambient conditions. J. Food Sci. Technol. 2015, 52, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Jithesh, N.M.; Shukla, S.P.; Kant, P.; Joshi, J.; Critchley, T.A.; Prithiviraj, B. Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 2018, 38, 463–478. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Schiavon, M.; Pizzeghello, D.; Muscolo, A.; Vaccaro, S.; Francioso, O.; Nardi, S. High molecular size humic substances enhance phenylpropanoid metabolism in maize (Zea mays L.). J. Chem. Ecol. 2010, 36, 662–669. [Google Scholar] [CrossRef]
- Altintas, S.; Bal, U. Effects of the commercial product based on Trichoderma harzianum on plant, bulb and yield characteristics of onion. Sci. Hortic. 2008, 116, 219–222. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Ali, M.; Al-Rashdan, A.; Ahmed, N. Onion (Allium cepa L.) is potentially a good source of important antioxidants. J. Food Sci. Technol. 2019, 56, 1811–1819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).