In Vitro Propagation and Phytochemical Composition of Centratherum punctatum Cass—A Medicinal Plant
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials, Surface Disinfection, and In Vitro Culture Establishment
2.2. Callus Induction Using Nodal Explant
2.3. Adventitious Shoot Induction from Callus
2.4. Axillary Shoot Induction Using Nodal Explants
2.5. In Vitro Rooting and Acclimatization
2.6. Preparation of Plant Methanolic Extract for GC-MS Analysis and Identification of Chemical Constituents
2.7. Experimental Design and Statistical Analysis
3. Results
3.1. Callus Induction
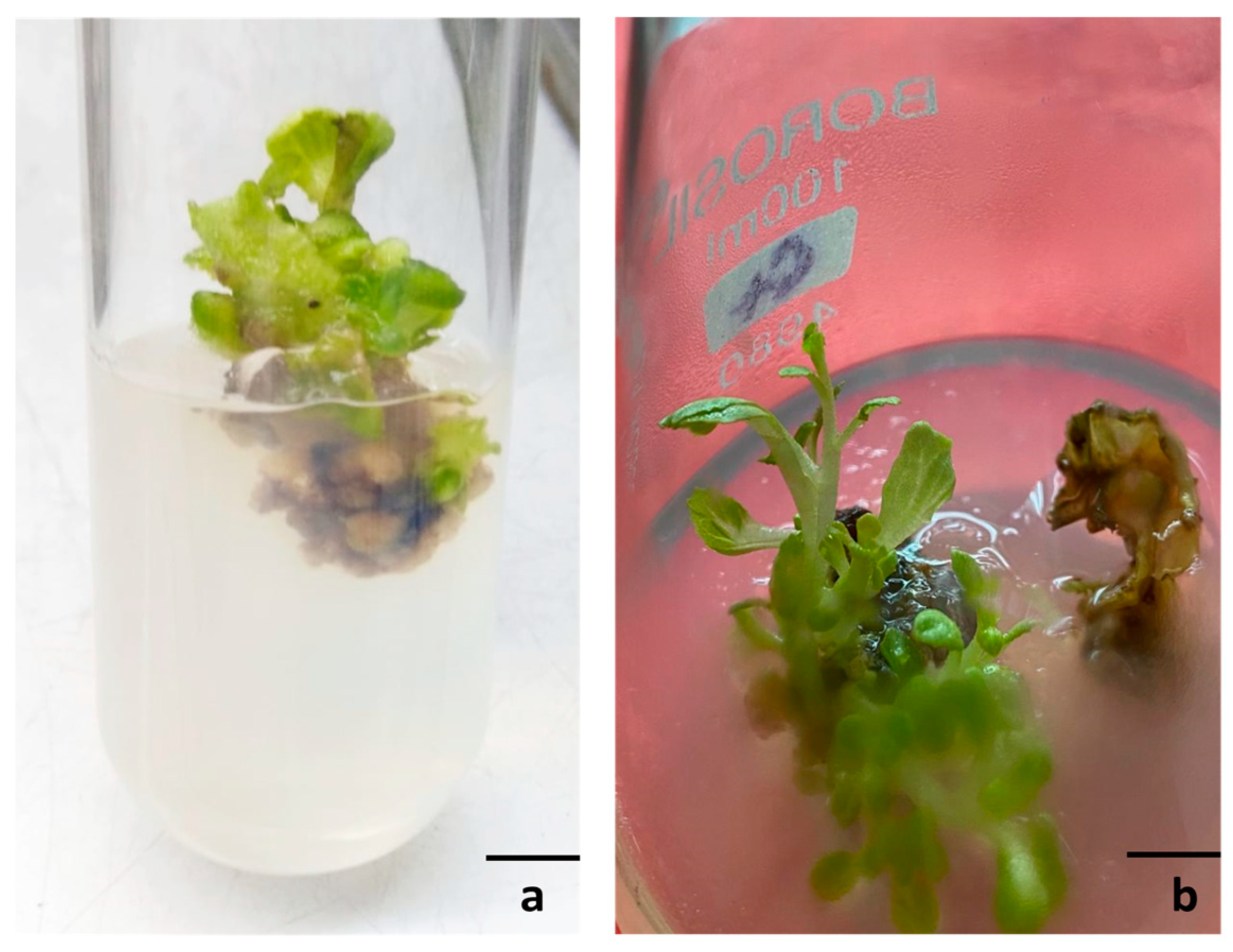
3.2. Adventitious Shoot Induction
3.3. Axillary (Direct) Shoot Regeneration
3.4. Induction of Roots in Regenerated Plantlets
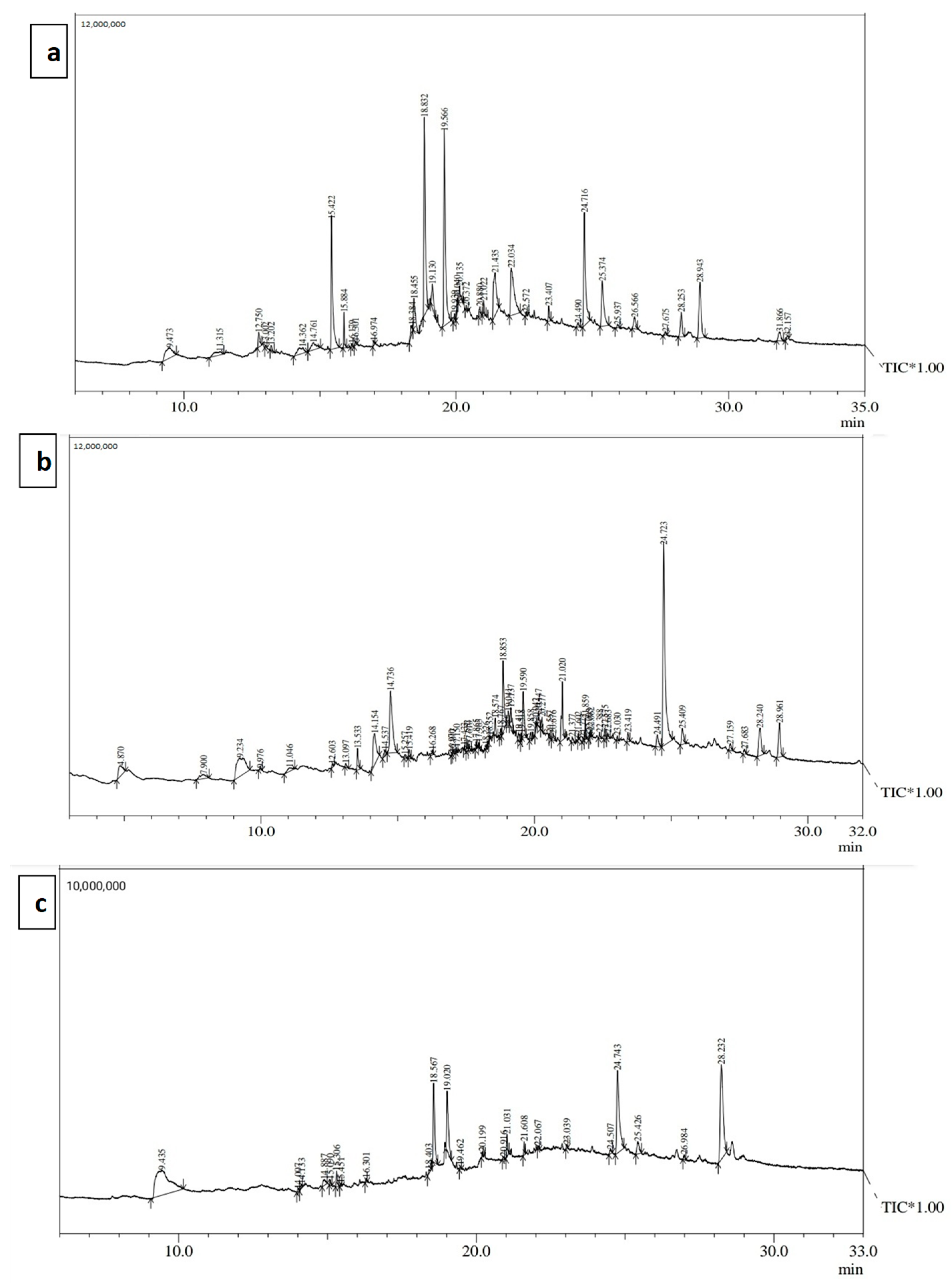
3.5. GC-MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahman, M.H.; Roy, B.; Chowdhury, G.M.; Hasan, A.; Saimun, M.S. Medicinal plant sources and traditional healthcare practices of forest-dependent communities in and around Chunati Wildlife Sanctuary in southeastern Bangladesh. Environ. Sustain. 2022, 5, 207–241. [Google Scholar] [CrossRef] [PubMed]
- Sivasubramanian, R.; Brindha, P. In vitro cytotoxic, antioxidant and GC-MS studies on Centratherum punctatum Cass. Int. J. Pharm. Pharm. Sci. 2013, 4, e8. [Google Scholar]
- Kirkman, L.K. Taxonomic revision of Centratherum and Phyllocephalum (Compositae: Vernonieae). Rhodora 1981, 83, 1–24. Available online: http://www.jstor.org/stable/23314048 (accessed on 26 October 2023).
- Gbolade, A.A.; Dzamic, A.M.; Ristic, M.S.; Marin, P.D. Essential oil composition of Centratherum punctatum from Nigeria. Chem. Nat. Compd. 2009, 45, 118–119. [Google Scholar] [CrossRef]
- Madhumitha, K.M.; Anbumalarmathi, J.; Sharmili, S.A.; Nandhini, G.; Priya, G.S. A comparative study of in vivo plant and in vitro callus extracts of Centratherum punctatum Cass. Annu. Res. Rev. Biol. 2020, 35, 1–13. [Google Scholar] [CrossRef]
- Smith, R.H. Plant Tissue Culture: Techniques and Experiments; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Syeed, R.; Mujib, A.; Malik, M.Q.; Gulzar, B.; Zafar, N.; Mamgain, J.; Ejaz, B. Direct somatic embryogenesis and flow cytometric assessment of ploidy stability in regenerants of Caladium × hortulanum ‘Fancy’. J. Appl. Genet. 2022, 63, 199–211. [Google Scholar] [CrossRef]
- Oseni, O.M.; Pande, V.; Nailwal, T.K. A review on plant tissue culture, a technique for propagation and conservation of endangered plant species. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3778–3786. [Google Scholar] [CrossRef]
- Qiang, B.; Miao, J.; Phillips, N.; Wei, K.; Gao, Y. Recent advances in the tissue culture of American ginseng (Panax quinquefolius). Chem. Biodivers. 2020, 17, e2000366. [Google Scholar] [CrossRef]
- Amiri, S.; Fotovat, R.; Tarinejad, A.R.; Panahi, B.; Mohammadi, S.A. In vitro regeneration of periwinkle (Catharanthus roseus L.) and fidelity analysis of regenerated plants with ISSR markers. J. Plant Physiol. Breed. 2019, 9, 129–135. [Google Scholar]
- Abbasin, Z.; Zamani, S.; Movahed, S.; Khaksar, G.; Tabatabaei, B.S. In vitro micropropagation of Yew (Taxus baccata) and production of plantlets. Biotechnology 2010, 9, 48–54. [Google Scholar] [CrossRef][Green Version]
- Maruyama, S.; Shibuya, N.; Kaku, H.; Desaki, Y. Arabidopsis cell culture for comparable physiological and genetic studies. Plant Signal. Behav. 2020, 15, 1781384. [Google Scholar] [CrossRef]
- Mohaddab, M.; El Goumi, Y.; Gallo, M.; Montesano, D.; Zengin, G.; Bouyahya, A.; Fakiri, M. Biotechnology and in vitro culture as an alternative system for secondary metabolite production. Molecules 2022, 27, 8093. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, N.V.; Thomas, T.D. Direct and indirect shoot regeneration from leaf explants of Centratherum punctatum Cass., A wild ornamental plant. Sci. Hortic. 2023, 320, 112201. [Google Scholar] [CrossRef]
- Aswathi, N.V.; Thomas, T.D. Transverse thin cell layer (tTCL) technology: A promising tool for micropropagation of Centratherum punctatum Cass. In Vitro Cell. Dev. Biol.—Plant 2023, 59, 340–353. [Google Scholar] [CrossRef]
- Mamgain, J.; Mujib, A.; Syeed, R.; Ejaz, B.; Malik, M.Q.; Bansal, Y. Genome size and gas chromatography-mass spectrometry (GC–MS) analysis of field-grown and in vitro regenerated Pluchea lanceolate plants. J. Appl. Genet. 2023, 64, 1–21. [Google Scholar] [CrossRef]
- Bansal, Y.; Mujib, A.; Mamgain, J.; Dewir, Y.H.; Rihan, H.Z. Phytochemical composition and detection of novel bioactives in anther callus of Catharanthus roseus L. Plants 2023, 12, 2186. [Google Scholar] [CrossRef]
- Hübschmann, H.J. Handbook of GC-MS: Fundamentals and Applications; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Olivia, N.U.; Goodness, U.C.; Obinna, O.M. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Future J. Pharm. Sci. 2021, 7, 59. [Google Scholar] [CrossRef]
- Arafa, N.M.; Girgis, N.; Ibrahem, M.; Mohamed, S.; El-Bahr, M.K. Phytochemical profiling by GC-MS analysis and antimicrobial activity potential of in vitro derived shoot cultures of some Egyptian herbal medicinal plants. Egypt. J. Chem. 2022, 65, 155–169. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Phys. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- El-Ashmawy, I.M.; Aljohani, A.S.; Soliman, A.S. Studying the bioactive components and phytochemicals of the methanol extract of Rhanterium epapposum Oliv. Appl. Biochem. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Fehér, A. Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim. Et Biophys. Acta (BBA)-Gene Regul. Mech. 2015, 1849, 385–402. [Google Scholar] [CrossRef]
- Foo, P.C.; Lee, Z.H.; Chin, C.K.; Subramaniam, S.; Chew, B.L. Shoot induction in white eggplant (Solanum melongena L. cv. BulatPutih) using 6-benzylaminopurine and kinetin. Trop. Life Sci. Res. 2018, 29, 119. [Google Scholar] [CrossRef]
- Zakaria, Z.B.; Zakaria, S.B.; Ishak, M.A.B.M. Induction of callus formation from different parts of Citrus grandis (Osbeck) flowers. Biotropia 2010, 17, 32. [Google Scholar] [CrossRef]
- Malwane, T.S.D. In Vitro Tissue Culture: Towards Conservation of Threatened Desiccation Sensitive Encephalartos cycads Seeds. Master’s Thesis, The University of Cape Town, Cape Town, South Africa, 2019. Available online: https://hdl.handle.net/11427/31805 (accessed on 26 October 2023).
- Sape, S.T.; Kandukuri, A.V.; Owk, A.K. Direct axillary shoot regeneration with nodal explants of Bacopa monnieri (L.) Pennell—A multi medicinal herb. J. Appl. Biol. Sci. 2020, 14, 190–197. Available online: https://www.jabsonline.org/index.php/jabs/article/view/726 (accessed on 26 October 2023).
- Bhat, M.S.; Rather, Z.A.; Nazki, I.T.; Banday, N.; Wani, T.; Rafiq, S.; Darwish, H. Standardization of in vitro micropropagation of Winter Jasmine (Jasminum nudiflorum) using nodal explants. Saudi J. Biol. Sci. 2022, 29, 3425–3431. [Google Scholar] [CrossRef] [PubMed]
- Oseni, O.M.; Nailwal, T.K.; Pande, V. Callus induction and multiple shoot proliferation from nodal explants of Mansonia altissima: Confirmation of genetic stability using ISSR and RAPD markers. In Vitro Cell. Dev. Biol.—Plant 2022, 58, 479–488. [Google Scholar] [CrossRef]
- Buah, J.N.; Danso, E.; Taah, E.A.; Abole, E.A.; Bediako, E.A.; Asiedu, J.; Baidoo, R. The Effects of Different Concentrations Cytokinins on the In Vitro Multiplication of Plantain (Musa sp.). 2010. Available online: http://hdl.handle.net/123456789/4934 (accessed on 26 October 2023).
- Singh, M.K.; Yadav, T.; Raman, R.K. A quick method for micro-propagation of Aloe vera L. from leaf explants via callus induction. J. Entomol. Zool. Stud. 2020, 8, 201–206. [Google Scholar]
- Hill, K.; Schaller, G.E. Enhancing plant regeneration in tissue culture: A molecular approach through manipulation of cytokinin sensitivity. Plant Signal. Behav. 2013, 8, 212–224. [Google Scholar] [CrossRef]
- Mujib, A.; Fatima, S.; Malik, M.Q. Gamma ray–induced tissue responses and improved secondary metabolites accumulation in Catharanthus roseus. Appl. Microbiol. Biotechnol. 2022, 106, 6109–6123. [Google Scholar] [CrossRef]
- Dilshad, E.; Asif, A.; Arooj, H.; Khan, S.H.; Bakhtiar, S.M. Impact of BAP on in vitro regeneration of potato (Solanum tuberosum L.). Curr. Trends OMICS 2021, 1, 67–79. [Google Scholar] [CrossRef]
- Mood, K.; Jogam, P.; Sirikonda, A.; Shekhawat, M.S.; Rohela, G.K.; Manokari, M.; Allini, V.R. Micropropagation, morpho-anatomical characterization, and genetic stability studies in Lippia javanica (Burm. f.) Spreng: A multipurpose medicinal plant. Plant Cell Tissue Organ. Cult. 2022, 150, 427–437. [Google Scholar] [CrossRef]
- Singh, C.K.; Raj, S.R.; Patil, V.R.; Jaiswal, P.S.; Subhash, N. Plant regeneration from leaf explants of mature sandalwood (Santalum album L.) trees under in vitro conditions. In Vitro Cell. Dev. Biol.—Plant 2013, 49, 216–222. [Google Scholar] [CrossRef]
- Martins, J.P.R.; Wawrzyniak, M.K.; Ley-López, J.M.; Kalemba, E.M.; Mendes, M.M.; Chmielarz, P. 6-Benzylaminopurine and kinetin modulations during in vitro propagation of Quercus robur (L.): An assessment of anatomical, biochemical, and physiological profiling of shoots. Plant Cell Tissue Organ. Cult. 2022, 151, 149–164. [Google Scholar] [CrossRef]
- Ahmad, S.; Jakhar, M.L.; Gothwal, D.K.; Ram, M.; Kumhar, B.L.; Kumawat, G.L. Effect of 6-Benzylaminopurine (BAP) and Kinetin (Kn) on callus induction under in vitro culture of Aloe vera. Biol. Forum 2022, 14, 890–894. [Google Scholar]
- Gerszberg, A.; Hnatuszko-Konka, K.; Kowalczyk, T.; Kononowicz, A.K. Efficient in vitro callus induction and plant regeneration protocol for different Polish tomato cultivars. Not. Bot. Horti Agrobot. 2016, 44, 452–458. [Google Scholar] [CrossRef][Green Version]
- Inceer, H.; Cuce, M.; Imamoglu, K.V.; Ergin, T.; Ucler, A.O. In vitro propagation and cytogenetic stability of Tripleurospermum insularum (Asteraceae)—A critically endanger redinsular endemic species from Turkey. Plant Biosyst. 2022, 156, 1213–1221. [Google Scholar] [CrossRef]
- Abdulhafiz, F.; Mohammed, A.; Kayat, F.; Zakaria, S.; Hamzah, Z.; Reddy Pamuru, R.; Gundala, P.B.; Reduan, M.F. Micropropagation of Alocasia longiloba Miq and comparative antioxidant properties of ethanolic extracts of the field-grown plant, in vitro propagated and in vitro-derived callus. Plants 2020, 29, 816. [Google Scholar] [CrossRef]
- Padma, M.; Ganesan, S.; Jayaseelan, T.; Azhagumadhavan, S.; Sasikala, P.; Senthilkumar, S.; Mani, P. Phytochemical screening and GC–MS analysis of bioactive compounds present in ethanolic leaves extract of Silybum marianum (L). J. Drug Deliv. Ther. 2019, 15, 85–89. Available online: https://jddtonline.info/index.php/jddt/article/view/2174 (accessed on 26 October 2023). [CrossRef]
- Jahan, I.; Tona, M.R.; Sharmin, S.; Sayeed, M.A.; Tania, F.Z.; Paul, A.; Chy, M.N.; Rakib, A.; Emran, T.B.; Simal-Gandara, J. GC-MS phytochemical profiling, pharmacological properties, and in silico studies of Chukrasia velutina leaves: A novel source for bioactive agents. Molecules 2020, 25, 3536. [Google Scholar] [CrossRef]
- Musa, A.M.; Ibrahim, M.A.; Aliyu, A.B.; Abdullahi, M.S.; Tajuddeen, N.; Ibrahim, H.; Oyewale, A.O. Chemical composition and antimicrobial activity of hexane leaf extract of Anisopus mannii (Asclepiadaceae). J. Intercult. Ethnopharmacol. 2015, 4, 129. [Google Scholar] [CrossRef]
- Sujatha, P.; Evanjaline, M.; Muthukumarasamy, S.; Mohan, V.R. Determination of bioactive components of Barleria courtallicanees (Acanthaceae) by gas chromatography–mass spectrometry analysis. Asian J. Pharm. Clin. Res. 2017, 10, 273. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sali, V.K.; Mani, S.; Vasanthi, H.R. Neophytadiene from Turbinariaornata suppresses LPS-induced inflammatory response in RAW 264.7 macrophages and Sprague Dawley rats. Inflammation 2020, 43, 937–950. [Google Scholar] [CrossRef]
- Vandana; Deora, G.S. Preliminary phytochemical screening and GC-MS analysis of methanolic leaf extract of Tephrosia falciformis ramaswami from Indian Thar Desert. Int. J. Pharm. Sci. Res. 2020, 61, 3040–3046. [Google Scholar] [CrossRef]
- Tyagi, T.; Agarwal, M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacogn. Phytochem. 2017, 6, 195–206. [Google Scholar]
- Yamuna, P.; Abirami, P.; Vijayashalini, P.; Sharmila, M. GC-MS analysis of bioactive compounds in the entire plant parts of ethanolic extract of Gomphrena decumbens Jacq. J. Med. Plants Stud. 2017, 5, 31–37. [Google Scholar]
- Kokaz, S.F.; Deb, P.K.; Abed, S.N.; Al-Aboudi, A.; Das, N.; Younes, F.A.; Salou, R.A.; Bataineh, Y.A.; Venugopala, K.N.; Mailavaram, R.P. Pharmacology of acetylcholine and cholinergic receptors. In Frontiers in Pharmacology of Neurotransmitters; Springer: Berlin/Heidelberg, Germany, 2020; pp. 69–105. [Google Scholar] [CrossRef]
- Srinivasan, S.; Priya, V. Phytochemical screening and GC-MS analysis of Cyperus dubius, Rottb. (Cyperaceae). J. Med. Plants 2019, 7, 89–98. [Google Scholar]
- Mujeeb, F.; Bajpai, P.; Pathak, N. Phytochemical evaluation, antimicrobial activity, and determination of bioactive components from leaves of Aegle marmelos. BioMed Res. Int. 2014, 2014, 497606. [Google Scholar] [CrossRef]
- Zhang, K.; He, W.; Du, Y.; Zhou, Y.; Wu, X.; Zhu, J.; Lu, Y. Inhibitory effect of lanosterol on cataractous lens of cynomolgus monkeys using a subconjunctival drug release system. Precis. Clin. Med. 2022, 5, pbac021. [Google Scholar] [CrossRef]
- De Almeida, P.; Boleti Rüdiger, A.L.; Lourenço, G.A.; da Veiga Junior, V.F.; Lima, E.S. Anti-inflammatory activity of triterpenes isolated from Protium paniculatum oil-resins. Evid.-Based Complement. Altern. Med. 2015, 2015, 293768. [Google Scholar] [CrossRef]
- Mozirandi, W.; Tagwireyi, D.; Mukanganyama, S. Evaluation of antimicrobial activity of chondrillasterol isolated from Vernonia adoensis (Asteraceae). BMC Complement. Altern. Med. 2019, 19, 249. [Google Scholar] [CrossRef]
- Bettio, L.E.; Gil-Mohapel, J.; Rodrigues, A.L.S. Guanosine and its role in neuropathologies. Purinergic Signal. 2016, 12, 411–426. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Parikh, J.R.; Patel, B.G. Formulation consideration and skin retention study of microemulsion containing tazarotene for targeted therapy of acne. J. Pharm. Investig. 2016, 46, 55–66. [Google Scholar] [CrossRef]
- Maurya, A.; Mohan, S.; Verma, S.C. Antidiabetic potential of naturally occurring sesquiterpenes: A review. Curr. Top. Med. Chem. 2021, 21, 851–862. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, X.; Song, D.; Liu, Y.; Kong, W. Anticancer activity of Eremanthin against the human cervical cancer cells is due to G2/M phase cell cycle arrest, ROS-mediated necrosis-like cell death and inhibition of PI3K/AKT signalling pathway. J. BUON 2020, 25, 1547–1553. [Google Scholar]
- Amudha, P.; Jayalakshmi, M.; Pushpabharathi, N.; Vanitha, V. Identification of bioactive components in Enhalusa coroides seagrass extract by gas chromatography-mass spectrometry. Asian J. Pharm. Clin. Res. 2018, 11, 313–315. [Google Scholar] [CrossRef]
- Rouvrais, C.; Bacqueville, D.; Bogdanowicz, P.; Haure, M.J.; Duprat, L.; Coutanceau, C.; Bessou-Touya, S. A new dermocosmetic containing retinaldehyde, delta- tocopherol glucoside and glycylglycineoleamide for managing naturally aged skin: Results from in vitro to clinical studies. In Clinical, Cosmetic and Investigational Dermatology; Taylor & Francis: Abingdon, UK, 2017; pp. 35–42. [Google Scholar] [CrossRef]
- Mugao, L.G.; Gichimu, B.M.; Muturi, P.W.; Mukono, S.T. Characterization of the volatile components of essential oils of selected plants in Kenya. Biochem. Res. Int. 2020, 2020, 8861798. [Google Scholar] [CrossRef] [PubMed]
- Nsofor, W.N.; Nwaoguikpe, R.N.; Ujowundu, F.N.; Keke, C.O.; Uba, M.T.U.; Edom, C.V. Phytochemical, GC-MS, FTIR and Amino acid profile of methanol extract of Tetrapleura tetraptera fruit. J. Drug Deliv. Ther. 2023, 13, 61–69. [Google Scholar] [CrossRef]
- Abubacker, M.N.; Devi, P.K. In vitro antifungal potentials of bioactive compound oleic acid, 3-(octadecyloxy) propyl ester isolated from Lepidagathis cristata Willd. (Acanthaceae) inflorescence. Asian Pac. J. Trop. Med. 2014, 7, S190–S193. [Google Scholar] [CrossRef]
- Subramanian, S.; Dowlath, M.J.H.; Karuppannan, S.K.; Saravanan, M.; Arunachalam, K.D. Effect of Solvent on the Phytochemical Extraction and GC-MS Analysis of Gymnema sylvestre. Pharmacogn. J. 2020, 12, 749–761. [Google Scholar] [CrossRef]
- Aodah, A.H.; Hashmi, S.; Akhtar, N.; Ullah, Z.; Zafar, A.; Zaki, R.M.; Ali, M.S. Formulation development, optimization by box–behnken design, and in vitro and ex vivo characterization of hexatriacontane-loaded transethosomal gel for antimicrobial treatment for skin infections. Gels 2023, 9, 322. [Google Scholar] [CrossRef]



| PGRs (mg/L) | Callus Induction (%) | Weight of Callus (g) | Type of Callus Observed | |||
|---|---|---|---|---|---|---|
| 2,4-D | NAA | BAP | Kn | |||
| 2.0 | 1.5 | 62.21 ± 1.28 c | 0.71 ± 0.08 c | White-brownish compact callus | ||
| 2.5 | 2.0 | 76.40 ± 0.81 c | 0.85 ± 0.32 c | |||
| 3.0 | 2.5 | 89.54 ± 0.31 b | 1.20 ± 0.09 b | |||
| 4.0 * | 3.5 * | 98.32 ± 0.47 a* | 2.02 ± 0.04 a* | |||
| 1.0 | 0.5 | 0 | 0 | Watery-friable callus | ||
| 1.5 | 1.0 | 67.35 ± 0.49 c | 0.19 ± 0.02 c | |||
| 2.0 | 1.5 | 78.13 ± 0.70 ab | 0.48 ± 0.01 ab | |||
| 2.5 * | 2.0 * | 82.27 ± 1.24 a* | 0.76 ± 0.21 a* | |||
| 3.0 | 2.5 | 70.63 ± 0.89 c | 0.32 ± 0.03 c | |||
| PGRs (mg/L) | Shoot Induction (%) | No. of Shoots/Gram of Callus | Mean Shoot Length (cm) | |
|---|---|---|---|---|
| BAP | Kn | |||
| 2.0 | 1.0 | 0 c | 0 c | 0c |
| 2.5 | 1.5 | 33.24 ± 0.13 b | 3.2 ± 1.27 b | 2.6 ± 0.35 b |
| 3.0 | 2.5 | 40.67 ± 0.38 b | 4.1 ± 0.63 b | 3.3 ± 0.17 b |
| 4.0 | 3.5 | 52.41 ± 0.24 a | 6.6 ± 0.35 a | 5.2 ± 0.69 a |
| 4.5 * | 4.0 * | 66.33 ± 0.44 a* | 8.3 ± 0.75 a* | 6.1 ± 0.63 a* |
| PGRs (mg/L) | Shoot Induction (%) | No. of Shoots | Mean Shoot Length (cm) | |
|---|---|---|---|---|
| BAP | Kn | |||
| 2.0 | 1.0 | 60.42 ± 0.87 d | 8.1 ± 0.57 d | 2.0 ± 0.12 d |
| 2.5 | 1.5 | 68.14 ± 1.17 c | 12.3 ± 0.94 c | 4.0 ± 0.06 c |
| 3.0 | 2.5 | 72.56 ± 1.44 c | 21.3 ± 1.0 c | 4.5 ± 0.29 c |
| 4.0 | 3.5 | 88.28 ± 0.90 b | 26.4 ± 1.15 b | 6.2 ± 0.17 b |
| 4.5 * | 4.0 * | 100.0 ± 2.89 a* | 30.2 ± 0.92 a* | 8.5 ± 0.28 a* |
| IAA (mg/L) | Root Induction (%) | No. of Roots/Shoot | Mean Root Length (cm) |
|---|---|---|---|
| 0.5 | 40.16 ± 1.18 c | 18.3 ± 0.81 c | 2.5 ± 0.33 c |
| 1.0 * | 95.27 ± 0.60 a* | 26.1 ± 0.58 a* | 6.2 ± 0.21 a* |
| 1.5 | 80.52 ± 0.52 b | 20.5 ± 1.25 b | 4.8 ± 0.11 b |
| Peak | R. Time | Area | Area% | Name |
|---|---|---|---|---|
| 1 | 9.473 | 2814136 | 3.38 | Sucrose |
| 2 | 11.315 | 1087916 | 1.31 | Pentanoic acid, 2-(methoxymethyl)-4-oxo- |
| 3 | 12.750 | 943200 | 1.13 | Neophytadiene |
| 4 | 13.002 | 89147 | 0.11 | Oxirane, hexadecyl- |
| 5 | 13.202 | 286531 | 0.34 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol |
| 6 | 14.362 | 1494158 | 1.79 | Decane, 1-bromo-2-methyl- |
| 7 | 14.761 | 1376385 | 1.65 | Palmitic Acid, TMS derivative |
| 8 | 15.422 | 8261961 | 9.92 | Phytol |
| 9 | 15.884 | 1054928 | 1.27 | Phytol, TMS derivative |
| 10 | 16.179 | 123837 | 0.15 | Eicosane, 7-hexyl- |
| 11 | 16.301 | 195717 | 0.24 | 1-Docosanol, acetate |
| 12 | 16.974 | 185984 | 0.22 | N1-Isopropyl-2-methyl-1,2-propanediamine |
| 13 | 18.384 | 708458 | 0.85 | Acetylcholine bromide |
| 14 | 18.455 | 2426875 | 2.91 | Crotonyl isothiocyanate |
| 15 | 18.832 | 11287490 | 13.56 | 1,6-Octadien, 3,5-Dimethyl-, Cis |
| 16 | 19.130 | 1705225 | 2.05 | Bicyclo[3.1.1]heptan-3-one, 2,6,6-trimethyl-, (1.alpha.,2.alp |
| 17 | 19.566 | 12486993 | 15.00 | 2(5H)-Furanone, 5-(2-methyl-3-methylene-4-butyl)- |
| 18 | 19.939 | 308148 | 0.37 | 2-Butenoic acid, 2-methyl-, 1,1a,1b,4,4a,5,7a,7b,8,9-decahy |
| 19 | 20.040 | 442568 | 0.53 | 4,4′-((p-Phenylene)diisopropylidene)diphenol |
| 20 | 20.135 | 1002999 | 1.20 | 2-Butenoic acid, 2-methyl-, 2-methyl-2-propenyl ester, (E)- |
| 21 | 20.372 | 172984 | 0.21 | 5A-Methyl-3,8-Dimethylene-2 Oxododecahydrooxireno[2′,3′:6,7]Naphtho[1,2B] Furan-6-YL 2-Methyl-2-B-Utenoate |
| 22 | 20.880 | 502397 | 0.60 | 1-Azatricyclo[3.3.1.13,7]Decane-4,6,10-Trione, |
| 23 | 21.022 | 621615 | 0.75 | Squalene |
| 24 | 21.435 | 4898059 | 5.88 | 4,8-Dimethylnona-3,8-dien-2-one |
| 25 | 22.034 | 6658350 | 8.00 | 2,6-Octadiene, 2,4-dimethyl- |
| 26 | 22.572 | 152066 | 0.18 | Stigmasta-4,7,22-trien-3.alpha.-ol |
| 27 | 23.407 | 650675 | 0.78 | Vitamin E |
| 28 | 24.490 | 269170 | 0.32 | 5-Cholesten-3.beta.-acetoxy-24-thiol |
| 29 | 24.716 | 8119706 | 9.75 | Stigmasta-5,22-Dien-3-Ol |
| 30 | 25.374 | 4164314 | 5.00 | Chondrillasterol |
| 31 | 25.937 | 145352 | 0.17 | .beta.-Amyrone |
| 32 | 26.566 | 1008550 | 1.21 | Noruns-12-Ene |
| 33 | 27.675 | 146023 | 0.18 | Acetic acid, 3-hydroxy-7-isopropenyl-1,4a-dimethyl-2,3,4,4 |
| 34 | 28.253 | 1764216 | 2.12 | Phytyl palmitate |
| 35 | 28.943 | 4585371 | 5.51 | Acetic Acid 17-(1,5-Dimethyl-Hex-4-Enyl)-4,4,1 |
| 36 | 31.866 | 893299 | 1.07 | 9,12-Octadecadienoic acid (Z,Z)-, octyl ester |
| 37 | 32.157 | 225509 | 0.27 | 9,10,12,13-Tetrabromooctadecanoic acid |
| 83260312 | 100.00 |
| Peak | R. Time | Area | Area% | Name |
|---|---|---|---|---|
| 1 | 4.870 | 1720817 | 2.56 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- |
| 2 | 7.900 | 991141 | 1.47 | 1,4-Diacetyl-3-acetoxymethyl-2,5-methylene-l-rhamnitol |
| 3 | 9.234 | 6098776 | 9.06 | Guanosine |
| 4 | 9.976 | 86895 | 0.13 | Butanoic Acid, 1-Methylhexyl Ester |
| 5 | 11.046 | 630043 | 0.94 | Alpha-L-Galactopyranoside, methyl 6-deoxy- |
| 6 | 12.603 | 154311 | 0.23 | Isopropyl myristate |
| 7 | 13.097 | 200311 | 0.30 | Benzene, (1-ethylundecyl)- |
| 8 | 13.533 | 1119716 | 1.66 | Benzene, (1-Methyldodecyl)- |
| 9 | 14.154 | 3854840 | 5.72 | Tridecanoic acid |
| 10 | 14.537 | 368787 | 0.55 | Benzene, (1-methyltridecyl)- |
| 11 | 14.736 | 6547794 | 9.72 | Azuleno[4,5-b]furan-2(3H)-one, 3a,4,6a,7,8,9,9a,9b-octahy |
| 12 | 15.257 | 148285 | 0.22 | 3-Methyl-2-Pent-2-Enyl-Cyclopent-2-Enone |
| 13 | 15.419 | 419102 | 0.62 | Phytol |
| 14 | 16.268 | 240870 | 0.36 | Tricosyl acetate |
| 15 | 16.970 | 72107 | 0.11 | 2-Butanamine, 2-methyl- |
| 16 | 17.017 | 141687 | 0.21 | 5-Decanone |
| 17 | 17.150 | 152870 | 0.23 | Silane, Trichlorooctadecyl- |
| 18 | 17.433 | 73677 | 0.11 | Tetracyclo[6.1.0.0(2,4).0(5,7)] Nonane, 3,3,6,6,9,9 |
| 19 | 17.532 | 280521 | 0.42 | 3,3,6,6,9,9-Hexaethyltetracyclo[6.1.0.0~2,4~.0~ |
| 20 | 17.610 | 233436 | 0.35 | Fumaric acid, 2-octyl 8-chlorooctyl ester |
| 21 | 17.865 | 170331 | 0.25 | Cyclopentanol, 3,3,4-Trimethyl-4-P-Tolyl-, ( |
| 22 | 17.965 | 110757 | 0.16 | Triamylbenzenes |
| 23 | 18.226 | 168774 | 0.25 | Benzene, Hexadecylpentyl- |
| 24 | 18.352 | 109276 | 0.16 | Tetracyclo[6.1.0.0(2,4).0(5,7)]nonane, 3,3,6,6,9,9-hexaethy |
| 25 | 18.574 | 711913 | 1.06 | Valeric acid, 2-methyloct-5-yn-4-yl ester |
| 26 | 18.767 | 273738 | 0.41 | Triamylbenzenes |
| 27 | 18.853 | 3914084 | 5.81 | 1,6-Octadien, 3,5-Dimethyl-, Cis |
| 28 | 19.041 | 698160 | 1.04 | Cyclooctanepropanal, 1-Nitro-2-Oxo-, (.+-.)- |
| 29 | 19.137 | 1193280 | 1.77 | 2,5-Furandione, 3-(Dodecenyl)Dihydro- |
| 30 | 19.417 | 341915 | 0.51 | 3-(4-Isopropylphenyl)-2-Methylpropanal # |
| 31 | 19.518 | 183490 | 0.27 | 1-(4-Undecylphenyl)Ethanone |
| 32 | 19.590 | 2565091 | 3.81 | 2(5H)-Furanone, 5-(2-methyl-3-methylene-4-butyl)- |
| 33 | 19.858 | 328123 | 0.49 | 3,3,6,6,9,9-Hexaethyltetracyclo[6.1.0.0~2,4~.0~ |
| 34 | 20.042 | 281338 | 0.42 | 4,4’-((p-Phenylene)diisopropylidene)diphenol |
| 35 | 20.147 | 715085 | 1.06 | Tetracosatetraene, 2,6,10,15,19,23-Hexameth |
| 36 | 20.277 | 528992 | 0.79 | 1h-Purin-6-Amine, [(2-Fluorophenyl)Methyl] |
| 37 | 20.557 | 195366 | 0.29 | Heptacosane, 1-chloro- |
| 38 | 20.676 | 457102 | 0.68 | 3-(4-Isopropylphenyl)-2-Methylpropanal # |
| 39 | 21.020 | 2965566 | 4.40 | Squalene |
| 40 | 21.377 | 317308 | 0.47 | 1-(4-Undecylphenyl)ethanone |
| 41 | 21.602 | 126037 | 0.19 | Tetratetracontane |
| 42 | 21.772 | 132976 | 0.20 | Tert-Butyl(Dimethyl)Silyl ([Tert-Butyl)Dim |
| 43 | 21.859 | 580965 | 0.86 | Oxirane, 2,2-dimethyl-3-(3,7,12,16,20-pentamethyl-3,7,11, |
| 44 | 22.003 | 154676 | 0.23 | Cyclododecasiloxane, Tetracosamethyl- |
| 45 | 22.062 | 149027 | 0.22 | .delta.-Tocopherol |
| 46 | 22.388 | 255473 | 0.38 | 2,2,4-Trimethyl-3-(3,8,12,16-Tetramethyl-Hept |
| 47 | 22.575 | 399552 | 0.59 | Stigmasta-4,7,22-trien-3.alpha.-ol |
| 48 | 22.683 | 279519 | 0.42 | (R)-6-Methoxy-2,8-dimethyl-2-((4R,8R)-4,8,12-trimethyltr |
| 49 | 23.030 | 173306 | 0.26 | 2-Methylhexacosane |
| 50 | 23.419 | 449827 | 0.67 | Vitamin E |
| 51 | 24.491 | 940173 | 1.40 | Ergost-5-en-3-ol, (3.beta.)- |
| 52 | 24.723 | 16732924 | 24.85 | Stigmasta-5,22-Dien-3-Ol |
| 53 | 25.409 | 1233682 | 1.83 | Stigmast-5-En-3-Ol, (3.Beta.,24s)- |
| 54 | 27.159 | 482631 | 0.72 | 9,19-Cyclolanostan-3-ol, 24-methylene-, (3.beta.)- |
| 55 | 27.683 | 332363 | 0.49 | 24-Norursa-3,12-diene |
| 56 | 28.240 | 2632334 | 3.91 | 4,4a,6b,8a,11,11,12b,14a-Octamethyl-Docosah |
| 57 | 28.961 | 2525755 | 3.75 | Acetic Acid 17-(1,5-Dimethyl-Hex-4-Enyl)-4,4,1 |
| 67346895 | 100.00 |
| Peak | R. Time | Area | Area% | Name |
|---|---|---|---|---|
| 1 | 9.435 | 9086621 | 28.50 | 4-tert-Butoxybutan-1-ol, TMS derivative |
| 2 | 14.007 | 101638 | 0.32 | Chrysantenyl 2-methuylbutanoate |
| 3 | 14.133 | 161145 | 0.51 | 1,2-Benzenedicarboxylic acid, bis(2-methylpropyl) ester |
| 4 | 14.887 | 388609 | 1.22 | Azuleno[4,5-b]furan-2(3H)-one, 3a,4,6a,7,8,9,9a,9b-octahy |
| 5 | 15.090 | 144661 | 0.45 | 6-Methyl-2-(4-methylcyclohex-3-en-1-yl)hepta-1,5-dien-4- |
| 6 | 15.306 | 464106 | 1.46 | Cedren-13-ol, 8- |
| 7 | 15.451 | 76948 | 0.24 | Octyl tetradecyl ether |
| 8 | 16.301 | 96897 | 0.30 | Oleic Acid, Propyl Ester |
| 9 | 18.403 | 138729 | 0.44 | Ethanamine, 2-[(4-Chlorophenyl)-2-Pyridiny |
| 10 | 18.567 | 3288142 | 10.31 | Chrysantenyl 2-methuylbutanoate |
| 11 | 19.020 | 2752301 | 8.63 | 8-Hydroxy-2,2-dimethyl-8-phenyl-oct-5-en-3-one |
| 12 | 19.462 | 73419 | 0.23 | Tridecane, 7-hexyl- |
| 13 | 20.199 | 105608 | 0.33 | Eicosane |
| 14 | 20.916 | 78320 | 0.25 | Tricosane, 2-Methyl- |
| 15 | 21.031 | 602490 | 1.89 | Squalene |
| 16 | 21.608 | 281207 | 0.88 | Hexatriacontane |
| 17 | 22.067 | 85059 | 0.27 | .delta.-Tocopherol |
| 18 | 23.039 | 81202 | 0.25 | Tetracontane |
| 19 | 24.507 | 173201 | 0.54 | Ergost-5-en-3-ol, (3.beta.)- |
| 20 | 24.743 | 5554604 | 17.42 | Stigmasta-5,22-Dien-3-Ol |
| 21 | 25.426 | 758195 | 2.38 | Chondrillasterol |
| 22 | 26.984 | 169076 | 0.53 | 24-Noroleana-3,12-diene |
| 23 | 28.232 | 7223088 | 22.65 | 4,4a,6b,8a,11,11,12b,14a-Octamethyl-Docosah |
| 31885266 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talan, A.; Mujib, A.; Ejaz, B.; Bansal, Y.; Dewir, Y.H.; Magyar-Tábori, K. In Vitro Propagation and Phytochemical Composition of Centratherum punctatum Cass—A Medicinal Plant. Horticulturae 2023, 9, 1189. https://doi.org/10.3390/horticulturae9111189
Talan A, Mujib A, Ejaz B, Bansal Y, Dewir YH, Magyar-Tábori K. In Vitro Propagation and Phytochemical Composition of Centratherum punctatum Cass—A Medicinal Plant. Horticulturae. 2023; 9(11):1189. https://doi.org/10.3390/horticulturae9111189
Chicago/Turabian StyleTalan, Anuradha, Abdul Mujib, Bushra Ejaz, Yashika Bansal, Yaser Hassan Dewir, and Katalin Magyar-Tábori. 2023. "In Vitro Propagation and Phytochemical Composition of Centratherum punctatum Cass—A Medicinal Plant" Horticulturae 9, no. 11: 1189. https://doi.org/10.3390/horticulturae9111189
APA StyleTalan, A., Mujib, A., Ejaz, B., Bansal, Y., Dewir, Y. H., & Magyar-Tábori, K. (2023). In Vitro Propagation and Phytochemical Composition of Centratherum punctatum Cass—A Medicinal Plant. Horticulturae, 9(11), 1189. https://doi.org/10.3390/horticulturae9111189









