Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Bioertilizers Used in the Experiment
2.3. Plant Growing Conditions
2.4. Plant Nutrition
2.5. Lettuce Harvest
2.6. Measurements of Plant Growth Parameters
2.7. Measurements of Total Soluble Solids (TSS), pH and Electrical Conductivity (EC) in Lettuce Leaves
2.8. Measurement of Nitrate Concentration
2.9. Measurement of Ascorbic Acid Content (Vitamin C)
2.10. Measurement of Total Phenolic and Flavonid Substances
2.11. Leaf Mineral Nutrient Analysis
2.12. Statistical Analyses
3. Results and Discussion
3.1. Effects of Biofertilizers on Plant Growth Parameters
3.2. Effects of Biofertilizers on Leaf Color Properties
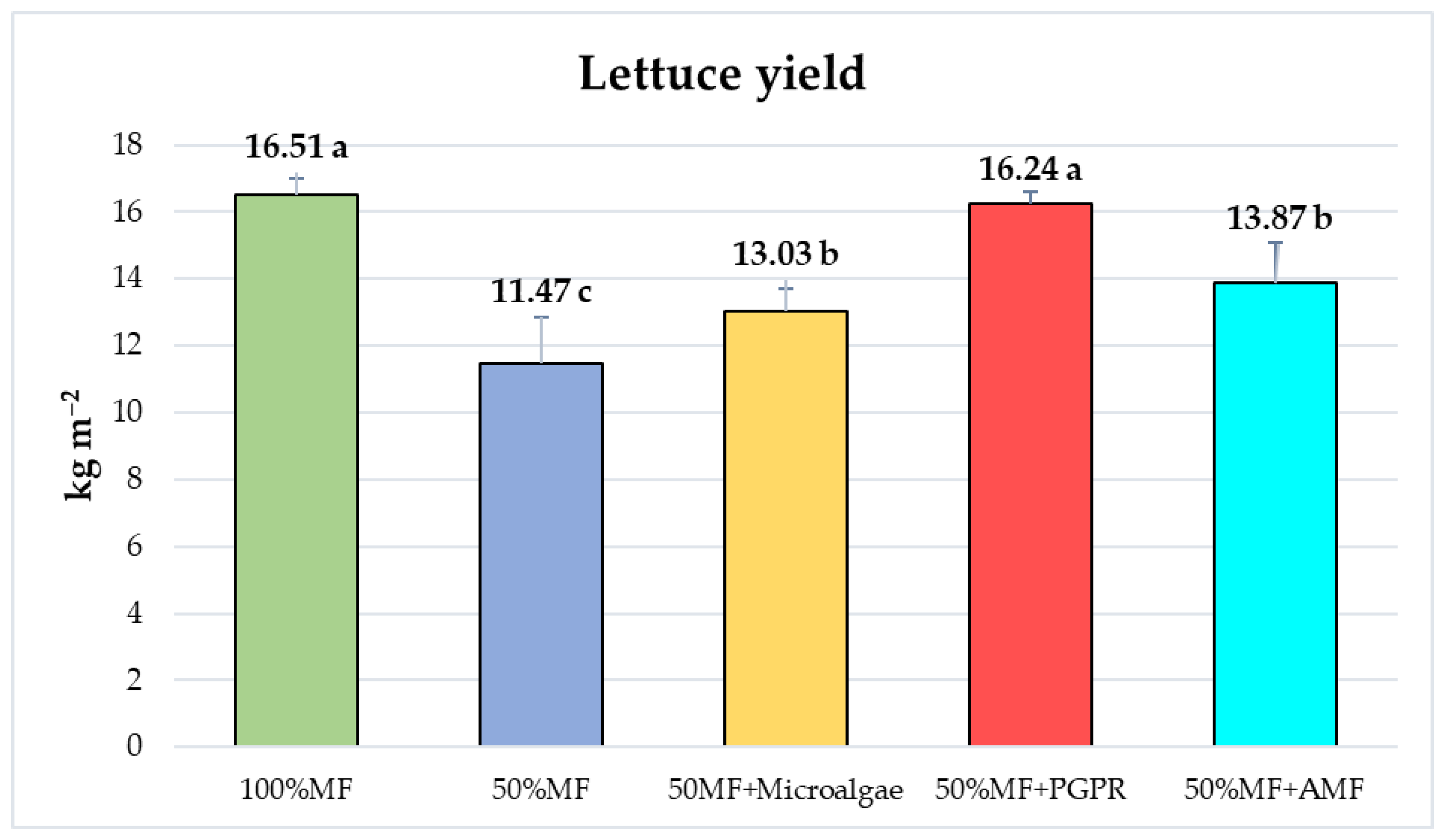
3.3. Effects of Biofertilizers on Lettuce Yield
3.4. Effects of Biofertilizers on Leaf Nutritional and Antioxidan Compounds
3.5. Nitrate Concentration in Lettuce Leaves
3.6. Effects of Biofertilizers on Macro and Micro Nutrients in Lettuce Leaves
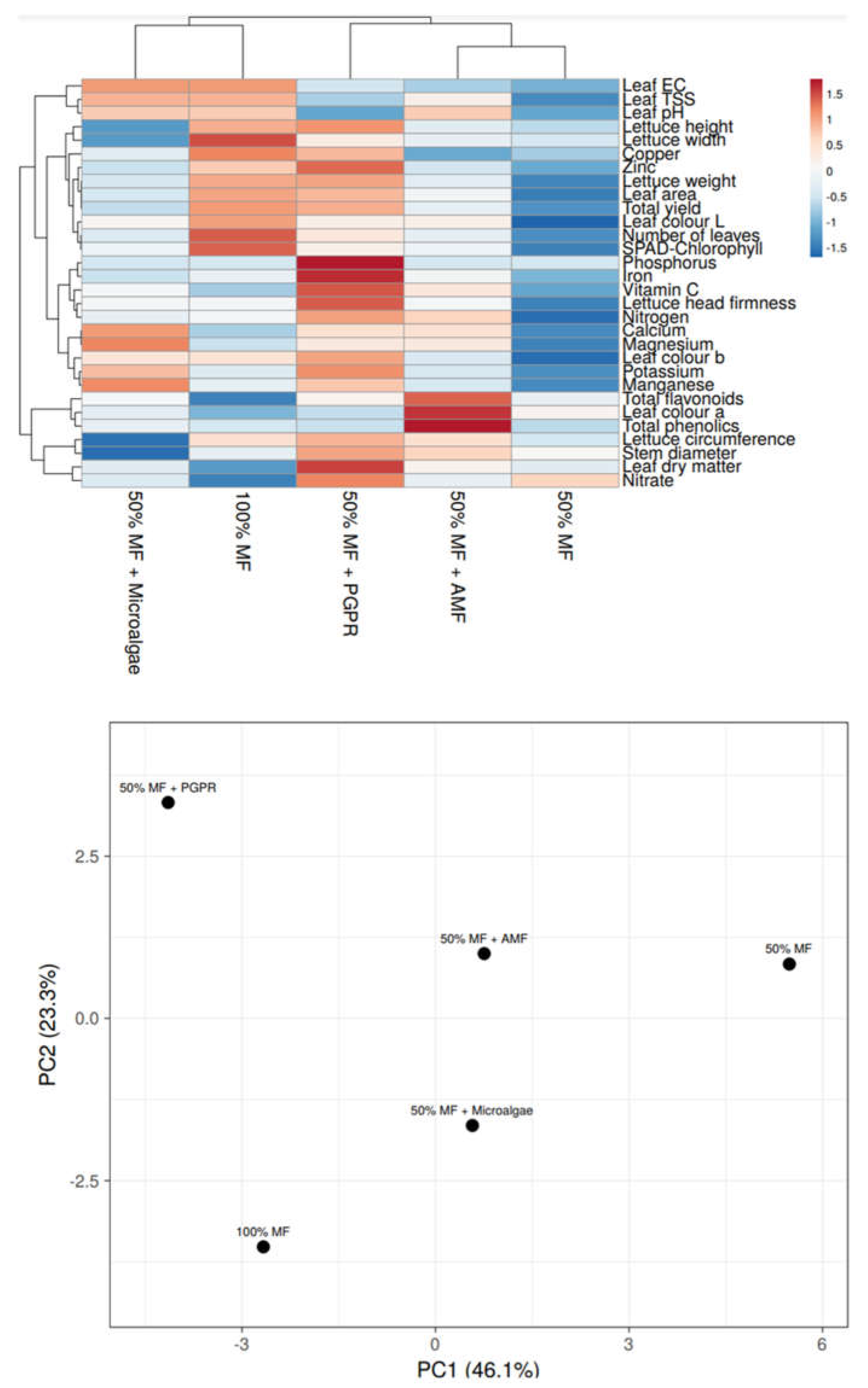
3.7. Heat Map and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Majid, M.; Khan, J.N.; Ahmad Shah, Q.M.; Masoodi, K.Z.; Afroza, B.; Parvaze, S. Evaluation of hydroponic systems for the cultivation of Lettuce (Lactuca sativa L., var. longifolia) and comparison with protected soil based cultivation. Agric. Water Manag. 2021, 245, 106572. [Google Scholar]
- Gruda, N.S. Increasing sustainability of growing media constituents and stand-alone substrates in soilless culture systems. Agronomy 2019, 9, 298. [Google Scholar] [CrossRef]
- Gumisiriza, M.S.; Ndakidemi, P.; Nalunga, A.; Mbega, E.R. Building sustainable societies through vertical soilless farming: A cost-effectiveness analysis on a small-scale non-greenhouse hydroponic system. Sustain. Cities Soc. 2022, 83, 103923. [Google Scholar] [CrossRef]
- Kloas, W.; Groß, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef]
- Murad, S.A.Z.; Harun, A.; Mohyar, S.N.; Sapawi, R.; Ten, S.Y. Design of aquaponics water monitoring system using arduino microcontroller. AIP Confer. Proc. 2017, 1885, 5002442. [Google Scholar]
- Mohammed, A.A.; Söylemez, S.; Sarhan, T.Z. Effect of biofertilizers, seaweed extract and inorganic fertilizer on growth and yield of lettuce (Lactuca sativa var. longifolia L.). Harran Agric. Food Sci. 2022, 26, 60–71. [Google Scholar] [CrossRef]
- Singh, A.; Ranawat, B.; Rank, M. Biofertilizer technologies for better crop nutrient—A sustainable smart agriculture. In Smart Agriculture for Developing Nations; Pakeerathan, K., Ed.; Advanced Technologies and Societal Change; Springer: Singapore, 2023; pp. 83–202. [Google Scholar]
- Zappelini, J.; Pescador, R.; Girardello, G.M.; de Souza, P.P.; Borghezan, M.; Oliveira, J.L. Physiological alterations in ‘Rubinela’lettuce (Lactuca sativa L.) cultivated in conventional and hydroponic systems. Acta Sci. Agron. 2024, 46, e62502. [Google Scholar]
- Savvas, D.; Gruda, N. Application of soilless culture technologies in the modern greenhouse industry—A review. Eur. J. Hortic. Sci. 2018, 83, 280–293. [Google Scholar] [CrossRef]
- Gruda, N.S. Advances in Soilless Culture and Growing Media in Today’s Horticulture—An Editorial. Agronomy 2022, 12, 2773. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Z.; Luo, Y. Advances and emerging trends in cultivation substrates for growing sprouts and microgreens toward safe and sustainable agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Gumisiriza, M.S.; Ndakidemi, P.A.; Mbega, E.R. Memoir and farming structures under soil-less culture (Hydroponic Farming) and the applicability for Africa: A Review. Agric. Rev. 2020, 41, 139–145. [Google Scholar] [CrossRef]
- Khater, E.S.; Bahnasawy, A.; Abass, W.; Morsy, O.; El-Ghobashy, H.; Shaban, Y.; Egela, M. Production of basil (Ocimum basilicum L.) under different soilless cultures. Sci. Rep. 2021, 11, 12754. [Google Scholar] [CrossRef] [PubMed]
- Wada, T. Theory and technology to control the nutrient solution of hydroponics. In Plant Factory Using Artificial Light; Elsevier: Amsterdam, The Netherlands, 2019; pp. 5–14. [Google Scholar]
- Santini, C.; Cavicchi, A. The challenging path towards a hydroponic indoor home cultivation system: The case of Nutritower. In Plant-Based Food Consumption; Woodhead Publishing: Sawston, UK, 2024; pp. 245–254. [Google Scholar]
- Mehra, M.; Saxena, S.; Sankaranarayanan, S.; Tom, R.J.; Veeramanikandan, M. IoT based hydroponics system using deep neural networks. Comput. Electron. Agric. 2018, 155, 473–486. [Google Scholar] [CrossRef]
- Rufí-Salís, M.; Calvo, M.J.; Petit-Boix, A.; Villalba, G.; Gabarrell, X. Exploring nutrient recovery from hydroponics in urban agriculture: An environmental assessment. Resour. Conserv. Recycl. 2020, 155, 104683. [Google Scholar] [CrossRef]
- Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic agriculture and microbial safety of vegetables: Promises, challenges, and solutions. Horticulturae 2023, 9, 51. [Google Scholar] [CrossRef]
- Harsela, C.N. Growth and yields of bima brebes shallot variety planted using a floating hydroponics system. Eduvest-J. Univers. Stud. 2023, 3, 1381–1388. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti Agrobot. 2016, 44, 603–612. [Google Scholar] [CrossRef]
- Velazquez-Gonzalez, R.S.; Garcia-Garcia, A.L.; Ventura-Zapata, E.; Barceinas-Sanchez, J.D.O.; Sosa-Savedra, J.C. A review on hydroponics and the technologies associated for medium- and small-scale operations. Agriculture 2022, 12, 646. [Google Scholar] [CrossRef]
- Fabek Uher, S.; Radman, S.; Opačić, N.; Dujmović, M.; Benko, B.; Lagundžija, D.; Mijić, V.; Prša, L.; Babac, S.; Šic Žlabur, J. Alfalfa, cabbage, beet and fennel microgreens in floating hydroponics—Perspective nutritious food? Plants 2023, 12, 2098. [Google Scholar] [CrossRef]
- Park, Y.; Williams, K.A. Organic hydroponics: A review. Sci. Hortic. 2024, 324, 112604. [Google Scholar] [CrossRef]
- Boubaker, H.; Da¸sgan, H.Y.; Tarchoun, N. Effects of the bio-fertilizers on potato mini tubers number and size produced from tissue culture plants. Int. J. Agric. Environ. Food Sci. 2021, 5, 514–523. [Google Scholar] [CrossRef]
- Singh, K.; Guleria, V.; Kaushal, S. Utilization of Biofertilizers and plant growth promoters in hydroponic production system. Curr. J. Appl. Sci. Technol. 2023, 42, 13–23. [Google Scholar] [CrossRef]
- Setiawati, M.R.; Afrilandha, N.; Hindersah, R.; Suryatmana, P.; Fitriatin, B.N.; Kamaluddin, N.N. The effect of beneficial microorganism as biofertilizer application in hydroponic-grown tomato. Sains Tanah J. Soil Sci. Agroclimatol. 2023, 20, 66–77. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Kumar, G.N.; Parekh, L.J.; Poole, P.S. Roleof soil microorganisms in improving P nutrition of plants. Plant Soil 2002, 245, 83–93. [Google Scholar] [CrossRef]
- Dhawi, F. The Role of plant growth-promoting microorganisms (PGPMs) and their feasibility in hydroponics and vertical farming. Metabolites 2023, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Boubaker, H.; Saadaoui, W.; Dasgan, H.Y.; Tarchoun, N.; Gruda, N.S. Enhancing seed potato production from in vitro plantlets and microtubers through biofertilizer application: Investigating effects on plant growth, tuber yield, size, and quality. Agronomy 2023, 13, 2541. [Google Scholar] [CrossRef]
- Menamoa, M.; Woldeb, Z. Effect of cyanobacteria application as biofertilizer on growth, yield and yield components of romaine lettuce (Lactuca sativa L.) on soils of Ethiopia. Amer. Sci. Res. J. Eng. Tech. Sci. 2013, 4, 50–58. [Google Scholar]
- Al-Taey, D.K.A.; Majid, Z.Z. The Activity of antioxidants enzymes and NPK contents as affected by water quality, kinetin, bio and organic fertilization in lettuce (Lactuca sativa L.). Iraqi J. Agric. Sci. 2018, 49, 506–518. [Google Scholar]
- Miceli, A.; Moncada, A.; Vetrano, F. Use of microbial biostimulants to increase the salinity tolerance of vegetable transplants. Agronomy 2021, 11, 1143. [Google Scholar] [CrossRef]
- Joshi, E.; Gupta, V.; Sasode, D.S.; Tiwari, S.; Sikarwar, R.S.; Singh, N. Liquid bıofertilizer and inorganic nutrients application impact on quality traits and physiology of kharif groundnut (Arachıs hypogea L.). Proc. PSMB 2018, 15, 67–74. [Google Scholar]
- Dasgan, H.Y.; Cetinturk, T.; Altuntas, O. The effects of biofertilisers on soilless organically grown greenhouse tomato. Acta Hortic. 2017, 1164, 555–561. [Google Scholar] [CrossRef]
- Dere, S.; Coban, A.; Akhoundnejad, Y.; Ozsoy, S.; Dasgan, H.Y. Use of mycorrhiza to reduce mineral fertilizers in soilless melon (Cucumis melo L.) cultivation. Not. Bot. Horti Agrobot. 2019, 47, 1331–1336. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, J.K. Complete reutilisation of mixed mackerel and brown seaweed wastewater as a high-quality biofertiliser in open-flow lettuce hydroponics. J. Clean. Prod. 2020, 247, 119081. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Aldiyab, A.; Elgudayem, F.; Ikiz, B.; Gruda, N.S. Effect of biofertilizers on leaf yield, nitrate amount, mineral content and antioxidants of basil (Ocimum basilicum L.) in a floating culture. Sci. Rep. 2022, 12, 20917. [Google Scholar] [CrossRef] [PubMed]
- Dasgan, H.Y.; Kacmaz, S.; Arpaci, B.B.; İkiz, B.; Gruda, N.S. Biofertilizers improve the leaf quality of hydroponically grown baby spinach (Spinacia oleracea L.). Agronomy 2023, 13, 575. [Google Scholar] [CrossRef]
- Ergun, O.; Dasgan, H.Y.; Isik, O. Effects of microalgae Chlorella vulgaris on hydroponically grown lettuce. Acta Hortic. 2020, 1273, 169–175. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Haroon, M.H.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Elgailani, I.E.H.; Elkareem, M.A.M.G.; Noh, E.A.A.; Adam, O.E.A.; Alghamdi, A.M.A. Comparison of two methods for the determination of vitamin C (Ascorbic Acid) in some fruits. Am. J. Chem. 2017, 2, 1–7. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of thompson seedless grape Juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Quettier-Deleu, C.; Gressier, B.; Vasseur, J.; Dine, T.; Brunet, C.; Luyckx, M.; Cazin, M.; Bailleul, F.; Trotin, F. Phenolic compounds and antioxidant activities of buckwheat (Fagopyrum esculentum Moench) hulls and flour. J. Ethnopharmacol. 2000, 72, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Arshad, M.; Hussain, S.; Bhatti, A.S. Perspective of plant growth promoting rhizobacteria (PGPR) containing ACC deaminase in stress agriculture. J. Ind. Microbiol. Biotechnol. 2007, 34, 635–648. [Google Scholar] [CrossRef] [PubMed]
- El-Tohamy, W.A.; El-Abagy, H.M.; El-Greadly, N.H.M.; Gruda, N. Hormonal changes, growth and yield of tomato plants in response to chemical and bio-fertilization application in sandy soils. J. Appl. Bot. Food Qual. 2009, 82, 179–182. [Google Scholar]
- Shabani, E. Improving the growth, P uptake and quality characteristics of ‘Lollo Rosso’lettuce in the nutrient solution by Bacillus subtilis in different phosphorus concentrations. J. Plant Nutr. 2023, 46, 971–983. [Google Scholar] [CrossRef]
- Jabborova, D.; Annapurna, K.; Paul, S.; Kumar, S.; Saad, H.A.; Desouky, S.; Ibrahim, M.F.M.; Elkelish, A. beneficial features of biochar and arbuscular mycorrhiza for ımproving spinach plant growth, root morphological traits, physiological properties, and soil enzymatic activities. J. Fungi 2021, 7, 571. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.; El-Tohamy, W.; Gruda, N. Increasing the productivity and product quality of vegetable crops using arbuscular mycorrhizal fungi: A review. Sci. Hortic. 2015, 187, 131–141. [Google Scholar] [CrossRef]
- Khan, A.G.; Kuek, C.; Chaudhry, T.M.; Khoo, C.S.; Hayes, W.J. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Azizoglu, U.; Yilmaz, N.; Simsek, O.; Ibal, J.C.; Tagele, S.B.; Shin, J.H. The fate of plant growth-promoting rhizobacteria in soilless agriculture: Future perspectives. 3 Biotech. 2021, 11, 382. [Google Scholar] [CrossRef]
- Prasad, M.R.; Srinivasan, M.; Chaudhary, M.; Choudhary, M.; Jat, L.K. Plant growth promoting rhizobacteria (PGPR) for sustainable agriculture: Perspectives and challenges. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Kumar, A., Singh, P.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 129–157. [Google Scholar]
- Bhat, T.A.; Ahmad, L.; Ganai, M.A.; Shams-Ul-Haq; Khan, O.A. Nitrogen fixing biofertilizers; mechanism and growth promotion: A review. J. Pure Appl. Microbiol. 2015, 9, 1675–1690. [Google Scholar]
- Dasgan, H.Y.; Yilmaz, M.; Dere, S.; İkiz, B.; Gruda, N.S. Bio-fertilizers reduced the need for the mineral fertilizers in soilless grown capia pepper. Horticulturae 2023, 9, 188. [Google Scholar] [CrossRef]
- Dasgan, H.Y.; Temtek, T. Impact of Biofertilizers on Plant Growth, Physiological and Quality Traits of Lettuce (Lactuca sativa L. var. longifolia) Grown under Salinity Stress; IntechOpen: London, UK, 2023; pp. 1–14. [Google Scholar]
- Özer Uyar, G.E.; Mismil, N. Symbiotic association of microalgae and plants in a deep water culture system. PeerJ 2022, 10, e14536. [Google Scholar] [CrossRef] [PubMed]
- Ng, Z.Y.; Ajeng, A.A.; Cheah, W.Y.; Ng, E.P.; Abdullah, R.; Ling, T.C. Towards circular economy: Potential of microalgae–bacterial-based biofertilizer on plants. J. Environ. Manag. 2024, 349, 119445. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Srivastava, P.K.; Singh, R.P. Growth promotion and zinc biofortification in lettuce (Lactuca sativa L.) by the application of Talaromyces strain as a biostimulant. Sci. Hortic. 2024, 323, 112534. [Google Scholar] [CrossRef]
- Gustab, M.; Ważny, R.; Jędrzejczyk, R.J.; Kalisz, A.; Domka, A.; Nosek, M.; Tokarz, K.; Rozpądek, P. Beneficial impact of multi-bacterial inoculation on growth of selected Brassicaceae seedlings in a greenhouse culture. Sci. Hortic. 2024, 324, 112575. [Google Scholar] [CrossRef]
- Coban, G.A.; Dasgan, H.Y.; Akhoundnejad, Y.; Cimen, B.A. Use of microalgae (Chlorella vulgaris) to save mineral nutrients in soilless grown tomato. Acta Hortic. 2020, 1273, 161–168. [Google Scholar] [CrossRef]
- Stojanović, M.; Petrović, I.; Žuža, M.; Jovanović, Z.; Moravčević, Đ.; Cvijanović, G.; Savić, S. The productivity and quality of lactuca sativa as influenced by microbiological fertilisers and seasonal conditions. Zemdirbyste 2020, 107, 345–352. [Google Scholar] [CrossRef]
- Alam, M.Z.; Choudhury, T.R.; Mridha, M.A.U. Arbuscular mycorrhizal fungi enhance biomass growth, mineral content, and antioxidant activity in tomato plants under drought stress. J. Food Qual. 2023, 2023, 2581608. [Google Scholar] [CrossRef]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef]
- Kaymak, H.Ç.; Aksoy, A.; Kotan, R. Inoculation with n2-fixing plant growth promoting rhizobacteria to reduce nitrogen fertilizer requirement of lettuce. Acta Sci. Pol. Hortorum Cultus 2020, 19, 23–35. [Google Scholar] [CrossRef]
- Vetrano, F.; Miceli, C.; Angileri, V.; Frangipane, B.; Moncada, A.; Miceli, A. Effect of bacterial inoculum and fertigation management on nursery and field production of lettuce plants. Agronomy 2020, 10, 1477. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Miceli, A. Alleviation of salt stress by plant growth? promoting bacteria in hydroponic leaf lettuce. Agronomy 2020, 10, 1523. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Nitrate in vegetables—Scientific opinion of the panel on contaminants in the food chain. Eur. Food Saf. Auth.-EFSA J. 2008, 6, 689. [Google Scholar]
- Briccoli, B.C.; Santilli, E.; Lombardo, L. Effect of arbuscular mycorrhizal fungi on growth and on micronutrient and macronutrient uptake and allocation in olive plantlets growing under high total Mn levels. Mycorrhiza 2015, 25, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, E.; Pirdashti, H.; Shahsavarpour Lendeh, K.; Gilani, Z.; Yaghoubi Khanghahi, M.; Crecchio, C. Effects of plant growth promoting microorganisms inoculums on mineral nutrition, growth and productivity of rice (Oryza sativa L.). J. Plant Nutr. 2020, 43, 1643–1660. [Google Scholar] [CrossRef]
- Begum, N.; Wang, L.; Ahmad, H.; Akhtar, K.; Roy, R.; Khan, M.I.; Zhao, T. Co-inoculation of arbuscular mycorrhizal fungi and the plant growth-promoting rhizobacteria improve growth and photosynthesis in tobacco under drought stress by up-regulating antioxidant and mineral nutrition metabolism. Microb. Ecol. 2022, 83, 971–988. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hamel, C.; Hamilton, R.I.; Ma, B.L.; Smith, D.L. Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 2000, 9, 331–336. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- Karlidag, H.; Esitken, A.; Turan, M.; Sahin, F. Effects of root inoculation of plant growth promoting rhizobacteria (PGPR) on yield, growth and nutrient element contents of leaves of apple. Sci. Hortic. 2007, 114, 16–20. [Google Scholar] [CrossRef]
- Pacheco, D.; Cotas, J.; Pereira, L.; Bahcevandziev, K. A Possible synergistic approach: Case study of Saccharina latissima extract and nitrifying bacteria in lettuce. J. Mar. Sci. Eng. 2023, 11, 1645. [Google Scholar] [CrossRef]
- Corrado, G.; Lucini, L.; Miras-Moreno, B.; Zhang, L.; Senizza, B.; Basile, B.; Rouphael, Y. Dataset on the effects of different pre-harvest factors on the metabolomics profile of lettuce (Lactuca sativa L.) leaves. Data 2020, 5, 119. [Google Scholar] [CrossRef]


| Stock A | Stock B |
|---|---|
| Calcium nitrate | Potassium sulfate |
| Fe—EDDHA | Mono potassium phosphate |
| Magnesium sulfate | |
| Potassium nitrate | |
| Microelements | |
| Zinc sulfate | |
| Boric acid | |
| Manganese sulfate | |
| Copper sulfate | |
| Ammonium molybdate |
| Element | mg L−1 |
|---|---|
| N | 150–220 |
| P | 30–40 |
| K | 270–312 |
| Ca | 170–210 |
| Mg | 50–65 |
| Fe | 3.00–5.00 |
| Zn | 0.30–0.55 |
| B | 0.70–0.97 |
| Cu | 0.20–0.30 |
| Mo | 0.10–0.20 |
| Mn | 0.55–0.96 |
| Treatments | Lettuce Height (cm) | Lettuce Circumference (cm) | Lettuce Width (cm) | Stem Diameter (mm) | Lettuce Head Firmness (kg cm−3) |
|---|---|---|---|---|---|
| 100% MF | 29.05 ± 0.87 a | 50.66 ± 1.8 a | 34.56 | 22.48 ± 0.82 ab | 0.603 ± 0.004 b |
| 50% MF | 26.81 ± 0.71 b | 47.81 ± 2.6 ab | 30.56 | 22.99 ± 1.52 ab | 0.477 ± 0.030 c |
| 50% MF + Microalgae | 25.86 ± 2.45 b | 44.40 ± 2.4 b | 29.10 | 20.19 ± 1.50 b | 0.611 ± 0.039 ab |
| 50% MF + PGPR | 29.35 ± 1.06 a | 51.76 ± 1.8 a | 32.10 | 24.64 ± 1.25 a | 0.654 ± 0.050 a |
| 50% MF + AMF | 27.30 ± 0.12 ab | 50.66 ± 1.4 a | 31.15 | 24.01 ± 3.26 a | 0.648 ± 0.034 a |
| LSD0.05 | 2.110 | 4.75 | ns | 2.923 | 0.0497 |
| p-value | 0.0156 | 0.0337 | 0.3156 | 0.0500 | <0.0001 |
| Treatments | Lettuce Weight (g plant−1) | Leaf Area (cm2 plant−1) | Number of Leaves (number plant−1) | Dry Matter Ratio in Leaf (%) |
|---|---|---|---|---|
| 100% MF | 389.1 ± 15.7 a | 5969 ± 297 a | 38.76 ± 1.1 a | 4.27 |
| 50% MF | 257.3 ± 13.3 c | 4445 ± 394 c | 34.30 ± 1.8 c | 4.51 |
| 50% MF + Microalgae | 312.4 ± 17.6 b | 5064 ± 299 bc | 35.90 ± 1.4 bc | 4.53 |
| 50% MF + PGPR | 391.6 ± 7.1 a | 5882 ± 765 ab | 37.10 ± 1.2 ab | 4.92 |
| 50% MF + AMF | 321.2 ± 22.4 b | 5321 ± 666 ab | 36.10 ± 1.8 bc | 4.63 |
| LSD0.05 | 51.108 | 829.86 | 2.531 | ns |
| p-value | 0.0004 | 0.0101 | 0.0278 | 0.100 |
| Treatments | L* | a* | b* | SPAD-Chlorophyll |
|---|---|---|---|---|
| 100% MF | 44.04 | −11.16 | 36.29 | 32.82 |
| 50% MF | 38.06 | −10.85 | 31.44 | 30.86 |
| 50% MF + Microalgae | 42.00 | −10.99 | 36.15 | 31.79 |
| 50% MF + PGPR | 42.30 | −11.11 | 37.62 | 32.00 |
| 50% MF + AMF | 42.16 | −10.51 | 34.34 | 31.82 |
| LSD0.05 | ns | ns | ns | ns |
| p-value | 0.070 | 0.2313 | 0.0536 | 2.446 |
| Treatments | TSS (%) | pH | EC (dS m−1) |
|---|---|---|---|
| 100% MF | 3.18 ± 0.15 ab | 6.09 | 8.52 ± 0.55 a |
| 50% MF | 2.50 ± 0.18 c | 5.99 | 7.71 ± 0.05 c |
| 50% MF + Microalgae | 3.23 ± 0.26 a | 6.05 | 8.49 ± 0.51 ab |
| 50% MF + PGPR | 2.70 ± 0.30 c | 6.04 | 7.91 ± 0.51 bc |
| 50% MF + AMF | 2.97 ± 0.43 b | 6.05 | 7.83 ± 0.70 c |
| LSD0.05 | 0.258 | ns | 0.669 |
| p-value | 0.0002 | 0.109 | 2.623 |
| Treatments | Vitamin C (mg 100 g FW−1) | Total Phenolics (mg GA 100 g FW−1) | Total Flavonoids (mg RU 100 g FW−1) | Nitrate (mg kg FW−1) |
|---|---|---|---|---|
| 100% MF | 7.80 ± 0.96 c | 62.45 ± 10.4 b | 96.70 ± 3.9 c | 462 ± 25 a |
| 50% MF | 7.00 ± 0.46 c | 61.39 ± 8.0 b | 134.81 ± 12.3 b | 168 ± 12 c |
| 50% MF + Microalgae | 9.70 ± 0.41 b | 64.69 ± 1.8 b | 139.97 ± 3.4 b | 320 ± 18 b |
| 50% MF + PGPR | 13.20 ± 0.13 a | 61.81 ± 3.0 b | 144.90 ± 1.6 b | 536 ± 19 a |
| 50% MF + AMF | 10.58 ± 1.26 b | 78.28 ± 5.2 a | 182.37 ± 9.9 a | 342 ± 26 b |
| LSD0.05 | 1.307 | 8.249 | 22.802 | 81.651 |
| p-value | <0.0001 | 0.0036 | <0.0001 | <0.0001 |
| Treatments | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| 100% MF | 5.37 ± 0.29 b | 0.23 | 8.42 ± 0.69 bc | 0.83 ± 0.44 bc | 1.10 ± 0.03 bc |
| 50% MF | 3.65 ± 0.69 c | 0.20 | 7.46 ± 0.73 d | 0.74 ± 0.35 c | 1.00 ± 0.07 c |
| 50% MF + Microalgae | 5.24 ± 0.15 b | 0.24 | 9.38 ± 0.91 ab | 1.06 ± 0.53 a | 1.28 ± 0.17 a |
| 50% MF + PGPR | 6.46 ± 0.32 a | 0.25 | 9.70 ± 0.75 a | 0.97 ± 0.79 ab | 1.21 ± 0.07 ab |
| 50% MF + AMF | 6.12 ± 0.80 a | 0.24 | 8.29 ± 0.73 cd | 0.96 ± 0.35 ab | 1.20 ± 0.07 ab |
| LSD0.05 | 0.720 | ns | 0.964 | 0.1960 | 0.1564 |
| p-value | <0.0001 | 0.0592 | 0.0020 | 0.0275 | 0.0195 |
| Treatments | Fe | Mn | Zn | Cu |
|---|---|---|---|---|
| 100% MF | 78.77 ± 6.84 b | 26.87 ± 2.33 bc | 65.80 ± 8.50 ab | 5.99 ± 0.58 a |
| 50% MF | 69.23 ± 1.28 b | 21.52 ± 3.35 c | 51.00 ± 3.63 c | 4.29 ± 0.61 bc |
| 50% MF + Microalgae | 74.29 ± 3.31 b | 33.38 ± 4.48 a | 55.44 ± 5.63 bc | 4.72 ± 0.38 b |
| 50% MF + PGPR | 101.42 ± 7.53 a | 31.52 ± 5.30 ab | 70.94 ± 6.97 a | 5.71 ± 0.40 a |
| 50% MF + AMF | 80.71 ± 8.64 b | 26.03 ± 3.04 bc | 56.12 ± 2.29 bc | 3.99 ± 0.06 c |
| LSD0.05 | 12.91 | 6.083 | 11.921 | 0.667 |
| p-value | 0.0016 | 0.0084 | 0.0188 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dasgan, H.Y.; Yilmaz, D.; Zikaria, K.; Ikiz, B.; Gruda, N.S. Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics. Horticulturae 2023, 9, 1274. https://doi.org/10.3390/horticulturae9121274
Dasgan HY, Yilmaz D, Zikaria K, Ikiz B, Gruda NS. Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics. Horticulturae. 2023; 9(12):1274. https://doi.org/10.3390/horticulturae9121274
Chicago/Turabian StyleDasgan, Hayriye Yildiz, Dilek Yilmaz, Kamran Zikaria, Boran Ikiz, and Nazim S. Gruda. 2023. "Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics" Horticulturae 9, no. 12: 1274. https://doi.org/10.3390/horticulturae9121274
APA StyleDasgan, H. Y., Yilmaz, D., Zikaria, K., Ikiz, B., & Gruda, N. S. (2023). Enhancing the Yield, Quality and Antioxidant Content of Lettuce through Innovative and Eco-Friendly Biofertilizer Practices in Hydroponics. Horticulturae, 9(12), 1274. https://doi.org/10.3390/horticulturae9121274










