Abstract
The storage of peach fruits at 4–5 °C can easily lead to chilling injury and greatly reduce the quality and commercial value of peach fruits. In this study, two kinds of peach fruits (CX and CM) were selected to analyze the mechanisms of chilling injury in fruits with different chilling sensitivity by means of their lipidomic, transcriptome, and dynamic changes in plant hormones. We found that the ethylene, abscisic acid (ABA), and lipid contents changed differently between CX and CM. The ABA and dilactosyl diacylglycerol (DGDG) contents significantly increased after refrigeration in CM fruit, leading to strong cold resistance. However, low temperatures induced a greater accumulation of ethylene, phospholipids, and ABA-GE in CX fruit than in CM fruit, eventually leading to more severe CI symptoms in CX fruit. Additionally, a transcriptional regulatory network for CM and CX fruits during cold storage was constructed, providing a new theoretical reference for the cultivation of cold-resistant peach cultivars and the development of postharvest preservation technology.
1. Introduction
The postharvest climacteric fruit will produce a lot of ethylene if it is stored at room temperature [1]. As a commonly used postharvest preservative technology, low-temperature storage can effectively delay the release rate of ethylene so as to delay tissue senescence and prolong the storage life of fruits to a certain extent. However, some fruits exposed to low temperatures below a certain threshold for long enough will suffer irreversible damage, which is called “chilling injury” (abbreviated as CI) [2]. Fruits with chilling injury usually have sunken epidermises [3], browning peel or pulp [4], etc. The internal browning of peach fruit caused by CI was most serious when stored at between 4 °C and 5 °C, but CI was not obvious at 0 °C [5].
While the fruits exhibited symptoms of chilling injury, the levels of endogenous hormones and metabolites were also affected to a certain extent. Chilling injury can increase the activity of Acs, the rate-limiting enzyme of ethylene biosynthesis in fruits and vegetables, accelerate the transformation of SAM into ACC, and lead to the accumulation of ACC. For example, the accumulation of ACC in cucumber fruits at 2.5 °C is much higher than 12.5 °C. [6]. While it is an important hormone in plants, ABA participates in the response to low-temperature stress, plays an important role in regulating plants’ cold resistance, and is considered to be the initiating factor of cold resistance gene expression [7]. ABA can eliminate excessive H2O2 by promoting antioxidant enzymes and the AsA-GSH pathway, thus reducing chilling injury in peach fruits [8]. Low temperatures or exogenous ABA treatment can reduce the degree of chilling injury to cold-sensitive fruits by changing the balance of ABA in plants [9]. In kiwifruit, appropriate concentrations of exogenous ABA promoted the synthesis of lignin in the pericarp during low-temperature storage, protected the fruit from the external environment, and reduced the accumulation of lignin in the pulp and the appearance of black spots on the pericarp [10].
Low-temperature stress leads to a decrease in cell membrane fluidity, a change in the cell membrane from a liquid crystal phase to a gel state, and, finally, to an increase in membrane permeability. Long-term low-temperature stress leads to symptoms of chilling injury [11,12]. The damage to cell membranes under low-temperature stress is accompanied by changes in fatty acid unsaturation and membrane lipid composition [13,14]. Under low-temperature stress, plants will synthesize more unsaturated fatty acids and improve the fluidity of their cell membranes [15]. As the most important component of the cell membrane, phospholipids mainly include PA (phosphatidic acid), PC (phosphatidylcholine), PE (phosphatidylethanol), PG (phosphatidylglycerol), PI (phosphatidylinositol), and PS (phosphoinositide synthase) [16]. Previous studies have shown that the increase in membrane lipid content is consistent with the increase in chilling tolerance, and the unsaturation of membrane fatty acids is positively correlated with the fluidity of membrane lipids [17]. The structure of MGDG in glycolipids changes from conical to cylindrical under low-temperature stress, thus maintaining membrane stability [18]. Peach fruits treated with CS can maintain high membrane fatty acid unsaturation and low phosphatidic acid content, which effectively alleviates CI and maintains the integrity of the cell membrane [19].
Metabolic group, transcriptome, and other combination techniques have been widely used in many horticultural crops to reveal their response mechanism to environmental stress during growth and development or maturation and softening For example, the physiological and biochemical changes in peach fruits in the dimensions of the transcriptome, proteome, and metabolic groups during postharvest chilling injury; the molecular mechanism of peach fruit responding to low-temperature stress; and a simple and non-destructive method for the detection and evaluation of fruit chilling injury during transportation and storage can be used [20]. In addition, the integration of metabolic groups and transcriptomics laid the foundation for elucidating the molecular mechanisms of plants’ responses to low-temperature stress. Arabidopsis E3 ligase PUB25/26 participates in plants’ responses to low-temperature signals by negatively regulating the protein stability of transcription factor MYB15 in the CBF signaling pathway [21]. Some fruits are prone to chilling injury when they are stored for a long time at unsuitably low temperatures. However, there have been few studies of the mechanism of fruit chilling injury combined with multi-group analysis.
Peach is a kind of fruit that is sensitive to cold, and its CI sensitivity depends on its genetic background, ripening stage, and orchard factors, such as its agronomic management and environmental conditions. The sensitivity levels of different peach varieties to 0 °C and 5 °C storage are very different [20]. In this study, we conducted a comprehensive analysis of lipid groups and transcriptional groups regarding the occurrence of chilling injury in fruits induced by low temperatures. According to the degree of chilling injury of fruits after low-temperature storage, two peach varieties with different chilling sensitivities, namely Chunxue (CX) and Chunmei (CM), were selected. Combined with lipid group and transcriptome analysis, the metabolic network related to fruit chilling injury was constructed, and the key transcription factors (TFs) that directly activate structural genes to affect the occurrence of chilling injury were screened, in order to further clarify the occurrence mechanism of fruit chilling injury and provide a theoretical basis for improving the commercial value of peach fruits.
2. Materials and Methods
2.1. Plant Materials and Treatments
Two peach varieties, namely CX and CM, were selected from the Zhengzhou Fruit Research Institute of the Chinese Academy of Agricultural Sciences. In the S4 I stage, fruits with the same size, uniform color, no diseases or insect pests, and no mechanical injury were picked, and at least 90 fruits were collected from each variety. The harvested fruits were immediately taken back to the laboratory and stored at 4 °C for 40 days. Samples were taken and physiological indices were measured at 0, 8, 16, 24, 32, and 40 days after storage. The pulp was then cut into small pieces, quickly frozen in liquid nitrogen, and stored at −80 °C for further use. Each sample had three biological replicas, and each repeat contained five fruits.
2.2. Determination of Ethylene and ABA Content and Firmness
Firstly, the fruit was transferred from 4 °C to room temperature (25 ± 2 °C) for 2 h, and the fruit was then placed in an airtight 2000 mL buckle box with a plug sealed at room temperature for 2 h; 1mL of gas was absorbed from the sample box using the injection needle, and the gas was then injected into a gas chromatograph (GC2010 Plus, Shimadzu, Kyoto, Japan) for the determination of ethylene production. Each box of samples was tested 3 times.
Two peach cultivars (CX 0 d, 8 d (ethylene release initiation L1), and 32 d (ethylene release peak L2) and CM 0 d, 24 d (ethylene release initiation L1), and 32 d (ethylene release peak L2)) were selected to determine the ABA contents. The 0.2 g liquid nitrogen frozen fruit was ground into powder using a high flux tissue grinder; then, a 1mL extract (methanol:isopropanol:acetic acid = 20:79:1 v/v/v) was added to extract overnight at 4 °C, 14,000× g centrifugal 5 min at 4 °C, precipitation and 1mL extract were extracted repeatedly, and the supernatants were combined twice and filtered through 0.45 μm disposable needle filter. Determination of ABA content by High performance liquid Chromatography (HPLC, LC-2030CD, Shimadzu, Japan).
The firmness of fruit repeatedly analyzed 5 times was detected using a TA-XTPLUS texture analyzer. A probe with a diameter of 5 mm (Godaming stable Microsystems, Godalming, UK) was also used. After removing the 1 mm peel, the firmness of each fruit was measured twice in the equatorial region.
2.3. Lipidomics Analysis
Three biological repetition lipid groups were sequenced in the 0 d, L1, and L2 periods for CX and CM. Lipid extraction was completed by Shanghai Applied Protein Technology Co., Ltd. (Shanghai, China). We ground the sample with liquid nitrogen, weighed the sample to 40 mg, added 200 μL water for mixing, added 240 μL pre-cooled methanol, performed vortex mixing, added 800 μL MTBE, repeated vortex mixing, placed the sample at room temperature for 20 min with 8000× g at 10 °C and centrifuged it for 15 min, took the upper organic phase, dried it with nitrogen, added 100 μL isopropanol solution for mass spectrometry analysis, vortexed it, centrifuged it for 15 min with 8000× g at 10 °C, and performed sample injection analysis. Lipids were separated via UHPLCNexeraLC-30A ultra-high-performance liquid chromatography. LipidSearch software version4.1 was used for peak identification, lipid identification (secondary identification), peak extraction, peak alignment, and quantification.
2.4. Transcriptome Sequencing
Transcript sequencing was used to analyze the differentially expressed genes of different cold-sensitive peaches during storage. According to the ethylene release rate and chilling injury symptoms of the two peach varieties during different storage periods, three repeated transcriptome sequences were carried out at the 0 d, L1, and L2 stages of CX and CM. The total RNA extraction, library construction, and RNA-Seq steps were completed by Nuoke Bioinformatics Co., Ltd. (Beijing, China). The library was sequenced via the Illumina NovaSeq6000 platform.
2.5. Analysis of Differential Genes by WGCNA
Based on the original data of ethylene release, firmness, ABA content, lipid content, and all gene expression determined via transcriptomics, the optimal beta value was determined via pretreatment using R language and Rstudio, and the visual cluster map and heatmap were constructed via a one-step method. According to the correlation degree between physiological data and gene expression, the color depth of different clustering modules in the heatmap was generated, i.e., the correlation degree. We used Gephi0.9.2 software to visualize the model network.
2.6. Prediction of Transcription Factors and Enrichment Analysis of Cis Motifs
The promoter sequence (2000 bp upstream of the transcriptional initiation site (TSS)) was retrieved from the peach genome (GDR; https://www.rosaceae.org/, accessed on 2 December 2023). Then, the TF-binding site (TFBS) of Arabidopsis thaliana was predicted using PlantTFDB v5.0 software (http://www.planttfdb.gao-lab.org/, accessed on 2 December 2023).
2.7. Data Analysis
The statistical significance of the differences was analyzed using Microsoft Office Excel 2019. We used GraphPad Prism 8 to draw the graphics and used TBtools to draw the heatmaps.
3. Results
3.1. Changes in Chilling Injury Symptoms and the Ethylene Contents of Peaches with Different Cold Sensitivities during Low-Temperature Storage
CM and CX, two different peach varieties, showed different cold sensitivities to low temperatures. CX began to release ethylene on day 8 after low-temperature storage, while CM began to release ethylene on day 24 after low-temperature storage. The fruits of the two peaches reached peak ethylene release at the same time, i.e., at 32 d (Figure 1B). At this time, the chilling injury symptoms of CX were more obvious than those of CM, and there was a watery change in the pulp (Figure 1A).
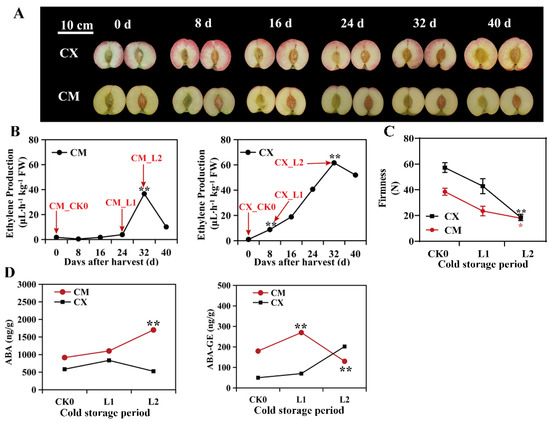
Figure 1.
Phenotypic and physiological indices of different cold-sensitive peaches during low-temperature storage: (A) fruit profiles and chilling injury phenotypes of CX and CM during low-temperature storage; (B) ethylene release rate; (C) firmness change; (D) changes in ABA contents (* p < 0.05, ** p < 0.01).
3.2. Changes in ABA Contents and Fruit Firmness in Peaches with Different Cold Sensitivities during Low-Temperature Storage
During low-temperature storage, the ABA contents of CM peaches were always higher than those of CX peaches, and the ABA contents increased greatly in the L2 stage, but the ABA contents of CX fruit remained at low levels and exhibited no significant changes. ABA-GE first increased and then decreased in CM peaches, but it continued to rise in CX peaches, and there was a significant difference between the two varieties in the L2 period (Figure 1D). By measuring the changes in fruit firmness in the two kinds of cold-sensitive peaches during low-temperature storage, it was found that the decrease in the firmness of the CX peach was significantly higher than that of CM from L1 to L2, which was related to the degree of chilling injury, and the chilling injury degree of CX peach was significantly higher than that of CM peach fruit (Figure 1C).
3.3. Changes in Lipid Compositions and Contents of Peaches with Different Cold Sensitivities during Low-Temperature Storage
The lipids in cell membranes are very important for plants to maintain cell-membrane fluidity and adapt to low-temperature stress. In order to analyze the lipid changes in different cold-sensitive peaches during low-temperature storage, lipidomics analysis was carried out on two peach varieties at the 0 d, L1, and L2 stages. Phospholipids were the main compounds in the CX and CM 0 d samples, and their components changed to some extent during low-temperature storage. The proportions of PA and diglyceride (DG) in CM peaches during L1 and L2 increased, while those of PC and PS decreased; the proportion of PA slightly decreased in L1 and L2 for CX peaches, while PC increased significantly in L2, accounting for about 40% of the total lipid content (Figure 2A).
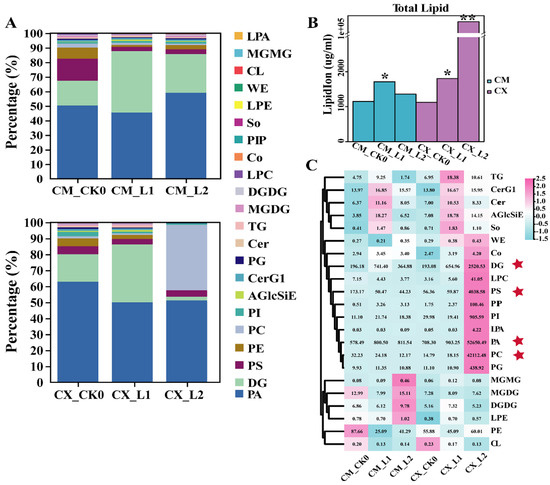
Figure 2.
Changes in the lipid compositions and contents of different cold-sensitive peaches during low-temperature storage: (A) changes in the lipid composition of CX and CM fruits during low-temperature storage; (B) changes in the total lipid contents of CX and CM fruits; (C) thermographic analysis of the changes in lipid contents in CX and CM fruits (* p < 0.05, ** p < 0.01).
The total lipid contents of CX peach fruits changed greatly during low-temperature storage, especially during the L2 stage, where the contents of glycerol phospholipids (PA, PS, PC, PI, PG, PIP, PE) and DG significantly increased (Figure 2A), but the total lipid contents of CM peaches were always at low levels during low-temperature storage (Figure 2B). Specifically, there were great differences in some lipid contents between the two peach varieties. For example, the contents of PC and PS in CX fruits greatly increased during low-temperature storage, but they decreased in CM fruits. The contents of monolactosyl diacylglycerol (MGDG) and digalactosyl diacylglycerol (DGDG) increased in CM fruits. However, no significant changes in these compounds were observed in CX fruits (Figure 2C). There were no significant changes in unsaturated lipids in CM fruits during low-temperature storage. The unsaturated contents of CX increased significantly during L2 storage at low temperatures (Figure S1).
3.4. Transcriptional Analysis of Differentially Expressed Genes
In order to understand the expression of genes related to chilling injury in different peach varieties during low-temperature storage, transcriptome sequencing was carried out in CX and CM at the 0 d, L1, and L2 periods. Correlation analysis showed that the Q30 score was more than 93%. More than 94% of the sequence readings could be aligned with the peach reference genome, and the accuracy and quality of the sequencing data were sufficient for further analysis. We compared CX to CM during low-temperature storage, and a total of 7860 differentially expressed genes were identified at 0 d, including 3938 upregulated genes and 3922 downregulated genes (Figure 3A). In the L1 period, a total of 6758 differentially expressed genes were identified, including 3286 upregulated genes and 3472 downregulated genes (Figure 3B). In the L2 period, the number of differentially expressed genes reached 11,037, including 5595 upregulated genes and 5442 downregulated genes (Figure 3C). We explain the differentially expressed genes using the Venn diagram below. The results showed that there were 4565 common genes between CX and CM, of which 219 genes were differentially expressed between CX and CM during low-temperature storage (Figure 3D).
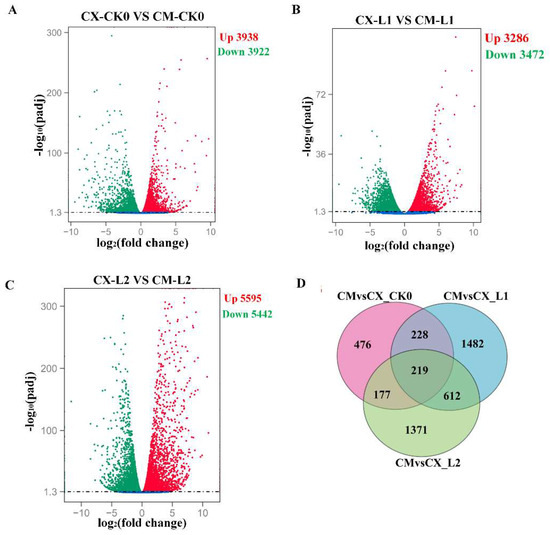
Figure 3.
Transcriptome analysis of CX and CM: (A) analysis of differentially expressed genes between CX and CM during low-temperature storage for 0 d; (B) analysis of differentially expressed genes between CX and CM during the L1 stage of low-temperature storage; (C) analysis of differentially expressed genes between CX and CM during the L2 stage of low-temperature storage; (D) Venn diagram analysis of common differentially expressed genes of the different cold-sensitive peaches noted during low-temperature storage.
3.5. Analysis of Differential Expression of Genes Related to Ethylene Biosynthesis and Signal Transduction in Peach Fruits during Low-Temperature Storage
According to the DEGs obtained via RNA-Seq, 11 differentially expressed genes related to ethylene biosynthesis and signal transduction were screened. The transcriptional abundance of these genes was estimated via FPKM (thousands of base fragments per million pieces of localized transcripts) from the RNA-Seq data. The FPKM value was used to construct a heatmap to estimate the expression levels of these selected genes. Among the 19 differentially expressed genes related to the ethylene pathway, the mRNA transcription of the ethylene biosynthesis genes SAMS, ACS1, ACO1, and ACO2 rapidly accumulated during L2 storage of CX, while the mRNA levels of SAMS, ACS2, and ACO1 in CM increased during L1 storage, and the gene expression levels of ACO2 and ACS2 were opposite to those found in CX. The expression of the ethylene signal transduction genes CTR1-2 and EBF-1 significantly increased during L1 storage in CX, and ETR and EBF-2 expression increased in the L2 stage, while CTR-1 expression decreased during CX cold storage. In CM, the expression of ETR significantly increased in the L1 stage, and the expression levels of CTR1, EIN2, and EBF increased in the L2 stage (Figure 4A).
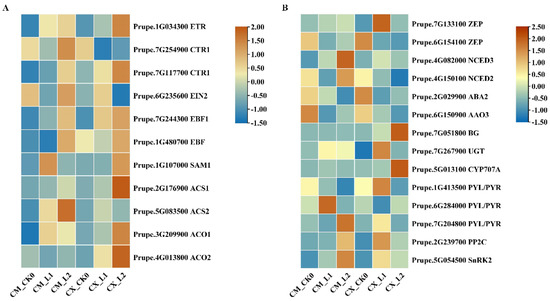
Figure 4.
(A) Gene expression analysis of the ethylene biosynthesis and signal transduction pathway. (B) Analysis of gene expression in the ABA biosynthesis and signal transduction pathway.
3.6. Differential Expression Analysis of Genes Related to ABA Metabolism and Signal Transduction during Low-Temperature Storage
Through stress signal transduction, ABA initiates the stress response in plants and improves the ability of plants to resist various stress factors. After screening and analysis, the expression levels of the ABA-synthesis-related genes NCED2 and NCED3 in CM fruits significantly increased during low-temperature storage, but there were no significant differences in CX fruits. The ABA-metabolism-related genes CYP707A and BG1 were significantly upregulated during the L2 stage in CX fruit, and the expression level of the UGT gene significantly increased during the L1 stage in CM fruit. The expression levels of genes related to the ABA signal transduction pathway also changed, and there were significant differences in the expression levels of PYL, PP2C, and SnRK2 between the two varieties (Figure 4B).
3.7. Differential Expression Analysis of Genes Related to Lipid Metabolism in Peach Fruits during Low-Temperature Storage
Lipids are important compounds in living organisms, as they can participate in and regulate a variety of life activities, and they play an important physiological role in the process of plants’ responses to low-temperature stress. In the transcriptome data related to the fatty acid metabolism pathway, seven genes encoding key enzymes were screened, including PDH, ACC, MCD, KAR, SAD, and ENR-2, which showed downregulated expression during the cold storage of CM fruits. This was highly positively related to the decrease in the contents of unsaturated fatty acids. During the low-temperature storage of CX peaches, the expression of MCD, ENR, and SAD decreased, while that of PDH and KAR first decreased and then increased, and that of ACC first increased and then decreased.
The contents of lipids (especially phospholipids) changed during the low-temperature storage of CX and CM fruits. Combined with the RNA-Seq data, we found that the transcription levels of PSS, CDS, DGK, PAP, FAD, PLC, PLP ζ, and AAPT were all upregulated in CX peaches, which were positively correlated with the increase in phospholipid content. The MGDG and DGDG contents increased during the low-temperature storage of CM peaches, while the related MGD and DGD contents increased at the gene level (Figure 5).
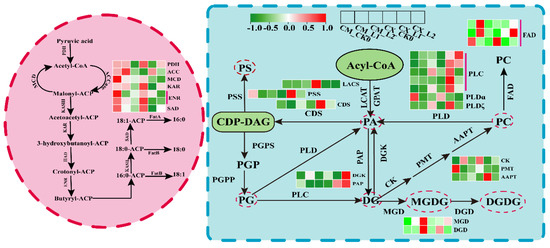
Figure 5.
Gene expression analysis of lipid metabolic pathways.
3.8. Analysis of Genes Related to Different Chilling Sensitivity Traits of Peach Fruits Based on WGCNA
A co-expression network was constructed using WGCNA to determine the differentially expressed genes of different cold-sensitive peach fruits during low-temperature storage. Through WGCNA analysis, the 4290 DEGs identified were divided into nine co-expression modules. The turquoise module was highly positively correlated with ethylene, ABA-GE, total lipid content, DG, PA, PC, and PS (with correlation coefficients of 0.58, 0.91, 0.89, 0.87, 0.9, and 0.78, respectively) and negatively correlated with ABA, MGDG, and DGDG (with correlation coefficients of −0.45, 0.33, and 0.26, respectively). The correlation analysis of the red module, except for the ethylene module, showed the opposite trend to that of the turquoise module. GO enrichment analysis showed that the genes in the turquoise module were responsive to stress, contained oxides, and participated in hormone and lipid metabolism in the cell membranes, while the red module’s genes were mainly involved in stress stimulation, ABA, lipids, and transcriptional regulation (Figure 6).
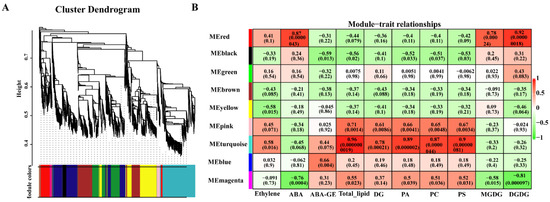
Figure 6.
Correlations of metabolites with physiological indices based on WGCNA: (A) clustering dendrogram of the average network adjacency for the identification of metabolite co-expression modules; (B) module–trait relationships.
Combined with the PlantTFDB database, 44 differentially expressed TFs were identified in the turquoise module. These TFs were divided into 19 families. There were 10 WRKY family genes, 6 ERF family genes, 4 NAC family genes, 3 bZIP family genes, 3 HSF family genes, and 3 MYB family genes. The expression of genes in the major TF families can be found in Figure 7A. Among all TF families, the expression of ERFs was the highest, followed by WRKYs and bZIPs. ERF5 (Prupe.5G061800), ERF2 (Prupe.4G055500), WRKY40 (Prupe.3G098100), and bZIP TF HY5 (Prupe.1G478400) had the highest expression levels and low-temperature responses in the two peach varieties, and the expression levels in CX were significantly higher than those in CM. ERF061 (Prupe.5G117800), WRKY35 (Prupe.8G265900), and ABF3 (Prupe.8G126600) were downregulated in CX and CM fruits during cold storage, but their transcription levels in CX were higher than those in CM fruits (Figure 7B,C). A correlation analysis showed that the expression levels of genes related to ethylene, ABA, and lipid metabolism were positively correlated with those of ERF5 (Prupe.5G061800), ERF2 (Prupe.4G055500), WRKY40 (Prupe.3G098100), and HY5 (Prupe.1G478400), as well as negatively correlated with those of ERF061 (Prupe.5G117800), WRKY35 (Prupe.8G265900), and ABF3 (Prupe.8G126600). In the red module, 34 TFs belonging to 21 families were identified. Four bHLH family genes, four bZIP genes, and four NAC family genes accounted for the largest proportion, of which the bZIP genes’ expression levels were the highest, followed by the bHLH genes. The transcriptional levels of PRE5 (Prupe.3G269500) and bZIP44 (Prupe.8G091600) were significantly higher than those of the other TF genes, and they were positively correlated with NCED2, NCED3, and MGD (Figure 7D,E).
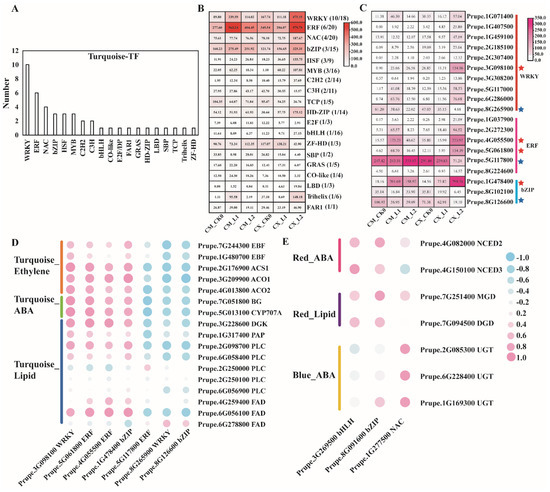
Figure 7.
Transcription factors and their correlations between different TF families and ABA, ethylene, and lipids: (A) the numbers of differentially expressed transcription factors in different categories; (B) the total FPKM heatmap of all transcription factors (TFs) of a specific TF family; (C) differential expression profiles of the WRKY, ERF, and bZIP families; (D) the correlation between differentially expressed WRKYs, ERFs, and bZIPs and differentially expressed genes related to ethylene, ABA, and lipid metabolism; (E) expression profiles of differentially expressed bHLH, bZIP, NAC, and ABA- and lipid-metabolism-related genes in the red and blue modules.
3.9. Analysis of Cis-Acting Elements of Key Structural Gene Promoters in Different Cold-Sensitive Peaches during Low-Temperature Storage
In order to further explore the relationships between TFs and ethylene-, ABA-, and lipid-metabolism-related genes, the cis element of the structural promoter was analyzed. The common binding sites of ERF, bZIP, and WRKY family genes were identified in the promoters of the ethylene biosynthesis genes ACS1, ACO1, and ACO2. The common binding sites of the bZIP, WRKY, ERF, bHLH, and NAC family genes were identified in the promoters of the ABA biosynthesis genes CYP707A, NCED2, NCED3, BG1, and UGT. The common binding sites of the bZIP, WRKY, ERF, and bHLH family genes were identified in the promoters of DGK, PAP, PLC, FAD, and DGD (Figure S8).
4. Discussion
4.1. The Levels of Endogenous Hormones in Peach Fruits Change with the Occurrence of Chilling Injury Symptoms
Low-temperature storage is the main way of prolonging the postharvest life of fresh fruits. When exposed to temperatures lower than the optimal growth temperature, cold-sensitive plants or specific plant organs may become damaged [20,22]. This study’s results showed that when peach fruits were stored at about 4–5 °C, there would be severe chilling injury symptoms, such as pulp browning and flavor loss [5,23,24]. The CX and CM fruits of two different cold-sensitive peaches were picked and stored at 4 °C during commercial ripening. The phenotypic observation showed that CX fruits showed serious cold injury symptoms, but this phenotype was not obvious in CM fruits (Figure 1A). This phenomenon shows that the sensitivity of different peach varieties to low temperatures is different. Low temperatures can induce the release of endogenous ethylene, which is related to the increase in membrane permeability, which, in turn, aggravates the membrane damage, resulting in increased membrane permeability and the destruction of cell partitions [25]. We measured the ethylene release rates of CX and CM fruits during low-temperature storage, and CX began to release ethylene on the 8th day of low-temperature storage. However, ethylene was released from CM fruits only on the 24th day of low-temperature storage (Figure 1B). After low-temperature storage, the SAMS, ACS1, ACO1, and ACO2 genes in CX fruits were significantly upregulated, and the expression of ACS1 and ACO1 in CM fruits was also upregulated, but to a significantly lower extent than that in CX fruits, which was positively correlated with the levels of ethylene production of the two varieties. It may be speculated that the great increase in endogenous ethylene during low-temperature storage may be a sign of chilling injury to some extent (Figure 4A).
Under low-temperature conditions, ABA accumulated in plants can regulate the cold-response genes [26], promote increases in the contents of some osmotic regulators in plants, and improve the stability of membranes, thus improving the cold resistance of plants [27]. The ABA contents of CM fruits were always at high levels during low-temperature storage, and there was a significant difference in ABA content between the L1 and L2 fruits of CX. (Figure 1D). Compared to CX, the expression levels of NCED2 and NCED3 in CM fruits were higher, indicating that the production of ABA between the two varieties was regulated by NCEDs (Figure 4B). At the same time, this result indicates that the two varieties of peach fruits have different cold resistances, as well as that the low-temperature resistance of CM fruits is significantly higher than that of CX fruits. ABA can combine with glucosyl to form inactive ABA-GE and rapidly release ABA through the action of β-glucosidase so as to regulate the contents of active ABA and make plants adapt to physiological and environmental changes [28]. During low-temperature storage, the contents of ABA-GE in CM fruits significantly decreased, but the contents of ABA-GE in CX fruits showed the opposite trend, which was dynamically balanced with the contents of ABA in the two peach fruits during low-temperature storage so as to regulate the resistance of peach fruits to low temperatures (Figure 1D). The expression of UGT was significantly high in CM fruits at the L1 stage, while the expression of BG1 in the L2 stage was the highest in CX fruits, indicating that the two different peach varieties maintained a steady state of ABA content through different ABA metabolic processes (Figure 4B).
4.2. The Changes in the Lipid Levels of Peach Fruits with Different Cold Sensitivities Were Different during Low-Temperature Storage
In this study, a liposome analysis of CX and CM fruits during low-temperature storage was carried out. We found that the lipid composition and contents of the two different peach varieties changed after low-temperature storage. As the original site of cell injury caused by low temperatures, the cell membrane is a semi-permeable membrane composed of two layers of phospholipid molecules. Predecessors have put forward two major theories on this phenomenon: one is the “membrane lipid phase transition” theory [29], and the other is that, under stress conditions, high levels of reactive oxygen species (ROS) will aggravate membrane lipid peroxidation and membrane protein polymerization, thus destroying the membrane’s structure and function [30]. The significant increase in PA contents during the low-temperature storage of CX fruits will inevitably lead to the accumulation of ROS, which will affect the integrity of the cell membranes [31]. In CX fruits, DGDG/MGDG decreased with the decrease in DGDG content (Figure 2). Increasing the ratio of DGDG/MGDG under low-temperature stress can maintain the fluidity of plasma membranes and reduce the damage caused to plants by low temperatures [32]. The MDG gene mediates the transformation from DG to MGDG, and DGD catalyzes the transformation from MGDG to DGDG. The expression level of DGD in CM fruits was significantly higher than that in CX fruits, which was consistent with the changes in DGDG contents. The contents of PA significantly increased during the L2 stage of CX fruits, and the expression of CDS and PSS in CX fruits increased during low-temperature storage, which may be related to the accumulation of phospholipids, indicating that low temperatures mediated and activated the PA biosynthesis pathway in phospholipids to promote PA accumulation, resulting in chilling injury, while the stable total lipid contents in CM peaches further showed that their cold sensitivity was lower than that of CX fruits. At the same time, we measured the fatty acid saturation of two varieties of peach fruits during low-temperature storage. In general, the shorter the fatty acid chain, the higher the degree of unsaturation, while the greater the fluidity of membrane lipids, the stronger the cold resistance of the plants [33]. Therefore, the rapid accumulation of unsaturated fatty acids in CX fruits during the L2 period represents their response to low temperatures and promotes their own protective mechanism (Figure S1), i.e., the accumulation of certain lipids to resist the damage stemming from the stressful environment [34].
4.3. Transcription Factors Play a Key Regulatory Role in Low-Temperature Stress
The peach genome was reported and has been widely used to explore the cold tolerance of peaches [35]. At present, the transcription factors known to be involved in low-temperature signal responses are AP2/ERF, NAC, WRKY, MYB, bZIP, and ZFPs [36]. The overexpression of TabZIP60 significantly improved the tolerance of plants to drought, salt, and freezing stress [37]). PpCBF6 participates in the mitigation of chilling injury in peach fruits by inhibiting the expression of lipoxygenase 5 (LOX5) and plant sulfur factor a (PSKa) [38]. The WRKY transcription factor increases ABA levels by directly activating NECD expression, which participates in the ABA-induced cold tolerance of banana fruits [39]. PpBZR1 regulates interactions between BR and glucose metabolism, thus regulating the mechanism of cold tolerance in peaches [40]. Based on genome and RNA-SEQ analyses, we revealed that C2H2 family transcription factor ZFP21 was involved in the response to low-temperature stress and was an important regulatory factor involved in the chilling injury mechanism of peach fruits [41]. In this study, we identified several families of transcription factors, among which several genes in the ERF, WRKY, bZIP, ABRE, and bHLH families are highly related to the ethylene, ABA, and lipid metabolism genes (Figure 7). In the promoters of key structural genes in the above three pathways, we also identified several common cis-acting elements of transcription factor families. After cold storage, the expression levels of the NCED and DGD genes in CM fruits increased with the increases in the ABA and DGDG contents. At the same time, a cis-element analysis of the NCED and DGD gene promoters revealed the existence of several bHLH and bZIP response elements (Figure S9). We speculated that transcriptional regulation may greatly affect the occurrence of chilling injury in peach fruits with different cold sensitivities during low-temperature storage. Testing this notion will also be the goal of our next study.
5. Conclusions
The results of lipogenomics and plant hormone determination showed that the CM of cold-insensitive peach fruits had strong cold resistance, and the contents of endogenous ABA and DGDG significantly increased during low-temperature storage. However, cold-sensitive peach fruits induced by low temperatures accumulated more ethylene, phospholipids, and ABA-GE than CM fruits, which also explained the severe symptoms of CI. At the same time, combined with transcriptome data, several transcription factor families, such as AP2/ERF.WRKY, were identified, and the transcriptional regulatory networks of CM and CX fruits during cold storage were constructed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae10010046/s1, Table S1: Statistics of RNA-seq data of ‘CM’ and ‘CX’ peach fruit sample. Table S2: Quality of reads obtained by RNA-seq analysis of ‘CM’ and ‘CX’ peach fruit sample. Table S3: Correlation matrix of RNA-seq samples. Table S4.: List of names and IDs of genes involved in ethylene and ABA biosynthesis and signaling, lipid metabolism. The abbreviations used are listed in the legend to Figure S3. Table S5: List of genes in the co-expression network of Figure S10A,B. Figure S1: Changes of firmness of CX and CM fruits during low temperature storage. Figure S2: Principal component analysis (PCA) of the lipidome data in ‘CM’ and ‘CX’ peaches stored at low temperature. Figure S3: Lipid type and saturation analysis in of ‘CM’ and ‘CX’ peaches stored at low temperature. (A) Changes in mainly lipid types; (B) Changes in lipid saturation of PC and PS. Blue, white, and red colors indicate low, medium, and high contents, respectively. The error bars represent the standard error (SE) calculated according to six independent biological replicates. Figure S4: Venn diagrams of DEGs among the different comparison groups. (A) CM responds to low temperature differential genes; (B) Spring snow responds to low temperature differential genes; (C) Spring beauty and spring beauty responds to low temperature differential genes Veen; (D) Different genes in each period of the two varieties. Figure S5: The gene significance (GS) for ethylene (y-axis), total lipid(y-axis), ABA(y-axis) and DGDG (y-axis) vs. the module membership (x-axis) in modules turquoise and red. (A,B) Gene significance (GS) for ethylene and total lipid vs. module membership in module turquoise. (C,D) Gene significance (GS) for ABA and DGDG vs. module membership in module red. Module membership (KME) is the module eigengene-based network connectivity. Figure S6: Expression heatmaps and profiles of DEGs and eigengenes in modules red, blue, turquoise and pink in response to cold storage in ‘CM’ and ‘CX’. Red indicates upregulated genes, and green indicates downregulated genes. The bar graph of eigengene expression shows the eigengene value variance calculated from the singular value composition for each module. Figure S7: GO enrichment analysis of DEGs in turquoise (A), red (B) and blue (C). Figure S8: Transcription factors and correlation between differentially TFs family and ABA, ethylene and lipid in red and blue modules. Figure S9: Prediction of the cis-acting elements in the 2000-bp ACS1, ACO1, ACO2, CYP707A, BG1, DGK, PAP, PLC, FAD, NCED2, NCED3, DGD and UGT promoter region was performed by searching the PLACE databases. Figure S10: Transcription factor and structural gene co-expression regulatory network. (A) Turquoise module differential TF and structural gene co-expression regulatory network; (B) Red module differential TF and structural gene co-expression regulatory network.
Author Contributions
Conceptualization, funding acquisition, and writing—review and editing, W.Z. (Wenfang Zeng) and Z.W.; methodology, investigation, and writing—original draft preparation, W.Z. (Wenduo Zhan) and Y.W.; data curation and formal analysis, W.D., A.L., Y.M., H.W. and J.M.; resources, H.L., L.N., L.P., S.S. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Excellent Youth Foundation of Henan Scientific Committee of China (212300410094), the National Natural Science Foundation of China (32271930), the Central Public-Interest Scientific Institution Basal Research Fund (no. ZGS202209), the Agricultural Science and Technology Innovation Program (ASTIP) (CAAS-ASTIP-2023-ZFRI), and the Special Fund of Henan Province for Agro-Scientific Research in the Public Interest (NO. 201300110500).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Seymour, G.B.; Ostergaard, L.; Chapman, N.H.; Knapp, S.; Martin, C. Fruit development and ripening. Annu. Rev. Plant Biol. 2013, 64, 219–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, K.; Wu, C.; Zhao, Y.; Yin, X.; Zhang, B.; Grierson, D.; Chen, K.; Xu, C. Effect of ethylene on cell wall and lipid metabolism during alleviation of postharvest chilling injury in peach. Cells 2019, 8, 1612. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Dal Cin, V.; Crisosto, C.H.; Labavitch, J.M. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot. 2004, 55, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Ketsa, S.; Chidtragool, S.; Lurie, S. Prestorage heat treatment and poststorage quality of mango fruit. HortScience 2000, 35, 247–249. [Google Scholar] [CrossRef]
- Zhang, C.; Ding, Z.; Xu, X.; Wang, Q.; Qin, G.; Tian, S. Crucial roles of membrane stability and its related proteins in the tolerance of peach fruit to chilling injury. Amino Acids 2010, 39, 181–194. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Saltveit, M.E. Physiological Response to Chilling Temperatures of Intermittently Warmed Cucumber Fruit. J. Am. Soc. Hortic. Sci. 2019, 115, 256–261. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F.; et al. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci. 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Tang, J.; Zhao, Y.; Qi, S.; Dai, Q.; Lin, Q.; Duan, Y. Abscisic acid alleviates chilling injury in cold-stored peach fruit by regulating ethylene and hydrogen peroxide metabolism. Front. Plant Sci. 2022, 13, 987573. [Google Scholar] [CrossRef]
- Deluc, L.G.; Quilici, D.R.; Decendit, A.; Grimplet, J.; Wheatley, M.D.; Schlauch, K.A.; Mérillon, J.M.; Cushman, J.C.; Cramer, G.R. Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genom. 2009, 10, 212. [Google Scholar] [CrossRef]
- Jin, M.; Jiao, J.; Zhao, Q.; Ban, Q.; Gao, M.; Suo, J.; Zhu, Q.; Rao, J. Dose effect of exogenous abscisic acid on controlling lignification of postharvest kiwifruit (Actinidia chinensis cv. hongyang). Food Control 2021, 124, 107911. [Google Scholar] [CrossRef]
- Chen, B.; Yang, H. 6-Benzylaminopurine alleviates chilling injury of postharvest cucumber fruit through modulating antioxidant system and energy status. J. Sci. Food Agric. 2013, 93, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Luengwilai, K.; Beckles, D.M.; Saltveit, M.E. Chilling-injury of harvested tomato (Solanum lycopersicum L.) cv. Micro-Tom fruit is reduced by temperature pre-treatments. Postharvest Biol. Technol. 2012, 63, 123–128. [Google Scholar] [CrossRef]
- Cao, S.; Zheng, Y.; Wang, K.; Jin, P.; Rui, H. Methyl jasmonate reduces chilling injury and enhances antioxidant enzyme activity in postharvest loquat fruit. Food Chem. 2009, 115, 1458–1463. [Google Scholar] [CrossRef]
- Wang, K.; Yin, X.R.; Zhang, B.; Grierson, D.; Xu, C.J.; Chen, K.S. Transcriptomic and metabolic analyses provide new insights into chilling injury in peach fruit. Plant Cell Environ. 2017, 40, 1531–1551. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.; Kaplan, F.; Kopka, J.; Selbig, J.; Hincha, D.K. Metabolomics of temperature stress. Physiol. Plant. 2008, 132, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Funnekotter, B.; Kaczmarczyk, A.; Turner, S.R.; Bunn, E.; Zhou, W.; Smith, S.; Flematti, G.; Mancera, R.L. Acclimation-induced changes in cell membrane composition and influence on cryotolerance of in vitro shoots of native plant species. Plant Cell Tissue Organ Cult. 2013, 114, 83–96. [Google Scholar] [CrossRef]
- Yamaki, S.; Uritani, I. Mechanism of chilling injury in sweet potatoes part V biochemical mechanism of chilling injury with special reference to mitochondrial lipid components. Agric. Biol. Chem. 1972, 36, 47–55. [Google Scholar] [CrossRef]
- Thalhammer, A.; Bryant, G.; Sulpice, R.; Hincha, D.K. Disordered cold regulated15 proteins protect chloroplast membranes during freezing through binding and folding, But do not stabilize chloroplast enzymes in vivo. Plant Physiol. 2014, 166, 190–201. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, S.; Chen, G.; Zheng, Y.; Jin, P. Cold shock treatment alleviates chilling injury in peach fruit by regulating antioxidant capacity and membrane lipid metabolism. Food Qual. Saf. 2022, 6, fyab026. [Google Scholar] [CrossRef]
- Franzoni, G.; Spadafora, N.D.; Sirangelo, T.M.; Ferrante, A.; Rogers, H.J. Biochemical and molecular changes in peach fruit exposed to cold stress conditions. Mol. Hortic. 2023, 3, 24. [Google Scholar] [CrossRef]
- Wang, X.; Ding, Y.; Li, Z.; Shi, Y.; Wang, J.; Hua, J.; Gong, Z.; Zhou, J.M.; Yang, S. PUB25 and PUB26 Promote Plant Freezing Tolerance by Degrading the Cold Signaling Negative Regulator MYB15. Dev. Cell 2019, 51, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Thomashow, M.F. Plant cold acclimation: Freezing tolerance genes and regulatory mechanisms. Annu. Rev. Plant Biol. 1999, 50, 571–599. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Yang, C.; Cao, X.; Wei, C.; Chen, K.; Li, X.; Zhang, B. Chilling-induced peach flavor loss is associated with expression and DNA methylation of functional genes. J. Adv. Res. 2022, 53, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Crisosto, C.H. Chilling injury in peach and nectarine. Postharvest Biol. Technol. 2005, 37, 195–208. [Google Scholar] [CrossRef]
- Wang, C.Y. Changes of polyamines and ethylene in cucumber seedlings in response to chilling stress. Physiol. Plant. 1987, 69, 253–257. [Google Scholar] [CrossRef]
- Barroso, C.; Romero, L.C.; Cejudo, F.J.; Vega, J.M.; Gotor, C. Salt-specific regulation of the cytosolic O-acetylserine(thiol)lyase gene from Arabidopsis thaliana is dependent on abscisic acid. Plant Mol. Biol. 1999, 40, 729–736. [Google Scholar] [CrossRef]
- Zuo, X.; Cao, S.; Zhang, M.; Cheng, Z.; Cao, T.; Jin, P.; Zheng, Y. High relative humidity (HRH) storage alleviates chilling injury of zucchini fruit by promoting the accumulation of proline and ABA. Postharvest Biol. Technol. 2021, 171, 111344. [Google Scholar] [CrossRef]
- Yoshida, T.; Christmann, A.; Yamaguchi-Shinozaki, K.; Grill, E.; Fernie, A.R. Revisiting the Basal Role of ABA—Roles Outside of Stress. Trends Plant Sci. 2019, 24, 625–635. [Google Scholar] [CrossRef]
- Lyons, J.M. Chilling Injury in Plants. Annu. Rev. Plant Physiol. 1973, 24, 445–466. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Biomembranes—Part C: Biological Oxidations. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Tan, W.J.; Yang, Y.C.; Zhou, Y.; Huang, L.P.; Xu, L.; Chen, Q.F.; Yu, L.J.; Xiao, S. Diacylglycerol acyltransferase and diacylglycerol kinase modulate triacylglycerol and phosphatidic acid production in the plant response to freezing stress. Plant Physiol. 2018, 177, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Moellering, E.R.; Benning, C. Galactoglycerolipid metabolism under stress: A time for remodeling. Trends Plant Sci. 2011, 16, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Wallace, P.A.; Teakle, N.L.; Colmer, T.D. Measuring Soluble Ion Concentrations (Na+, K+, Cl−) in Salt-Treated. In Plants BT—Plant Stress Tolerance: Methods and Protocols; Springer: New York, NY, USA, 2010. [Google Scholar] [CrossRef]
- Song, C.; Wang, K.; Xiao, X.; Liu, Q.; Yang, M.; Li, X.; Feng, Y.; Li, S.; Shi, L.; Chen, W.; et al. Membrane lipid metabolism influences chilling injury during cold storage of peach fruit. Food Res. Int. 2022, 157, 111249. [Google Scholar] [CrossRef] [PubMed]
- Cao, K.; Yang, X.; Li, Y.; Zhu, G.; Fang, W.; Chen, C.; Wang, X.; Wu, J.; Wang, L. New high-quality peach (Prunus persica L. Batsch) genome assembly to analyze the molecular evolutionary mechanism of volatile compounds in peach fruits. Plant J. 2021, 108, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Tweneboah, S.; Oh, S.K. Biological roles of NAC transcription factors in the regulation of biotic and abiotic stress responses in solanaceous crops. J. Plant Biotechnol. 2017, 44, 1–11. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Xia, C.; Zhao, G.; Liu, J.; Jia, J.; Kong, X. A novel wheat bZIP transcription factor, TabZIP60, confers multiple abiotic stress tolerances in transgenic Arabidopsis. Physiol. Plant. 2015, 153, 538–554. [Google Scholar] [CrossRef]
- Jiao, C. PpCBF6 Is Involved in Phytosulfokine α-Retarded Chilling Injury by Suppressing the Expression of PpLOX5 in Peach Fruit. Front. Plant Sci. 2022, 13, 874338. [Google Scholar] [CrossRef]
- Luo, D.L.; Ba, L.J.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Involvement of WRKY Transcription Factors in Abscisic-Acid-Induced Cold Tolerance of Banana Fruit. J. Agric. Food Chem. 2017, 65, 3627–3635. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, K.; Wei, Y.; Jiang, S.; Ye, J.; Xu, F.; Chen, Y.; Shao, X. PpBZR1, a BES/BZR transcription factor, enhances cold stress tolerance by suppressing sucrose degradation in peach fruit. Plant Physiol. Biochem. 2023, 202, 107972. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, Z.; Wang, H.; Zhang, W.; Li, S.; Xu, M. Transcriptome and genome analysis to identify C2H2 genes participating in low-temperature conditioning-alleviated postharvest chilling injury of peach fruit. Food Qual. Saf. 2022, 6, fyac059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).