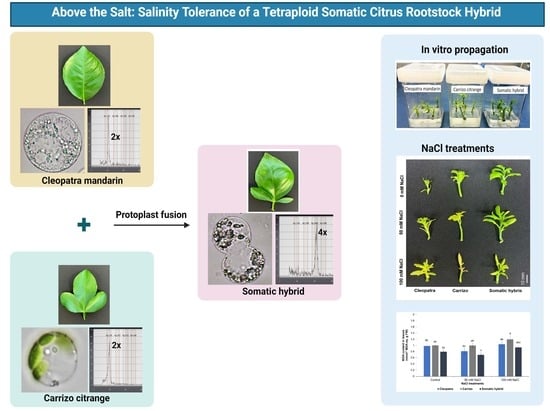
Physiological and Biochemical Evaluation of Salt Stress Tolerance in a Citrus Tetraploid Somatic Hybrid
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Flow Cytometry and Leaf Morphology Analysis
2.3. Somatic Fusion Confirmation Using Simple Sequence Repeat (SSR) Marker Analysis
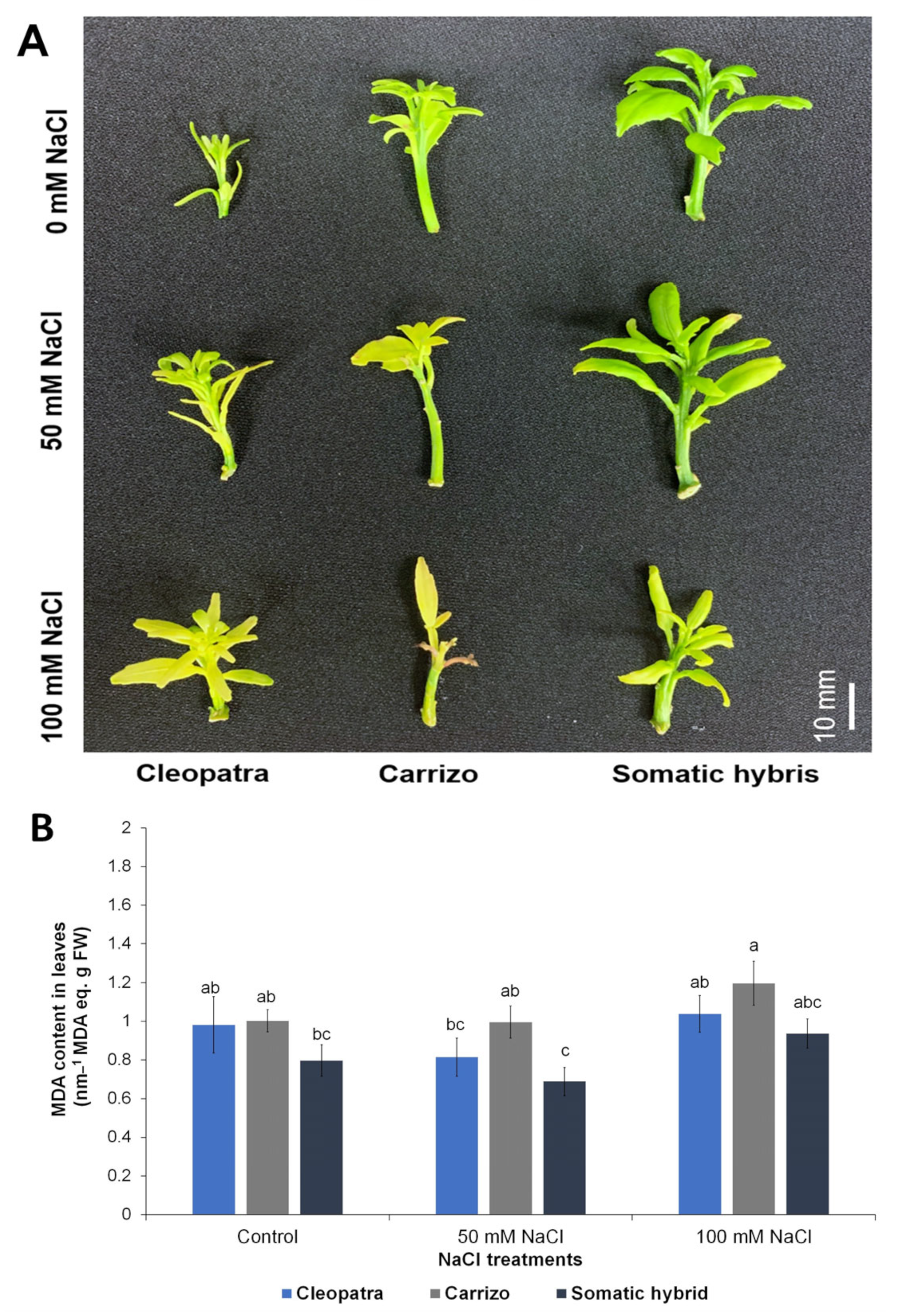
2.4. In Vitro Propagation and NaCl Treatments
2.5. Physiological and Biochemical Variables
2.6. Statistical Analysis
3. Results
3.1. Leaf Morphology and Ploidy Confirmation
3.2. Molecular Characterization of Donor Parents and the Somatic Hybrid Using SSR Markers
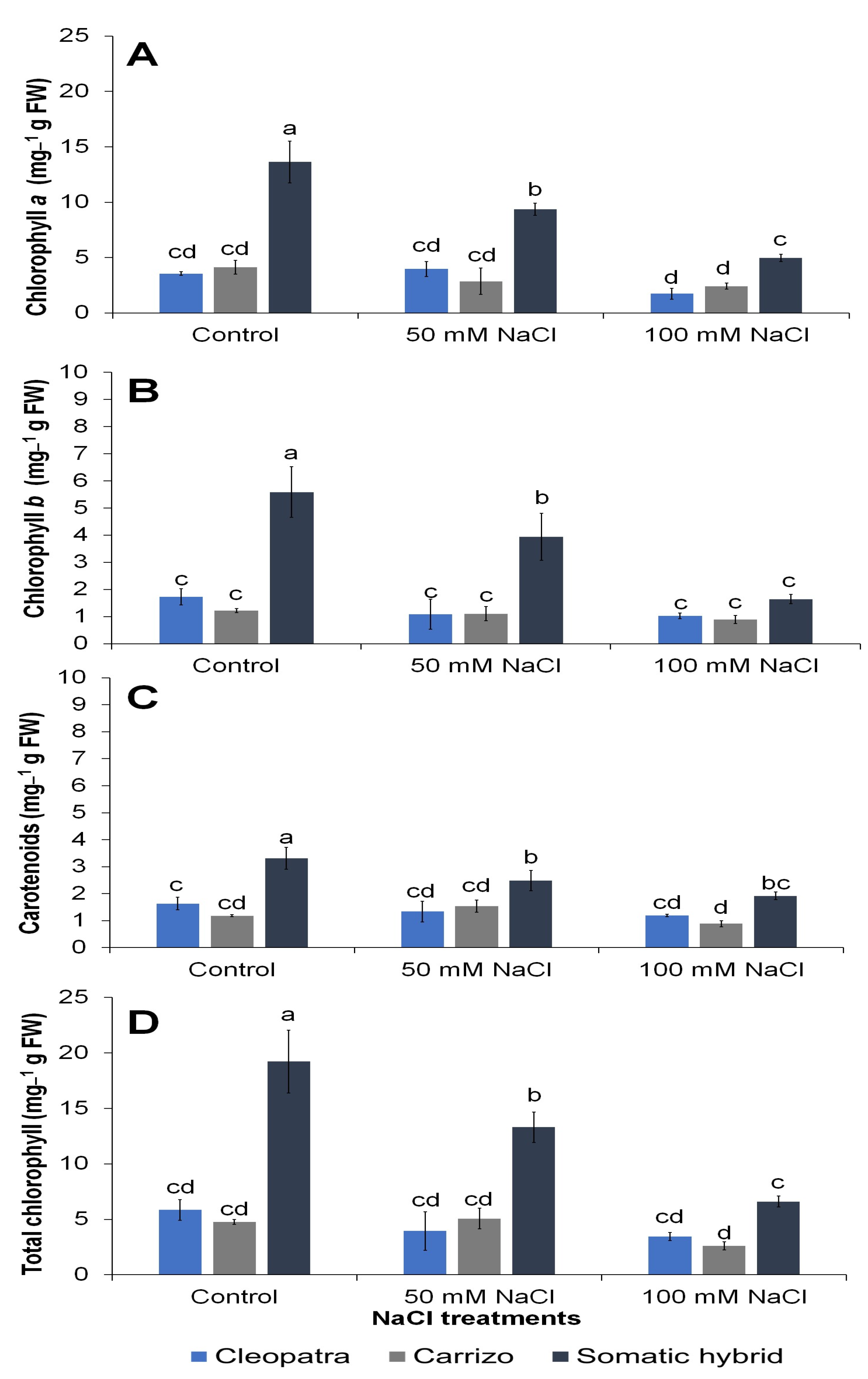
3.3. Physiological and Biochemical Variables
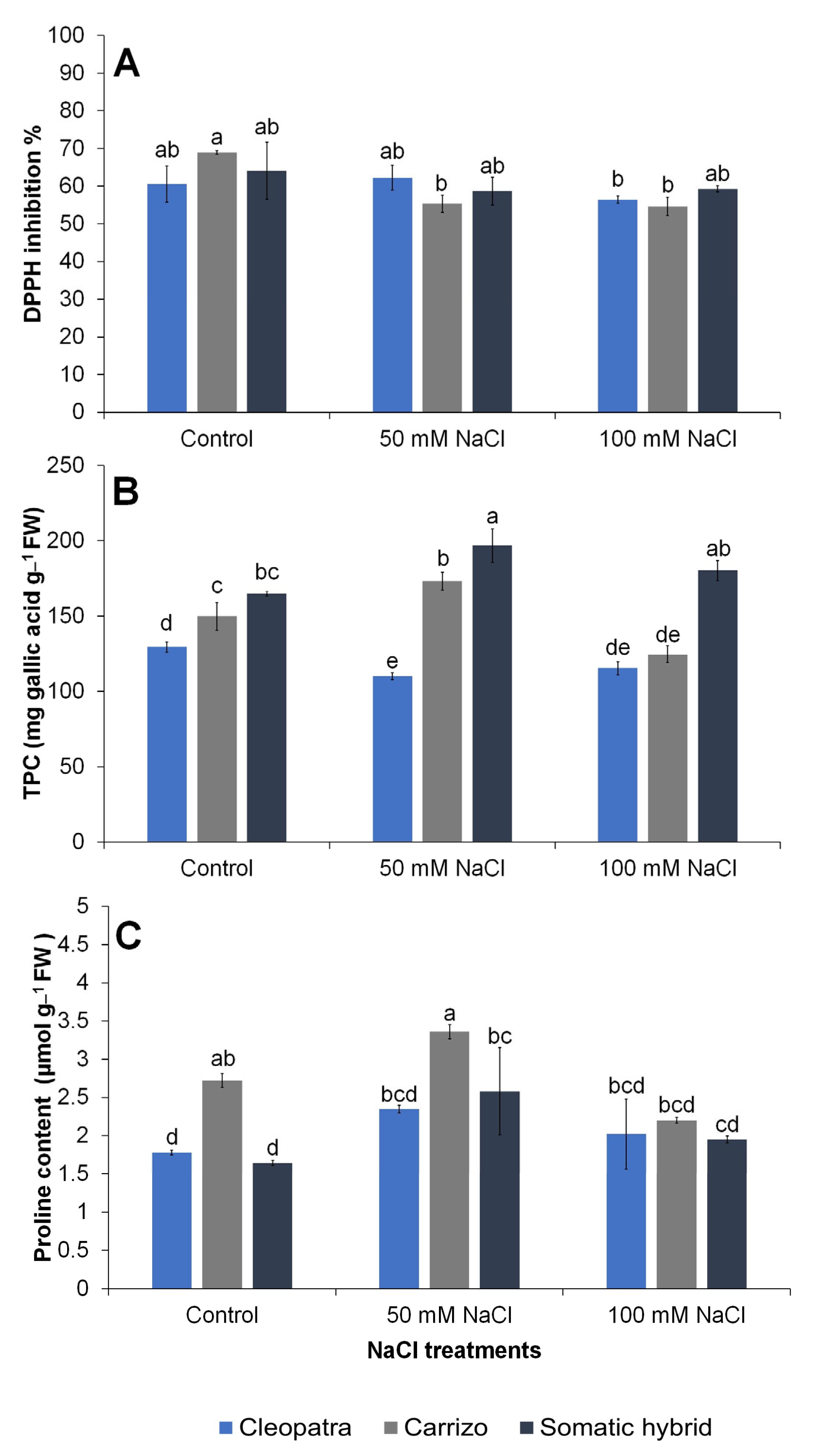
3.4. DPPH Radical Scavenging Activity, Total Phenolic Compounds, and Proline Content
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Talón, M.; Wu, G.A.; Gmitter, F.G., Jr.; Rokhsar, D.S. The origin of citrus. In The Genus Citrus; Elsevier: Amsterdam, The Netherlands, 2020; pp. 9–31. [Google Scholar] [CrossRef]
- Ṣahin-Çevik, M.; Moore, G.A. Identification and expression analysis of cold-regulated genes from the cold-hardy Citrus relative Poncirus trifoliata (L.) Raf. Plant Mol. Biol. 2006, 62, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, C.; Iglesius, A.; Yang, X.-B.; Epstein, P.R.; Chivian, E. Climate change and extreme weather events-Implications for food production, plant diseases, and pests. In National Aeronautics and Space Administration; University of Nebraska-Lincoln: Lincoln, NE, USA, 2001. [Google Scholar]
- Kodra, E.; Steinhaeuser, K.; Ganguly, A.R. Persisting cold extremes under 21st-century warming scenarios. Geophys. Res. Lett. 2011, 38, 8. [Google Scholar] [CrossRef]
- Cohen, J.; Jones, J.; Furtado, J.C.; Tziperman, E. Warm Arctic, Cold Continents: A Common Pattern Related to Arctic Sea Ice Melt, Snow Advance, and Extreme Winter Weather. Oceanography 2013, 26, 150–160. Available online: https://www.jstor.org/stable/24862104 (accessed on 7 October 2023). [CrossRef]
- Trouet, V.; Babst, F.; Meko, M. Recent enhanced high-summer North Atlantic Jet variability emerges from three-century context. Nat. Commun. 2018, 9, 180. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, L.M.; Dutt, M.; Vincent, C.I.; Grosser, J.W. Salinity-induced physiological responses of three putative salt tolerant citrus rootstocks. Horticulturae 2020, 6, 90. [Google Scholar] [CrossRef]
- Romero, P.; Dodd, I.C.; Martinez-Cutillas, A. Contrasting physiological effects of partial root zone drying in field-grown grapevine (Vitis vinifera L. cv. Monastrell) according to total soil water availability. J. Exp. Bot. 2012, 63, 4071–4083. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Khan, Y.; Mumtaz, R.; Shinwari, Z.K.; Khan, A.L.; Khan, A.; Ahmed, A.-H. Plant growth promoting bacteria as an alternative strategy for salt tolerance in plants: A review. Microbiol. Res. 2018, 209, 21–32. [Google Scholar] [CrossRef]
- Dourmap, C.; Roque, S.; Morin, A.; Caubrière, D.; Kerdiles, M.; Béguin, K.; Perdoux, R.; Reynoud, N.; Bourdet, L.; Audebert, P.-A. Stress signalling dynamics of the mitochondrial electron transport chain and oxidative phosphorylation system in higher plants. Ann. Bot. 2020, 125, 721–736. [Google Scholar] [CrossRef]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Oukarroum, A.; Bussotti, F.; Goltsev, V.; Kalaji, H.M. Correlation between reactive oxygen species production and photochemistry of photosystems I and II in Lemna gibba L. plants under salt stress. Environ. Exp. Bot. 2015, 109, 80–88. [Google Scholar] [CrossRef]
- Rady, M. A novel organo-mineral fertilizer can mitigate salinity stress effects for tomato production on reclaimed saline soil. S. Afr. J. Bot. 2012, 81, 8–14. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Dutt, M.; Shalan, A.M.; El-Kady, M.E.; El-Boray, M.S.; Shabana, Y.M.; Grosser, J.W. Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S. Afr. J. Bot. 2020, 132, 155–163. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Shalan, A.M.; El-Boray, M.S.; Vincent, C.I.; El-Kady, M.E.; Grosser, J.W.; Dutt, M. Application of silicon nanoparticles enhances oxidative stress tolerance in salt stressed ‘Valencia’sweet orange plants. Sci. Hortic. 2022, 295, 110856. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Stanton, D.; Amin, B.H.; Grosser, J.W.; Dutt, M. Overexpression of the Arabidopsis NPR1 gene confers enhanced salt tolerance by regulating antioxidant and starch accumulation in citrus. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 150, 695–707. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Huyck, P.J.; Vincent, C.I.; Gmitter, F.G.; Grosser, J.W.; Dutt, M. Physiological Responses and Gene Expression Patterns in Open-Pollinated Seedlings of a Pummelo-Mandarin Hybrid Rootstock Exposed to Salt Stress and Huanglongbing. Plants 2021, 10, 1439. [Google Scholar] [CrossRef] [PubMed]
- Castle, W.; Bowman, K.; Grosser, J.; Futch, S.; Graham, J. Florida Citrus Rootstock Selection Guide. 2016. Available online: https://crec.ifas.ufl.edu/extension/citrusrootstock (accessed on 7 October 2023).
- Sattler, M.C.; Carvalho, C.R.; Clarindo, W.R. The polyploidy and its key role in plant breeding. Planta 2016, 243, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Ollitrault, P.; Germanà, M.A.; Froelicher, Y.; Cuenca, J.; Aleza, P.; Morillon, R.; Grosser, J.W.; Guo, W. Ploidy Manipulation for Citrus Breeding, Genetics, and Genomics. In The Citrus Genome; Springer: Berlin/Heidelberg, Germany, 2020; pp. 75–105. [Google Scholar] [CrossRef]
- Grosser, J.; Jiang, J.; Louzada, E.; Chandler, J.; Gmitter, F. Somatic hybridization, an integral component of citrus cultivar improvement: II. Rootstock improvement. HortScience 1998, 33, 1060–1061. [Google Scholar] [CrossRef]
- Grosser, J.; Jiang, J.; Mourao-Fo, F.; Louzada, E.; Baergen, K.; Chandler, J.; Gmitter, F. Somatic hybridization, an integral component of citrus cultivar improvement: I. Scion improvement. HortScience 1998, 33, 1057–1059. [Google Scholar] [CrossRef]
- Grosser, J.W.; An, H.J.; Calovic, M.; Lee, D.H.; Chen, C.; Vasconcellos, M.; Gmitter, F.G. Production of new allotetraploid and autotetraploid citrus breeding parents: Focus on zipperskin mandarins. HortScience 2010, 45, 1160–1163. [Google Scholar] [CrossRef]
- Guo, W.W.; Xiao, S.X.; Deng, X.X. Somatic cybrid production via protoplast fusion for citrus improvement. Scientia Horticulturae 2013, 163, 20–26. [Google Scholar] [CrossRef]
- Grosser, J.W. Citrus Rootstock Named ‘UFR-1’. U.S. Patent USPP27,277 P3, 18 October 2016. [Google Scholar]
- Stover, E.; Hall, D.G.; Grosser, J.; Gruber, B.; Moore, G.A. Huanglongbing-related Responses of ‘Valencia’Sweet Orange on Eight Citrus Rootstocks during Greenhouse Trials. HortTechnology 2018, 28, 776–782. [Google Scholar] [CrossRef]
- Dutt, M.; Mahmoud, L.M.; Chamusco, K.; Stanton, D.; Chase, C.D.; Nielsen, E.; Quirico, M.; Yu, Q.; Gmitter, F.G., Jr.; Grosser, J.W. Utilization of somatic fusion techniques for the development of HLB tolerant breeding resources employing the Australian finger lime (Citrus australasica). PLoS ONE 2021, 16, e0255842. [Google Scholar] [CrossRef] [PubMed]
- Grosser, J.; Gmitter, F.; Tusa, N.; Chandler, J. Somatic hybrid plants from sexually incompatible woody species: Citrus reticulata and Citropsis gilletiana. Plant Cell Rep. 1990, 8, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Shafieizargar, A.; Awang, Y.; Ajamgard, F.; Juraimi, A.S.; Othman, R.; Ahmadi, A.K. Assessing five citrus rootstocks for NaCl salinity tolerance using mineral concentrations, proline and relative water contents as indicators. Asian J. Plant Sci. 2015, 14, 20–26. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Modulation of antioxidant defense system is associated with combined drought and heat stress tolerance in citrus. Front. Plant Sci. 2017, 8, 953. [Google Scholar] [CrossRef] [PubMed]
- Grosser, J.W.; Gmitter, F.G. Protoplast fusion for production of tetraploids and triploids: Applications for scion and rootstock breeding in citrus. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 104, 343–357. [Google Scholar] [CrossRef]
- Grosser, J.W.; Gmitter, F.G., Jr. Protoplast fusion and citrus improvement. Plant Breed. Rev. 1990, 8, 339–374. [Google Scholar] [CrossRef]
- Mahmoud, L.M.; Grosser, J.W.; Dutt, M. Silver compounds regulate leaf drop and improve in vitro regeneration from mature tissues of Australian finger lime (Citrus australasica). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 141, 455–464. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.a.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Qin, C.; Ahanger, M.; Zhou, J.; Ahmed, N.; Wei, C.; Yuan, S.; Ashraf, M.; Zhang, L. Beneficial role of acetylcholine in chlorophyll metabolism and photosynthetic gas exchange in Nicotiana benthamiana seedlings under salinity stress. Plant Biol. 2020, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.F.; Hussain, S.; Anjum, M.A.; Ahmad, S.; Ali, M.A.; Ejaz, S.; Morillon, R. Better salinity tolerance in tetraploid vs diploid volkamer lemon seedlings is associated with robust antioxidant and osmotic adjustment mechanisms. J. Plant Physiol. 2020, 244, 153071. [Google Scholar] [CrossRef]
- Saleh, B.; Allario, T.; Dambier, D.; Ollitrault, P.; Morillon, R. Tetraploid citrus rootstocks are more tolerant to salt stress than diploid. Comptes Rendus Biol. 2008, 331, 703–710. [Google Scholar] [CrossRef]
- Van de Peer, Y.; Maere, S.; Meyer, A. The evolutionary significance of ancient genome duplications. Nat. Rev. Genet. 2009, 10, 725–732. [Google Scholar] [CrossRef]
- Doyle, J.J.; Coate, J.E. Polyploidy, the nucleotype, and novelty: The impact of genome doubling on the biology of the cell. Int. J. Plant Sci. 2019, 180, 1–52. [Google Scholar] [CrossRef]
- Hias, N.; Leus, L.; Davey, M.W.; Vanderzande, S.; Van Huylenbroeck, J.; Keulemans, J. Effect of polyploidization on morphology in two apple (Malus × domestica) genotypes. Hortic. Sci. 2017, 44, 55–63. [Google Scholar] [CrossRef]
- Teng, S.; Qian, Q.; Zeng, D.; Kunihiro, Y.; Fujimoto, K.; Huang, D.; Zhu, L. QTL analysis of leaf photosynthetic rate and related physiological traits in rice (Oryza sativa L.). Euphytica 2004, 135, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Nian, J.; Shen, N.; Lai, K.; Hu, J.; Zeng, D.; Ge, C.; Fang, Y.; Zhu, L. Characterization and fine mapping of the rice gene OsARVL4 regulating leaf morphology and leaf vein development. Plant Growth Regul. 2016, 78, 345–356. [Google Scholar] [CrossRef]
- Xue, H.; Zhang, B.; Tian, J.R.; Chen, M.M.; Zhang, Y.Y.; Zhang, Z.H.; Ma, Y. Comparison of the morphology, growth and development of diploid and autotetraploid ‘Hanfu’ apple trees. Sci. Hortic. 2017, 225, 277–285. [Google Scholar] [CrossRef]
- Liu, X.P.; Hawkins, C.; Peel, M.D.; Yu, L.X. Genetic loci associated with salt tolerance in advanced breeding populations of tetraploid alfalfa using genome-wide association studies. Plant Genome 2019, 12, 180026. [Google Scholar] [CrossRef] [PubMed]
- Münzbergová, Z.; Haisel, D. Effects of polyploidization on the contents of photosynthetic pigments are largely population-specific. Photosynth. Res. 2019, 140, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Behzadi Rad, P.; Roozban, M.R.; Karimi, S.; Ghahremani, R.; Vahdati, K. Osmolyte accumulation and sodium compartmentation has a key role in salinity tolerance of pistachios rootstocks. Agriculture 2021, 11, 708. [Google Scholar] [CrossRef]
- Ruiz, M.; Quinones, A.; Martínez-Cuenca, M.R.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martínez-Alcántara, B. Tetraploidy enhances the ability to exclude chloride from leaves in carrizo citrange seedlings. J. Plant Physiol. 2016, 205, 1–10. [Google Scholar] [CrossRef]
- Oustric, J.; Quilichini, Y.; Morillon, R.; Herbette, S.; Luro, F.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid citrus seedlings subjected to long-term nutrient deficiency are less affected at the ultrastructural, physiological and biochemical levels than diploid ones. Plant Physiol. Biochem. 2019, 135, 372–384. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, Z.; Fan, G.; Dong, Y.; Deng, M.; Xu, E.; Zhai, X.; Cao, H. A comparison of the transcriptomes between diploid and autotetraploid Paulownia fortunei under salt stress. Physiol. Mol. Biol. Plants 2019, 25, 1–11. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Liu, S.; Zhao, Y.; Pang, X.; Li, Y. Autotetraploidization in Ziziphus jujuba Mill. var. spinosa enhances salt tolerance conferred by active, diverse stress responses. Environ. Exp. Bot. 2019, 165, 92–107. [Google Scholar] [CrossRef]

| Primer | Forward and Reverse Primer Sequences (5′ to 3′) |
|---|---|
| CX6F04 | AGTGAACTGTCCATTGGATTTTCG |
| GTGTTGAATCCCGACCTTCTACC | |
| CX6F29 | TTCACCACAAACGAAGACTCAGAC |
| CTGTAATCCACTCGGTAATCCGAC | |
| CX5F57 | CCTCGCCAATGACCTTTGTATTTA |
| CAATACGTTTGGGTTCTAGTTCCG | |
| CX0010 | AACCGAAGATGGAGGGAACT |
| ACATTCATGGCCACATCTCA | |
| CX0035 | CCATTAACGAGAAAACCAAACA |
| CAAAAAGGGGTTGCAAAGAA | |
| CX2021 | AAGGTCATGTCTTTAGCACTTTGA |
| CAAGTTGCCAATTCAGGAGG | |
| CX6F02 | AACAGTGTAGCATCGCACTTTCAC |
| GATACAAGGGACTTGCCCATCTC | |
| CX6F16 | GTCTTCACCCTCTCCATCTTCATC |
| GGGACTATGGCAACAATAACTCCA | |
| CX6F07 | CTGTTACCGTTGAGGAAACCAAAG |
| CTCTTCAGCTGGTTTCTCTTCCTG | |
| CX6F13 | AAACCCAAGTCATAAACGTCAGGA |
| ATCTTCAATGCTTTTGGAGCAAAC | |
| CX6F17 | GATACAAATTAGCATTTGATTGAATGGA |
| ATCGGGACTCGCATTAGGGT | |
| CX6F21 | CTACAAGTTCCCCAGTTATCCCG |
| ACTTGACCCGCTCTAGGAGTGAC | |
| CX6F18 | GTCTTCAACGAAGTTGCAGGCT |
| TACTATTTCGAGAGAGCAGCAGCA | |
| CX2007 | AAATCGGCTAGTTGCAAACG |
| CCTTGACATTGTCGATGGTG |
| Genotype/EST-SSR Marker | CX6F04 * | CX6F29 | ||||||
|---|---|---|---|---|---|---|---|---|
| Carrizo citrange | 157 | 162 | 149 | 156 | ||||
| Cleopatra mandarin | 162 | 169 | 156 | 156 | ||||
| Somatic hybrid | 157 | 162 | 162 | 169 | 149 | 156 | 156 | 156 |
| CX5F57 | CX0010 | |||||||
| Carrizo citrange | 156 | 166 | 222 | 229 | ||||
| Cleopatra mandarin | 156 | 156 | 219 | 219 | ||||
| Somatic hybrid | 156 | 156 | 156 | 166 | 219 | 219 | 222 | 229 |
| CX0035 | CX2021 | |||||||
| Carrizo citrange | 172 | 186 | 150 | 157 | ||||
| Cleopatra mandarin | 172 | 172 | 150 | 150 | ||||
| Somatic hybrid | 172 | 172 | 172 | 186 | 150 | 150 | 150 | 157 |
| CX6F02 | CX6F16 | |||||||
| Carrizo citrange | 168 | 175 | 170 | 175 | ||||
| Cleopatra mandarin | 168 | 168 | 164 | 164 | ||||
| Somatic hybrid | 168 | 168 | 168 | 175 | 164 | 164 | 170 | 175 |
| CX6F07 | CX6F13 | |||||||
| Carrizo citrange | 104 | 110 | 172 | 178 | ||||
| Cleopatra mandarin | 104 | 104 | 178 | 178 | ||||
| Somatic hybrid | 104 | 104 | 104 | 110 | 172 | 178 | 178 | 178 |
| CX6F17 | CX6F21 | |||||||
| Carrizo citrange | 133 | 133 | 155 | 155 | ||||
| Cleopatra mandarin | 139 | 158 | 149 | 155 | ||||
| Somatic hybrid | 133 | 133 | 139 | 158 | 155 | 155 | 155 | 155 |
| CX6F18 | CX2007 | |||||||
| Carrizo citrange | 161 | 161 | 172 | 177 | ||||
| Cleopatra mandarin | 155 | 166 | 174 | 174.6 | ||||
| Somatic hybrid | 161 | 167 | 172 | 177 | ||||
| Variables | Genotype | NaCl Treatments | Interaction |
|---|---|---|---|
| MDA content * | 0.0077 | 0.0222 | 0.923 |
| Chlorophyll a | <0.0001 | <0.0001 | 0.001 |
| Chlorophyll b | <0.0001 | 0.0008 | 0.0063 |
| Carotenoids | <0.0001 | 0.0067 | NS |
| Total Chlorophyll | <0.0001 | <0.0001 | 0.0015 |
| DPPH inhibition | NS | 0.034 | NS |
| Total phenolic compounds | <0.0001 | 0.0018 | <0.0001 |
| Proline content | 0.0013 | 0.0013 | NS |
| Variables * | Chl a | Chl b | Caro | T Chl | DPPH | TPC | Proline | MDA |
|---|---|---|---|---|---|---|---|---|
| Chl a | 1 | |||||||
| Chl b | 0.9512 | 1 | ||||||
| Caro | 0.9153 | 0.7867 | 1 | |||||
| T Chl | 0.9953 | 0.9767 | 0.8845 | 1 | ||||
| DPPH | 0.2445 | 0.1653 | 0.3311 | 0.2221 | 1 | |||
| TPC | 0.5295 | 0.4503 | 0.4859 | 0.5102 | −0.0104 | 1 | ||
| Proline | −0.2261 | −0.3848 | −0.0665 | −0.2785 | 0.1009 | 0.184 | 1 | |
| MDA | −0.4446 | −0.3946 | −0.4399 | −0.4336 | −0.4312 | −0.2828 | 0.0005 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, L.M.; Killiny, N.; Holden, P.; Gmitter, F.G., Jr.; Grosser, J.W.; Dutt, M. Physiological and Biochemical Evaluation of Salt Stress Tolerance in a Citrus Tetraploid Somatic Hybrid. Horticulturae 2023, 9, 1215. https://doi.org/10.3390/horticulturae9111215
Mahmoud LM, Killiny N, Holden P, Gmitter FG Jr., Grosser JW, Dutt M. Physiological and Biochemical Evaluation of Salt Stress Tolerance in a Citrus Tetraploid Somatic Hybrid. Horticulturae. 2023; 9(11):1215. https://doi.org/10.3390/horticulturae9111215
Chicago/Turabian StyleMahmoud, Lamiaa M., Nabil Killiny, Paige Holden, Frederick G. Gmitter, Jr., Jude W. Grosser, and Manjul Dutt. 2023. "Physiological and Biochemical Evaluation of Salt Stress Tolerance in a Citrus Tetraploid Somatic Hybrid" Horticulturae 9, no. 11: 1215. https://doi.org/10.3390/horticulturae9111215
APA StyleMahmoud, L. M., Killiny, N., Holden, P., Gmitter, F. G., Jr., Grosser, J. W., & Dutt, M. (2023). Physiological and Biochemical Evaluation of Salt Stress Tolerance in a Citrus Tetraploid Somatic Hybrid. Horticulturae, 9(11), 1215. https://doi.org/10.3390/horticulturae9111215