Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Chemicals
2.3. Experiment Design and Plant Materials
2.4. Determination of C, N and P Contents
2.5. Determination of Protein and Amino Acid Content
2.6. Determination of NSC Content
2.7. Determination of Total Phenol (TP) and Total Flavonoid (TF) Contents
2.8. Assay of DPPH Radical-Scavenging Activity
2.9. Assay of ABTS Radical-Scavenging Activity
2.10. Assay of Ferric-Reducing Antioxidant Power (FRAP)
2.11. Statistical Analysis
3. Results
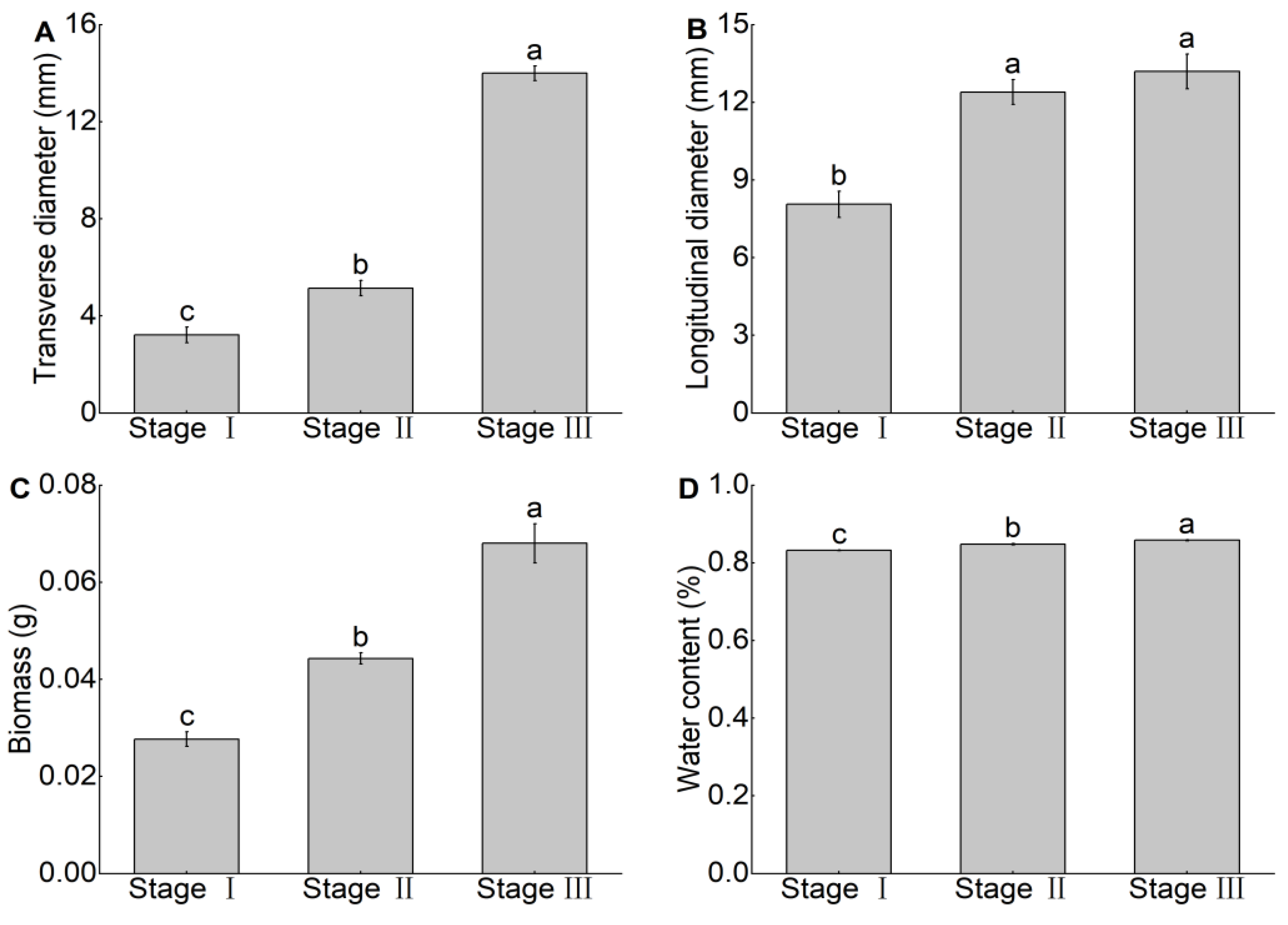
3.1. Changes in Morphological Features and Biomass, and Water Content
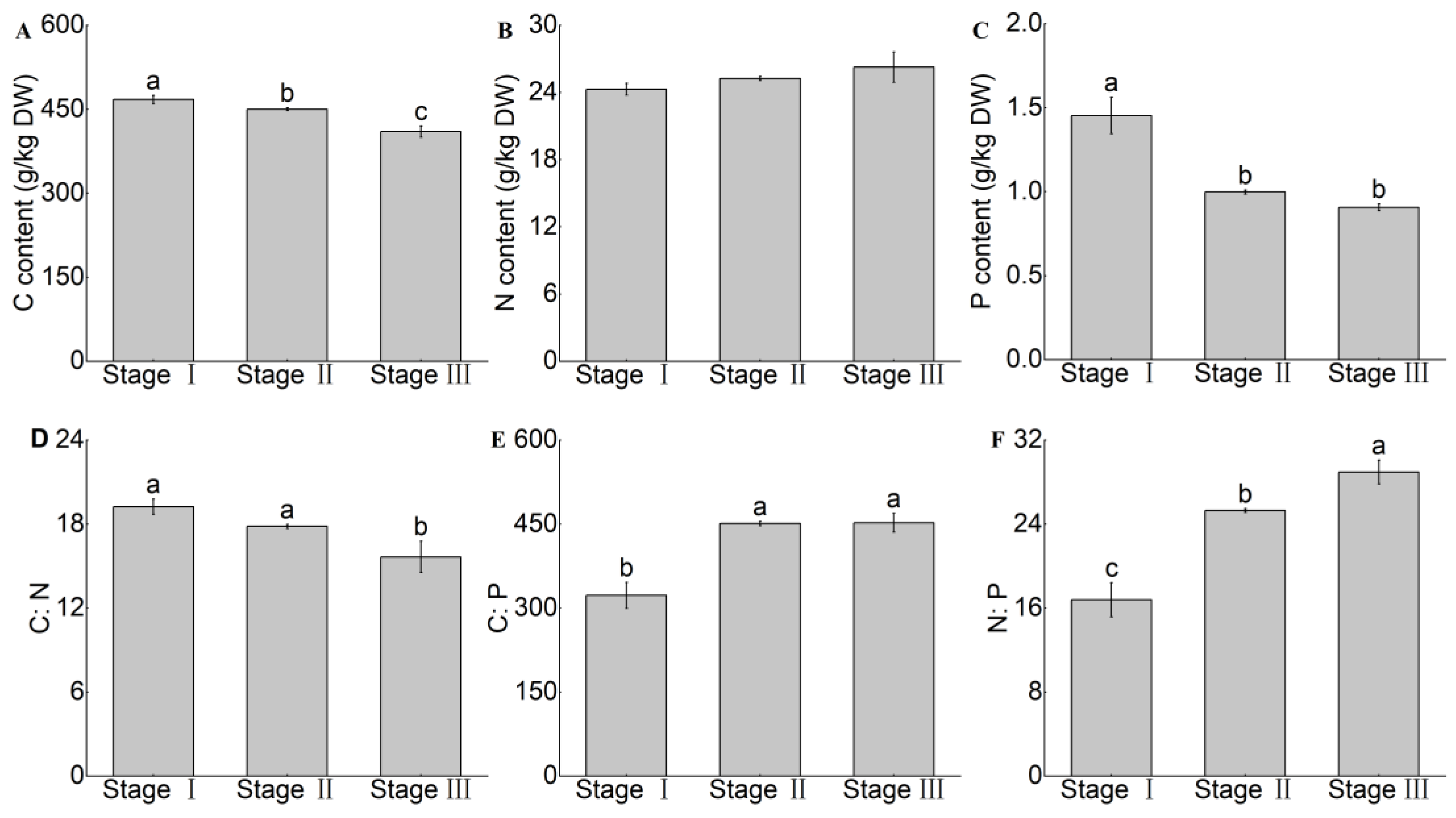
3.2. Changes in C, N, and P Content and the C:N:P Stoichiometric Ratio
3.3. Changes in NSC Content
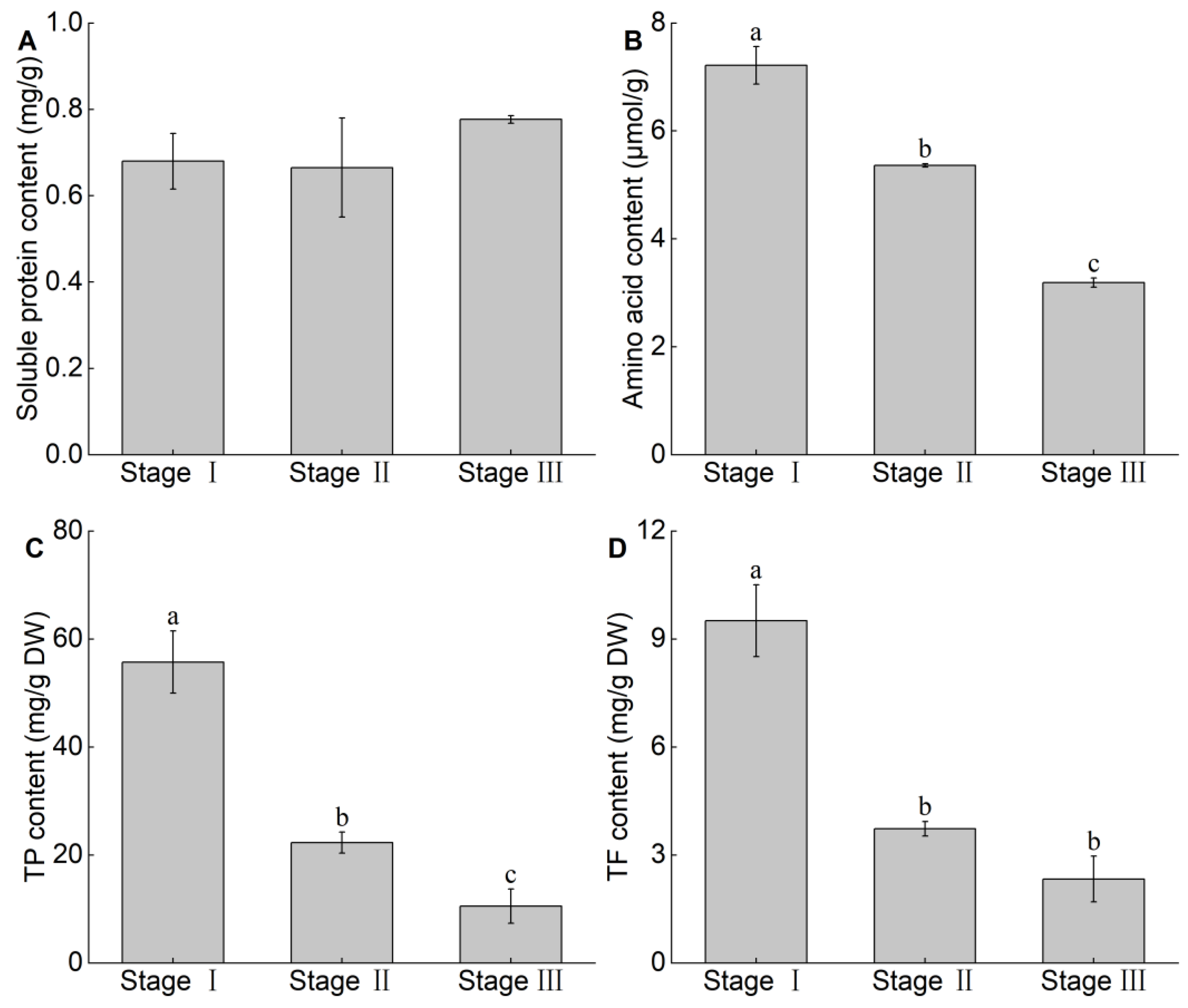
3.4. Changes in Soluble Protein, Amino acid, TP and TF Content
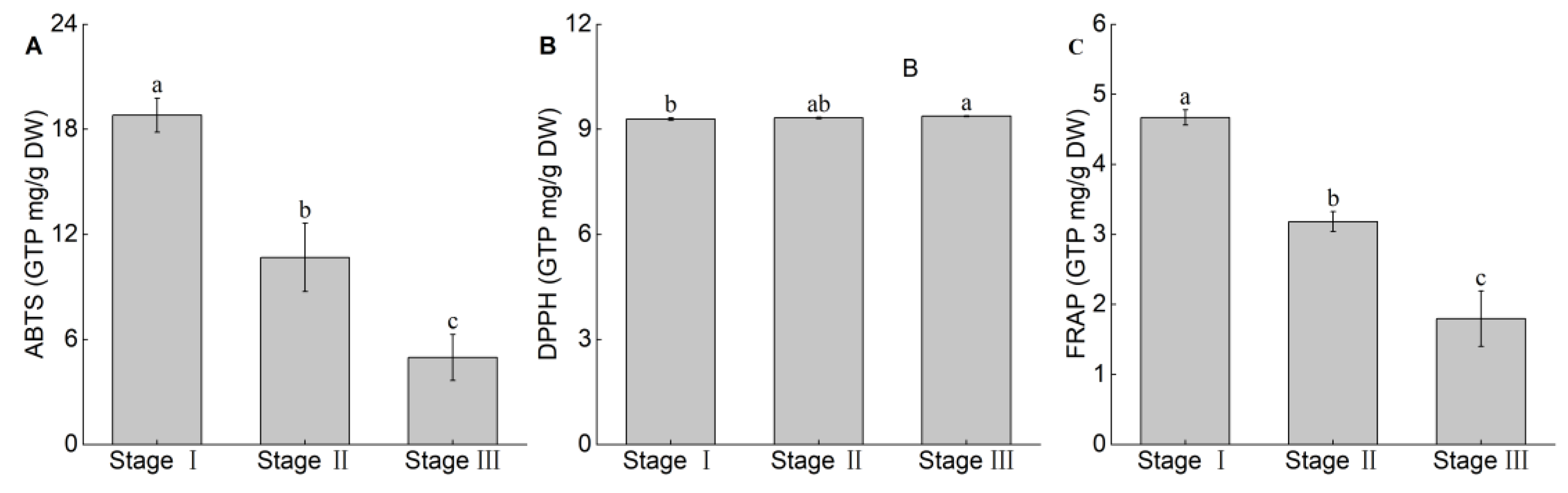
3.5. Changes in Antioxidant Activity
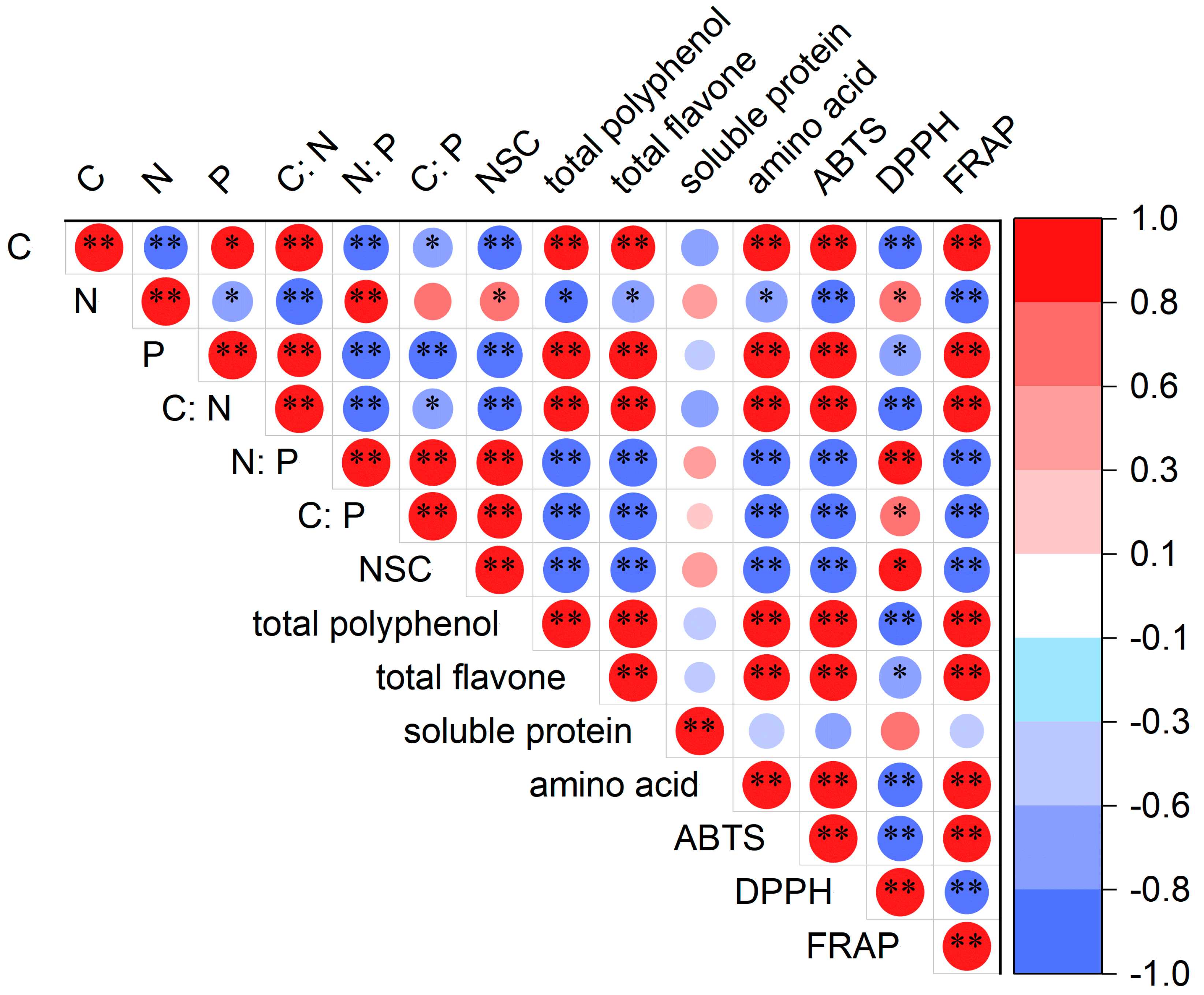
3.6. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Zhang, D.; Yu, S. A new stilbene from Cercis chinensis Bunge. J. Integr. Plant Biol. 2005, 47, 1021–1024. [Google Scholar] [CrossRef]
- Mu, L.; Li, J.; Yang, J.; Zhang, D. New dibenz[b, f]oxepins from Cercis chinensis Bunge. J. Asian Nat. Prod. Res. 2007, 9, 649–653. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Cui, L.; Liu, Z.; Wei, J.; Kang, W. Antioxidant and α-glucosidase inhibitiory activity of Cercis chinensis flowers. Food Sci. Hum. Wellness 2020, 9, 313–319. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, J.; Guo, Q.; Sun, K.; Chen, L.; Zhang, W.; Yang, B.; Kang, W. Two novel heteroglycan with coagulant activity from flowers of Cercis chinensis Bunge. J. Mol. Struct. 2021, 1243, 130756. [Google Scholar] [CrossRef]
- Hong, Y.; Liao, X.; Chen, Z. Determination of bioactive components in the fruits of Cercis chinensis Bunge by HPLC-MS/MS and quality evaluation by principal components and hierarchical cluster analyses. J. Pharm. Anal. 2021, 11, 465–471. [Google Scholar] [CrossRef]
- Pinakin, D.J.; Kumar, V.; Suri, S.; Sharma, R.; Kaushal, M. Nutraceutical potential of tree flowers: A comprehensive review on biochemical profile, health benefits, and utilization. Food Res. Int. 2020, 127, 108724. [Google Scholar] [CrossRef]
- Monaci, F.; Ancora, S.; Paoli, L.; Loppi, S.; Franzaring, J. Differential elemental stoichiometry of two Mediterranean evergreen woody plants over a geochemically heterogeneous area. Perspect. Plant Ecol. Evol. Syst. 2022, 55, 125672. [Google Scholar] [CrossRef]
- Shamsi, S.R.H.A.; Rabert, G.A.; Kurup, S.S.; Alyafei, M.A.M.; Jaleel, A. Biochemical changes and antioxidant variations in date palm (Phoenix dactylifera L.) varieties during flower induction and development. Plants 2021, 10, 2550. [Google Scholar] [CrossRef]
- Önder, S.; Tonguç, M.; Erbaş, S.; önder, D.; Mutlucan, M. Investigation of phenological, primary and secondary metabolites changes during flower developmental of Rosa damascena. Plant Physiol. Biochem. 2022, 192, 20–34. [Google Scholar] [CrossRef]
- Anwesha, C.; Rim, C.; Smritikana, D.; Mridushree, B.; Sonali, D.; Anton, R.S.; Malay, D. Role of metabolites in flower development and discovery of compounds controlling flowering time. Plant Physiol. Biochem. 2022, 190, 109–118. [Google Scholar] [CrossRef]
- Reid, M.S. Flower development: From bud to bloom. Acta Hortic. 2005, 669, 105–110. [Google Scholar] [CrossRef]
- Fernandes, L.; Ramalhosa, E.; Baptista, P.; Pereira, J.A.; Saraiva, J.A.; Casal, S.I.P. Nutritional and nutraceutical composition of pansies (Viola × wittrockiana) during flowering. J. Food Sci. 2019, 84, 490–498. [Google Scholar] [CrossRef]
- Shahri, W.; Tahir, I.; Islam, S.T.; Bhat, M.A. Physiological and biochemical changes associated with flower development and senescence in so far unexplored Helleborus orientalis Lam. cv. Olympicus. Physiol. Mol. Biol. Plants 2011, 17, 33–39. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Ni, M.; Yu, F. A Study on petal morphological and physiological characteristics of Styrax japonicus during the flowering period. Agronomy 2021, 11, 1498. [Google Scholar] [CrossRef]
- Çetinbaş-Genç, A.; Yanık, F.; Vardar, F. Histochemical and biochemical alterations in the stigma of Hibiscus syriacus (Malvaceae) during flower development. Caryologia 2019, 72, 3–13. [Google Scholar] [CrossRef]
- Yu, P.; Pu, D.; Zhou, W. Analysis of soluble proteins during flowering of Petunia hybrida. J. Plant Physiol. Mol. Biol. 2004, 30, 179–182. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Park, Y.E.; Hyeon, H.; Kim, J.K.; Lee, S.Y.; Park, S.U. Metabolic profiling and antioxidant activity during flower development in Agastache rugosa. Physiol. Mol. Biol. Plants 2021, 27, 445–455. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Minden, V.; Kleyer, M. Internal and external regulation of plant organ stoichiometry. Plant Biol. (Stuttg.) 2014, 16, 897–907. [Google Scholar] [CrossRef]
- Agren, G.I. Stoichiometry and nutrition of plant growth in natural communities. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 153–170. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef]
- Qian, W.; Hu, Y.; Lin, X.; Yu, D.; Jia, S.; Ye, Y.; Mao, Y.; Yi, L.; Gao, S. Phenological growth stages of Abelmoschus manihot: Codification and description according to the BBCH Scale. Agronomy 2023, 13, 1328. [Google Scholar] [CrossRef]
- Mishchenko, S.; Mokher, J.; Laiko, I.; Burbulis, N.; Kyrychenko, H.; Dudukova, S. Phenological growth stages of hemp (Cannabis sativa L.): Codification and description according to the BBCH scale. Žemės Ūkio Mokslai 2017, 24, 31–36. [Google Scholar] [CrossRef]
- Rana, N.; Singh, S.; Dhakad, A.K.; Dhatt, K.K. Coding phenological growth stages of yellow bells (Tecoma stans (L.) Juss. ex Kunth) based on BBCH scale and its implications for urban greening. Curr. Plant Biol. 2023, 34, 100284. [Google Scholar] [CrossRef]
- Zhi, X.; Gao, S.; Zhi, X.; Zou, J.; Ma, N.; Liu, T.; Hu, Y.; Yao, Y.; Hong Rui, W. Responses of the growth and nutrient stoichiometry in Ricinus communis seedlings on four soil types. J. Elem. 2022, 27, 223–238. [Google Scholar] [CrossRef]
- Jhanji, S.; Kaur, G.; Kaur, R.; Dhatt, U.K. Physiological and biochemical changes during flower development and senescence in Chrysanthemum and Gladiolus. Acta Physiol. Plant. 2023, 45, 14. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Z.; Wang, Z.; Chen, Y.; Wen, Z.; Liu, B.; Tigabu, M. Responses of leaf morphology, NSCs contents and C:N:P stoichiometry of Cunninghamia lanceolata and Schima superba to shading. BMC Plant Biol. 2020, 20, 354. [Google Scholar] [CrossRef]
- Flávia de Abreu Pinheiro, F.; Ferreira Elias, L.; de Jesus Filho, M.; Uliana Modolo, M.; Gomes Rocha, J.D.C.; Fumiere Lemos, M.; Scherer, R.; Soares Cardoso, W. Arabica and Conilon coffee flowers: Bioactive compounds and antioxidant capacity under different processes. Food Chem. 2021, 336, 127701. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, T.G. Optimization of phenolics and flavonoids extraction from the fruit of Empetrum nigrum var. japonicum from Jeju Island in South Korea. J. Ind. Eng. Chem. 2021, 98, 350–357. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Sreelatha, S.; Padma, P.R. Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of Maturity. Plant Food. Hum. Nutr. 2009, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- El Karkouri, J.; Kchibale, A.; Chroho, M.; Eddamsyry, B.; Touijer, H.; El Makhoukhi, F.; Handaq, N.; Eto, B.; Salamatullah, A.; Bourhia, M.; et al. Phytochemical profile, antioxidant activity, anti-hyperglycemic effect and toxicity assessment of Ridolfia segetum (L.) moris extract. Life 2023, 13, 44. [Google Scholar] [CrossRef] [PubMed]
- Motti, R.; Paura, B.; Cozzolino, A.; Falco, B.D. Edible flowers used in some countries of the Mediterranean basin: An ethnobotanical overview. Plants 2022, 11, 3272. [Google Scholar] [CrossRef]
- Dujmović, M.; Radman, S.; Opačić, N.; Fabek Uher, S.; Mikuličin, V.; Voća, S.; šic žlabur, J. Edible flower species as a promising source of specialized metabolites. Plants 2022, 11, 2529. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, L.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E.; Casal, S. Phytochemical characterization of Borago officinalis L. and Centaurea cyanus L. during flower development. Food Res. Int. 2019, 123, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Kim, N.; Han, W.; Guo, Y.; Han, T.; Du, E.; Fang, J. Effects of nitrogen and phosphorus supply on growth rate, leaf stoichiometry, and nutrient resorption of Arabidopsis thaliana. Plant Soil 2015, 388, 147–155. [Google Scholar] [CrossRef]
- Huang, J.; Wang, P.; Niu, Y.; Yu, H.; Ma, F.; Xiao, G.; Xu, X. Changes in C:N:P stoichiometry modify N and P conservation strategies of a desert steppe species Glycyrrhiza uralensis. Sci. Rep.-UK 2018, 8, 12668. [Google Scholar] [CrossRef]
- Xuan, L.; Wang, Q.; Liu, Z.; Xu, B.; Cheng, S.; Zhang, Y.; Lu, D.; Dong, B.; Zhang, D.; Zhang, L.; et al. Metabolic analysis of the regulatory mechanism of sugars on secondary flowering in Magnolia. BMC Mol. Cell Biol. 2022, 23, 56. [Google Scholar] [CrossRef]
- Li, X.; Cui, J.; Qin, X.; Wang, J.; Wang, M. Metabolic and transcriptional regulatory mechanisms of differential carbohydrates formation from flower buds to flowers of Hemerocallis citrina. Sci. Hortic. 2023, 308, 111553. [Google Scholar] [CrossRef]
- Le, T.N.; Luong, H.Q.; Li, H.; Chiu, C.; Hsieh, P. Broccoli (Brassica oleracea L. var. italica) sprouts as the potential food source for bioactive properties: Acomprehensive study on in vitro disease models. Foods 2019, 8, 532. [Google Scholar] [CrossRef]
- Zhang, W.; Li, J.; Zhang, W.; Njie, A.; Pan, X. The changes in C/N, carbohydrate, and amino acid content in leaves during female flower bud differentiation of Juglans sigillata. Acta Physiol. Plant. 2022, 44, 19. [Google Scholar] [CrossRef]
- Li, J.; Ye, G.; Liu, H.; Wang, Z. New insights on Abelmoschusmanihot flower development: Dynamic changes of flavonoids based on a metabolomic approach. J. Plant Biochem. Biot. 2022, 31, 351–360. [Google Scholar] [CrossRef]
- Han, M.; Zhao, Y.; Meng, J.; Yin, J.; Li, H. Analysis of physicochemical and antioxidant properties of Malus spp. petals reveals factors involved in flower color change and market value. Sci. Hortic. 2023, 310, 111688. [Google Scholar] [CrossRef]
- Guo, C.; Liu, K.; Li, E.; Chen, Y.; He, J.; Li, W.; Dong, W.; Suo, Z. Maternal donor and genetic variation of Lagerstroemia indica cultivars. Int. J. Mol. Sci. 2023, 24, 3606. [Google Scholar] [CrossRef] [PubMed]
- Kozicka, M.; Hallmann, E. Identification and quantification of bioactive compounds in organic and conventional edible pansy flowers (Viola × wittrockiana) and their antioxidant activity. Plants 2023, 12, 1264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, A.; Yang, X.; Yang, L.; Shi, Y.; Dong, L.; Lei, F.; Sun, L.; Bao, M.; Sun, X. Analysis of physiological and biochemical factors affecting flower color of herbaceous peony in different flowering periods. Horticulturae 2023, 9, 502. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.-Y.; Qian, W.-Z.; Yi, L.; Ye, Y.-L.; Gu, T.; Gao, S.; Cao, G.-X. Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages. Horticulturae 2023, 9, 961. https://doi.org/10.3390/horticulturae9090961
Ren H-Y, Qian W-Z, Yi L, Ye Y-L, Gu T, Gao S, Cao G-X. Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages. Horticulturae. 2023; 9(9):961. https://doi.org/10.3390/horticulturae9090961
Chicago/Turabian StyleRen, Hong-Yu, Wen-Zhang Qian, Lu Yi, Yu-Lin Ye, Tao Gu, Shun Gao, and Guo-Xing Cao. 2023. "Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages" Horticulturae 9, no. 9: 961. https://doi.org/10.3390/horticulturae9090961
APA StyleRen, H.-Y., Qian, W.-Z., Yi, L., Ye, Y.-L., Gu, T., Gao, S., & Cao, G.-X. (2023). Nutrient Composition and Antioxidant Activity of Cercis chinensis Flower in Response to Different Development Stages. Horticulturae, 9(9), 961. https://doi.org/10.3390/horticulturae9090961




