Dynamic Changes in Physicochemical and Microbiological Qualities of Coconut Water during Postharvest Storage under Different Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fresh Coconut Water Collection and Storage Conditions
2.2. Microbial Profile and Population Determination
2.3. Physicochemical and Compound Analysis
2.4. Analysis of Volatile Metabolites Using Headspace SPME-GC/MS
2.5. Comparison between Sensory Criteria and Laboratory Indexes for Fresh Coconut Water Quality
2.6. Statistics
3. Results and Discussion
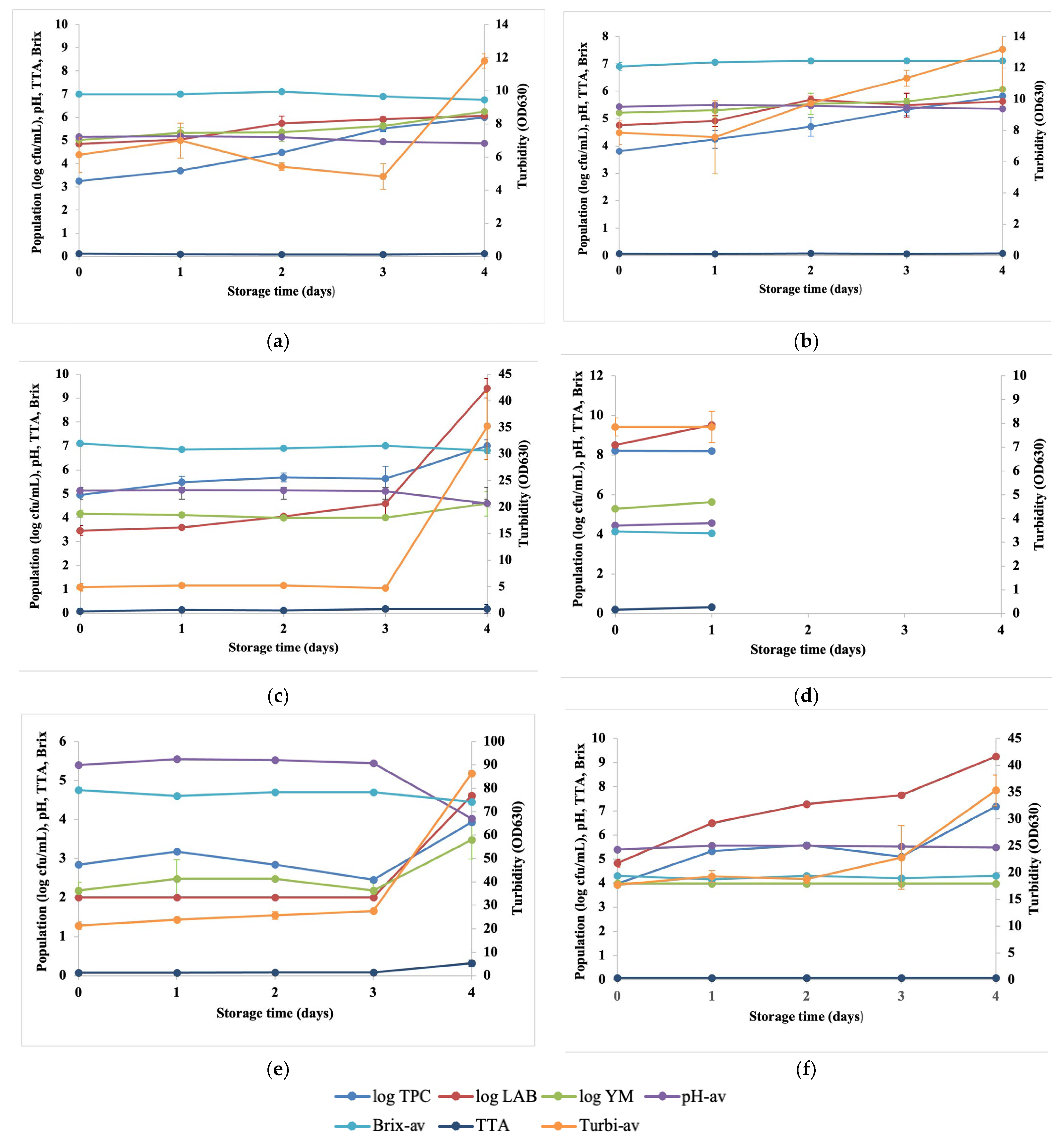
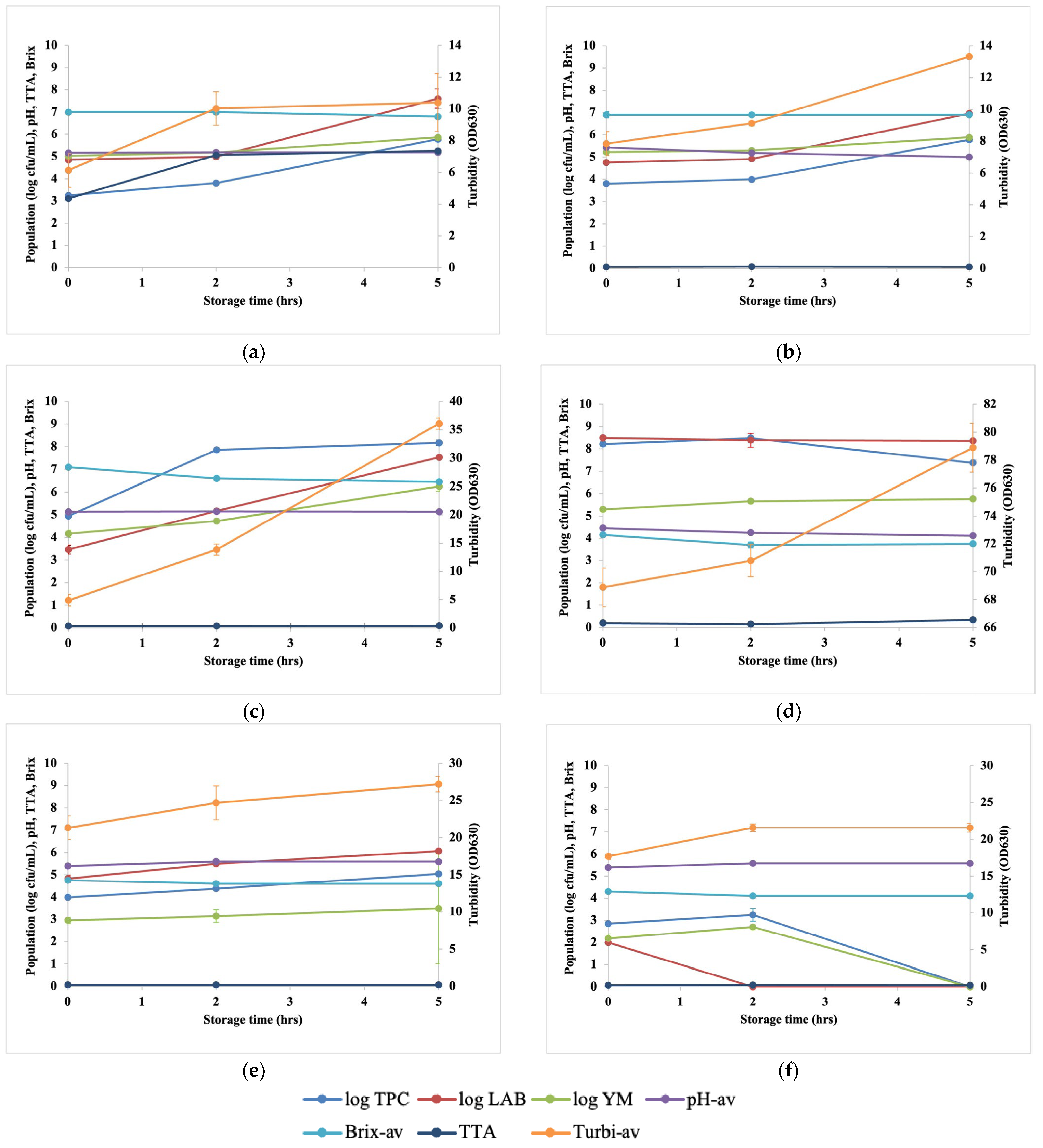
3.1. Changes in Microbial and Physicochemical Properties of Fresh Coconut Water under Different Storage Conditions
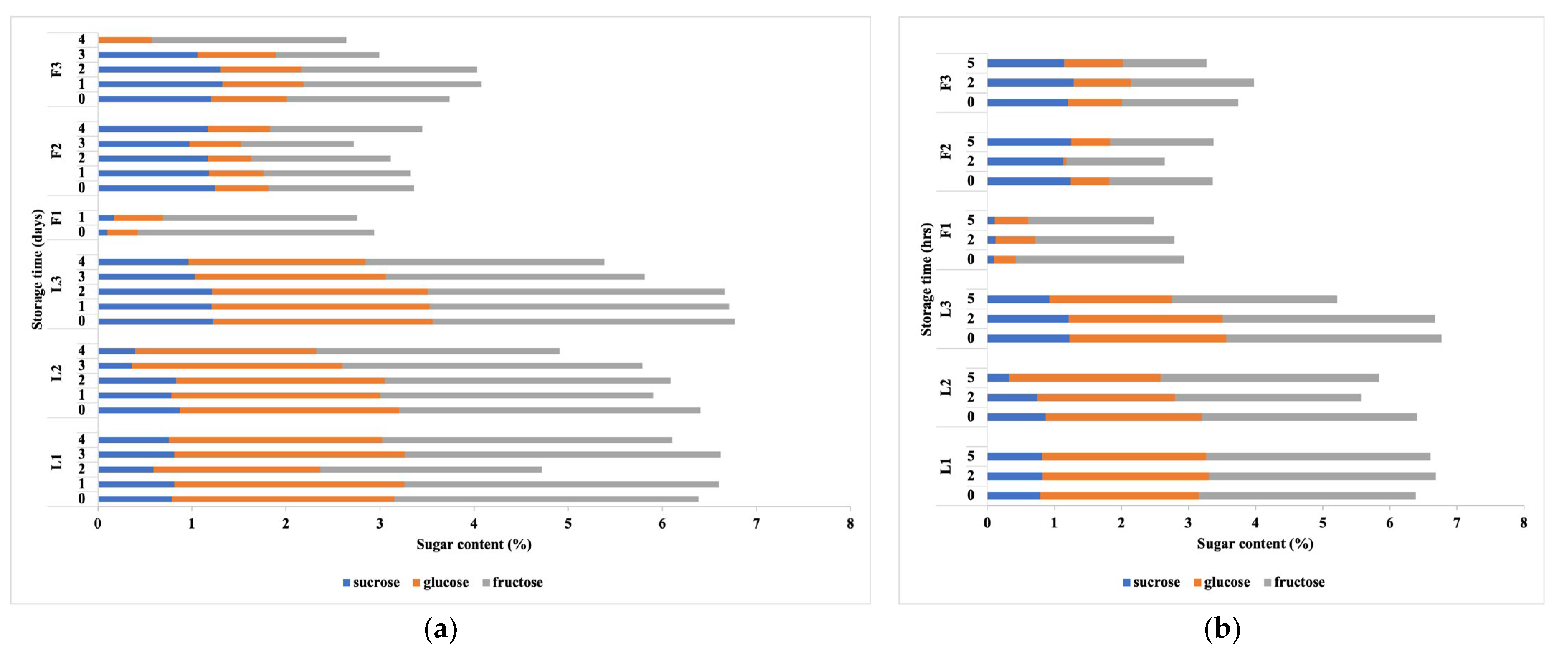
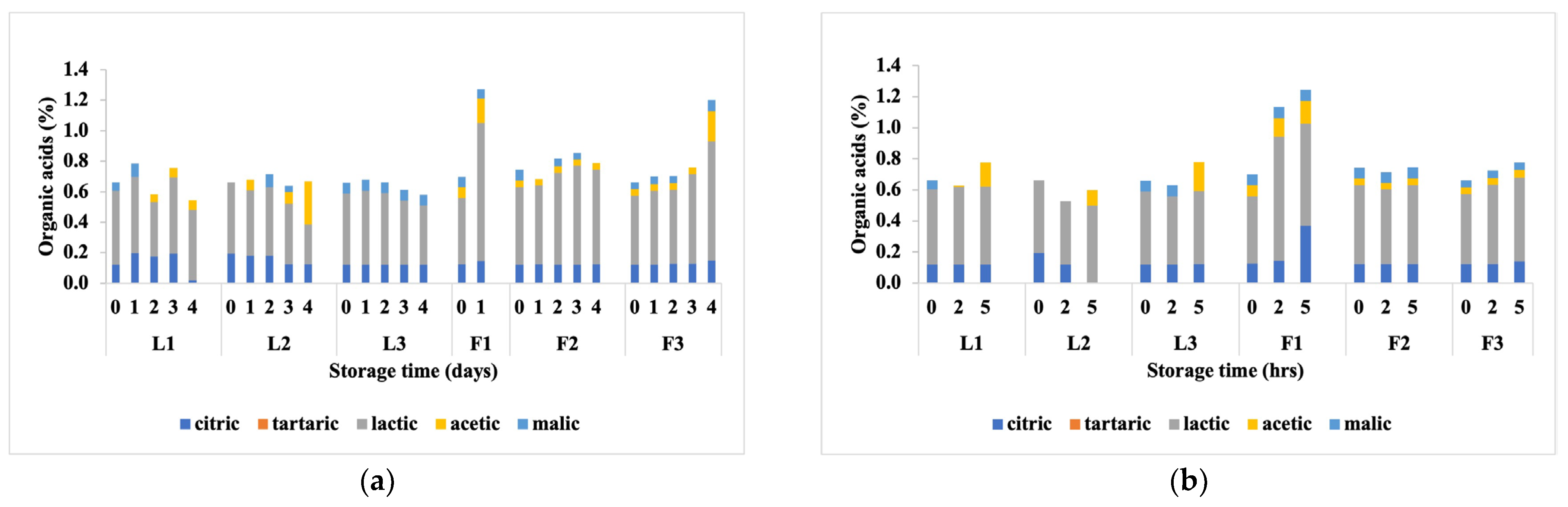
3.2. Changes in Sugars, Organic Acids and Volatile Metabolite Compounds under Different Storage Conditions
3.3. Properties of Fresh Coconut Water before and after Storage as Compared to Sensory Grading System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medeiros, A.C.; de Paiva, V.D.F.L. Therapeutic Use of Coconut Water. J. Surg. Res. 2013, 3, 83–91. [Google Scholar] [CrossRef]
- Jackson, J.C.; Gordon, A.; Wizzard, G.; McCook, K.; Rolle, R. Changes in Chemical Composition of Coconut (Cocos Nucifera) Water During Maturation of the Fruit. J. Sci. Food. Agric. 2004, 84, 1049–1052. [Google Scholar] [CrossRef]
- Rolle, R.S. Good Practice for the Small-Scale Production of Bottled Coconut Water; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Volume 1. [Google Scholar]
- Prades, A.; Manuel, D.; Nafissatou, D.; Pain, J.-P. Coconut Water Uses, Composition and Properties: A Review. Fruits 2012, 67, 87–107. [Google Scholar] [CrossRef]
- Yong, J.W.H.; Ge, L.; Ng, Y.F.; Tan, S.N. The Chemical Composition and Biological Properties of Coconut (Cocos nucifera L.) Water. Molecules 2009, 14, 5144–5164. [Google Scholar] [CrossRef] [PubMed]
- Awua, A.K.; Doe, E.D.; Agyare, R. Exploring the Influence of Sterilisation and Storage on Some Physicochemical Properties of Coconut (Cocos nucifera L.) Water. BMC Res. Notes 2011, 4, 451. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, C.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Alternatives to Conventional Thermal Treatments in Fruit-Juice Processing. Part 2: Effect on Composition, Phytochemical Content, and Physicochemical, Rheological, and Organoleptic Properties of Fruit Juices. Crit. Rev. Food Sci. Nutr. 2017, 57, 637–652. [Google Scholar] [CrossRef]
- Rydzak, L.; Kobus, Z.; Nadulski, R.; Wilczyński, K.; Pecyna, A.; Santoro, F.; Starek-Wójcicka, A.; Krzywicka, M. Analysis of Selected Physicochemical Properties of Commercial Apple Juices. Processes 2020, 8, 1457. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. Ph and Titratable Acidity. In Food Analysis; Springer International Publishing: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar] [CrossRef]
- Mao, D.-P.; Zhou, Q.; Chen, C.-Y.; Quan, Z.-X. Coverage Evaluation of Universal Bacterial Primers Using the Metagenomic Datasets. BMC Microbiol. 2012, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Cocolin, L.; Bisson, L.F.; Mills, D.A. Direct Profiling of the Yeast Dynamics in Wine Fermentations. FEMS Microbiol. Lett. 2000, 189, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical Evaluation of Two Primers Commonly Used for Amplification of Bacterial 16s rRNA Genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, H.; Chen, Z.; Lv, Z.; Xie, Y.; Lu, F. Profiling of Dynamic Changes in the Microbial Community During the Soy Sauce Fermentation Process. Appl. Microbiol. Biotechnol. 2013, 97, 9111–9119. [Google Scholar] [CrossRef] [PubMed]
- Chahorm, K.; Prakitchaiwattana, C. Application of Reverse Transcriptase-PCR-DGGE as a Rapid Method for Routine Determination of Vibrio Spp. in Foods. Int. J. Food Microbiol. 2018, 264, 46–52. [Google Scholar] [CrossRef] [PubMed]
- AOAC. International A: Official Methods of Analysis of the AOAC International; Horwitz, W., Ed.; The Association: Arlington County, VA, USA, 2000. [Google Scholar]
- Tan, T.-C.; Cheng, L.-H.; Bhat, R.; Rusul, G.; Easa, A.M. Composition, Physicochemical Properties and Thermal Inactivation Kinetics of Polyphenol Oxidase and Peroxidase from Coconut (Cocos Nucifera) Water Obtained from Immature, Mature and Overly-Mature Coconut. Food Chem. 2014, 142, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Nelson, N. A Photometric Adaptation of the Somogyi Method for the Determination of Glucose. J. Biol. Chem. 1944, 153, 375–380. [Google Scholar] [CrossRef]
- Chanprasartsuk, O.; Prakitchaiwattana, C.; Sanguandeekul, R.; Fleet, G.H. Autochthonous Yeasts Associated with Mature Pineapple Fruits, Freshly Crushed Juice and Their Ferments; and the Chemical Changes During Natural Fermentation. Bioresour. Technol. 2010, 101, 7500–7509. [Google Scholar] [CrossRef] [PubMed]
- Det-udom, R.; Settachaimongkon, S.; Chancharoonpong, C.; Suphamityotin, P.; Suriya, A.; Prakitchaiwattana, C. Factors Affecting Bacterial Community Dynamics and Volatile Metabolite Profiles of Thai Traditional Salt Fermented Fish. Food Sci. Technol. Int. 2023, 29, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Metsalu, T.; Vilo, J. Clustvis: A Web Tool for Visualizing Clustering of Multivariate Data Using Principal Component Analysis and Heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef]
- Reddy, K.V.; Das, M.; Das, S.K. Filtration resistances in non-thermal sterilization of green coconut water. J. Food Eng. 2005, 69, 381–385. [Google Scholar] [CrossRef]
- Gordon, A.; Jackson, J. Case Study: Application of Appropriate Technologies to Improve the Quality and Safety of Coconut Water. In Food Safety and Quality Systems in Developing Countries; Academic Press: Cambridge, MA, USA, 2017; pp. 185–216. [Google Scholar] [CrossRef]
- Elavarasi, V.; Pugazhendhi, A.; Priyadharsani, T.K.P.; Valsala, H.; Thamaraiselvi, K. Screening and Characterization of Weissella cibaria Isolated from Food Source for Probiotic Properties. Int. J. Comp. Appl. 2014, 1, 29–32. [Google Scholar]
- Yu, H.S.; Jang, H.J.; Lee, N.K.; Paik, H.D. Evaluation of the Probiotic Characteristics and Prophylactic Potential of Weissella cibaria Strains Isolated from Kimchi. LWT 2019, 112, 108229. [Google Scholar] [CrossRef]
- Dong, S.; Fan, L.; Ma, Y.; Du, J.; Xiang, Q. Inactivation of polyphenol oxidase by dielectric barrier discharge (DBD) plasma: Kinetics and mechanisms. LWT 2021, 145, 111322. [Google Scholar] [CrossRef]
- Pipliya, S.; Kumar, S.; Srivastav, P.P. Inactivation kinetics of polyphenol oxidase and peroxidase in pineapple juice by dielectric barrier discharge plasma technology. Innov. Food Sci. Emerg. Technol. 2022, 80, 103081. [Google Scholar] [CrossRef]
- Giri, S.S.; Sukumaran, V.; Sen, S.S.; Park, S.C. Use of a Potential Probiotic, Lactobacillus casei L4, in the Preparation of Fermented Coconut Water Beverage. Front. Microbiol. 2018, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- Othaman, M.; Sharifudin, S.; Mansor, A.; Abdul Kahar, A.; Long, K. Coconut Water Vinegar: New Alternative with Improved Processing Technique. J. Eng. Sci. Technol. 2014, 9, 293–302. [Google Scholar]
- Sucupira, N.R.; Alves Filho, E.G.; Silva, L.M.A.; de Brito, E.S.; Wurlitzer, N.J.; Sousa, P.H.M. NMR Spectroscopy and Chemometrics to Evaluate Different Processing of Coconut Water. Food Chem. 2017, 216, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, W.; Chen, W.; Chen, H. Improving the Quality of Matured Coconut (Cocos Nucifera Linn.) Water by Low Alcoholic Fermentation with Saccharomyces cerevisiae: Antioxidant and Volatile Profiles. J. Food Sci. Technol. 2018, 55, 964–976. [Google Scholar] [CrossRef]
- Xiang, W.L.; Zhang, N.D.; Lu, Y.; Zhao, Q.H.; Xu, Q.; Rao, Y.; Liu, L.; Zhang, Q. Effect of Weissella cibaria co-inoculation on the quality of Sichuan Pickle fermented by Lactobacillus plantarum. LWT 2020, 121, 108975. [Google Scholar] [CrossRef]
- Wang, D.; Chen, G.; Tang, Y.; Ming, J.; Huang, R.; Li, J.; Ye, M.; Fan, Z.; Chi, Y.; Zhang, Q.; et al. Study of bacterial community succession and reconstruction of the core lactic acid bacteria to enhance the flavor of paocai. Int. J. Food Microbiol. 2022, 375, 109702. [Google Scholar] [CrossRef]
- De Souza, A.S.; Coutinho, J.P.; de Souza, L.B.B.C.; Barbosa, D.P.; da Silva Júnior, A.L.S.; Paixão, M.V.S. Physical-Chemical Characterization of Fermented Coconut Water (Cocos nucifera L). Int. J. Adv. Eng. Res. Sci. 2020, 7, 247–255. [Google Scholar] [CrossRef]
- Kinderlerer, J.L.; Hatton, P.V.; Chapman, A.J.; Rose, M.E. Essential Oil Produced by Chrysosporium xerophilum in Coconut. Phytochemistry 1988, 27, 2761–2763. [Google Scholar] [CrossRef]
- Lin, F.M.; Wilkens, W.F. Volatile Flavor Components of Coconut Meat. J. Food Sci. 2006, 35, 538–539. [Google Scholar] [CrossRef]
- Jirapakkul, W.; Rodkwan, N.; Nasution, Z. Effect of Heat Treatment and Storage on Volatile Compounds of Coconut Milk. Ital. J. Food Sci. 2018, 5, 62–66. [Google Scholar]
- Tinchan, P.; Lorjaroenphon, Y.; Cadwallader, K.R.; Chaiseri, S. Changes in the Profile of Volatiles of Canned Coconut Milk During Storage. J. Food Sci. 2015, 80, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Jayalekshmy, A.; Narayanan, C.S.; Mathew, A.G. Identification of Volatile Flavor Compounds in Roasted Coconut. J. Am. Oil. Chem. Soc. 1991, 68, 873–880. [Google Scholar] [CrossRef]
- Gamero, A.; Quintilla, R.; Groenewald, M.; Alkema, W.; Boekhout, T.; Hazelwood, L. High-Throughput Screening of a Large Collection of Non-Conventional Yeasts Reveals Their Potential for Aroma Formation in Food Fermentation. Food Microbiol. 2016, 60, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The Potential Correlation between Bacterial Diversity and the Characteristic Volatile Flavour of Traditional Dry Sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Salmerón, I.; Thomas, K.; Pandiella, S.S. Effect of Potentially Probiotic Lactic Acid Bacteria on the Physicochemical Composition and Acceptance of Fermented Cereal Beverages. J. Funct. Foods 2015, 15, 106–115. [Google Scholar] [CrossRef]
- Chun, J.; Kwon, D.-Y.; Kim, J.S.; Kim, J.-H. Sensory Properties of Soy Yoghurts Prepared from Yellow and Black Soymilk Using Streptococcus Infantarius 12 and Weisellia sp. 4. J. Sci. Food Agric. 2008, 88, 1845–1849. [Google Scholar] [CrossRef]
- Bongers, R.S.; Hoefnagel, M.H.N.; Kleerebezem, M. High-Level Acetaldehyde Production in Lactococcus Lactis by Metabolic Engineering. Appl. Environ. Microbiol. 2005, 71, 1109–1113. [Google Scholar] [CrossRef]
- Hahn, F. An on-Line Detector for Efficiently Sorting Coconut Water at Four Stages of Maturity. Biosyst. Eng. 2012, 111, 49–56. [Google Scholar] [CrossRef]

| Sample | Initial Microbes | Temperature | Main Microbes at the End of Storage |
|---|---|---|---|
| Young coconut water (street food-type preparation) L1 | Weissella cibaria Weissella spp. Leuconostoc spp. | AT (5 h) | Weissella cibaria Weissella spp. Leuconostoc spp. |
| 4 °C (4 days) | Weissella cibaria Weissella spp. Leuconostoc sp | ||
| Mature coconut water (street food-type preparation) L2 | Weissella cibaria Weissella spp. Leuconostoc spp. | AT (5 h) | Weissella cibaria Weissella spp. |
| 4 °C (4 days) * with low-intensity band | Weissella cibaria Weissella spp. Leuconostoc spp. | ||
| Mature coconut water (street food-type preparation) L3 | Weissella cibaria Weissella spp. Klebsiella pneumoniae | AT (5 h) | Weissella cibaria Weissella spp. Leuconostoc spp. Erwinia spp. |
| 4 °C (4 days) | Weissella cibaria Weissella spp. Leuconostoc spp. | ||
| Industrial supplied coconut water F1 | Weissella cibaria Weissella spp. Leuconostoc spp. Klebsiella pneumoniae | AT (5 h) | Weissella spp. |
| 4 °C (1 day) | Weissella spp. | ||
| Industrial supplied coconut water F2 | Weissella cibaria Weissella spp. Leuconostoc spp. Escherichia coli | AT (5 h) | Weissella cibaria Weissella spp. Leuconostoc spp. |
| 4 °C (4 days) | Weissella cibaria Weissella spp. Leuconostoc spp. | ||
| Industrial supplied coconut water F3 | Weissella cibaria Weissella spp. Leuconostoc spp. Klebsiella pneumoniae | AT (5 h) | Weissella spp. |
| 4 °C (4 days) | Weissella spp. |
| TPC | Turbidity | TTA | LAB | Turbidity | TTA | YM | Turbidity | TTA | Grade Range | Sense Based Spoilage |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.97 ± 0.29 e | 19.91 ± 3.08 b | 0.08 ± 0.02 c | 2.50 ± 0.64 f | 19.91 ± 3.08 c | 0.07 ± 0.01 d | 2.33 ± 0.15 d | 20.24 ± 3.45 ab | 0.07 ± 0.00 b | 2–5 | Clear to slightly turbid and coconut-like aroma |
| 4.04 ± 0.27 d | 12.87 ± 7.71 b | 0.08 ± 0.01 c | 4.08 ± 0.50 e | 5.05 ± 0.28 c | 0.08 ± 0.03 bc | 3.18 ± 0.25 c | 23.50 ± 3.12 ab | 0.08 ± 0.02 b | 2–5 | |
| 5.09 ± 0.25 c | 14.62 ± 10.20 b | 0.09 ± 0.03 c | 5.01 ± 0.28 d | 12.89 ± 6.85 c | 0.08 ± 0.02 cd | 3.98 ± 0.21 c | 5.07 ± 0.26 b | 0.17 ± 0.09 a | 2 | Slightly turbid and less coconut-like aroma |
| 5.72 ± 0.15 b | 11.14 ± 6.93 b | 0.11 ± 0.04 b | 5.94 ± 0.29 c | 13.71 ± 8.95 c | 0.09 ± 0.02 bcd | 5.17 ± 0.28 b | 11.44 ± 8.37 b | 0.11 ± 0.04 ab | 0 | Turbid and ferment odor |
| 7.81 ± 0.55 a | 58.66 ± 26.50 a | 0.21 ± 0.10 a | 7.78 ± 0.57 b | 41.44 ± 27.37 b | 0.11 ± 0.04 b | 5.86 ± 0.25 a | 32.98 ± 32.98 a | 0.15 ± 0.10 ab | 0 | |
| 8.83 ± 0.00 a | 82.58 ± 0.00 a | 0.34 ± 0.00 a | 9.25 ± 0.3 a | 70.85 ± 23.9 a | 0.29 ± 0.08 a | 7.94 ± 0.00 a | 82.58 ± 0.00 a | 0.34 ± 0.00 a | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Detudom, R.; Deetae, P.; Wei, H.; Boran, H.; Chen, S.; Siriamornpun, S.; Prakitchaiwattana, C. Dynamic Changes in Physicochemical and Microbiological Qualities of Coconut Water during Postharvest Storage under Different Conditions. Horticulturae 2023, 9, 1284. https://doi.org/10.3390/horticulturae9121284
Detudom R, Deetae P, Wei H, Boran H, Chen S, Siriamornpun S, Prakitchaiwattana C. Dynamic Changes in Physicochemical and Microbiological Qualities of Coconut Water during Postharvest Storage under Different Conditions. Horticulturae. 2023; 9(12):1284. https://doi.org/10.3390/horticulturae9121284
Chicago/Turabian StyleDetudom, Rachatida, Pawinee Deetae, Hu Wei, Hu Boran, Shiguo Chen, Sirithon Siriamornpun, and Cheunjit Prakitchaiwattana. 2023. "Dynamic Changes in Physicochemical and Microbiological Qualities of Coconut Water during Postharvest Storage under Different Conditions" Horticulturae 9, no. 12: 1284. https://doi.org/10.3390/horticulturae9121284
APA StyleDetudom, R., Deetae, P., Wei, H., Boran, H., Chen, S., Siriamornpun, S., & Prakitchaiwattana, C. (2023). Dynamic Changes in Physicochemical and Microbiological Qualities of Coconut Water during Postharvest Storage under Different Conditions. Horticulturae, 9(12), 1284. https://doi.org/10.3390/horticulturae9121284







