Prediction of Growth and Quality of Chinese Cabbage Seedlings Cultivated in Different Plug Cell Sizes via Analysis of Image Data Using Multispectral Camera
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultivations
2.2. Growth Measurements
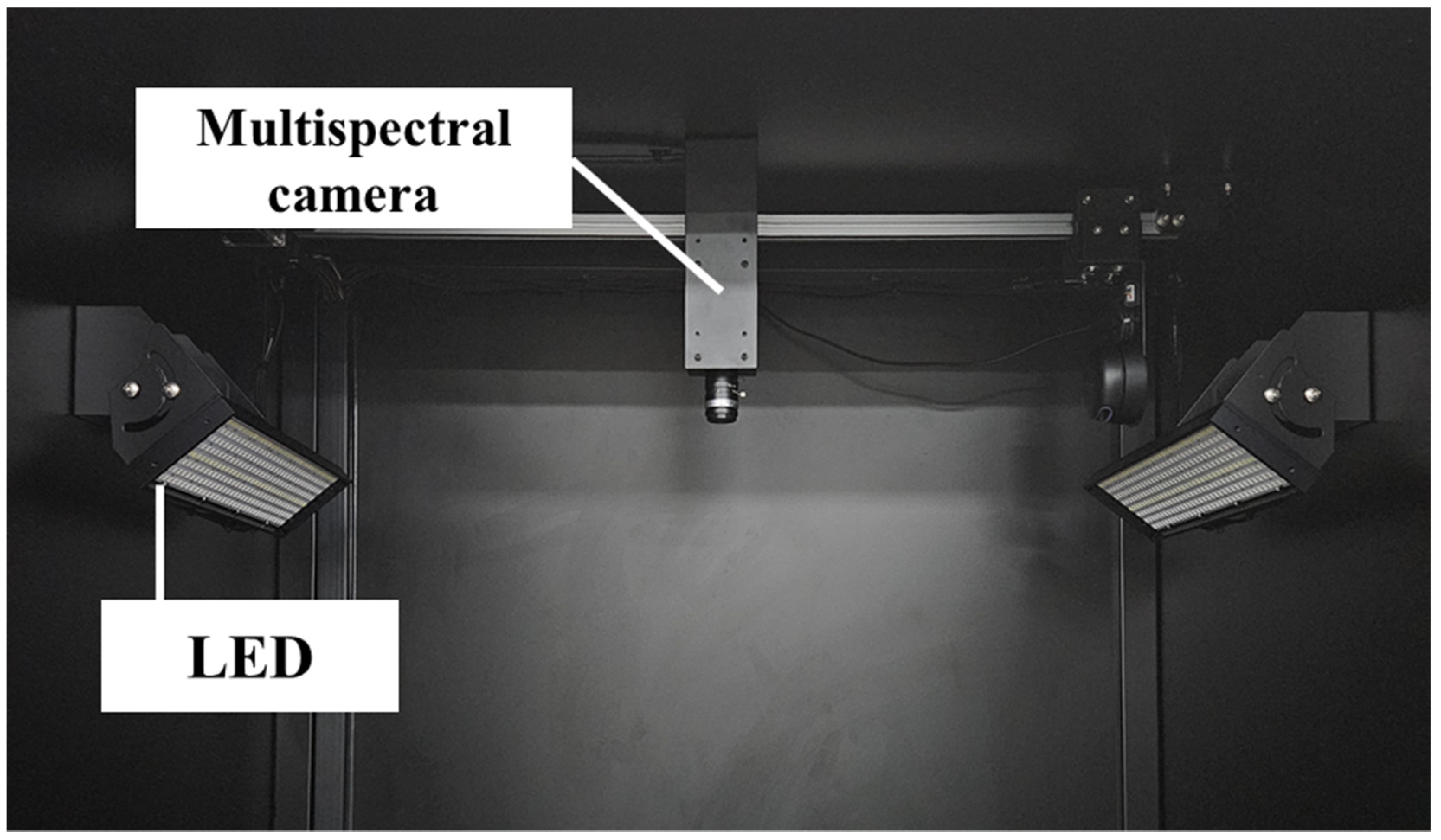
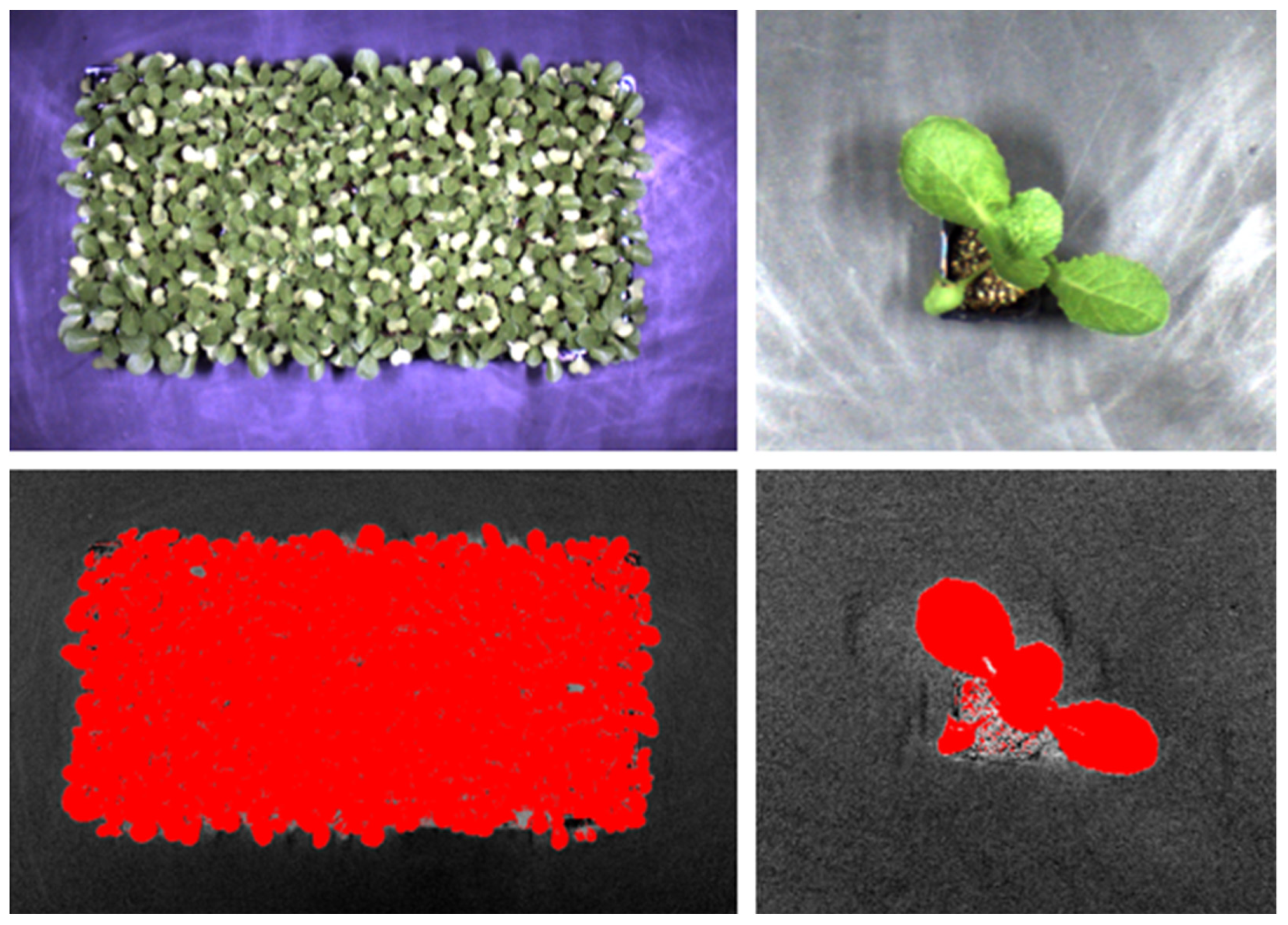
2.3. Image-Based Measurements and Analysis
2.4. Image-Based Measurements and Analysis
3. Results and Discussion
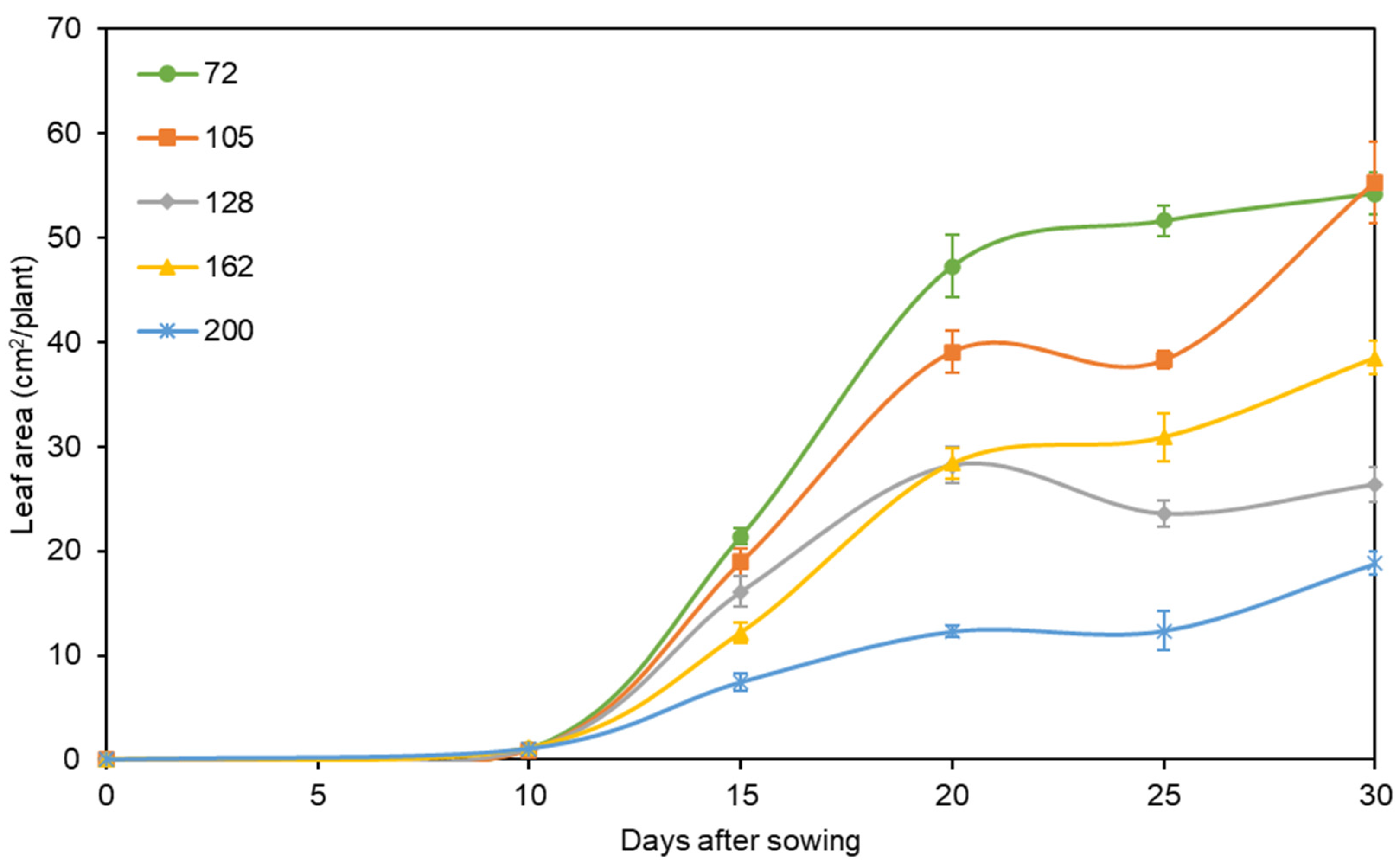
3.1. Seedling Growth in Chinese Cabbage as Affected by Cell Size of Plug Tray
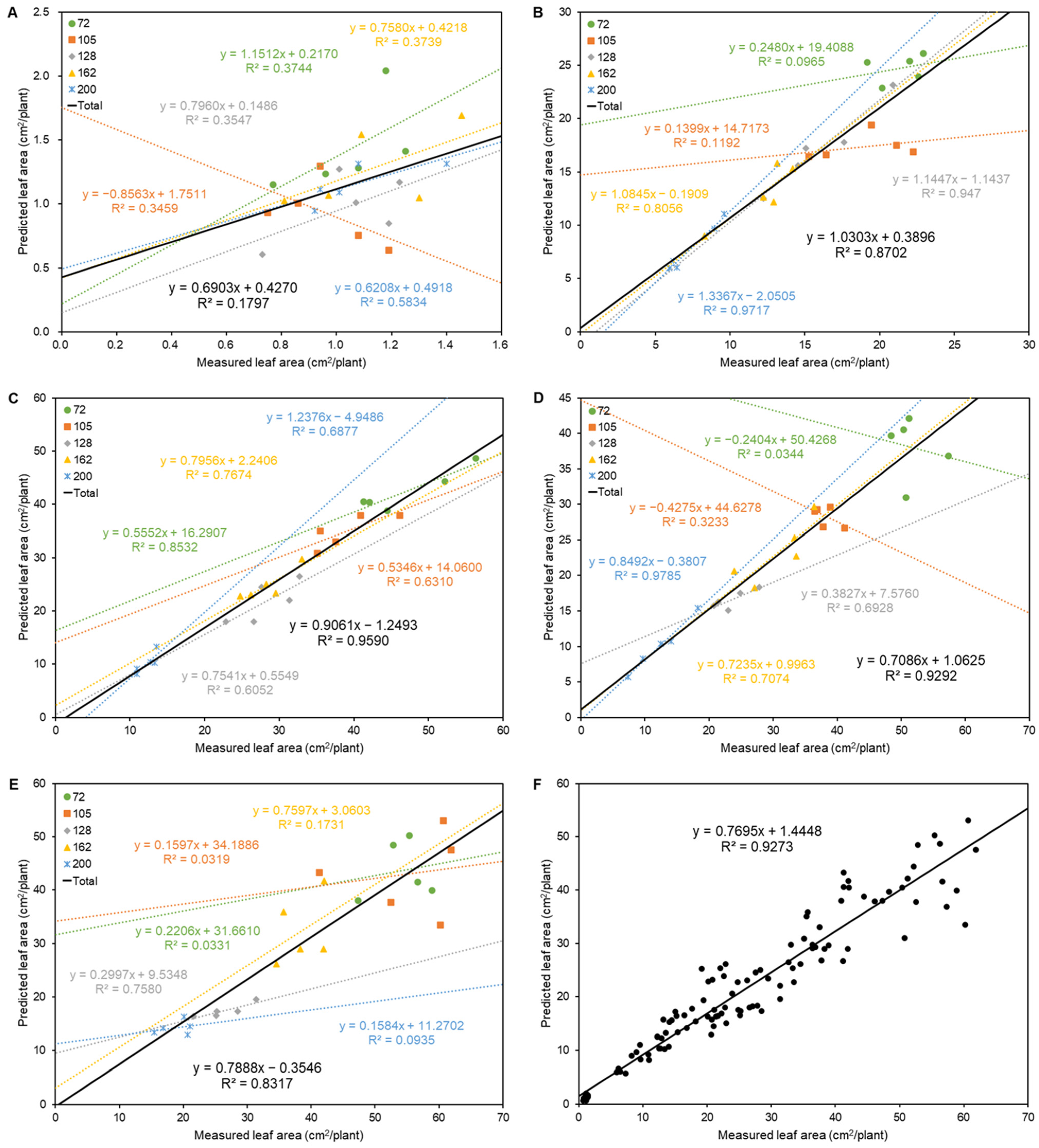
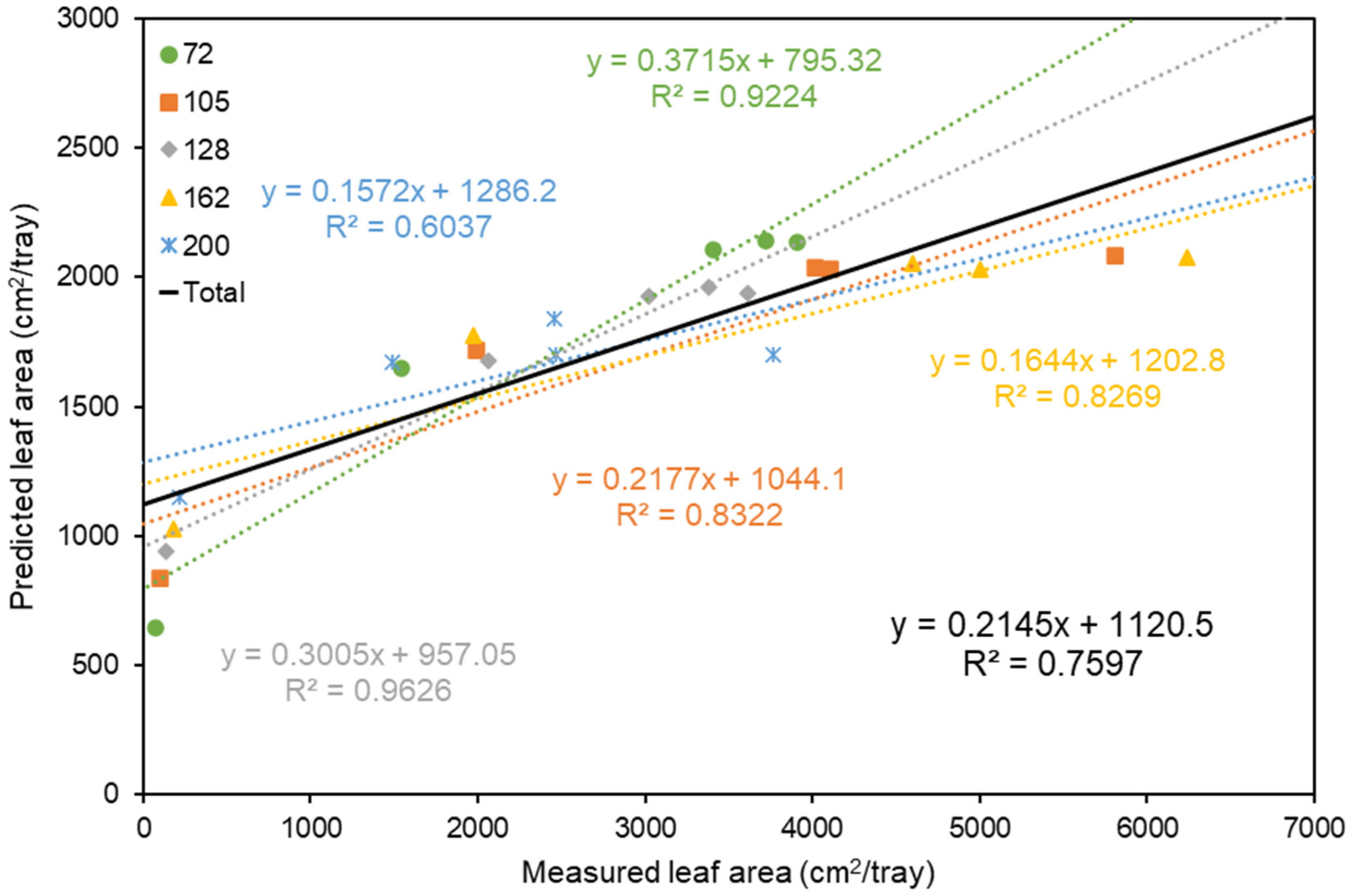
3.2. Correlation Analysis between Measured and Predicted Leaf Area
3.3. Correlation Analysis between Vegetation Indices and Growth Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Vegetables Production Performance in 2021. Available online: https://data.mafra.go.kr/opendata/data/indexOpenDataDetail.do?data_id=20220216000000001975 (accessed on 27 October 2023).
- Ministry of Agriculture, Food and Rural Affairs (MAFRA). Survey on the Consumption of Raw Material in the Food Industry in 2021. Available online: https://data.mafra.go.kr/opendata/data/indexOpenDataDetail.do?data_id=20141015000000000209 (accessed on 27 October 2023).
- Jeong, B.R.; Hwang, S.J.; Kang, N.J. Plug Seedling Production, 1st ed.; Gyeongsang National University Press Department: Jinju, Republic of Korea, 2016; pp. 13–22. [Google Scholar]
- Park, G.H.; Park, H.T.; Han, H.S. A Study on the Current State and Development Strategies of Raising Seedlings Industry, 1st ed.; Korea Rural Economic Institute: Naju, Republic of Korea, 2011; pp. 15–87. [Google Scholar]
- Yeoung, Y.R.; Jeon, J.Y.; Shim, S.Y. Characteristics of root development and seedling quality during pepper seedling growth in copper coated plug trays. J. Korean Soc. Hortic. Sci. 2002, 43, 151–154. [Google Scholar]
- Korea Agro-Fisheries & Food Trade Corporation (aT). Distribution of Agricultural Products in 2021. Available online: https://www.kamis.or.kr/customer/circulation/domestic/analysis.do (accessed on 27 October 2023).
- Kim, S.E.; Lee, M.H.; Ahn, B.J.; Kim, Y.S. Effects of Spacing and Plug Cell Size on Seedling Quality and Yield and Qualities of Tomatoes. Prot. Hortic. Plant Fact. 2013, 22, 256–261. [Google Scholar] [CrossRef]
- Oh, H.J.; Park, Y.G.; Park, J.E.; Jeong, B.R. Effect of Cell Size on Growth and Development of Plug Seedlings of Three Indigenous Medicinal Plants. Prot. Hortic. Plant Fact. 2014, 23, 71–76. [Google Scholar] [CrossRef]
- Jeong, H.W.; Kim, H.M.; Lee, H.R.; Kim, H.M.; Hwang, S.J. Growth of Astragalus membranaceus during Nursery Period as Affected by Different Plug Tray Cell Size, Number of Seeds per Cell, Irrigation Interval, and EC Level of Nutrient Solution. HortScience Techonol. 2020, 38, 210–217. [Google Scholar] [CrossRef]
- Maynard, E.T.; Vavrina, C.S.; Scott, W.D. Containerized muskmelon transplants: Cell volume effects on pretransplant development and subsequent yield. HortScience 1996, 31, 58–61. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, P.; Zhang, Q.A.; Yan, C.; Yu, F.; Yi, J.; Fang, L. Effect of different levels of nitrogen, phosphorus, and potassium on root activity and chlorophyll content in leaves of Brassica oleracea seedlings grown in vegetable nursery substrate. Hortic. Environ. Biotechnol. 2017, 58, 5–11. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of different NO3−: NH4+ ratios on the photosynthesis and ultrastructure of lettuce seedlings. Hortic. Environ. Biotechnol. 2020, 61, 459–472. [Google Scholar] [CrossRef]
- Khaeim, H.; Kende, Z.; Balla, I.; Gyuricza, C.; Eser, A.; Tarnawa, Á. The Effect of Temperature and Water Stresses on Seed Germination and Seedling Growth of Wheat (Triticum aestivum L.). Sustainability 2022, 14, 3887. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.; Menexes, G.; Dangitsis, C.; Kintzonidis, D. Assessing quantitative criteria for characterization of quality categories for grafted watermelon seedlings. Horticulturae 2019, 5, 16. [Google Scholar] [CrossRef]
- Kim, H.C.; Cho, Y.H.; Ku, Y.G.; Bae, J.H. Seedling qualities of hot pepper according to seedling growth periods and growth and yield after planting. Hortic. Sci. Technol. 2015, 33, 839–844. [Google Scholar] [CrossRef]
- Michelon, N.; Pennisi, G.; Myint, N.O.; Orsini, F.; Gianquinto, G. Optimization of Substrate and Nutrient Solution Strength for Lettuce and Chinese Cabbage Seedling Production in the Semi-Arid Environment of Central Myanmar. Horticulturae 2021, 7, 64. [Google Scholar] [CrossRef]
- Min, M.; Lee, W.S.; Kim, Y.H.; Bucklin, R.A. Nondestructive detection of nitrogen in Chinese cabbage leaves using VIS–NIR spectroscopy. HortScience 2006, 41, 62–166. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.; Lu, X.; Ma, F.; Chen, W.; Yang, J.; Zheng, L. Application of multispectral imaging to determine quality attributes and ripeness stage in strawberry fruit. PLoS ONE 2014, 9, e87818. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, D.; Cai, Z.; Wang, L.; Wang, J.; Sun, L.; Fan, X.; Shen, X.; Zhao, J. Spectral technology and multispectral imaging for estimating the photosynthetic pigments and SPAD of the Chinese cabbage based on machine learning. Comput. Electron. Agric. 2022, 195, 106814. [Google Scholar] [CrossRef]
- Ihuoma, S.O.; Madramootoo, C.A. Sensitivity of spectral vegetation indices for monitoring water stress in tomato plants. Comput. Electron. Agric. 2019, 163, 104860. [Google Scholar] [CrossRef]
- Rubo, S.; Zinkernagel, J. Exploring hyperspectral reflectance indices for the estimation of water and nitrogen status of spinach. Biosyst. Eng. 2022, 214, 58–71. [Google Scholar] [CrossRef]
- Lopez-Fornieles, E.; Brunel, G.; Rancon, F.; Gaci, B.; Metz, M.; Devaux, N.; Taylor, J.; Tisseyre, B.; Roger, J.M. Potential of Multiway PLS (N-PLS) regression method to analyse time-series of multispectral images: A case study in agriculture. Remote Sens. 2022, 14, 216. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, S.D. Assessment of spinach seedling health status and chlorophyll content by multivariate data analysis and multiple linear regression of leaf image features. Comput. Electron. Agric. 2018, 152, 281–289. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.L.; Lenz-Wiedemann, V.I.S.; Rascher, U.; et al. Satellite remote sensing-based in-season diagnosis of rice nitrogen status in Northeast China. Remote Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahan, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obs. Geoinf. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Bian, C.; Shi, H.; Wu, S.; Zhang, K.; Wei, M.; Zhao, Y.; Sun, Y.; Zhuang, H.; Zhang, X.; Chen, S. Prediction of field-scale wheat yield using machine learning method and multi-spectral UAV data. Remote Sens. 2022, 14, 1474. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Niu, Y.; Han, W. Mapping maize water stress based on UAV multispectral remote sensing. Remote Sens. 2019, 11, 605. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Li, D.; Zhou, X.; Yao, X.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of RGB, color-infrared and multispectral images acquired from unmanned aerial systems for the estimation of nitrogen accumulation in rice. Remote Sens. 2018, 10, 824. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agron. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Padilla, F.M.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Threshold values of canopy reflectance indices and chlorophyll meter readings for optimal nitrogen nutrition of tomato. Ann. Appl. Biol. 2015, 166, 271–285. [Google Scholar] [CrossRef]
- Sun, G.; Wang, X.; Sun, Y.; Ding, Y.; Lu, W. Measurement method based on multispectral three-dimensional imaging for the chlorophyll contents of greenhouse tomato plants. Sensors 2019, 19, 3345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, X.; Zhang, L.; Xu, S.; Li, H.; Xia, X.; Dai, L.; Xu, L.; Yu, J.; Liu, X. A method of high throughput monitoring crop physiology using chlorophyll fluorescence and multispectral imaging. Front. Plant Sci. 2018, 9, 407. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W. Monitoring vegetation systems in the Great Plains with ERTS. NASA Spec. Publ. 1974, 351, 309–317. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between leaf chlorophyll content and spectral reflectance and algorithms for non-destructive chlorophyll assessment in higher plant leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Ciganda, V.; Rundquist, D.C.; Arkebauer, T.J. Remote estimation of canopy chlorophyll content in crops. Geophys. Res. Lett. 2005, 32, 271–282. [Google Scholar] [CrossRef]
- Broge, N.H.; Leblanc, E. Comparing prediction power and stability of broadband and hyperspectral vegetation indices for estimation of green leaf area index and canopy chlorophyll density. Remote Sens. Environ. 2000, 76, 156–172. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Roujean, J.L.; Breon, F.M. Estimating PAR absorbed by vegetation from bidirectional reflectance measurements. Remote Sens. Environ. 1995, 51, 375–384. [Google Scholar] [CrossRef]
- Marsh, D.B.; Paul, K.B. Influence of container type and cell size on cabbage transplant development and field performance. HortScience 1988, 23, 310–311. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, Y.G.; Jung, B.R. Seedling Quality, and Early Growth and Fruit Productivity after Transplanting of Squash as Affected by Plug Cell Size and Seedling Raising Period. J. Bio-Environ. Control 2019, 28, 185–196. [Google Scholar] [CrossRef]
- Singh, B.; Kumar, S.; Elangovan, A.; Vasht, D.; Arya, S.; Duc, N.T.; Swami, P.; Pawar, G.S.; Raju, D.; Krishna, H.; et al. Phenomics based prediction of plant biomass and leaf area in wheat using machine learning approaches. Front. Plant Sci. 2023, 14, 1214801. [Google Scholar] [CrossRef] [PubMed]
- Nyakwende, E.; Paull, C.J.; Atherton, J.G. Non-destructive determination of leaf area in tomato plants using image processing. J. Hortic. Sci. 1997, 72, 255–262. [Google Scholar] [CrossRef]
- Mohammadi, V.; Minaei, S.; Mahdavian, A.R.; Khoshtaghaza, M.H.; Gouton, P. Estimation of Leaf Area in Bell Pepper Plant using Image Processing techniques and Artificial Neural Networks. In Proceedings of the 2021 IEEE International Conference on Signal and Image Processing Applications (IEEE ICSIPA 2021), Kuala Terengganu, Malaysia, 13–15 September 2021; Institute of Electrical and Electronic Engineers (IEEE): Piscataway, NJ, USA, 2021; pp. 173–178. [Google Scholar]
- Gang, M.S.; Kim, H.J.; Kim, D.W. Estimation of Greenhouse Lettuce Growth Indices Based on a Two-Stage CNN Using RGB-D Images. Sensors 2022, 22, 5499. [Google Scholar] [CrossRef]
- Tong, J.H.; Li, J.B.; Jiang, H.Y. Machine vision techniques for the evaluation of seedling quality based on leaf area. Biosyst. Eng. 2013, 115, 369–379. [Google Scholar] [CrossRef]
- Kim, C.H.; van Iersel, M.W. Morphological and Physiological Screening to Predict Lettuce Biomass Production in Controlled Environment Agriculture. Remote Sens. 2022, 14, 316. [Google Scholar] [CrossRef]
- De Souza, R.; Grasso, R.; Peña-Fleitas, M.T.; Gallardo, M.; Thompson, R.B.; Padilla, F.M. Effect of cultivar on chlorophyll meter and canopy reflectance measurements in cucumber. Sensors 2020, 20, 509. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, L.; Niu, Z.; Gao, S.; Wu, M. Nondestructive estimation of canopy chlorophyll content using Hyperion and Landsat/TM images. Int. J. Remote Sens. 2010, 31, 2159–2167. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesus, J.; Araus, J.L.; Royo, C. Spectral vegetation indices as nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Carlson, T.N.; Ripley, D.A. On the relation between NDVI, fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997, 62, 241–252. [Google Scholar] [CrossRef]
- Curran, P.J. Multispectral remote sensing for the estimation of green leaf area index. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1983, 309, 257–270. [Google Scholar] [CrossRef]
- Sellers, P.J. Canopy reflectance, photosynthesis and transpiration. Int. J. Remote Sens. 1985, 6, 1335–1372. [Google Scholar] [CrossRef]
- Solari, F.; Shanahan, J.; Ferguson, R.; Schepers, J.; Gitelson, A. Active sensor reflectance measurements of corn nitrogen status and yield potential. Agron. J. 2008, 100, 571–579. [Google Scholar] [CrossRef]
- Tang, Z.; Guo, J.; Xiang, Y.; Lu, X.; Wang, Q.; Wang, H.; Cheng, M.; Wang, H.; Wang, X.; An, J.; et al. Estimation of Leaf Area Index and Above-Ground Biomass of Winter Wheat Based on Optimal Spectral Index. Agronomy 2022, 12, 1729. [Google Scholar] [CrossRef]
- Li, J.; Shi, Y.; Veeranampalayam-Sivakumar, A.N.; Schachtman, D.P. Elucidating sorghum biomass, nitrogen and chlorophyll contents with spectral and morphological traits derived from unmanned aircraft system. Front. Plant Sci. 2018, 9, 1406. [Google Scholar] [CrossRef]
- Elvanidi, A.; Katsoulas, N.; Bartzanas, T.; Ferentinos, K.P.; Kittas, C. Crop water status assessment in controlled environment using crop reflectance and temperature measurements. Precis. Agric. 2017, 18, 332–349. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Pattey, E.; Zarco-Tejada, P.J.; Strachan, I.B. Hyperspectral vegetation indices and novel algorithms for predicting green LAI of crop canopies: Modeling and validation in the context of precision agriculture. Remote Sens. Environ. 2004, 90, 337–352. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Badagliacca, G.; Messina, G.; Praticò, S.; Lo Presti, E.; Preiti, G.; Monti, M.; Modica, G. Multispectral Vegetation Indices and Machine Learning Approaches for Durum Wheat (Triticum durum Desf.) Yield Prediction across Different Varieties. AgriEngineering 2023, 5, 2032–2048. [Google Scholar] [CrossRef]
| Index | Equation | Reference |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | (RNIR − RRED)/(RNIR + RRED) | [33] |
| Green Normalized Difference Vegetation Index (GNDVI) | (RNIR − RGREEN)/(RNIR + RGREEN) | [34] |
| Green Chlorophyll Index (CIgreen) | (RNIR/RGREEN) − 1 | [35,36] |
| Triangle Vegetation Index (TVI) | 0.5 × (120 × (RNIR − RGREEN) − 200 × (RRED − RGREEN)) | [37] |
| Modified Red Edge Normalized Difference Vegetation Index (mrNDVI) | (R750 − R650)/(R750 + R650 − 2 × R450) | [38] |
| Renormalized Difference Vegetation Index (RDVI) | (RNIR − RRED)/(RNIR + RRED)1/2 | [39] |
| DAS z | Cells of Plug Tray | Plant Height (cm) | Number of Leaves (/Plant) | SPAD Value | Shoot Fresh Weight (g/Plant) | Shoot Dry Weight (g/Plant) |
|---|---|---|---|---|---|---|
| 10 | 72 cell | 2.50b y | 1.8a | 22.20a | 0.251a | 0.016a |
| 105 cell | 2.24b | 1.0b | 24.66a | 0.231ab | 0.016a | |
| 128 cell | 2.22b | 1.2ab | 22.40a | 0.212b | 0.017a | |
| 162 cell | 2.82a | 1.4ab | 22.62a | 0.242ab | 0.015a | |
| 200 cell | 3.06a | 1.2ab | 25.42a | 0.235ab | 0.016a | |
| 15 | 72 cell | 6.26a | 3.2a | 28.32a | 0.916a | 0.068a |
| 105 cell | 5.98a | 3.2a | 28.64a | 0.760b | 0.062ab | |
| 128 cell | 5.54ab | 3.2a | 28.12a | 0.724b | 0.058bc | |
| 162 cell | 5.00bc | 3.0a | 29.08a | 0.559c | 0.051c | |
| 200 cell | 4.40c | 2.6a | 28.08a | 0.404d | 0.035d | |
| 20 | 72 cell | 8.54a | 4.8a | 27.06a | 1.678a | 0.170a |
| 105 cell | 8.12a | 4.2b | 26.10a | 1.562a | 0.168a | |
| 128 cell | 7.24b | 3.8b | 26.86a | 1.189b | 0.125b | |
| 162 cell | 6.76b | 4.2b | 25.64a | 1.167b | 0.118b | |
| 200 cell | 5.28c | 3.0c | 27.90a | 0.631c | 0.081c | |
| 25 | 72 cell | 8.30a | 5.4a | 28.40a | 1.879a | 0.271a |
| 105 cell | 7.42b | 5.2ab | 26.62a | 1.397b | 0.197b | |
| 128 cell | 6.94b | 4.6b | 26.12a | 0.960c | 0.139c | |
| 162 cell | 6.04c | 4.8ab | 26.78a | 1.221b | 0.177b | |
| 200 cell | 5.30d | 3.6c | 27.24a | 0.561d | 0.088d | |
| 30 | 72 cell | 7.24b | 6.0a | 27.76a | 1.987a | 0.368a |
| 105 cell | 8.16a | 6.0a | 27.90a | 2.141a | 0.348a | |
| 128 cell | 6.32c | 4.8bc | 26.02a | 1.025c | 0.159c | |
| 162 cell | 7.28b | 5.4ab | 28.24a | 1.572b | 0.246b | |
| 200 cell | 5.64c | 4.2c | 27.98a | 0.694d | 0.127c |
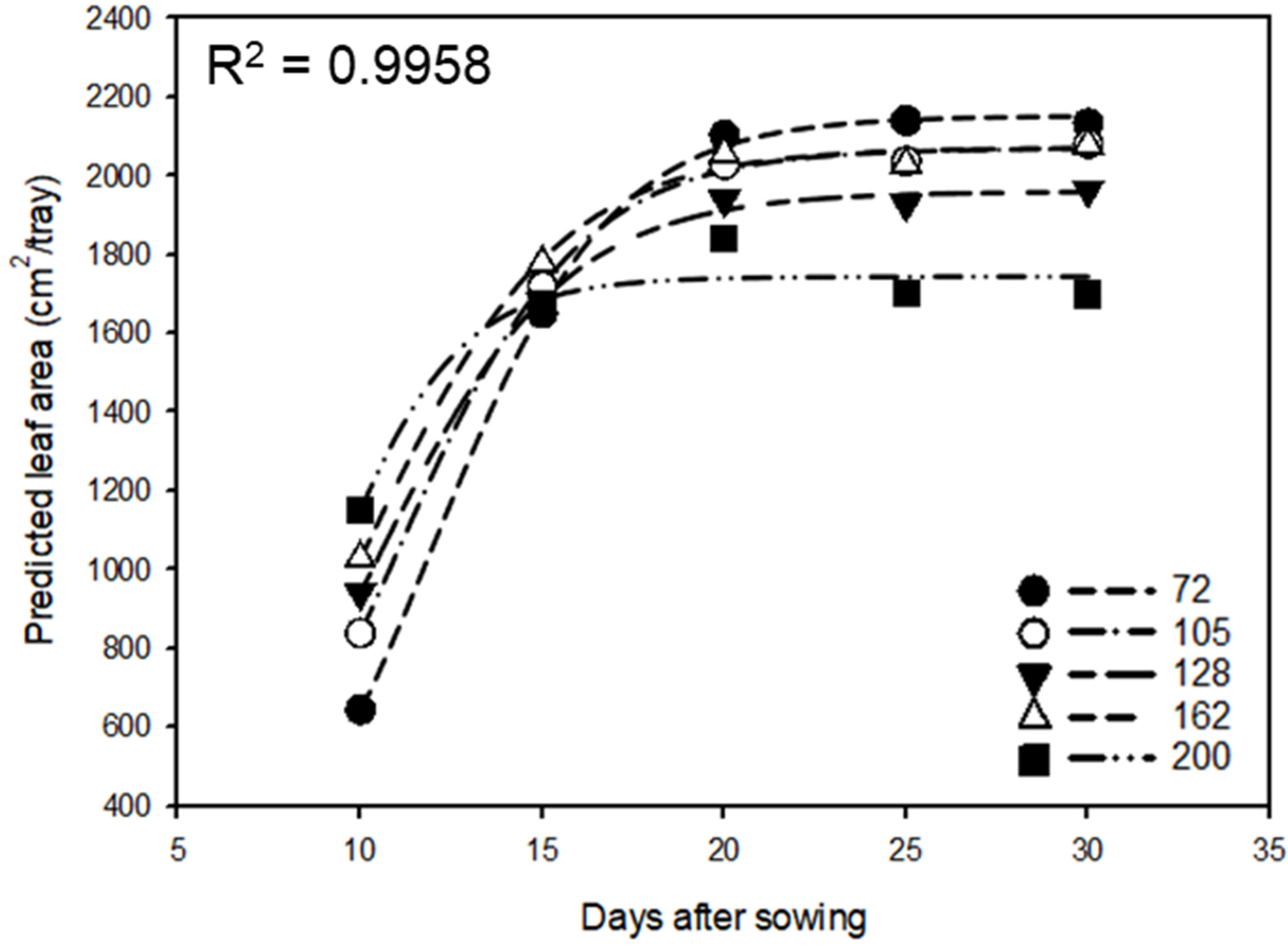
| Treatment | Equation | R2 |
|---|---|---|
| 72 cell | PLA = 2152.58/(1 + e−(x−12.07)/2.41) | 0.999 |
| 105 cell | PLA = 2069.76/(1 + e−(x−10.97)/2.51) | 0.999 |
| 128 cell | PLA = 1960.13/(1 + e−(x−10.22)/2.63) | 0.998 |
| 162 cell | PLA = 2071.66/(1 + e−(x−10.05)/2.68) | 0.997 |
| 200 cell | PLA = 1744.24/(1 + e−(x−8.79)/1.84) | 0.952 |
| NDVI | GNDVI | CIgreen | TVI | mrNDVI | RDVI | |
|---|---|---|---|---|---|---|
| LAI | 0.2753 | 0.2652 | 0.2654 | 0.1619 | 0.2775 | 0.2418 |
| SPAD value | 0.0237 | 0.0200 | 0.0215 | 0.2380 | 0.1009 | 0.1492 |
| Shoot dry weight | 0.5674 | 0.6078 | 0.6156 | 0.3480 | 0.6068 | 0.5221 |
| NDVI | GNDVI | CIgreen | TVI | mrNDVI | RDVI | |
|---|---|---|---|---|---|---|
| LAI | 0.0339 | 0.0315 | 0.0310 | 0.1164 | 0.2152 | 0.0913 |
| SPAD value | 0.0878 | 0.1106 | 0.1101 | 0.0829 | 0.2424 | 0.1093 |
| Shoot dry weight | 0.4832 | 0.4773 | 0.4706 | 0.6414 | 0.6095 | 0.7018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, S.; Hong, I.; Kwack, Y. Prediction of Growth and Quality of Chinese Cabbage Seedlings Cultivated in Different Plug Cell Sizes via Analysis of Image Data Using Multispectral Camera. Horticulturae 2023, 9, 1288. https://doi.org/10.3390/horticulturae9121288
Ban S, Hong I, Kwack Y. Prediction of Growth and Quality of Chinese Cabbage Seedlings Cultivated in Different Plug Cell Sizes via Analysis of Image Data Using Multispectral Camera. Horticulturae. 2023; 9(12):1288. https://doi.org/10.3390/horticulturae9121288
Chicago/Turabian StyleBan, Sehui, Inseo Hong, and Yurina Kwack. 2023. "Prediction of Growth and Quality of Chinese Cabbage Seedlings Cultivated in Different Plug Cell Sizes via Analysis of Image Data Using Multispectral Camera" Horticulturae 9, no. 12: 1288. https://doi.org/10.3390/horticulturae9121288
APA StyleBan, S., Hong, I., & Kwack, Y. (2023). Prediction of Growth and Quality of Chinese Cabbage Seedlings Cultivated in Different Plug Cell Sizes via Analysis of Image Data Using Multispectral Camera. Horticulturae, 9(12), 1288. https://doi.org/10.3390/horticulturae9121288









