Improves the Resilience of Cucumber Seedlings under High-Light Stress through End-of-Day Addition of a Low Intensity of a Single Light Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Treatments
2.3. Item Determination
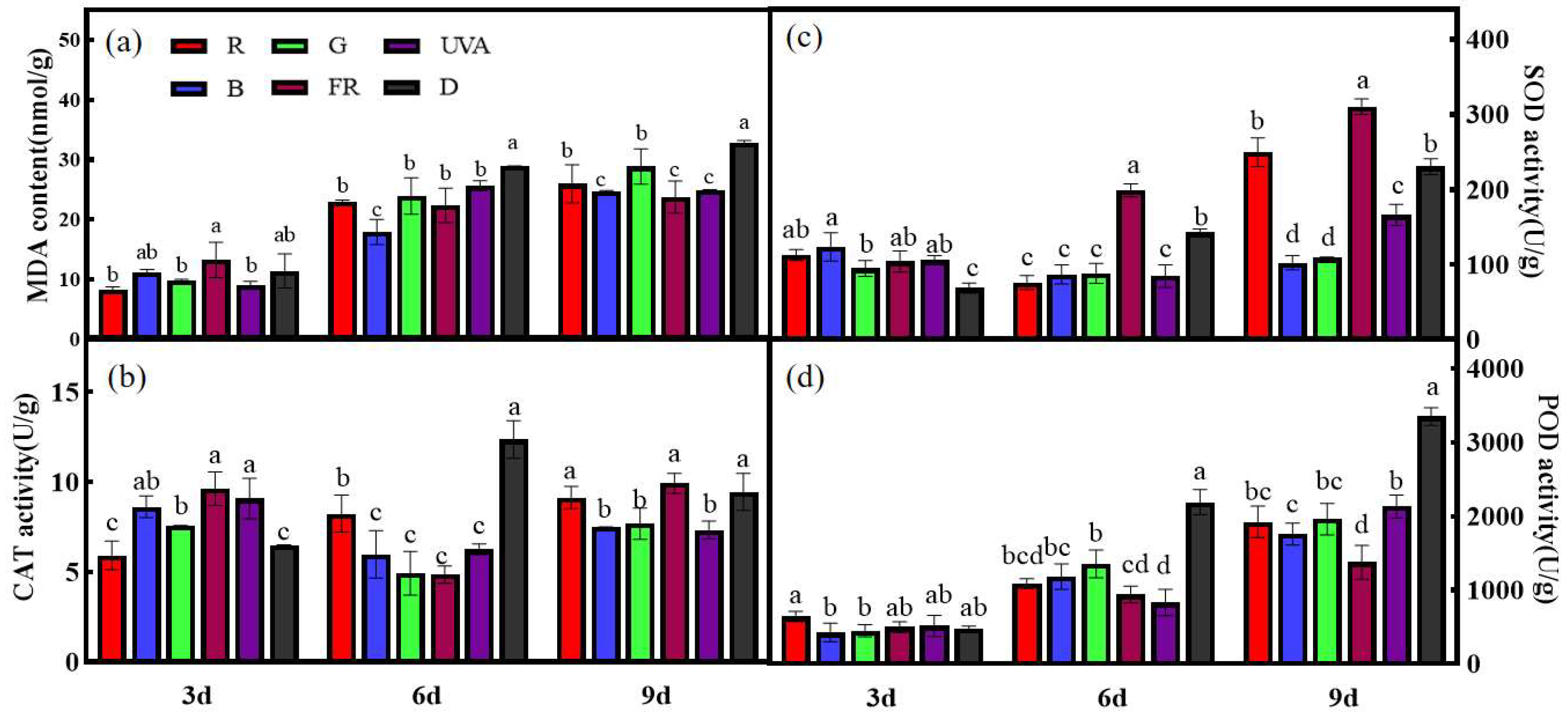
2.3.1. Malondialdehyde Content
2.3.2. Antioxidant Enzyme Activity
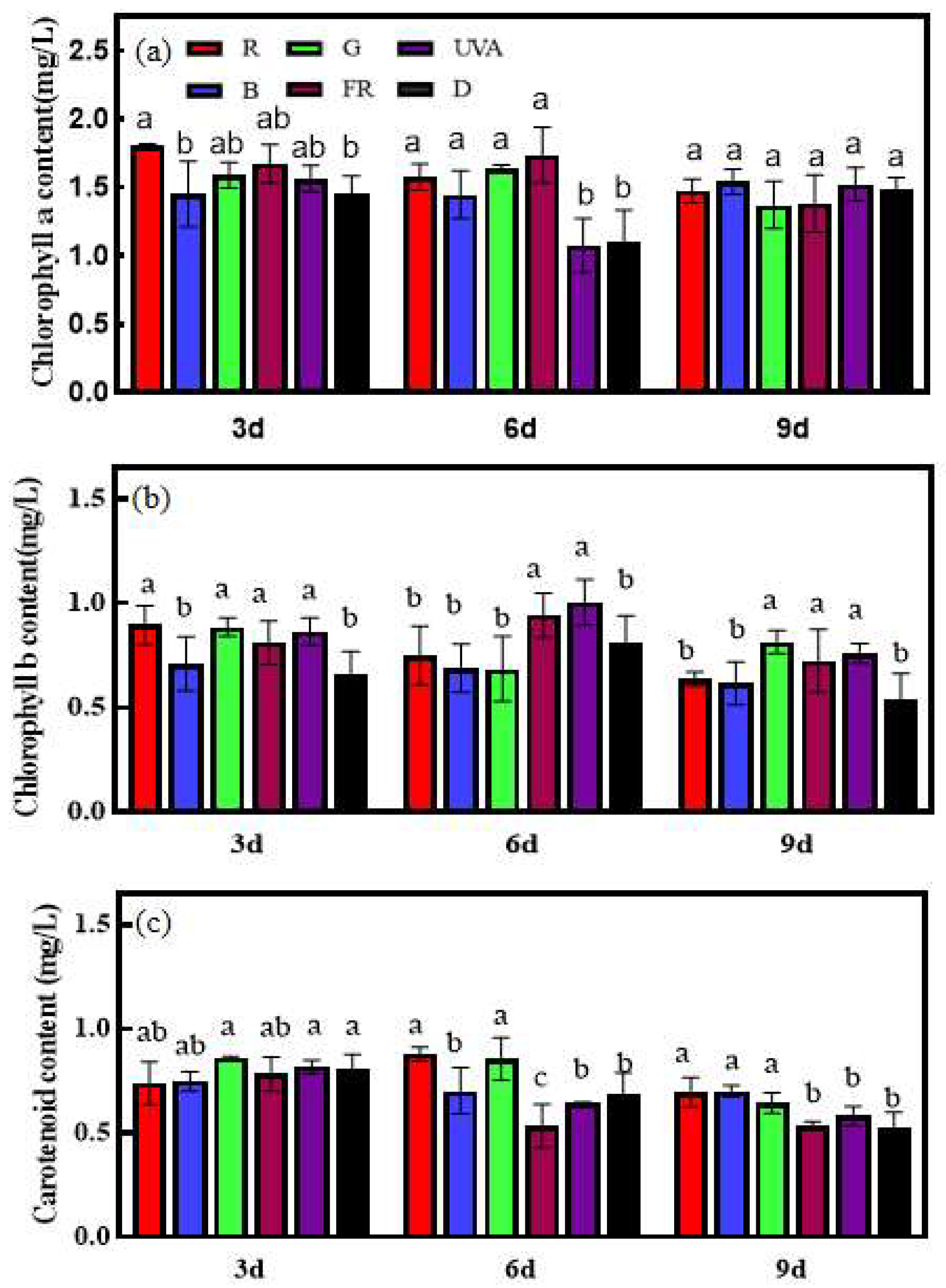
2.3.3. Chlorophyll Content and Its Synthesis and Degradation Key Enzyme Activities
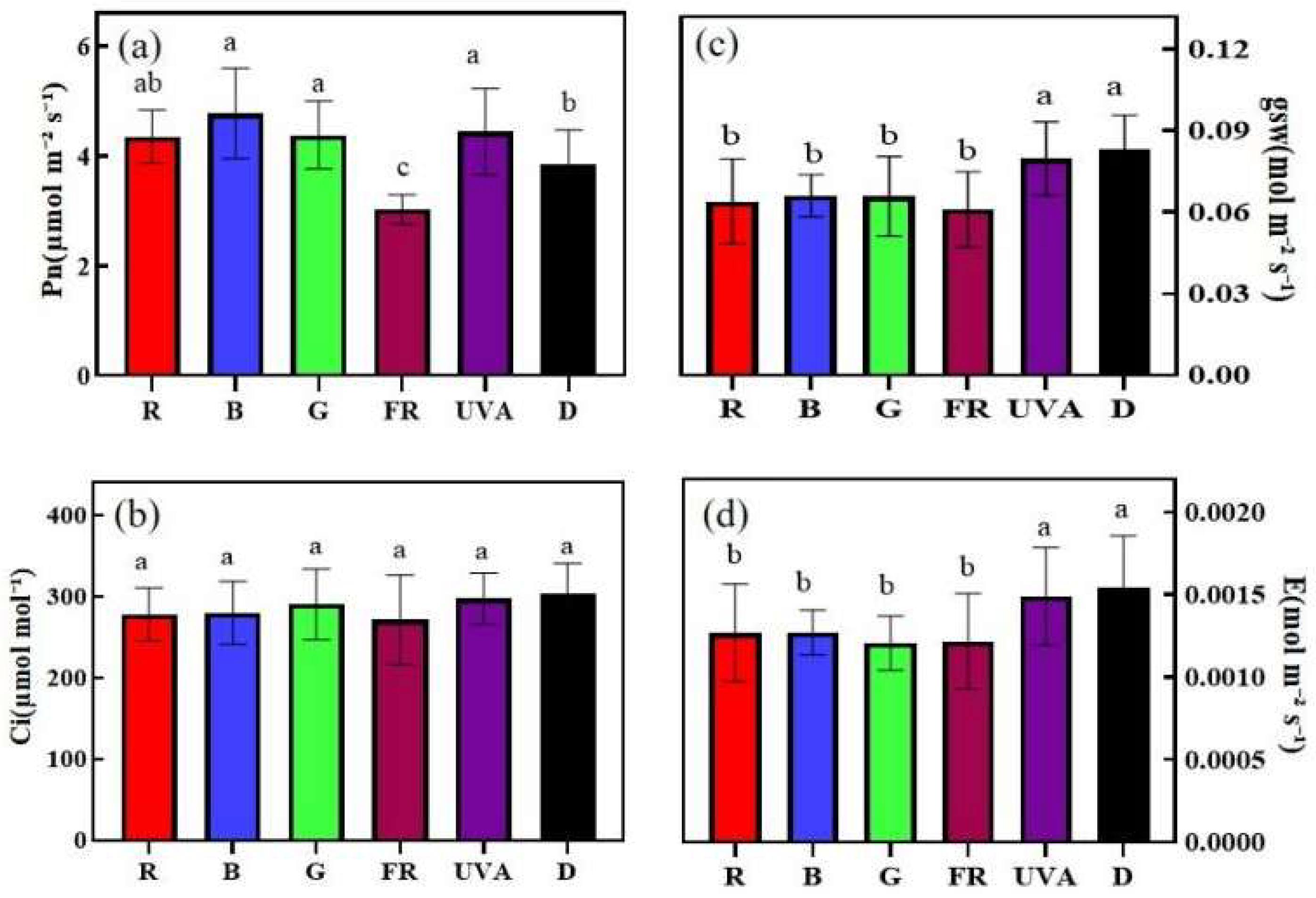
2.3.4. Determination of Parameters Related to Plant Photosynthetic Efficiency
2.3.5. Determination of Chlorophyll a Fluorescence Parameters
2.3.6. Determination of Secondary Metabolites
2.4. Statistical Analyses
3. Results
3.1. Effects of EOD Addition of Different Single Light Qualities before Dark on Antioxidant Content of Cucumber Seedling Leaves under High-Light Stress
3.2. Effects of EOD Addition of Different Single Light Qualities before Dark on Chlorophyll Content of Cucumber Seedlings under High-Light Stress
3.3. Effects of EOD Addition of Different Single Light Qualities before Dark on Chlorophyll-Related Enzyme Activities in Cucumber Seedlings under High-Light Stress
3.4. Effects of EOD Addition of Different Single Light Qualities before Dark on Photosynthesis of Cucumber Seedlings under High-Light Stress
3.5. Effects of EOD Addition of Different Single Light Qualities on Chlorophyll a Fluorescence Parameters and Photosystem Energy Conversion Efficiency in Cucumber Seedlings under High-Light Stress
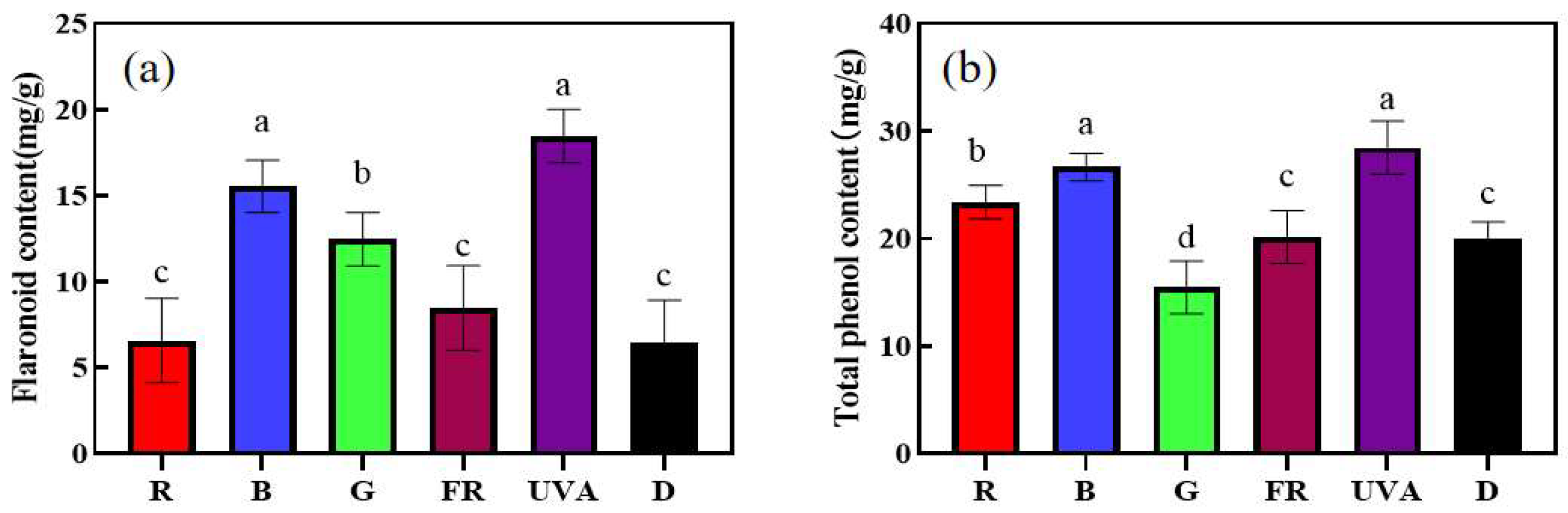
3.6. Effect of Adding Different Single Light Qualities before Darkness on Flavonoids and Total Phenol Content of Cucumber Seedling Leaves under High-Light Stress
4. Discussion
4.1. Different EOD Light Quality Affects Antioxidant and Secondary Metabolite Contents of Cucumber Seedlings
4.2. Different EOD Light Quality Affects Chlorophyll Content and Synthase and Degradative Enzyme Activities in Cucumber Seedlings
4.3. Different EOD Light Quality Affects Photosynthesis and Chlorophyll Fluorescence of Cucumber Seedlings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Ke, X.; Yang, X.; Liu, Y.; Hou, X. Plants response to light stress. J. Genet. Genom. 2022, 49, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, A.-S.; Fankhauser, C. Plant strategies for enhancing access to sunlight. Curr. Biol. 2017, 27, R931–R940. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2004, 56, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in Excess: The molecular mechanisms. Annu. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-McSweeney, A.; González-Gordo, S.; Aranda-Sicilia, M.N.; Rodríguez-Rosales, M.P.; Venema, K.; Palma, J.M.; Corpas, F.J. Loss of function of the chloroplast membrane k+/h+ antiporters ATKEA1 and ATKEA2 alters the ROS and no metabolism but promotes drought stress resilience. Plant Physiol. Biochem. 2021, 160, 106–119. [Google Scholar] [CrossRef]
- Szymańska, R.; Ślesak, I.; Orzechowska, A.; Kruk, J. Physiological and biochemical responses to high light and temperature stress in plants. Environ. Exp. Bot. 2017, 139, 165–177. [Google Scholar] [CrossRef]
- Roach, T.; Krieger-Liszkay, A. Regulation of Photosynthetic Electron Transport and photoinhibition. Curr. Protein Pept. Sci. 2014, 15, 351–362. [Google Scholar] [CrossRef]
- Faseela, P.; Puthur, J.T. Chlorophyll a fluorescence changes in response to short and long term high light stress in rice seedlings. Indian J. Plant Physiol. 2016, 22, 30–33. [Google Scholar] [CrossRef]
- Bian, Z.; Cheng, R.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Mawphlang, O.I.; Kharshiing, E.V. Photoreceptor mediated plant growth responses: Implications for photoreceptor engineering toward improved performance in crops. Front. Plant Sci. 2017, 8, 1181. [Google Scholar] [CrossRef]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current status and recent achievements in the field of horticulture with the use of light-emitting diodes (leds). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical Farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A.; Boylan, M.; Vierstra, R.D.; Quail, P.H. Is the far-red-absorbing form of Avena phytochrome a that is present at the end of the day able to sustain stem-growth inhibition during the night in transgenic tobacco and tomato seedlings? Planta 1995, 197, 225–232. [Google Scholar] [CrossRef]
- Frąszczak, B. Effect of short-term exposure to red and blue light on Dill Plants Growth. Hortic. Sci. 2013, 40, 177–185. [Google Scholar] [CrossRef]
- Yang, Z.-C.; Kubota, C.; Chia, P.-L.; Kacira, M. Effect of end-of-day far-red light from a movable led fixture on squash rootstock hypocotyl elongation. Sci. Hortic. 2012, 136, 81–86. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, H.; Mei, Y.; Li, Q.; Bai, Y.; Yu, H.; Xu, X.; Ma, J.; Wu, Y.; Yang, Z. Integrated transcriptome and metabolome analysis reveals an essential role for auxin in hypocotyl elongation during end-of-day far-red treatment of Cucurbita moschata (Duch. Ex Lam.). Agronomy 2021, 11, 853. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Prasad, K. Plant Secondary Metabolites; Apple Academic Press, Inc.: Palm Bay, FL, USA, 2017. [Google Scholar]
- Rodriguez, C.; Torre, S.; Solhaug, K.A. Low levels of ultraviolet-B radiation from fluorescent tubes induce an efficient flavonoid synthesis in Lollo Rosso lettuce without negative impact on growth. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 178–184. [Google Scholar] [CrossRef]
- Goto, E.; Hayashi, K.; Furuyama, S.; Hikosaka, S.; Ishigami, Y. Effect of UV light on phytochemical accumulation and expression of anthocyanin biosynthesis genes in red leaf lettuce. Acta Hortic. 2016, 1134, 179–186. [Google Scholar] [CrossRef]
- Vaštakaitė, V.; Viršilė, A.; Brazaitytė, A.; Samuolienė, G.; Jankauskienė, J.; Sirtautas, R.; Duchovskis, P. The effect of UV-a supplemental lighting on antioxidant properties of Ocimum basilicum L. Microgreens in Greenhouse. In Proceedings of the 7th International Scientific Conference Rural Development 2015, Kaunas, Lithuania, 19–20 November 2015. [Google Scholar] [CrossRef]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A role for barley CRYPTOCHROME1 in light regulation of grain dormancy and germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef]
- Ouzounis, T.; Razi Parjikolaei, B.; Fretté, X.; Rosenqvist, E.; Ottosen, C.-O. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in Lactuca sativa. Front. Plant Sci. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.; Chory, J. Light control of Plant Development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Zeng, Z.; Zhang, X.; Xie, D.; Li, X.; Ma, L.; Liu, M.; Liu, H. Green means go: Green light promotes hypocotyl elongation via brassinosteroid signaling. Plant Cell 2023, 35, 1304–1317. [Google Scholar] [CrossRef] [PubMed]
- Bayat, L.; Arab, M.; Aliniaeifard, S.; Seif, M.; Lastochkina, O.; Li, T. Effects of growth under different light spectra on the subsequent high light tolerance in Rose Plants. AoB Plants 2018, 10, ply052. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.F. Experimental Guidance of Plant Physiology; Higher Education Press: Beijing, China, 2006. [Google Scholar]
- Winkel-Shirley, B. Biosynthesis of Flavonoids and Effects of Stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Kołton, A.; Wojciechowska, R. Modifying folate and polyphenol concentrations in lamb’s lettuce by the use of LED supplemental lighting during cultivation in greenhouses. J. Funct. Foods 2016, 26, 228–237. [Google Scholar] [CrossRef]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Park, S.-A.; Park, B.-J.; Lee, Y.; Oh, M.-M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Mohanty, B.; Lakshmanan, M.; Lim, S.-H.; Kim, J.K.; Ha, S.-H.; Lee, D.-Y. Light-specific transcriptional regulation of the accumulation of carotenoids and phenolic compounds in rice leaves. Plant Signal. Behav. 2016, 11, 3002–3020. [Google Scholar] [CrossRef]
- Soares, C.; de Sousa, A.; Pinto, A.; Azenha, M.; Teixeira, J.; Azevedo, R.A.; Fidalgo, F. Effect of 24-epibrassinolide on ROS content, antioxidant system, lipid peroxidation and Ni uptake in Solanum nigrum L. under Ni stress. Environ. Exp. Bot. 2016, 122, 115–125. [Google Scholar] [CrossRef]
- Fridovich, I. Biological effects of the superoxide radical. Arch. Biochem. Biophys. 1986, 247, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative damage, lipid peroxidation and antioxidant protection in chloroplasts. Chem. Phys. Lipids 1987, 44, 327–340. [Google Scholar] [CrossRef]
- Wise, R.R.; Naylor, A.W. Chilling-enhanced photooxidation. Plant Physiol. 1987, 83, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A.; Linn, S. DNA damage and oxygen radical toxicity. Science 1988, 240, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. HortScience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Wu, M.; Hou, C.; Jiang, C.; Wang, Y.; Wang, C.; Chen, H.; Chang, H. A novel approach of LED light radiation improves the antioxidant activity of pea seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in Sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Wang, M.; Liu, H. Green light enhances growth, photosynthetic pigments and CO2 assimilation efficiency of lettuce as revealed by “knock out” of the 480–560 nm spectral waveband. Photosynthetica 2017, 55, 144–152. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Viršilė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Duchovskis, P. Nutrient levels in Brassicaceae microgreens increase under tailored light-emitting diode spectra. Front. Plant Sci. 2019, 10, 1475. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Wojciechowska, R.; Kruczek, M.; Habela, A. Supplemental lighting with leds improves the biochemical composition of two Valerianella locusta (L.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 441–449. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; El-Nakhel, C.; Pannico, A.; Graziani, G.; Soteriou, G.A.; Giordano, M.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Genotype-specific modulatory effects of select spectral bandwidths on the nutritive and phytochemical composition of microgreens. Front. Plant Sci. 2019, 10, 1501. [Google Scholar] [CrossRef] [PubMed]
- Büchert, A.M.; Civello, P.M.; Martínez, G.A. Chlorophyllase versus pheophytinase as candidates for chlorophyll dephytilation during senescence of broccoli. J. Plant Physiol. 2011, 168, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Guan, J. Involvement of pheophytinase in ethylene-mediated chlorophyll degradation in the peel of harvested ‘yali’ pear. J. Plant Growth Regul. 2013, 33, 364–372. [Google Scholar] [CrossRef]
- Guyer, L.; Hofstetter, S.S.; Christ, B.; Lira, B.S.; Rossi, M.; Hörtensteiner, S. Different mechanisms are responsible for chlorophyll dephytylation during fruit ripening and leaf senescence in Tomato. Plant Physiol. 2014, 166, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin improved anthocyanin accumulation by regulating gene expressions and resulted in high reactive oxygen species scavenging capacity in cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef] [PubMed]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Chen, H.; Hwang, J.E.; Lim, C.J.; Kim, D.Y.; Lee, S.Y.; Lim, C.O. Arabidopsis DREB2C functions as a transcriptional activator of HsfA3 during the heat stress response. Biochem. Biophys. Res. Commun. 2010, 401, 238–244. [Google Scholar] [CrossRef]
- Chinchilla, S.; Izzo, L.; van Santen, E.; Gómez, C. Growth and physiological responses of lettuce grown under pre-dawn or end-of-day sole-source light-quality treatments. Horticulturae 2018, 4, 8. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, Y.; Zhang, Y.; Bian, Z.; Fanourakis, D.; Yang, Q.; Li, T. Morphological and physiological properties of indoor cultivated lettuce in response to additional far-red light. Sci. Hortic. 2019, 257, 108725. [Google Scholar] [CrossRef]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 k among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Dhanya Thomas, T.T.; Dinakar, C.; Puthur, J.T. Effect of UV-B priming on the abiotic stress tolerance of stress-sensitive rice seedlings: Priming imprints and cross-tolerance. Plant Physiol. Biochem. 2020, 147, 21–30. [Google Scholar] [CrossRef]
- Erickson, E.; Wakao, S.; Niyogi, K.K. Light stress and photoprotection in chlamydomonas reinhardtii. Plant J. 2015, 82, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Faseela, P.; Puthur, J.T. The imprints of the High Light and UV-B stresses in Oryza sativa L. ‘kanchana’ seedlings are differentially modulated. J. Photochem. Photobiol. B Biol. 2018, 178, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Janeeshma, E.; Johnson, R.; Amritha, M.S.; Noble, L.; Aswathi, K.P.; Telesiński, A.; Kalaji, H.M.; Auriga, A.; Puthur, J.T. Modulations in chlorophyll a fluorescence based on intensity and spectral variations of light. Int. J. Mol. Sci. 2022, 23, 5599. [Google Scholar] [CrossRef] [PubMed]
- Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as early indicators of light stress in Barley. J. Photochem. Photobiol. B Biol. 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Gururani, M.A.; Venkatesh, J.; Tran, L.S. Regulation of photosynthesis during abiotic stress-induced photoinhibition. Mol. Plant 2015, 8, 1304–1320. [Google Scholar] [CrossRef]
- Osmond, C.B.; Grace, S.C. Perspectives on photoinhibition and photorespiration in the field: Quintessential inefficiencies of the light and dark reactions of photosynthesis? J. Exp. Bot. 1995, 46, 1351–1362. [Google Scholar] [CrossRef]

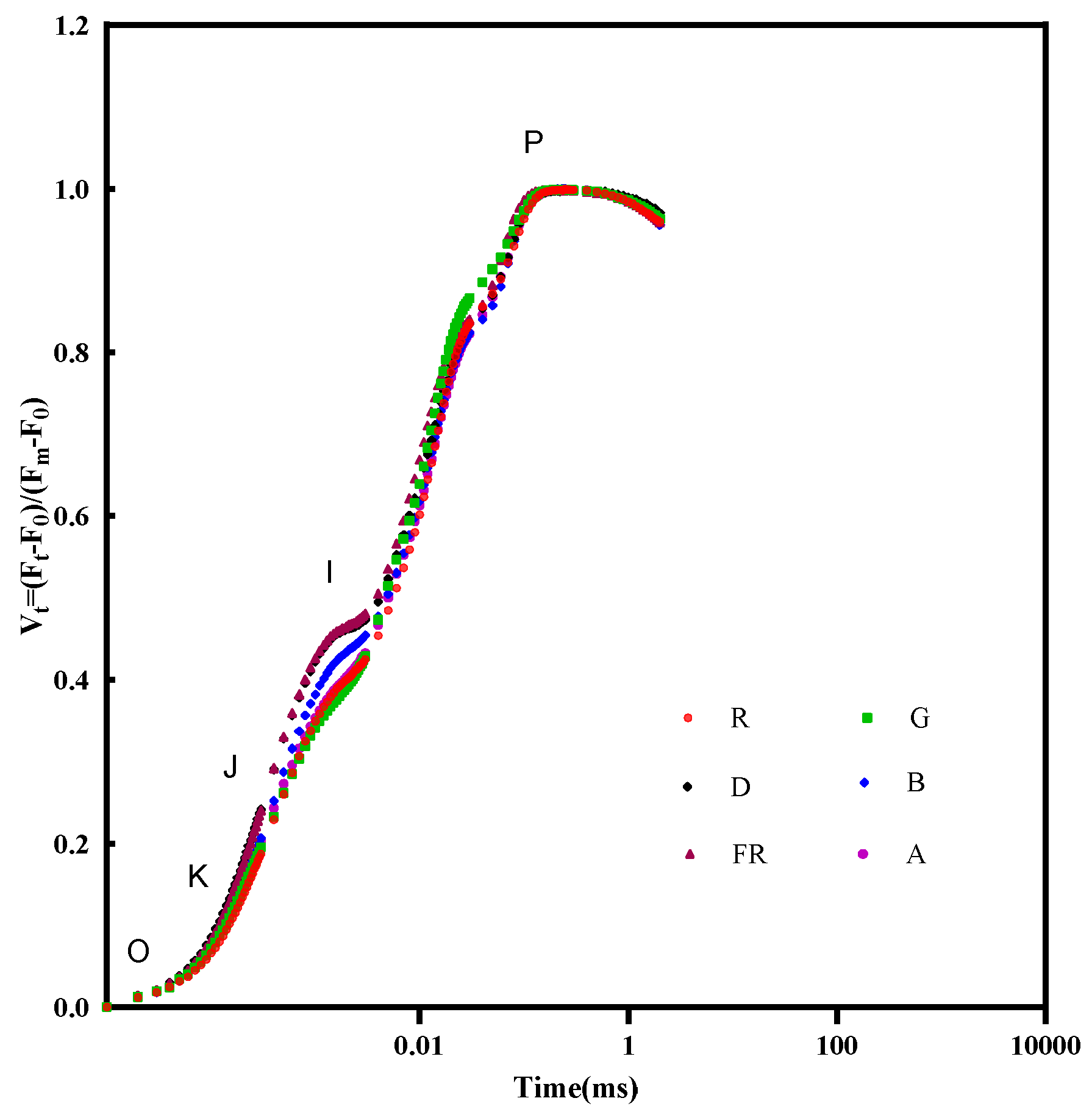
| Chlorophyll a Fluorescence Parameters | An Explanation of the Meaning of Words or Phrases |
|---|---|
| FV/FM | It represents the maximum quantum yield of PSII |
| FV/FO | It represents the maximum efficiency of the water-splitting complex |
| SM = Area/(FM − Fo) | It represents the multiple turnovers of QA reductions |
| VJ | Relative variable fluorescence at phase J of the fluorescence induction curve |
| VI | Relative variable fluorescence at phase I of the fluorescence induction curve |
| PIABS = γRC/(1 − γRC) × φPo/(1 − φPo) × ψo/(1 − ψo) | Performance index of PS I on absorption basis |
| PITOTAL = PIABS × δRo/(1 − δRo) | Performance index of electron flux to the final PS I electron acceptors |
| φPo | Maximum quantum yield of primary PSII photochemistry (at t = 0) |
| φ(Eo) | Quantum yield (at t = 0) for electron transport from QA- to plastoquinone |
| ψo | Probability (at t = 0) that a trapped exciton moves an electron into the electron transport chain beyond QA |
| YRC | The probability that PSII chlorophyll molecule functions as RC |
| δ(Ro) = (1 − VJ)/(1 − VI) | Efficiency/probability (at t = 0) with which an electron from the intersystem carriers moves to reduce end electron acceptors at the PSI acceptor side |
| ABS/RC = (1 − γRC)/γRC | Absorption flux per RC corresponding directly to its apparent antenna size |
| TRo/RC = ΔV/Δt0 × (1/Vj) | Trapping flux leading to QA reduction per RC at t = 0 |
| ETo/RC = ΔV/Δt0 × (1/Vj) ψ0 | Electron transport flux from QA- to plastoquinone per RC at t = 0 |
| DIo/RC = (ABS/RC − TR0/RC) | Dissipated energy flux per RC at the initial moment of the measurement, i.e., at t = 0 |
| ABS/RC = (1 − γRC)/γRC | Absorption flux per RC corresponding directly to its apparent antenna size |
| ABS/CSm | Absorption of energy per excited cross-section (CS) |
| approximated by FM | |
| TRo/CSm | Excitation energy flux trapped by PSII of a |
| Photosynthesising sample cross-section (CS) approximated by FM | |
| ETo/CSm | Electron flux transported by PSII of a |
| Treatments | FV/FM | FV/FO | SM | VJ | VI | PIABS | PITOTAL |
|---|---|---|---|---|---|---|---|
| R | 0.805 ± 0.002 ab | 4.404 ± 0.059 a | 17.932 ± 1.331 a | 0.404 ± 0.053 a | 0.837 ± 0.009 ab | 3.394 ± 0.584 a | 1.272 ± 0.166 a |
| G | 0.811 ± 0.007 a | 4.285 ± 0.178 a | 15.139 ± 1.387 bc | 0.394 ± 0.013 a | 0.868 ± 0.013 a | 3.185 ± 0.218 ab | 0.891 ± 0.099 b |
| FR | 0.806 ± 0.005 ab | 4.18 ± 0.399 a | 14.457 ± 1.165 c | 0.471 ± 0.017 a | 0.843 ± 0.028 ab | 2.16 ± 0.153 b | 0.923 ± 0.039 b |
| D | 0.798 ± 0.006 b | 4.19 ± 0.146 a | 17.915 ± 0.316 a | 0.467 ± 0.001 a | 0.836 ± 0.001 ab | 2.171 ± 0.147 b | 0.967 ± 0.064 b |
| B | 0.815 ± 0.009 a | 4.409 ± 0.246 a | 17.574 ± 2.448 ab | 0.438 ± 0.083 a | 0.825 ± 0.026 b | 3.013 ± 1.091 ab | 1.365 ± 0.521 a |
| UVA | 0.813 ± 0.007 a | 4.355 ± 0.201 a | 17.737 ± 0.715 a | 0.408 ± 0.034 a | 0.825 ± 0.027 b | 3.054 ± 0.529 ab | 1.28 ± 0.214 a |
| Treatments | φPo | φ(Eo) | ψo | YRC | δ(Ro) |
|---|---|---|---|---|---|
| R | 0.815 ± 0.002 a | 0.486 ± 0.043 a | 0.596 ± 0.053 a | 0.133 ± 0.007 ab | 0.274 ± 0.01 a |
| G | 0.811 ± 0.006 a | 0.491 ± 0.008 a | 0.606 ± 0.013 a | 0.107 ± 0.01 b | 0.219 ± 0.024 a |
| FR | 0.806 ± 0.015 a | 0.426 ± 0.011 a | 0.529 ± 0.017 a | 0.127 ± 0.022 ab | 0.296 ± 0.045 a |
| D | 0.807 ± 0.005 a | 0.431 ± 0.002 a | 0.533 ± 0.001 a | 0.133 ± 0.001 ab | 0.308 ± 0.001 a |
| B | 0.815 ± 0.009 a | 0.457 ± 0.066 a | 0.562 ± 0.083 a | 0.142 ± 0.021 a | 0.311 ± 0.009 a |
| UVA | 0.813 ± 0.007 a | 0.482 ± 0.031 a | 0.592 ± 0.034 a | 0.142 ± 0.021 a | 0.298 ± 0.065 a |
| Treatments | ABS/RC | TRo/RC | ETo/RC | DIo/RC |
|---|---|---|---|---|
| R | 1.944 ± 0.071 b | 1.585 ± 0.059 c | 0.947 ± 0.114 a | 0.360 ± 0.013 b |
| G | 2.073 ± 0.126 ab | 1.681 ± 0.115 bc | 1.018 ± 0.055 a | 0.392 ± 0.011 ab |
| FR | 2.171 ± 0.040 a | 1.750 ± 0.064 ab | 0.925 ± 0.023 a | 0.420 ± 0.026 a |
| D | 2.212 ± 0.079 a | 1.785 ± 0.052 a | 0.952 ± 0.029 a | 0.427 ± 0.027 a |
| B | 1.971 ± 0.157 b | 1.606 ± 0.122 bc | 0.897 ± 0.105 a | 0.365 ± 0.039 b |
| UVA | 2.094 ± 0.074 ab | 1.602 ± 0.058 bc | 1.009 ± 0.084 a | 0.391 ± 0.022 ab |
| Treatments | ABS/CSm | TRo/CSm | ETo/CSm | DIo/CSm |
|---|---|---|---|---|
| R | 46,167.333 ± 2495.077 ab | 37,622.667 ± 2032.765 a | 22,508.333 ± 3124.695 ab | 8544.667 ± 474.031 a |
| G | 46,075.333 ± 2686.795 ab | 37,361.000 ± 2471.864 a | 22,620.333 ± 1154.449 ab | 8714.333 ± 222.194 a |
| FR | 44,793.667 ± 2660.432 ab | 36,137.000 ± 2811.193 a | 19,093.667 ± 1105.913 ab | 8656.667 ± 204.270 a |
| D | 43,224.000 ± 619.000 b | 34,892.000 ± 733.000 a | 18,613.000 ± 369.000 b | 8332.000 ± 114.000 a |
| B | 46,007.667 ± 1062.27 ab | 37,486.000 ± 715.199 a | 21,022.000 ± 2840.544 ab | 8521.667 ± 538.000 a |
| UVA | 47,812.333 ± 2680.798 a | 38,887.333 ± 2508.309 a | 23,084.000 ± 2732.314 a | 8925.000 ± 195.049 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, S.; Qiu, C.; Cao, Q.; Xu, P.; Zhang, G.; Wu, Y.; Yang, Z. Improves the Resilience of Cucumber Seedlings under High-Light Stress through End-of-Day Addition of a Low Intensity of a Single Light Quality. Horticulturae 2023, 9, 1237. https://doi.org/10.3390/horticulturae9111237
Li X, Zhao S, Qiu C, Cao Q, Xu P, Zhang G, Wu Y, Yang Z. Improves the Resilience of Cucumber Seedlings under High-Light Stress through End-of-Day Addition of a Low Intensity of a Single Light Quality. Horticulturae. 2023; 9(11):1237. https://doi.org/10.3390/horticulturae9111237
Chicago/Turabian StyleLi, Xue, Shiwen Zhao, Chun Qiu, Qianqian Cao, Peng Xu, Guanzhi Zhang, Yongjun Wu, and Zhenchao Yang. 2023. "Improves the Resilience of Cucumber Seedlings under High-Light Stress through End-of-Day Addition of a Low Intensity of a Single Light Quality" Horticulturae 9, no. 11: 1237. https://doi.org/10.3390/horticulturae9111237
APA StyleLi, X., Zhao, S., Qiu, C., Cao, Q., Xu, P., Zhang, G., Wu, Y., & Yang, Z. (2023). Improves the Resilience of Cucumber Seedlings under High-Light Stress through End-of-Day Addition of a Low Intensity of a Single Light Quality. Horticulturae, 9(11), 1237. https://doi.org/10.3390/horticulturae9111237





