Abstract
To elucidate the physiological mechanisms underlying the impact of exogenous nickel ions (Ni2+) on the adaptability of tomato (Solanum lycopersicum L.) seedling roots to low-nitrogen levels, the cultivar ‘Micro Tom’ was selected as the experimental material and cultivated hydroponically in the cultivation room of the Fujian Agriculture and Forestry University. Two distinct nitrogen concentrations (7.66 and 0.383 mmol·L−1) and two different levels of Ni2+ (0 and 0.1 mg·L−1 of NiSO4·6H2O) were employed as treatments. On the 9th day of cultivation, we measured the root biomass, the concentrations of antioxidant compounds, and the activities of antioxidant enzymes in the tomato seedlings. The study showed that when the nitrogen levels were low, the growth and development of the tomato seedling roots were hindered. This led to a significant increase in the levels of hydrogen peroxide (H2O2), superoxide anion (O2−), and malondialdehyde (MDA), indicating oxidative damage to the roots. Conversely, treatment with Ni2+ induced a notable increase in the activity of antioxidant enzymes in the seedlings and augmented the accumulation of nonenzymatic antioxidants, such as ascorbic acid (ASA) and reduced glutathione (GSH), thereby enhancing the operational efficiency of the ascorbate–glutathione cycle (ASA–GSH). Consequently, this led to substantial reductions in the H2O2 and MDA levels, ultimately mitigating the oxidative damage inflicted on the tomato seedling roots subjected to low-nitrogen stress. In conclusion, exogenous Ni2+ can reduce the peroxidative damage of tomato seedlings by promoting antioxidase activity in tomato seedlings under low-nitrogen stress, improve the tolerance of tomato seedlings to low-nitrogen stress, and maintain the normal growth and development of tomato seedlings.
1. Introduction
Nitrogen (N) is an essential plant nutrient and an important component of proteins, nucleic acids, phospholipids, and certain growth hormones in plants, accounting for 40–50% of the final crop yield [1,2,3]. Because the plant’s demand for N is the highest among all mineral elements, N deficiency will inevitably limit normal plant growth and development [4,5,6]. The application of N fertilizer in agricultural production tends to increase crop yield. Vegetable farmers often use excessive N fertilizers in actual production, applying much more than required for crop growth to improve economic efficiency [7,8]. However, a previous study revealed that excessive nitrogen fertilizer inputs not only did not improve crop yield and quality but also reduced the efficiency of nitrogen fertilizer utilization, which increased the loss of nitrogen fertilizer and caused serious environmental pollution problems [9]. However, a reduction in nitrogen application to a certain level can produce low-nitrogen stress, which can lead to the excessive production of reactive oxygen species (ROS) in plants [10,11]. ROS chiefly include hydrogen peroxide (H2O2), and superoxide anion (O2−), and they are mainly produced in subcellular organelles such as chloroplasts, mitochondria, and the cytosol [12,13]. These ROS are highly reactive and toxic and can cause damage to proteins, lipids, carbohydrates, and DNA [14]. ROS include free radicals and non-free radicals (molecules), which can eventually lead to oxidative stress [15]. These ROS are highly active and toxic and prone to cause damage to proteins, lipids, carbohydrates, and DNA, which ultimately leads to oxidative stress [16]. ROS include both free radical and non-free radical (molecular) forms, and under conditions of adversity stress, plants have very effective enzymatic and nonenzymatic antioxidant defense systems [17], which work synergistically to scavenge excess ROS, control cascade oxidation, and protect plant cells from oxidative damage [18,19]. In recent years, mitigating the damage of low-nitrogen stress on crops by adding exogenous substances has become an effective way to overcome nitrogen deficiency in the environment. Nickel (Ni) is widely acknowledged as an essential micronutrient for plants due to its multifaceted biological roles and functions [18]. When cultivated in nutrient solutions with insufficient nickel levels, plants may exhibit noticeable symptoms associated with nickel deficiency, exemplified by the occurrence of “mouse ears or leaflets” (ME-LL) observed in hickory [19,20]. Moreover, it is worth noting that nickel serves as the sole known activator of urease, an enzyme responsible for the hydrolysis of urea into ammonia and carbon dioxide. This activation mechanism effectively prevents the toxic accumulation of urea [21].
Currently, improving plant adaptability to low-nitrogen environments by inducing physiological and biochemical changes through the application of exogenous substances has become a crucial research direction due to the increasing prominence of fertilizer issues. Previous studies have shown that a particular concentration of Ni2+ can enhance lettuce growth, resulting in improved nitrogen use efficiency and related antioxidant enzyme activities. Additionally, the application of an appropriate amount of Ni2+ externally has been shown to promote the photosynthesis of tomato seedlings, resulting in increased growth and a reduction in the negative effects of low-nitrogen stress. Furthermore, Ni2+ has the ability to regulate the carbon and nitrogen metabolism pathways of tomato roots, thereby mitigating the effects of low-nitrogen levels [22,23,24]. Notably, these investigations have primarily focused on aboveground processes and carbon and nitrogen metabolic pathways. However, the precise physiological mechanisms underlying whether exogenous Ni2+ can alleviate the adverse consequences of low-nitrogen stress on the growth and development of tomato seedlings by modulating the antioxidant system within the root system remain incompletely understood. Given that the root system functions as the primary component responsible for water and nutrient absorption, alterations in plant defense systems occur when nitrogen concentrations decline, consequently impinging upon normal growth and development processes [25]. However, there remains a dearth of research on the physiological mechanism of exogenous nickel ion (Ni2+) promoting low-nitrogen adaptability in tomato seedlings. To address this gap, the present experiment aims to investigate the impacts of Ni2+ on the growth and antioxidant enzyme activities of tomato seedling roots subjected to low-nitrogen stress. Therefore, in this experiment, we used the prescreened Ni2+ concentration in our laboratory. The outcomes of this investigation are expected to provide a solid theoretical foundation for enhancing the low-nitrogen tolerance of tomatoes through the application of exogenous Ni2+ in practical agricultural production.
2. Materials and Methods
2.1. Plant Growth and Sampling
This experiment was conducted in the plant culture facility of the Vegetable Research Institute, located in the Horticulture Science and Technology Building at the Fujian Agriculture and Forestry University (119.23° E, 26.08° N). The selected tomato variety for the experiment was ‘Micro Tom’. Once the seedlings reached the stage of 5 leaves and 1 apex, seedlings of a similar size were transferred to hydroponic tanks for cultivation. Each hydroponic tank accommodated 9 tomato seedlings, and after 3 days of seedling acclimation, 5 tomato seedlings of a uniform size were selected from each tank for the formal treatment.
In the experiment, two concentration levels were used for Ni2+: 0 mg/L (Ni0) and 0.1 mg/L (Ni0.1), while the nitrogen was set at 0.383 mmol/L (low nitrogen, LN) and 7.66 mmol/L (normal nitrogen, NN). This resulted in a total of four treatment combinations. Among them, the Ni2+ was supplied by NiSO4-6H2O, with the sulfur (S) content of 0.1 mg·L−1 NiSO4 accounted for only 0.07% of the S content in the nutrient solution, rendering the impact of S content in the NiSO4 negligible. The nitrogen treatment was performed using a complete nitrogen nutrient solution formulated according to the Yamazaki nutrient solution formula. The nutrient solution formulas for the two nitrogen treatments with different nitrogen concentrations included identical concentrations of CaCl2, KH2PO4, KCl, and MgSO4-7H2O: 1.425, 0.635, 3.168 mmol·L−1, and 0.998 mmol·L−1, respectively. In the NN treatment, the concentrations of Ca(NO3)2-4H2O, KNO3, and NH4H2PO4 were 1.499, 3.996, and 0.669 mmol·L−1, respectively. In the LN treatment, these concentrations were reduced to 0.075, 0.200, and 0.033 mmol·L−1, respectively. During the experiment, the nutrient solution was replaced every three days for a duration of nine days, commencing on day 0 of the treatment. The pertinent indices were assessed by taking samples at the conclusion of the experiment, with each index being measured thrice.
2.2. Root Growth Index
Three tomato seedlings exhibiting uniform and consistent growth were meticulously chosen. The root systems of these seedlings were thoroughly rinsed with deionized water and allowed to drain. Subsequently, the roots were carefully excised from the root–stem union. The fresh weight of the underground portion was accurately measured. Following this step, the samples were subjected to heat treatment at 105 °C for a duration of 15 min, and dried at 80 °C for 48 h to constant weight. The dry weight of the samples was subsequently determined. Simultaneously, three tomato seedlings with consistent growth were selected to assess the surface area of their root systems using an advanced LA2400 root scanner.
2.3. Root Antioxidant Index
The superoxide dismutase (SOD) activity was determined by the nitrogen blue tetrazolium (NBT) method [26], the peroxidase (POD) activity by the guaiacol method [27], the catalase (CAT) activity by the UV-absorbance method [28], and the malondialdehyde (MDA) content by the thiobarbituric acid (TBA) colorimetric method [29]. Hydrogen peroxide (H2O2) (catalog #H2O2-2-Y), superoxide anion (O2−) (catalog #SA-2-G), reduced glutathione (GSH) (catalog #GSH-2-W), oxidized glutathione (GSSG) (catalog# GSSG-2-W), ascorbic acid (ASA) (catalog #ASA-2A-W), dehydroascorbic acid (DHA) (catalog #DHA-2-W), ascorbate peroxidase (APX) (catalog #APX-2-W), monodehydroascorbate reductase (MDHAR) (catalog #MDHAR-2-W), dehydroascorbate reductase (DHAR) (catalog #DHAR-2-W), glutathione S-transferase (GST) (catalog #GST-2-W), thioredoxin peroxidase (TPX) (catalog #TPX-2-W), glutathione reductase (GR) (catalog #GR-2-W), and glutathione peroxidase (GPX) (catalog #GPX-2-W) activities were also calculated by the kit (Comin Biotechnology Co., Ltd., Suzhou, China).
2.4. Statistical Analysis
The data were processed using Microsoft Excel 2016, plotted with SigmaPlot 10.0, and subjected to two-way ANOVA with Duncan’s multiple range test (p < 0.05) using the statistical software DPS 17.10.
3. Results
3.1. Effect of Nickel Ions on Root Growth Indices of Tomato Seedlings under Low-Nitrogen Stress
As shown in Table 1, under the same nickel concentration treatment, the fresh weight, dry weight, and surface area of tomato seedling roots in the LN treatment were all reduced to different degrees compared to those in the NN treatment. In particular, the fresh weights decreased significantly under both the 0 (Ni0) and 0.1 (Ni0.1) mg·L−1 Ni2+ concentrations, which were 40.38% and 28.36%, respectively; the dry weights also decreased significantly under the Ni0.1 treatment, but none of the root surface areas were significantly affected. Under the same nitrogen treatment conditions, the Ni0.1-treated tomato seedling root fresh weight, dry weight, and surface area all increased to different degrees compared to the Ni0 treatment. In particular, under LN stress, the root fresh weight, dry weight, and surface area increased up to 54.83%, 31.25%, and 34.83%, respectively; the increase in the root fresh weight reached a significant level and approached the level of the NN treatment. In the NN concentration, the Ni0.1-treated root dry weight also increased significantly by 33.33% over the Ni0 treatment, but neither the root dry weight nor the surface area was significantly affected. The above results indicate that low-nitrogen stress significantly inhibited the accumulation of the root fresh weight in tomato seedlings, and the application of 0.1 mg·L−1 Ni2+ significantly alleviated this inhibition and restored it to a level close to that of the NN treatment.

Table 1.
The root growth of tomato seedlings under treatments with different concentrations of N and Ni2+.
3.2. Effects of Nickel Ions on O2−, H2O2, and MDA Contents and SOD, POD, and CAT Activities in the Root System of Tomato Seedlings under Low-Nitrogen Stress
As depicted in Figure 1, the levels of the O2−, H2O2, MDA contents, as well as the SOD and CAT activities in the tomato seedling roots subjected to the LN treatment exhibited varying degrees of increase compared to those treated with normal nitrogen under the same nickel concentration conditions. Notably, at Ni2+ concentrations of 0 (Ni0) and 0.1 (Ni0.1) mg·L−1, the O2− content and SOD and CAT activities were significantly increased, reaching 59.01%,14.69%, and 54.76%, respectively. Moreover, under the Ni0 treatment, the H2O2 and MDA contents significantly increased. Furthermore, in comparison to the Ni0 treatment under the same nitrogen treatment conditions, the Ni0.1 treatment exhibited varying degrees of enhancement in the O2− content and the SOD, POD, and CAT activities, particularly demonstrating significant increases of 14.82%, 41.69%, and 54.84% in the root system under LN stress. Conversely, there were significant increases in the O2−, H2O2, and MDA contents under the NN treatment, while no significant changes were observed in the SOD, POD, and CAT activities. However, under LN stress, the H2O2 and MDA contents in the tomato seedling roots treated with Ni0.1 exhibited substantial decreases of 71.70% and 70.10% compared to the N0 treatment, though the O2− content did not demonstrate a significant variation. These findings suggest that the application of 0.1 mg·L−1 Ni2+ induced the enhancement of SOD, POD, and CAT activities in the root system of tomato seedlings, thereby improving their capacity to scavenge reactive oxygen species and mitigating the peroxidative injuries caused by low-nitrogen stress.
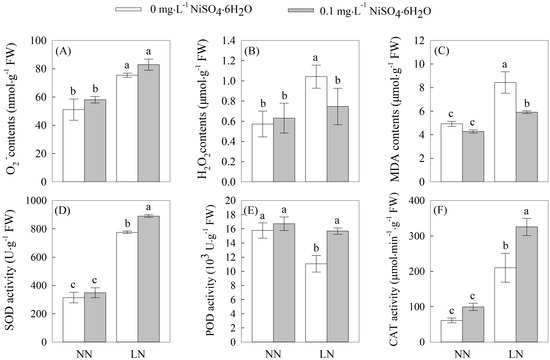
Figure 1.
The contents of (A) O2−, (B) H2O2, and (C) MDA and activities of (D) SOD, (E) POD, and (F) CAT in the root system of tomato seedlings under treatments with different concentrations of N and Ni2+. Different letters above bars indicate a significant difference at p < 0.05.
3.3. Effects of Nickel Ions on Root ASA and Its Metabolic Enzymes in Tomato Seedlings under Low-Nitrogen Stress
3.3.1. Changes in ASA and DHA Levels
Within the ascorbate–glutathione cycle, the ASA/DHA ratio serves as an indicator of plant in vivo resistance, with a positive correlation. As depicted in Figure 2A,E, under comparable nickel concentration treatments, the ASA content in the tomato seedling roots subjected to the LN treatment was notably higher than in those treated with normal nitrogen, while the DHA content exhibited a significant decrease. Both the ASA and DHA contents exhibited significant increases at Ni2+ concentrations of 0 (Ni0) and 0.1 (Ni0.1) mg·L−1, reaching levels of significance. Moreover, the increases in the ASA content reached 108.73%, and 95.59%, respectively, whereas the decreases in the DHA content reached 62.86% and 70.73%, respectively. Consequently, the ASA/DHA ratios were significantly higher. Under equivalent nitrogen treatment conditions, the Ni0.1 treatment induced noteworthy alterations in the root ASA and DHA contents compared to the Ni0 treatment. Specifically, the increases in the root ASA content reached 35.08% and 44.15% under LN stress and NN concentration, respectively, while the decreases in the DHA content reached 38.46% and 21.90%, respectively. These changes led to substantial elevations in the ASA/DHA ratios. Taken together, these findings demonstrate that the application of 0.1 mg·L−1 Ni2+ can modulate the ASA/DHA ratio and facilitate an increase in the ASA content within the root system of tomato seedlings, thus reinforcing their resistance capabilities.
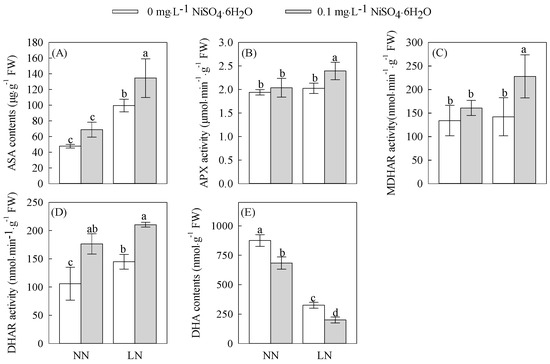
Figure 2.
The ascorbic acid system changes in the roots of tomato seedling under treatments with different concentrations of N and Ni2+.(A) ASA content. (B) APX activity. (C) MDHAR activity. (D) DHAR activity. (E) DHA content. Different letters above bars indicate a significant difference at p < 0.05.
3.3.2. Changes in APX, DHAR, and MDHAR Activities
As shown in Figure 2B–D, under the same nickel concentration treatment, the APX, DHAR, and MDHAR activities of tomato seedling roots in the LN treatment all increased to different degrees compared to those in the NN treatment, and the APX and MDHAR activities increased significantly at the 0.1 (Ni0.1) mg·L−1 Ni2+ concentration. The increases reached significant levels of 17.54% and 41.67%, respectively, and the DHAR activity also rose significantly under the Ni0 treatment. Under the same nitrogen treatment conditions, the APX, DHAR, and MDHAR activities increased to different degrees in the Ni0.1 treatment compared to the Ni0 treatment, especially DHAR activity, which increased significantly in the LN and NN treatments, reaching 45.34% and 66.67%, respectively. The increases in the root APX and MDHAR activities reached significant levels of 18.24% and 60.38% in LN stress, while no significant changes were observed in the APX and MDHAR activities under the NN treatment. The above results indicate that the application of 0.1 mg·L−1 Ni2+ improved the enhancement of APX, DHAR, and MDHAR activities in the root system of tomato seedlings, promoted the reduction of DHA to ASA in the root system, and facilitated the regeneration of ASA in the ascorbate–glutathione cycling system in the root system of tomato seedlings, which was conducive to the enhancement of resistance.
3.4. Effects of Nickel Ions on GSH and Its Metabolic Enzymes in the Root System of Tomato Seedlings under Low-Nitrogen Stress
3.4.1. Changes in GSH and GSSG Levels
GSH/GSSG can be used as a measure of the operational efficiency of the ascorbate–glutathione cycle in plants with a positive relationship. Figure 3A,B shows that under the same nickel concentration treatment, LN treatment tomato seedling roots showed a significant increase in the GSH content compared to the NN treatment and a significant decrease in the GSSG content. The ASA and DHA contents were significantly increased at both the 0 (Ni0) and 0.1 (Ni0.1) mg·L−1 Ni2+ concentrations and varied at significant levels, with the GSH content increasing by 45.28% and 58.64%, respectively, and the GSSG content decreasing by 22.25% and 37.47%, respectively, with both GSH/GSSG ratios increasing significantly. Under the same nitrogen treatment conditions, the ASA and DHA contents of the Ni0.1-treated tomato seedlings had significant changes compared to the Ni0-treated seedlings. The GSH content of the root system increased by 22.93%, the GSSG content decreased by 22.70% under LN stress, and the GSH/GSSG ratios were all significantly elevated. However, there was no significant change in either the GSH or GSSG content or the GSH/GSSG ratio at the NN concentration. The above results indicate that the application of 0.1 mg·L−1 Ni2+ increased the GSH/GSSG ratio and contributed to the increase in the GSH content in the root system of the tomato seedlings under low-nitrogen stress, promoting the operation efficiency of the ascorbate–glutathione cycle, which is favorable for the improvement of the low-nitrogen tolerance of tomato seedlings.
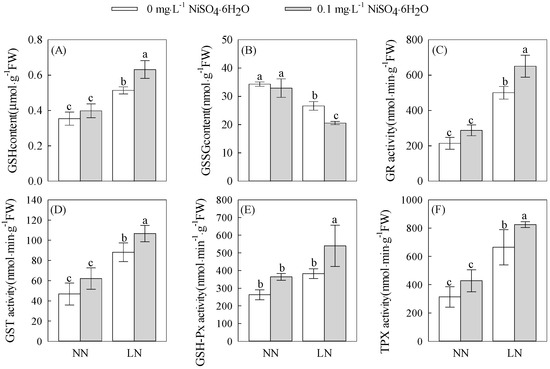
Figure 3.
The changes in the glutathione system in the roots of tomato seedlings under treatments with different concentrations of N and Ni2+. (A) GSH content.(B) GSSG content. (C) GR activity. (D) GST activity. (E) GSH-Px activity. (F) TPX activity. Different letters above bars indicate a significant difference at p < 0.05.
3.4.2. Changes in GR, GST, GPX, and TPX Activities
As illustrated in Figure 3C–F, it is evident that the activities of GR, GST, GPX, and TPX in the tomato seedling roots exposed to the LN treatment were significantly elevated compared to those under the NN treatment, even at the same nickel concentration. Specifically, the activities of GR, GST, and TPX increased markedly by 133.33%, 88.52%, and 112.20% at Ni2+ concentrations of 0 (Ni0) and 0.1 mg·L Ni2+, respectively. Moreover, GPX activity also increased significantly under the Ni0.1 treatment. In contrast, no significant changes were observed in the GR, GST, GPX, or TPX activities under the NN treatment conditions. Remarkably, in the presence of the Ni0.1 treatment, the activities of GR, GST, GPX, and TPX showed even more pronounced increases compared to the Ni0 treatment under the same nitrogen levels. Specifically, under LN stress, the activities of GR, GST, GPX, and TPX significantly increased by 30.00%, 20.87%, 40.12%, and 20.14%, respectively. Conversely, there were no significant alterations in the activities of GR, GST, GPX, and TPX under the NN treatment. These findings highlight that the application of 0.1 mg·L−1 Ni2+ can effectively enhance the activities of GR, GST, and TPX in the root system of tomato seedlings subjected to low-nitrogen stress. Additionally, this treatment also promoted an increase in the GSH content. Furthermore, GPX activity facilitates the reaction between GSH and ROS, resulting in the generation of oxidized glutathione GSSG. This enzymatic reaction plays a crucial role in safeguarding the root system from ROS-induced damage, thus ensuring the maintenance of normal cellular functioning.
4. Discussion
Nitrogen (N) is an essential and widely recognized limiting factor in plant growth and development, playing pivotal roles in diverse metabolic processes [30]. Due to its crucial involvement in nitrogen uptake and responses to environmental challenges, studying the physiological and biochemical changes in the root system holds great research significance and implications for plant growth [31]. Nickel, an essential micronutrient for plants, has been extensively studied for its ability to promote plant growth [18]. As a fundamental indicator that reflects the growth status of plants, the dry and fresh weights of plant roots can provide an overall indication of the impact of environmental stress on plant roots. The present study discovered that low-nitrogen stress significantly impeded the overall plant dry and fresh weight, root dry and fresh weight, root surface area, and other indices of tomato seedlings. However, the introduction of the Ni2+ treatment resulted in varying degrees of increases in both the dry fresh weight and root surface area of the tomato seedling roots, consistent with previous findings [22].
Reactive ROS are often generated as a result of abiotic stresses in the natural environment. These ROS are highly reactive and toxic, and can cause oxidative stress in plant tissues [32,33]. However, plants have their own antioxidant defense systems, which include non-enzymatic components, such as glutathione, proline, α-tocopherol, carotenoids, and flavonoids, as well as enzymatic components, such as SOD, CAT, GPX, and GR. Defense mechanisms play a pivotal role in mitigating the harmful effects of ROS accumulation [34,35]. SOD, POD, and CAT are key enzymatic scavenging systems for ROS in plants, and their activities exert a crucial influence on the plant’s antioxidant defense system [36]. Low-nitrogen stress is considered an environmental stress that can lead to the accumulation of reactive ROS in plants. Several studies have demonstrated that low-nitrogen levels can decrease the capacity of ROS scavenging, leading to the excessive accumulation of H2O2 and subsequent membrane lipid peroxidation [37]. In instances where there is a deficiency in antioxidant capacity, the introduction of exogenous substances through supplementation can aid in enhancing tolerance to particular stress conditions [38]. The presence of Ni2+ as a trace element in plants has also been shown to enhance the ability of plants to withstand stress [24]. In the present study, under low-nitrogen conditions, the levels of MDA, H2O2, and O2− in tomato root cells exhibited an increasing trend. In response, tomato roots activated their own antioxidant defense system by increasing the activities of SOD and CAT to mitigate the damage caused by ROS to plant cells. However, this ability to self-regulate is limited. When treated with Ni2+ under low-nitrogen conditions, the activities of SOD, POD, and CAT increased significantly, while the levels of MDA and H2O2 decreased significantly, reducing the excessive accumulation of ROS. The results indicate that Ni2+ had a mitigating effect on the accumulation of reactive oxygen species and membrane lipid peroxidation in tomato roots under low-nitrogen stress. This led to an improvement in the low-nitrogen tolerance of the tomato seedlings to some extent.
The ASA–GSH cycling system, which functions as a vital pathway for ROS scavenging in plants, holds great importance in maintaining normal plant growth [15]. In the ASA–GSH cycle, ascorbate peroxidase (APX) utilizes ASA as a substrate to effectively scavenge H2O2. This enzymatic process involves the utilization of two molecules of ASA to efficiently reduce H2O2 to water, consequently generating monodehydroascorbate (MDHA) [17]. MDHA, known as a transient radical, can become spontaneously disproportionate to form dehydroascorbate (DHA) and regenerate ASA. Another important enzyme in the cycle is dehydroascorbate reductase (DHAR), which reduces DHA back to ASA using glutathione (GSH) as a potent reducing agent [39].
In the research conducted, notable findings were observed in the root system of tomato seedlings subjected to low-nitrogen stress. The activity of MDHAR and DHAR was significantly higher in this condition, leading to a considerable increase in the ASA content and a decrease in the DHA content, which is a byproduct of the reaction between ASA and H2O2. Consequently, the ASA/DHA ratio was considerably higher compared to the seedlings treated with normal nitrogen levels. These results demonstrate that the application of Ni2+ positively influenced the antioxidant enzyme activities and antioxidant contents in the root system of tomato seedlings under low-nitrogen stress. The addition of Ni2+ significantly amplified MDHAR and DHAR activities, thereby enhancing the rate of ASA production. Although there was a significant decrease in the DHA content, it was postulated that Ni2+ could bolster the regenerative capacity of ASA within the root system of tomato seedlings. This, in turn, resulted in a noticeable increase in the ASA content and an improved ASA/DHA ratio.
APX is considered to be an essential antioxidant enzyme that utilizes ASA as an electron acceptor to reduce H2O2. During stressful conditions, APX plays a crucial role in eliminating excess H2O2. The activity of APX is indicative of the plant’s ability to scavenge H2O2 through its ascorbic acid–glutathione circulatory system. The experimental outcomes revealed a marked elevation in APX activity within the root system of tomato seedlings exposed to low-nitrogen stress, and this activity was further increased by the addition of exogenous Ni2+. These findings suggest that Ni2+ supplementation can enhance the low-nitrogen tolerance ability of tomato seedlings by fortifying their root system.
Plants establish a comprehensive GSH redox system to mitigate the negative impacts of ROS on plant physiology by utilizing the redox capacity of GSH itself and integrating GSH metabolism with other antioxidant pathways [40]. The GSH/GSSG ratio plays a key role in redox signaling pathways within this system [41], engaging in interactions with hormonal and redox molecules while participating in signal transduction processes [42,43,44]. Our study found that Ni2+ treatment under low-nitrogen stress increased the GSH content and the GSH/GSSG ratio in tomato seedling roots. We also observed changes in the activities of GR, GST, GPX, and TPX, along with a decrease in the GSSG content. These results suggest that the Ni2+ treatment enhanced the conversion of GSSG to GSH, leading to lower GSSG levels and a higher GSH/GSSG ratio. Supplementation with Ni2+ enhanced GSH synthesis and improved the conversion of GSSG to GSH under low-nitrogen conditions.
5. Conclusions
The objective of this study was to investigate the effects of reactive ROS accumulation on the growth and root morphology of tomato plants under low-nitrogen stress. To achieve this, an appropriate concentration of Ni2+ was added, and the activity of antioxidant enzymes and the contents of antioxidant substances in the roots of tomato seedlings were observed to increase. The efficiency and capacity of the antioxidant system were improved through this supplementation. This, in turn, inhibited the accumulation of ROS and enhanced the plants’ ability to tolerate low-nitrogen stress. As a result, the oxidative damage to the roots of tomato seedlings was alleviated.
Author Contributions
S.R. and K.Z. designed the research plan. K.Z., S.R. and Y.Z. performed the experiments. S.R. and K.Z. wrote the manuscript. K.Z. also performed the data analysis for physiological experiments. W.H. revised the manuscript. F.Z. helped modified the manuscript. F.Z. agreed to serve as the author responsible for contact and ensure communication. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Seed Industry Innovation and Industrialization Project in Fujian Province (zycxny2021008).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liang, Y.; Zhao, X.; Jones, A.M.; Gao, Y. G proteins sculp root architecture in response to nitrogen in rice and Arabidopsis. Plant Sci. 2018, 274, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Poudel, N.S.; Bhusal, G.; Simkhada, S.; Regmi, B.R.; Adhikari, B.; Poudel, S. Growth parameter and yield attributes of rice (Oryza sativa) as influenced by different combination of nitrogen sources. World J. Agric. Res. 2018, 2, 58–64. [Google Scholar]
- Stein, L.Y.; Klotz, M.G. The nitrogen cycle. Curr. Biol. 2016, 26, R94–R98. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Crawford, N.M.; Coruzzi, G.M.; Tsay, Y.F. Nitrate signaling: Adaptation to fluctuating environments. Curr. Opin. Plant Biol. 2010, 13, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Coruzzi, G.; Bush, D.R. Nitrogen and carbon nutrient and metabolite signaling in plants. Plant Physiol. 2001, 125, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Shin, R. Nutrient sensing and signaling: NPKS. Annu. Rev. Plant Biol. 2007, 58, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Yangx, L.; Lu, Y.; Ding, Y.; Yin, X.; Raza, S. Optimising nitrogen fertilisation: A key to improving nitrogen-use efficiency and minimising nitrate leaching losses in an intensive wheat/maize rotation (2008–2014). Field Crops Res. 2017, 206, 1–10. [Google Scholar] [CrossRef]
- Saleque, M.A.; Naher, U.A.; Islam, A.; Pathan, A.B.M.B.U.; Hossain, A.T.M.S.; Meisner, C.A. Inorganic and organic Phosphorus fertilizer effects on the Phosphorus fractionation in wetland rice soils. Soil Sci. Soc. Am. J. 2004, 68, 1635–1644. [Google Scholar] [CrossRef]
- Jensen, L.S.; Schjoerring, J.K.; Van Der Hoek, K.W.; Damgaard Poulsen, H.; Zevenbergen, J.F.; Pallière, C.; Lammel, J.; Brentrup, F.; Jongbloed, A.W.; Willems, J.; et al. Benefits of nitrogen for food, fibre and industrial production. In The European Nitrogen Assessment; Cambridge University Press: Cambridge, UK, 2011; pp. 32–61. [Google Scholar]
- Xiong, L.L.; Lu, Y.Z.; Liu, Y.Y.; Peng, H.L.; Huang, X.M.; Wang, J.J. Exogenous zinc: Effects on the growth and antioxidant system of Coix lachryma-jobi L.Under nitrogen stress. Chin. Agric. Sci. Bull. 2021, 37, 24–29. [Google Scholar]
- Chen, Y.; Wang, Q.; Chen, X.; Wang, X. Effects of nitrogen deficiency stress on physiological characteristics of Festuca arundinacea at seedling stage. Chin. J. Grassl. 2022, 44, 9–15. [Google Scholar]
- Geng, X.M.; Xiao, L.Y.; Zhao, H.; Liu, P. Sub-cellular localization of ROS-scavenging system in Rhododendron leaves under heat stress and H2O2 pretreatment. Acta Bot. Boreal. Occident. Sin. 2019, 39, 791–800. [Google Scholar]
- Guo, H.G.; Zhao, C.J.; Wu, Y.B. Effects of Rhizoctonia solani on reactive oxygen species and antioxidant enzyme activities of Sorghum seedlings. Acta Bot. Boreal. Occident. Sin. 2022, 42, 796–802. [Google Scholar]
- Zhao, C.F.; Yang, M.; Li, H.J.; Hao, M.Y.; Wang, G.X.; Zhang, R.H. Effect of foliar spraying melatonin on photosynthesis and antioxidant system of maize leaves under drought stress and rewatering. Acta Bot. Boreal. Occident. Sin. 2021, 41, 1526–1534. [Google Scholar]
- Wang, Z.; Zhang, Q.F.; Hu, Y.J.; Wang, C.R. Relationship between ascorbic acid and plant stress resistance. Mod. Agric. 2013, 31–32. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxidative Med. Cell. Longev. 2016, 2016, 9130976. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Zhao, Y.R.; Huang, S.Q.; Wang, Y.J.; Qi, J.E.; Wei, Y.Q. Physiological and biochemical mechanism of exogenous trehalose on drought resistance in sweet sorghum seedlings. Plant Physiol. J. 2022, 58, 654–666. [Google Scholar]
- Rahman, H.; Sabreen, S.; Alam, S.; Kawai, S. Effects of nickel on growth and composition of metal micronutrients in barley plants grown in nutrient solution. J. Plant Nutr. 2005, 28, 393–404. [Google Scholar] [CrossRef]
- Wood, B.W.; Reilly, C.C.; Nyczepir, A.P. Mouse-ear of pecan: I. symptomatology and occurrence. HortScience 2004, 39, 87–94. [Google Scholar] [CrossRef]
- Wood, B.W.; Reilly, C.C.; Nyczepir, A.P. Mouse-ear of pecan: II. influence of nutrient applications. HortScience 2004, 39, 95–100. [Google Scholar] [CrossRef]
- Sirko, A.; Brodzik, R. Plant ureases: Roles and regulation. Acta Biochim. Pol. 2000, 47, 1189–1195. [Google Scholar] [CrossRef]
- Yang, D.Q.; He, X.L.; Li, J.; Li, S.H.; Du, Z.J.; Zhang, K.; Zhong, F.L. Effects of interaction between exogenous nickel and nitrogen on growth and photosynthetic characteristics of tomato seedlings. Jiangsu J. Agric. Sci. 2021, 37, 936–943. [Google Scholar]
- Li, S.H.; Yang, D.; Tian, J.; Wang, S.; Yan, Y.; He, X.; Du, Z.; Zhong, F. Physiological and transcriptional response of carbohydrate and nitrogen metabolism in tomato plant leaves to nickel ion and nitrogen levels. Sci. Hortic. 2022, 292, 110620. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yan, Y.N.; Shang, C.Y.; Chen, L.; Li, J.M.; Zhong, F.L.; Lin, Y.Z. Effect of nickel ion on growth, physiology and nitrogen absorption in Lactuca sativa L. seedling. Acta Bot. Boreal. Occident. Sin. 2018, 38, 2060–2071. [Google Scholar]
- Li, Y.M.; He, X.R.; Li, Q.M.; Liu, B.B.; Li, S.H.; Ai, X.Z.; Wei, M.; Zhang, D.L. Effect of CO2 enrichment on antioxidant system in cucumber seedling root system under drought stress. Plant Physiol. J. 2019, 55, 1011–1019. [Google Scholar]
- Stewart, R.R.; Bewley, J.D. Lipid peroxidation associated with accelerated aging of soybean axes. Plant Physiol. 1980, 65, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Kochba, J.; Lavee, S.; Spiegelroy, P. Differences in peroxidase activity and iso-enzymes in embryogenic and non-embryogenic’ Shamouti’ orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Vandenlangenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nu-trient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Gao, J.F. Experimental Guide to Plant Physiology; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Yan, H.; Shi, H.; Hu, C.; Luo, M.; Xu, C.; Wang, S.; Li, N.; Tang, W.; Zhou, Y.; Wang, C.; et al. Transcriptome differences in response mechanisms to low-nitrogen stress in two wheat varieties. Int. J. Mol. Sci. 2021, 22, 12278. [Google Scholar] [CrossRef]
- Wei, X.W.; Lv, J.; Wu, H.; Gou, C.; Xu, H.W.; Zhou, X.F. Research advances on plant roots. North. Hortic. 2012, 206–209. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.Y.; Huang, J.J.; Zhou, Y.J. Effects of exogenous melatonin on growth and physiological indexes of tomato seedlings under NaHCO3 stress. North. Hortic. 2022, 9, 1–9. [Google Scholar]
- Wang, K.H.; Zhu, B.; Zhu, Z.J. Review of the role of GSH/GSSG in plant abiotic stress response. Acta Hortic. Sin. 2021, 48, 647–660. [Google Scholar]
- Huo, Z.Y.; Cao, P.; Ma, X.X.; Wang, S.B.; Li, S.H.; Shao, G.R.; Zhong, F.L. Physiological response of epidermal wax in non-heading Chinese cabbage under high temperature stress. Acta Bot. Boreal. Occident. Sin. 2022, 42, 89–97. [Google Scholar]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.D.; Liu, H.Y.; Zhang, X.Q.; Liu, Y.P.; Diao, M. Effect of different nitrogen levels on growth, photosynthetic characteristics and reactive oxygen scavenging capacity of Cichorium endivia L. North. Hortic. 2016, 15, 21–26. [Google Scholar]
- Nawaz, M.A.; Huang, Y.; Bie, Z.; Ahmed, W.; Reiter, R.J.; Niu, M. Melatonin: Current status and future perspectives in plant science. Front. Plant Sci. 2016, 6, 1230. [Google Scholar] [CrossRef] [PubMed]
- Gapper, C.; Dolan, L. Control of plant development by reactive oxygen species. Plant Physiol. 2006, 141, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.S.; Yan, M.; Cheng, H.Y.; Zhang, H.B.; Wang, X.J.; Chang, J.N.; Huang, F.; He, X.F.; Su, L.; Gao, J.Y. Effects of spent mushroom substrate biochar on soil Cu form and physiological characteristics of sugar beet in sewage irrigation areas. J. Henan Agric. Sci. 2020, 49, 60–68. [Google Scholar]
- Li, J.M.; Jin, H. Regulation of brassinosteroid signaling. Trends Plant Sci. 2007, 12, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Srivalli, S.; Khanna-Chopra, R. Delayed wheat flag leaf senescence due to removal of spikelets is associated with increased activities of leaf antioxidant enzymes, reduced glutathione/oxidized glutathione ratio and oxidative damage to mitochondrial proteins. Plant Physiol. Biochem. 2009, 47, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.B.; Chu, L.Y.; Lu, Z.H.; Kang, C.M. Primary antioxidant free radical scavenging and redox signaling pathways in higher plant cells. Plant Signal. Behav. 2007, 4, 8–14. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).