Abstract
The introduction of new ornamental species is a challenge for the floriculture industry. Ebenus sibthorpii is an endemic species of Greece, with a strong ornamental potential. To the best of our knowledge, there are no studies on the in vitro propagation of this species. Therefore, the current study aimed to determine the possibility of micropropagation of Ebenus sibthorpii as a first step for its introduction into the floriculture industry. A preliminary study on the germination ability of the species was also conducted on 1/2-strength Murashige and Skoog medium (MS/2), in the range of 5–35 °C. Seeds germinated at 46–64% indifferently of temperature between the range of 10–30 °C. In vitro cultures were successfully established (77–80% explant response) from single-node explants excised from seedlings, on MS medium either hormone-free (Hf) or supplemented with 6-benzyladenine (BA) at 0.5 and 1 mg L−1. The subsequent multiplication stage involved subcultures in MS medium either Hf or supplemented with various cytokinin types and concentrations, while the combination of 0.01 mg L−1 naphthaleneacetic acid (NAA) with BA was also tested. Τhe highest multiplication indices (3.6–4.6) were observed in media containing BA at 0.1–0.5 mg L−1, regardless of NAA presence, and in those with 0.5 mg L−1 ZEA or 1 mg L−1 TDZ. The highest number of shoots were formed in TDZ media, but almost half of them did not elongate. To address this, a two-fold culture was developed, where micro-shoots produced on TDZ media were transferred to Hf, MS or MS/2 medium to elongate. Thus, the highest multiplication index (16.4) was achieved when micro-shoots from 1 mg L−1 TDZ medium were transferred to MS medium. The present study could be the basis of further exploitation and conservation of E. sibthorpii.
1. Introduction
Ebenus sibthorpii DC. 1825 (f. Fabaceae) (Figure 1) is one of the two Ebenus species endemic to Greece [1]. The genus includes 18 species with a center of diversity in the eastern Mediterranean area [2]. E. sibthorpii grows on stony and gravelly sites as a constituent of phrygana, road embankments and open woodlands, mainly on limestone, from 100 to 800 m altitude, in south-eastern continental Greece (Attica, northeastern Peloponnese, eastern Central Greece and Magnesia), as well as the nearby island of Evia (Euboea) and, surprisingly, the remote island of Rhodes in the SE Aegean region [3]. It is an evergreen, small, procumbent, perennial bush with a woody base and a height of 50–60 cm. The leaves are compound (imparipinnate), consisting of two to five leaflets, 1 to 3 cm long, 4 to 6 mm wide [2]. It is very attractive during flowering, bearing pink-purple flowers in dense, showy, broadly cylindrical racemes on long peduncles, averaging 30–50 flowers per raceme, from April to July (Figure 1) [3]. The flower buds of the species are covered in dense hairs with a silvery sheen, being very attractive even before opening [4]. All the above morphological characteristics make evident the expediency of its inclusion in the floricultural industry for the production of potted plants, landscape plants and cut flowers, as similar shrubs of small stature [5,6,7,8,9,10,11,12,13]. E. cretica is the second species of the genus native to Greece, endemic but quite common on the island of Crete, which also has strong potential for use as a new floricultural crop [14] and has been studied as a member of plant communities for use in urban, Mediterranean, green roofs [15].

Figure 1.
E. sibthorpii inflorescence (A), plant (B) and habitat (C) in Tourkovounia, Attica, at the peak of the flowering season (May 2021).
The production of new ornamental species is a challenge for the floriculture industry. ‘Special’ cut flowers have been exponentially growing in popularity over the last 15 years, favoring sustainability with minimum energy and agrochemical inputs during their production [16]. Nevertheless, pot-plant and cut flower sales in the European Union increased by 7% from 2006 to 2016, overcoming the global economic status [17]. Contemporary, large-scale commercial floriculture is in dire need of the introduction and utilization of novel crops, with the market seeking new species suitable for the new climatic conditions and challenges of the Anthropocene. Hence, there is great interest in native, drought-tolerant species of Mediterranean flora, and numerous studies have been published in recent years on the introduction of drought-tolerant Greek native species into professional horticulture [5,7,8,9,10,12,13,18,19,20,21]. Thus, E. sibthorpii could be introduced as a new alternative species with the added value of its adaptation to low levels of soil moisture.
The exploitation of such new species as ornamental crops can be facilitated by the existence of a simple, productive and low-cost propagation protocol. Micropropagation using in vitro grown seedlings as starting material is a widespread method for the establishment of in vitro cultures, as it overcomes culture infection problems, reduces time needed for the establishment of in vitro cultures and usually leads to high proliferation rates [9,10,13,20,21,22,23,24]. On the other hand, in vitro propagation initiated from seedlings can have a positive effect on the genetic diversity of the produced material compared to the use of other type of explants. Thus, it permits the concurrent multiplication of different genotypes, enhancing the selection of multiple ornamental and commercial clones, a procedure especially important in cases of novel horticultural crops [25,26,27].
The occurrence of seeds with a hard exocarp is common for Fabaceae species [28]. The water-impermeable seed coat in Fabaceae species involves a specified structure that facilitates the entrance of the water, allowing imbibition after mechanical and/or chemical injury [29,30]. Many species of Fabaceae are high-temperature-treated to break their dormancy [31,32].
Murashige and Skoog (MS) medium, especially in its half-strength preparation (MS/2) [33] has been used in a previous micropropagation study of E. cretica [34]. There are no studies on the effectiveness of MS medium on other Ebenus species. Still, both the type and concentration of selected cytokinin effect on different responses in other Fabaceae species during the culture establishment and multiplication stages have been studied [35,36,37]. It must be mentioned that the micropropagation of E. cretica presented several difficulties due to hyperhydridicity [38]. In the present study, in addition to other cytokinins, the effect of thidiazuron (TDZ) (non-purine cytokinin), an artificially modified phenyl-urea (N-phenyl-N′-1,2,3-thidiazol-5-yl-urea) was also tested for its effect on shoot proliferation. TDZ has been used in the in vitro propagation of various plant species at low concentrations [21,22,39]. The morphogenic response on TDZ media varies depending on the concentration, the type of explant, and genotype. Despite decades of research, the mechanism of action of TDZ in regulating the growth of explants remains unknown [40].
In a recent meta-analysis assessment of the extinction risk of the Greek endemic taxa, E. sibthorpii was listed among the endangered (EN) Greek endemics due to the human-induced degradation and alteration of its habitat [41], with the species remaining non-evaluated by IUCN and the Greek Red Data Book for Plants. In vitro methods can be critically important in plant conservation of desirable but threatened plant taxa, enhancing their propagation, and thus relieving propagule collection pressure from natural populations, while functioning as an alternative way of storing plant germplasm in living collections. An efficient clonal propagation method with the employment of molecular methods could enhance the use of E. sibthorpii in the ornamental plant market, with respect to the genetic diversity and the preservation of intraspecific variability.
To our knowledge, there is no documentation in the literature concerning the micropropagation of E. sibthorpii. The present study is assessing the in vitro shoot proliferation of the species starting from seedlings grown in vitro, aiming to develop an efficient micropropagation protocol. Furthermore, the in vitro germinability of the species was studied, aiming to enhance the performance and success rates of its propagation.
2. Materials and Methods
2.1. Plant Material
Fully ripened seeds were collected from a population of E. sibthorpii located within the urban core of Athens, growing in remnants of degraded Mediterranean shrubland (phrygana) on stony, calcareous soil, near an abandoned quarry (38°00′53.2″ N, 23°45′46.9″ E, Attica, Greece) in July 2021. The seeds were isolated from the fruits (pods) which had been left to mature and dry on the mother plants, dehiscing readily for seed dispersal, indicating their complete ripening [42]. Then, the seeds were dry-stored in the dark for 3 months in paper bags (25 °C, 30% relative humidity); the pericarp was removed by hand before the disinfection of the seeds. A total of 420 seeds were used for the studies on in vitro germination.
For the micropropagation experiments, seedlings grown in vitro were used as starting plant material.
2.2. In Vitro Germination
The seeds were immersed in hot water with an initial temperature of 90 °C for 12 h in order to achieve scarification of the seed coat and breaking of the dormancy. Afterwards, the seeds were surface-sterilized with a solution of ethyl alcohol (90%, v/v) for 10 s followed by a 20% (v/v) solution of commercial bleach (4.6% w/v sodium hypochlorite) for 10 min. Then, the seeds were rinsed three times, for 3 min each, with sterile, distilled water, and cultured in vitro, in plastic Petri dishes (9 cm), containing a solid (8 g L−1 agar) half-strength Murashige and Skoog [33] medium (MS/2). Seed incubation was carried out in constant-conditions growth chambers at seven different temperatures, i.e., 5, 10, 15, 20, 25, 30 and 35 °C and a 16-h cool white fluorescent light 37.5 μmol m−2 s−1/8-h dark photoperiod.
Germination data were recorded every second for 30 days. Germination was defined as the emergence of the radicle that would be at least 2 mm long [43], and T50 was defined as the needed time for germination to reach up to 50% of its maximum [44]. A total of 60 seeds were used per treatment (six Petri dishes per treatment, 10 seeds per Petri dish). The formula proposed by Maguire [45] was used for the calculation of the germination speed index (GSI).
in which: G1, G2 and Gn = number of normal seedlings counted during the 1st, 2nd and final count; N1, …, Nn = number of days elapsed from the start of incubation during the first and last count. A cut-test on the 30th day was used for the viability assessment of those seeds that did not emerge: seeds that contained a well-formed and white–greenish embryo were considered viable [46].
GSI = G1/N1 + G2/N2 + … + Gn/Nn
2.3. Establishment of In Vitro Cultures
Two–three days after the end of the germination experiment, E. sibthorbii seedlings grown in vitro were transferred to a solid (8 g L−1 agar) Hf MS medium for further growth. Single-node explants, 0.6 cm at length, derived from 60-day-old seedlings were cultured on Hf MS medium or on MS medium supplemented with 6-benzyladenine (BA) at 0.5 or 1 mg L−1 (initial culture).
2.4. Multiplication Stage
At the multiplication stage, single-node explants (0.6 cm long) were cultured on MS medium, either Hf or supplemented with different cytokinins, i.e., BA, zeatin (ZEA), kinetin (KIN), at concentrations 0.1–1 mg L−1; BA was also used in combination with naphthaleneacetic acid (ΝAA) at 0.01 mg L−1.
The effect of thidiazuron (TDZ) on multiplication was also tested; single-node explants were cultured on MS medium containing 0.1, 0.5 or 1 mg L−1 TDZ, followed by a transfer of TDZ-origin micro-shoots on Hf, MS or MS/2 medium.
2.5. In Vitro Rooting and Ex Vitro Acclimatization
Micro-shoots, 1.5 cm long, produced by sub-culturing on media with various plant growth regulators during the multiplication stage, were transferred for rooting onto half-strength MS (MS/2) medium, Hf or containing 0, 0.5, 1 or 2 mg L−1 indole-3-butyric acid (IBA) or 1 or 2 mg L−1 indole-3-acetic acid (IAA).
Twelve well-formed rooted micro-shoots 1.5 cm long, selected from a total of twenty micro-shoots that had rooted, were well rinsed with tap water. Then, they were transferred to containers (500 mL) with a peat: perlite (1:1 v/v) substrate and covered with transparent plastic wrap (Sanitas; Sarantis S.A., Maroussi, Greece) to maintain humidity. The containers were placed for one week in a growth chamber (25 °C, 16-h cool white fluorescent light 37.5 μmol m−2 s−1/8-h dark photoperiod). Then, they were uncovered and transferred for two more weeks into a heated glasshouse (37°58′58.0″ N, 23°42′19.2″ E). The peat used was peat moss (Klasmann-Deilmann Gmbh, Geeste, Germany), with pH 5.5–6.5; the perlite had particle diameters from 0.1–0.5 cm (Perloflor; Isocon S.A., Athens, Greece).
2.6. In Vitro Culture Conditions and Data Collection
In vitro cultures took place in 100 mL Magenta GA-7 vessels (7.2 cm × 7.2 cm × 10 cm; Sigma-Aldrich, St. Louis, MO, USA), containing four explants each. The cultures were maintained at 25 °C and a 16-h cool white fluorescent light 37.5 μmol m−2 s−1/8-h dark photoperiod. The media contained 30 g L−1 sucrose, were solidified with 8 g L−1 agar (M. Roumboulakis SA, Athens, Greece). The media pH was adjusted to 5.7–5.8 following by agar addition and autoclaving at 12 °C for 20 min.
Measurements in the vitro experiments consisted of shoot formation percentage, total number of shoots per explant, number of long (longer than 0.5 cm, LS) and short (shorter than 0.5 cm, SS) shoots, length of LS, number of nodes per LS, as well as percentage of rooting, root number and root length. All establishment, multiplication and rooting data were collected after 30 d of culture. The acclimatization percentage was recorded 21 d after plantlets were transferred to ex vitro conditions. The “multiplication index” (MI) was calculated by multiplying the percentage of explants that had produced shoots by the mean shoot number per responding explant, then dividing by 0.6 (explant length during subculture), to obtain the potential of the culture proliferation.
2.7. Experimental Design and Statistical Analysis
Τhe completely randomized design was used and the significance of the results was tested by one-way analysis of variance (ANOVA). Two-way ANOVA was applied for the factorial study on TDZ effect on multiplication to highlight differences and interactions between the culture medium (MS/2, MS) and TDZ concentration in the medium of shoot origin (0.1, 0.5 or 1 mg L−1). Arcsine transformation was used for the data of germination, shoot formation and rooting percentage prior to statistical analysis; the means were compared by the Tukey test at p < 0.05 (JMP 14.0 software, SAS Institute Inc., Cary, NC, USA, 2013). The replicate number per each treatment is shown in the data tables. Experiments in the multiplication stage involved five subcultures, and data for the purposes of the statistical analysis were pooled. Germination percentages were expressed as mean ± standard error.
3. Results
3.1. In Vitro Germination
The method of seed sterilization had a success percentage of 95%. Seeds germinated at percentages of from 45.6–64% in the 10–30 °C range without difference (Table 1; Figure 2). At 5 °C and 35 °C, no germination took place. T50 was 4–6 d, while the time for full germination was 28 d at 10 °C and 15 °C gradually decreasing to 20 d at 30 °C (Table 1; Figure 2). The GSI was 48.8–69.7, with no difference between temperature treatments (Table 1).

Table 1.
In vitro germination of E. sibthorpii seeds, time taken for 50% of the final germination percentage (T50), time for full germination and germination speed index (GSI), at 5, 10, 15, 20, 25, 30 and 35 °C.
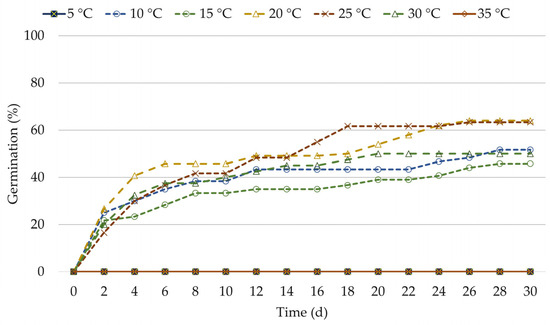
Figure 2.
Germination time-course curves of E. sibthorpii seeds, in Petri dishes containing half-strength, hormone-free MS medium, at 5, 10, 15, 20, 25, 30 and 35 °C, under 16-h light/8-h darkness photoperiod. Six Petri dishes per treatment (60 seeds/treatment) were used.
3.2. Establishment of In Vitro Cultures and Multiplication Stage
At the end of the germination experiments, E. sibthorpii young seedlings derived from in vitro-germinated seeds were transferred onto a half-strength Hf MS medium for further growth. Forty days after transfer, the seedlings were 4–4.5 cm tall, with 4–5 nodes of vigorous growth of 5–7 leaves (Figure 3B).

Figure 3.
E. sibthorpii seed germination in Petri dishes containing half-strength MS medium (MS/2) at 15 °C and 16 h light/8 h darkness photoperiod after 30 days (A) and sixty-day-old in vitro-grown young seedlings of E. sibthorpii on MS/2 medium (B). Bars show a length of 1 cm.
Subsequently, the establishment of the initial in vitro cultures was successful on different MS media either Hf or supplemented with BA at concentrations of 0.5 or 1 mg L−1 (Figure 4). No differences were observed in terms of explant response to form shoots (72–80%), long and short shoot number (1.1–1.4 and 1–1.3, respectively) between different media tested (Table 2). On the other hand, shoot length was higher (2.3–2.4 cm) in media supplemented with BA (Table 2; Figure 4), and MI increased to 4.2 in media containing 0.5 mg L−1 ΒA, with no difference, though, from media with 1 mg L−1 BA. Regarding the node number per micro-shoot, it was higher for the longer shoots (4.4) on 0.5 mg L−1 BA-medium, with no difference from shoots on 1 mg L−1 BA-medium (3.4).

Figure 4.
Response of E. sibthorpii single-node explants at the in vitro establishment stage. Shoots formed on explants after 4 weeks of culture on Hf MS medium (A) or MS with 0.5 or 1 mg L−1 BA at ((B,C), respectively). Bars show a length of 1 cm.

Table 2.
Establishment of E. sibthorpii initial in vitro culture on Hf MS medium or MS supplemented with 0.5 and 1 mg L−1 6-benzyladenine (BA), from single-node explants excised from in vitro young seedlings grown on MS/2 medium.
At the multiplication stage, the percentage of explants that formed shoots was over 76.7%; it was highest (95%) on TDZ media (Table 3) with no difference from BA-media (82–84%) and the medium containing 0.5 mg L−1 ZEA (85.5%). The highest long-shoot number (2.7) was formed on the medium with 1 mg L−1 TDZ, with no difference (2.1) from medium with 0.1 mg L−1 TDZ (Table 3; Figure 4). TDZ media produced the highest number of shoots (LS plus SS); however, shoot proliferation on these media was characterized by callus formation and morphological abnormalities, i.e., hyperhydrated and deformed shoots as well as a high number of non-elongated shoots (Figure 5). Thus, on TDZ media the SS number was highest (1.7–2), although with no difference from 0.5 mg L−1 ZEA medium (1.5) (Table 3). Shoot length of LS was highest on 0.1/0.01 mg L−1 BA/NAA medium (2.6), with no difference from 0.5 mg L−1 ZEA medium (Table 3). The shoots on 0.5 mg L−1 ZEA medium had the highest number of nodes (4.7) (Table 3). The MI was highest for media containing 0.5 mg L−1 ZEA, with no differences from 1 mg L−1 TDZ (4.2) and BA-media (3.6–4.2) and 1 mg L−1 KIN medium or medium with 0.5 mg L−1 ZEA (Table 3).

Table 3.
Multiplication stage: effect of different cytokinins, i.e., 6-benzyladenine (BA), kinetin (KIN), zeatin (ZEA), thidiazuron (TDZ), at various concentrations, and auxin, naphthaleneacetic acid (NAA), on shoot proliferation from single-node explants excised from micro-shoots of E. sibthorpii grown on Hf ΜS medium or MS containing 0.1, 0.5 and 1 mg L−1 BA.
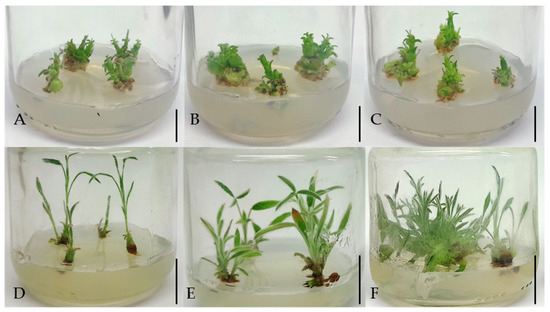
Figure 5.
Multiplication stage: In vitro response of E. sibthorpii single-node explants during a two-fold culture approach, i.e., shoots and callus formed on explants after 4 weeks of culture on ΜS medium supplemented with TDZ at 0.1, 0.5 and 1 mg L−1 ((A–C), respectively), and subsequent transfer of micro-shoots formed in each of the previous media to Hf ΜS medium for elongation ((D–F), respectively). Bars show a length of 1 cm.
3.3. Effect of TDZ Two-Fold Culture on Multiplication
A two-fold culture approach was tested, aiming to overcome the TDZ inhibitory effect on micro-shoot elongation at the multiplication stage. Micro-shoots produced on TDZ media were transferred onto a Hf MS or MS/2 medium. The two-way ANOVA (main factors: TDZ concentration in the origin medium, MS medium strength of the subculture medium) showed that different-origin explants shared the same shooting performance between different strengths of subculture media. The LS number was strongly affected by the TDZ concentration in the origin medium and was highest when 1 mg L−1 TDZ was used, with no difference, however, from 0.5 mg L−1 TDZ-origin medium (Figure 5; Table 4). A significant interaction of the two main factors was observed regarding shoot length, which was highest for the 0.1 mg L−1 TDZ-origin medium in both MS and MS/2 medium (3.2 cm and 2.6 cm, respectively) and 1.0 mg L−1 TDZ-origin medium in MS medium (2.4 cm). There were no differences for the node number, while a significant interaction between the two main factors was observed regarding the multiplication index. The highest MI (16.4) was observed when micro-shoots produced on 1 mg L−1 TDZ medium were transferred onto MS medium (Figure 5; Table 4).

Table 4.
Response of single-node explants derived from micro-shoots produced at the multiplication stage on TDZ media, as affected by the concentration of TDZ (0.0, 0.5 or 1 mg L−1) in the original medium and the strength of ΜS medium (full or half) at the subsequent transfer of shoots on Hf medium.
3.4. In Vitro Rooting and Ex Vitro Acclimatization
Micro-shoots rooted at low percentages; the rooting percentage was highest (30.0%) on MS/2 medium supplemented with 2 mg L−1 IBA, with no difference from the rooting percentage observed in 1 mg L−1 IBA and 2 mg L−1 IAA-media (10%) (Table 5, Figure 6). A few (2.5–4.5) short (0.5–1.5 cm) roots were produced with no differences between different treatments. The rooted micro-shoots were gradually decreased in growth, withered between the 7th and 14th day, and they failed to acclimatize.

Table 5.
In vitro rooting of E. sibthorpii micro-shoots on half-strength MS medium as affected by auxin type (IBA: indole-3-butyric acid, IAA: indole-3-acetic acid) and concentration (mg L−1).

Figure 6.
In vitro-rooted E. sibthorpii micro-shoots on MS medium with 2 mg L−1 IBA (A) or IAA (B). Arrows indicate roots; bars show a length of 1 cm.
4. Discussion
The present study focused on the in vitro propagation ability of E. sibthorpii, an endemic shrub in Greece, with potential uses as a new, ornamental species, using in vitro-grown young seedlings as starting plant material. To achieve this goal of introducing the species into the floriculture industry, a fast and efficient propagation method is desirable. Micropropagation could be applied in any season, overcoming the disadvantages of traditional propagation techniques, producing short, uniform and stable plantlets using limited space and water resources [47].
Concerning the natural regeneration of Ebenus sibthorpii populations, anecdotal evidence points to the sporadic germination of a persistent seedbank after a disturbance (e.g., fire) and/or the ongoing weakening of the hard seed coat due to the dry hot summers. At the same time, the production of seeds in situ is observed to be usually very low, with the population sampled in the current study exhibiting just a mere 10% reproductive success (percentage of flowers successfully producing seeds), while the growth rate of seedlings has been observed to be low and erratic, discouraging the use of traditional propagation methods. The use of seeds as parental material in combination with other in vitro tools and ex situ/in situ conservation measures can contribute to mitigating the extinction risk of plant populations, being very useful for the rare and endangered species [48,49,50]. The in vitro plant propagation sometimes is the only choice for conservation of highly endangered species [51,52]. Still, it can be used as an invaluable tool for the ex vitro conservation of “exceptional species”, whose propagules are unavailable or unsuitable for long-term storage in conventional seed banks [53,54].
Many members of Fabaceae have seeds with seed coat dormancy, a valuable adaptation for the survival of plant species growing under adverse conditions in fire- and drought-prone habitats [55,56]. This type of physiological dormancy prevents seeds from completing germination even under favorable conditions, with impermeable structures surrounding and constraining the embryo prior to their removal, blocking water imbibition [57,58]. In the present study, hot water immersion was used, which had a favorable effect and led to satisfactory germination percentages of up to 64 ± 6.7%. Τhe thermal pretreatment of hard-coated seeds with hot water shows distinctly different promotive patterns with those of mechanical scarification, with the heat shock inducing cracks on the lens of hard-seeded Fabaceae [59]. The limited number of wild-collected seeds did not allow other dormancy-breaking treatments to be tested in germination experimentation, but in a previous preliminary experiment, seeds not treated with hot water did not germinate. The present germination scores are lower compared to the germination percentages of E. cretica previously reported by Vlahos and Dragassaki [38], who measured 93% germination at 22 °C, with seeds receiving no prior treatment. The differences in germination percentages of these two Ebenus species suggest species-specific differences in the anatomy and ecophysiology of their seeds, with a strong genetic and evolutionary basis as products of natural selection in different habitats [60,61]. Variations in seed size, germination rate and viability have been observed between different species of the same genus, e.g., in Onobrychis and Salvia [62,63], and sometimes even within the same genotype of a species [64,65,66]. Nevertheless, the environmental parameters during seed production, maturation and storage strongly affect seed behavior and should always be assessed, if possible, as they have been proven to significantly affect the germination of other hard-seeded Fabaceae genera, such as Hedysarum [67,68]. The germination percentages of E. sibthorpii are similar to those of other Fabaceae species, i.e., Acacia mearnsii [69], Senna artemisioides ssp. × coriacea [32], Senna spectabilis [70] and Senna artemisioides [36] in which a hot water pre-treatment of the seeds had been suggested as well.
The minimum cardinal temperature was defined at 10 °C, while the maximum cardinal temperature was defined at 30 °C, with no differences in both the germination percentages and GSI (Table 1). It could, thus, be claimed that E. sibthorpii has lower and upper thresholds close to 5 °C and 40 °C, respectively, indicating the indifference of the species towards germination temperature, a common trait between hard-seeded species [30]. The present hot water treatment had a positive effect, but more research could lead to an increase in the germination percentages.
The establishment of E. sibthorpii in vitro initial cultures was successful, being of paramount importance for its subsequent in vitro culture, as a rapid and efficient micropropagation protocol of the species could accelerate efforts both for conservation and exploitation. Several regionally restricted species such as Origanum dictamnus, Neoregelia cruenta and Brighmania insignis have been introduced in commercial ornamental horticulture despite their endangered status, easing demand and lessening the impact of overharvesting on their natural populations [53,71,72,73]. The in vitro response of node explants depends on the nutrient media and the effect of plant growth regulators that control the physiological alteration in the plant tissue. Different types and concentrations of cytokinins were found to be effective at stimulating axillary shoot development, with the formation and development of normal, elongated shoots on most media tested. In a number of species native to thermo-Mediterranean plant communities, such as phrygana, the addition of BA to the substrate, at rather low concentrations, favors the proliferation of shoots, increasing their number [74]. An increase in the BA concentration causes an increase in the number of shoots produced but at the same time a decrease in their length [7,9,10,13,20,23,75]. In the present work, this did not seem to be verified, while the combination of low concentration of BA with NAA was favorable in shoot elongation, possibly due to the effect of NAA.
Ιn media containing TDZ, more shoots were formed, which were shorter in length, and more solid callus growth was observed compared to other media. The effects of the TDZ supplementation into the MS media can be varied, its addition in growth media being linked with either higher multiplication rates [76,77] or/and adverse effects, such as the formation of abnormal shoots [78,79,80]. Thus, in addition to experimentation with different types and concentrations of cytokinins, a two-fold culture was developed during the multiplication stage. In the first stage, TDZ was used for the establishment of single-node explants in vitro cultures, with the aim to produce multiple shoots, while the second stage consisted of the subculture of single micro-shoots produced in the previous stage in hormone-free medium for their elongation. This method has been previously used successfully for the micropropagation of ×Malosorbus florentina Zucc., a rare and endangered native tree of Greece [81], and of Sphaerophysa kotschyana, an endangered, monotypic herbaceous Fabaceae endemic to Turkey [82].
In the current study, the root formation in micro-shoots was low, a common problem observed during micropropagation of woody species, which can create considerable obstacles in the economic viability of micropropagation protocols [83]. In some cases, the cytokinin content of the proliferation media can affect micro-shoot rooting (carry-over effect). It has been observed that in Lens culinaris (lentil), the longer the shoots were exposed to cytokinins, the stronger was the latters’ inhibitory effect on the in vitro rooting of the shoots [84,85]. Furthermore, TDZ in the regenerated shoots of Sophora flavescens inhibited root organogenesis [86], a phenomenon also observed in the micropropagation of various other species [87].
Due to the low rooting percentages, there was not an adequate quantity of plantlets to proceed to ex vitro acclimatization experiments. Only 12 plantlets had good morphological characteristics and were tested for acclimatization. From those, none survived the acclimatization stage. Different factors could affect the growth and lead to plantlet death during their transfer from the in vitro to ex vitro conditions, such as the morphological, anatomic, and physiological aberrancies and inadequacies of the rooted micro-shoots [88,89,90].
In vitro rhizogenesis has been previously assessed as a complex phenomenon during the study of multiple ornamental crops, with various factors, both intrinsic and external, affecting the induction and growth of adventitious roots at the basal end of micro-shoots [83]. Still, in vitro species recalcitrance has often been studied for a series of species [90,91,92]. At the same time, the ex vitro acclimatization of the plantlets produced in vitro can have a crucial impact in the success of any proposed micropropagation protocol, with an intricate interplay of morphological, physiological and environmental parameters affecting the survival of the acclimatizing rooted micro-shoots [90,91,92]. Consequently, the lack of success at the stage of rooting and ex vitro acclimatization of E. sibthorpii, a problem often encountered during the introduction of novel floricultural species into in vitro propagation, shall be studied in detail in future research stemming from the findings of the current paper.
5. Conclusions
To our knowledge, the present research is the first on in vitro seed germination and culture of E. sibthorpii. Three-month-old E. sibthorpii seeds germinated, registering good percentages, the cardinal germination temperatures defining at 10 °C to 30 °C. Regarding the efficiency of the micropropagation method, successful establishment of cultures was carried out on MS medium containing 0.5 mg L−1 and 1 mg L−1 BA. The two-fold culture at the multiplication stage, i.e., culture on 0.5 or 1 mg L−1 TDZ medium followed by micro-shoot transfer on Hf medium, was efficient in securing satisfactory multiplication rates and shoots of good quality.
An efficient germination method is reported, with a subsequent successful establishment of in vitro cultures and a good shoot multiplication. The rooting capacity of the produced micro-shoots was very low, while the ex vitro acclimatization of regenerated E. sibthorpii plantlets was unsuccessful. Further studies are needed to improve the in vitro rooting and acclimatization efficiency of E. sibthorpii microplants. The currently described method could serve as a promising starting point for the commercial exploitation and conservation of this species, whose characteristics make it a potential new floricultural crop, given that the problems encountered in the final stages of its micropropagation are overcome.
Author Contributions
Conceptualization, K.B.; methodology, K.B. and A.-E.B.; formal analysis, K.B. and M.P.; investigation, K.B., D.V.-V. and A.-E.B.; data curation, K.B. and M.P.; writing—original draft preparation, K.B., A.-E.B. and M.P.; writing—review and editing, K.B., A.-E.B. and M.P.; visualization, K.B. and D.V.-V.; supervision, K.B.; project administration, K.B. and M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All data are presented in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zografidis, A. New floristic records in Greece. Parnass. Arch. 2017, 5, 61–66. [Google Scholar]
- Huxley, A.; Taylor, W. Flowers of Greece and the Aegean; Chatto and Windus: London, UK, 1977; p. 186. [Google Scholar]
- Strid, A. Atlas of the Aegean Flora; Botanischer Garten und Botanisches Museum Berlin-Dahlem: Berlin, Germany, 2016; Volume 33, p. 1578. [Google Scholar]
- Sficas, G. Wildflowers of Greece; Efstathiades Group SA: Athens, Greece, 1987; p. 116. [Google Scholar]
- Darras, A.I.; Spiliopoulos, I.; Kartsonas, E.; Assimomitis, P.; Karras, S. Antioxidant profile, propagation and cultivation of Nepeta camphorata, the endemic species of Mt Taygetos (Greece). S. Afr. J. Bot. 2020, 131, 391–397. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent Development in Micropropagation Techniques for Rare Plant Species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]
- Martini, A.N.; Papafotiou, M. In Vitro Propagation and NaCl Tolerance of the Multipurpose Medicinal Halophyte Limoniastrum monopetalum. HortScience 2020, 55, 436–443. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Maloupa, E.; Tsoktouridis, G. Propagation and ex-situ conservation of Lomelosia minoana subsp. minoana and Scutellaria hirta—Two ornamental and medicinal Cretan endemics (Greece). Not. Bot. Horti Agrobot. 2021, 49, 12168. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, Μ.; Bertsouklis, K.F. Studies on seed germination and micropropagation of Clinopodium nepeta a medicinal and aromatic plant. HortScience 2019, 54, 1558–1564. [Google Scholar] [CrossRef]
- Vlachou, G.; Papafotiou, Μ.; Bertsouklis, K.F. Seed germination, micropropagation from adult and juvenile origin explants and address of hyperhydricity of the Cretan endemic herb Calamintha cretica. Not. Bot. Horti Agrobot. 2020, 48, 1504–1518. [Google Scholar] [CrossRef]
- Kostas, S.; Kaplani, A.; Koulaouzidou, E.; Kotoula, A.-A.; Gklavakis, E.; Tsoulpha, P.; Hatzilazarou, S.; Nianiou-Obeidat, I.; Kanellis, A.K.; Economou, A. Sustainable Exploitation of Greek Rosmarinus officinalis L. Populations for Ornamental Use through Propagation by Shoot Cuttings and In Vitro Cultures. Sustainability 2022, 14, 4059. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Tsopela, S. In Vitro Propagation of Three Populations of the Endangered, Greek Endemic Cerastium candidissimum and Short-Term Storability of Alginate-Encapsulated Shoot Explants for Exploitation and Conservation. Horticulturae 2023, 9, 273. [Google Scholar] [CrossRef]
- Papafotiou, M.; Vlachou, G.; Martini, A.N. Investigation of the Effects of the Explant Type and Different Plant Growth Regulators on Micropropagation of Five Mediterranean Salvia spp. Native to Greece. Horticulturae 2023, 9, 96. [Google Scholar] [CrossRef]
- Syros, T.; Yupsanis, T.; Zafiriadis, H.; Economou, A. Activity and isoforms of peroxidases, lignin and anatomy, during adventitious rooting in cuttings of Ebenus cretica L. J. Plant Physiol. 2004, 161, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Varela-Stasinopoulou, D.S.; Nektarios, P.A.; Ntoulas, N.; Trigas, P.; Roukounakis, G.I. Sustainable Growth of Medicinal and Aromatic Mediterranean Plants Growing as Communities in Shallow Substrate Urban Green Roof Systems. Sustainability 2023, 15, 5940. [Google Scholar] [CrossRef]
- Darras, A. Overview of the Dynamic Role of Specialty Cut Flowers in the International Cut Flower Market. Horticulturae 2021, 7, 51. [Google Scholar] [CrossRef]
- Eurostat. Horticultural Products. Flowers and Ornamental Plants. Statistics 2006–2016; Eurostat: Luxembourg, 2017. [Google Scholar]
- Bertsouklis, K.; Papafotiou, M. Seed germination of Arbutus unedo, A. andrachne and their natural hybrid A. andrachnoides in relation to temperature and period of storage. HortScience 2013, 48, 347–351. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae 2022, 8, 1114. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N. In Vitro Seed and Clonal Propagation of the Mediterranean Aromatic and Medicinal Plant Teucrium capitatum. HortScience 2016, 51, 403–411. [Google Scholar] [CrossRef]
- Papafotiou, M.; Bertsouklis, K.F.; Trigka, M. Micropropagation of Arbutus unedo, A. andrachne, and their natural hybrid, A.xandrachnoides from seedling explants. J. Hortic. Sci. Biotechnol. 2013, 6, 768–775. [Google Scholar] [CrossRef]
- Ahmad, N.; Faisal, M.; Ahmad, A.; Alatar, A.A.; Qahtan, A.A.; Alok, A. Thidiazuron Induced In Vitro Clonal Propagation of Lagerstroemia speciosa (L.) Pers.—An Important Avenue Tree. Horticulturae 2022, 8, 359. [Google Scholar] [CrossRef]
- Martini, A.N.; Vlachou, G.; Papafotiou, M. Effect of Explant Origin and Medium Plant Growth Regulators on In Vitro Shoot Proliferation and Rooting of Salvia tomentosa, a Native Sage of the Northeastern Mediterranean Basin. Agronomy 2022, 12, 1889. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Growing of the Cretan Therapeutic Herb Origanum Dictamnus in the Urban Fabric: The Effect of Substrate and Cultivation Site on Plant Growth and Potential Toxic Element Accumulation. Plants 2023, 12, 336. [Google Scholar] [CrossRef]
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing over-harvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Generoso, A.L.; Carvalho, V.S.; Walter, R.; Campbell, G.; Araújo, L.S.; Santana, J.G.S.; Cunha, M. Mature-embryo culture in the cryopreservation of passion fruit (Passifora edulis Sims) seeds. Sci. Hortic. 2019, 256, 108638. [Google Scholar] [CrossRef]
- Silva, S.S.S.; Souza, E.H.; Souza, F.V.D.; Max, D.A.S.; Rossi, M.L.; Costa, M.A.P.C. Post-seminal development and cryopreservation of endemic or endangered bromeliads. An. Da Acad. Bras. Ciências 2021, 93, 20191133. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Classification, biogeography, and phylogenetic relationships of seed dormancy. In Seed Conservation: Turning Science into Practice; Smith, R., Dickie, J., Linington, S., Pritchard, H., Probert, R., Eds.; The Royal Botanic Gardens, Kew: London, UK, 2003; pp. 518–544. [Google Scholar]
- Baskin, J.M.; Baskin, C.C. A classification system for seed dormancy. Seed Sci. Res. 2004, 14, 1–16. [Google Scholar] [CrossRef]
- Auld, T.D.; O’Connell, M.A. Predicting patterns of post-fire germination in 35 eastern Australian Fabaceae. Aust. J. Ecol. 1991, 16, 53–70. [Google Scholar] [CrossRef]
- Pound, L.M.; Ainsley, P.J.; Facelli, M. Dormancy-breaking and germination requirements for seeds of Acacia papyrocarpa, Acacia oswaldii and Senna artemisioides ssp. coriacea, three Australian arid-zone Fabaceae species. Aust. J. Bot. 2014, 62, 546–557. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phys. Plan. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Vlahos, J.C. Ebenus cretica L.; An attractive endemic plant of Crete with potential for floricultural use. HortScience 1996, 31, 769–774. [Google Scholar] [CrossRef]
- Gbadamosi, A.E.; Hassan, K.O. In vitro propagation of Senna alata (linn) on WPM and MS media with varying PGR. J. Sustain. Dev. 2013, 2, 1997–2007. [Google Scholar] [CrossRef]
- Bertsouklis, K.; Naksi, K.; Aretaki, P.-E. In vitro germination and regeneration of Senna artemisioides, a valuable leguminous ornamental shrub. Not. Bot. Horti Agrobo. 2023, 51, 12992. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. In Vitro Seed and Clonal Propagation of the Mediterranean Bee Friendly Plant Anthyllis hermanniae L. Sustainability 2023, 15, 4025. [Google Scholar] [CrossRef]
- Vlahos, J.C.; Dragassaki, M. In vitro regeneration of Ebenus cretica L. Acta Hortic. 2000, 541, 305–309. [Google Scholar] [CrossRef]
- Gupta, S.; Mao, A.A.; Sarma, S. Effects of Thidiazuron (TDZ) on Direct Shoot Organogenesis of Gymnocladus assamicus: A Threatened and Critically Endangered Species from Northeast India. Natl. Acad. Sci. Lett. 2020, 43, 85–91. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Giebelhaus, R.T.; Victor, J.M.R.; Murch, S.J.; Saxena, P.K. The Morphoregulatory Role of Thidiazuron: Metabolomics-Guided Hypothesis Generation for Mechanisms of Activity. Biomolecules 2020, 10, 1253. [Google Scholar] [CrossRef] [PubMed]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Βiology 2021, 10, 195. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998; p. 666. [Google Scholar]
- Ιnternational Seed Testing Association. International rules for seed testing. Seed Sci. Tech. 1999, 27, 333. [Google Scholar]
- Soltani, A.; Galeshi, S.; Zeinali, E.; Latifi, N. Genetic variation for and interrelationships among seed vigor traits in wheat from the Caspian Sea coasts of Iran. Seed Sci. Technol. 2001, 29, 653662. [Google Scholar]
- Maguire, J.D. Speed of germination-Aid in selection and evaluation for seedling emergence and vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Carruggio, F.; Onofri, A.; Impelluso, C.; Giusso del Galdo, G.; Scopece, G.; Cristaudo, A. Seed dormancy breaking and germination in Bituminaria basaltica and B. bituminosa (Fabaceae). Plants 2020, 9, 1110. [Google Scholar] [CrossRef]
- Mani, M.; Mathiyazhagan, C.; Dey, A.; Faisal, M.; Alatar, A.A.; Alok, A.; Shekhawat, M.S. Micro-morpho-anatomical transitions at various stages of in vitro development of Crinum malabaricum Lekhak and Yadav: A critically endangered medicinal plant. Plant Biol. 2023, 25, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Barnicoat, H.; Cripps, R.; Kendon, J.; Sarasan, V. Conservation in vitro of rare and threatened ferns—Case studies of biodiversity hotspot and island species. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 37–45. [Google Scholar] [CrossRef]
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic plant species conservation: Biotechnological approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Magrini, S.; Azzella, M.M.; Bolpagni, R.; Zucconi, L. In Vitro Propagation of Isoëtes sabatina (Isoetaceae): A Key Conservation Challenge for a Critically Endangered Quillwort. Plants 2020, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Sarasan, V.; Cripps, R.; Ramsay, M.M.; Atherton, C.; McMichen, M.; Prendergast, G.; Rowntree, J.K. Conservation in vitro of threatened plants—Progress in the past decade. Vitr. Cell. Dev. Biol.-Plant 2006, 42, 206–214. [Google Scholar] [CrossRef]
- Engelmann, F. Use of biotechnologies for the conservation of plant biodiversity. Vitr. Cell. Dev. Biol.-Plant 2011, 47, 5–16. [Google Scholar] [CrossRef]
- Werden, L.K.; Sugii, N.C.; Weisenberger, L.; Keir, M.J.; Koob, G.; Zahawi, R.A. Ex situ conservation of threatened plant species in island biodiversity hotspots: A case study from Hawai‘i. Biol. Conserv. 2020, 243, 108435. [Google Scholar] [CrossRef]
- Pence, V.C.; Meyer, A.; Linsky, J.; Gratzfeld, J.; Pritchard, H.W.; Westwood, M.; Bruns, E.B. Defining exceptional species—A conceptual framework to expand and advance ex situ conservation of plant diversity beyond conventional seed banking. Biol. Conserv. 2022, 266, 109440. [Google Scholar] [CrossRef]
- Teketay, D. Germination ecology of twelve indigenous and eight exotic multipurpose leguminous species from Ethiopia. For. Ecol. Manag. 1996, 80, 209–223. [Google Scholar] [CrossRef]
- Smykal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef]
- Bewley, J.D. Seed germination and dormancy. Plant Cell 1997, 9, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef] [PubMed]
- Thanos, C.A.; Georghiou, K. Ecophysiology of fire-stimulated seed germination in Cistus incanus ssp. creticus (L.) Hey wood and C. salvifolius L. Plant Cell Environ. 1988, 11, 841–849. [Google Scholar] [CrossRef]
- Estrelles, E.; Güemes, J.; Riera, J.; Boscai, U.; Ibars, A.; Costa, M. Seed germination behavior in Sideritis from different Iberian habitats. Not. Bot. Horti Agrobot. 2010, 38, 9–13. [Google Scholar]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Uzun, S.; Yükselgüngör, D. Micropropagation of some Onobrychis species through in vitro shoot regeneration. Acta Sci. Pol. Hortorum Cultus 2020, 19, 45–52. [Google Scholar] [CrossRef]
- Kanellou, E.; Vlachou, G.; Martini, A.N.; Bertsouklis, K.F.; Papafotiou, M. Seed germination of five sage species (Salvia sp.) of populations native to Greece. Acta Hortic. 2022, 1345, 439–444. [Google Scholar] [CrossRef]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B 2009, 364, 1059–1074. [Google Scholar] [CrossRef]
- Donohue, K.; De Casas, R.R.; Burghardt, L.T.; Kovach, K.; Willis, C.G. Germination, post germination adaptation, and species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. Available online: https://www.annualreviews.org/doi/10.1146/annurev-ecolsys-102209-144715 (accessed on 28 June 2023). [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Zheng, Y.; Xie, Z.; Gao, Y.; Yu, Y.; Shimizu, H. Influence of light, temperature and water stress on germination of Hedysarum Fruticosum. South Afr. J. Bot. 2005, 71, 167–172. [Google Scholar] [CrossRef]
- Hu, X.W.; Wang, Y.R.; Wu, Y. Effects of the pericarp on imbibition, seed germination, and seedling establishment in seeds of Hedysarum scoparium Fisch. et Mey. Ecol. Res. 2008, 24, 559–564. [Google Scholar] [CrossRef]
- São José, J.F.B.; Volpiano, C.G.; Vargas, L.K.; Hernandes, M.A.S.; Lisboa, B.B.; Schlindwein, G.; Beneduzi, A.; Longoni, L.S.; Sampaio, J.A.T. Influence of hot water on breaking dormancy, incubation temperature and rhizobial inoculation on germination of Acacia mearnsii seeds. Aust. For. 2019, 82, 157–161. [Google Scholar] [CrossRef]
- Zembele, E.R.; Ngulube, E.S. Effect of seed pre-treatment methods on germination and early seedling growth of Senna spectabilis. Int. J. For. Res. 2022, 2022, 6731479. [Google Scholar] [CrossRef]
- Carneiro, L.; Araújo, R.; Brito, G.; Fonseca, M.H.P.B.; Costa, A.; Crocomo, O.J.; Mansur, E. In Vitro regeneration from leaf explants of Neoregelia cruenta (R. Graham) L.B. Smith, an endemic bromeliad from Eastern Brazil. Plant Cell Tissue Organ. 1998, 55, 79–83. [Google Scholar] [CrossRef]
- Tassoula, L.; Papafotiou, M.; Liakopoulos, G.; Kargas, G. Water use efficiency, growth and anatomic-physiological parameters of Mediterranean xerophytes as affected by substrate and irrigation on a green roof. Not. Bot. Horti Agrobot. 2021, 49, 12283. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Cretan Dittany (Origanum dictamnus L.), a Valuable Local Endemic Plant: In Vitro Regeneration Potential of Different Type of Explants for Conservation and Sustainable Exploitation. Plants 2023, 12, 182. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Vlachou, G. In Vitro propagation as a tool to enhance the use of native ornamentals in Archaeological sites of Greece. Acta Hortic. 2017, 1155, 301–308. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Maloupa, E. Micropropagation and salt tolerance of in vitro grown Crithmum maritimum L. Plant Cell Tissue Organ Cult. 2008, 94, 209–217. [Google Scholar] [CrossRef]
- Siddique, I.; Anis, M. Rapid micropropagation of Ocimum basilicum using shoot tip explants pre-cultured in thidiazuron supplemented in liquid medium. Biol. Plant 2007, 51, 787–790. [Google Scholar] [CrossRef]
- Faisal, M.; Alatar, A.A.; Hegazy, A.K.; Alharbi, S.A.; El-Sherikh, M.; Okla, M.K. Thidiazuron induced in vitro multiplication of Mentha arvensis and evaluation of genetic stability by flow cytometry and molecular markers. Ind. Crop Prod. 2014, 62, 100–106. [Google Scholar] [CrossRef]
- Papafotiou, M.; Kalantzis, A. Seed germination and in vitro propagation of Sideritis athoa. Acta Hortic. 2009, 813, 471–476. [Google Scholar] [CrossRef]
- Marco-Medina, A.; Casas, J.L. In vitro multiplication and essential oil composition of Thymus moroderi Pau ex Martinez, an endemic Spanish plant. Plant Cell Tissue Organ Cult. 2015, 120, 99–108. [Google Scholar] [CrossRef]
- Juan-Vicedo, J.; Ramírez-Luna, J.E.; Piqueras, A.; Casas, J.L. Micropropagation and cryopreservation by vitrification of the Spanish endemic medicinal plant Sideritis leucantha Cav. subsp. leucantha (Lamiaceae). Vitr. Cell. Dev. Biol.-Plant 2021, 57, 1057–1065. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M. Effects of plant growth regulators and environmental factors on in vitro propagation of X Malosorbus florentina L. Propag. Ornam. Plants 2013, 13, 112–122. [Google Scholar]
- Erişen, S.; Öncel, Z. In vitro propagation of the threatened plant Sphaerophysa kotschyana (Fabaceae): Inter simple-sequence-repeat (ISSR) analysis and salt tolerance of the regenerants. Aust. J. Bot. 2013, 61, 67. [Google Scholar] [CrossRef]
- Wiszniewska, A.; Nowak, B.; Kołton, A.; Sitek, E.; Grabski, K.; Dziurka, M.; Długosz-Grochowska, O.; Dziurka, K.; Tukaj, Z. Rooting response of Prunus domestica L. micro-shoots in the presence of phytoactive medium supplements. Plant Cell Tissue Organ. 2016, 125, 163–176. [Google Scholar] [CrossRef]
- Polanco, M.C.; Ruiz, M.L. Effect of benzylaminopurine on in vitro and in vivo root development in lentil (Lens culinaris Medik). Plant Cell Rep. 1997, 17, 22–26. [Google Scholar] [CrossRef]
- Polanco, M.C.; Ruiz, M.L. Factors that affect plant regeneration from in vitro culture of immature seeds in four lentil cultivars. Plant Cell Tissue Organ Cult. 2001, 66, 133–139. [Google Scholar] [CrossRef]
- Zhao, D.L.; Guo, G.Q.; Wang, X.Y.; Zheng, G.C. In vitro micropropagation of a medicinal plant species Sophora flavescens. Biol. Plant. 2003, 7, 117–120. [Google Scholar] [CrossRef]
- Guo, B.; Bilal, H.A.; Zeb, A.; Xu, L.; Wei, Y. Thidiazuron: A multi-dimensional plant growth regulator. Afr. J. Sci. Technol. Innov. Dev. 2011, 10, 8984–9000. [Google Scholar] [CrossRef]
- Amoo, S.O.; Finnie, J.F.; Van Staden, J. The role of meta-topolins in alleviating micropropagation problems. Plant Growth Regul. 2011, 63, 197–206. [Google Scholar] [CrossRef]
- Bunn, E.; Turner, S.R.; Dixon, K.W. Biotechnology for saving rare and threatened flora in a biodiversity hotspot. Vitr. Cell. Dev. Biol.-Plant. 2011, 47, 188–200. [Google Scholar] [CrossRef]
- Carra, A.; Catalano, C.; Badalamenti, O.; Carimi, F.; Pasta, S.; Motisi, A.; Abbate, L.; La Bella, F.; Fazan, L.; Kozlowski, G.; et al. Overcoming sexual sterility in conservation of endangered species: The prominent role of biotechnology in the multiplication of Zelkova sicula (Ulmaceae), a relict tree at the brink of extinction. Plant Cell Tissue Organ. 2019, 137, 139–148. [Google Scholar] [CrossRef]
- Benson, E.E. Sepecial symposium: In vitro plant recalcitrance in vitro plant recalcitrance: An introduction. Vitr. Cell. Dev. Biol.-Plant 2000, 36, 141–148. [Google Scholar] [CrossRef]
- Stevens, M.E.; Pijut, P.M. Rapid in vitro shoot multiplication of the recalcitrant species Juglans nigra L. Vitr. Cell. Dev. Biol.-Plant 2005, 54, 309–317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).