Abstract
Drought is one of the leading abiotic factors limiting morphological and physiological activities in blackberry cultivation. In this study, the effects of silver nanoparticles (AgNPs) on some morphological and biochemical processes in boysenberry (Rubus ursinus Chamisso and Schlenhtendal) under drought stress were investigated. The experiment was performed with three drought stress levels simulated with Polyethylene Glycol (PEG) (0, 4, 8%) and three AgNP treatments (0, 0.1, 0.2 mg L−1) in vitro conditions. Drought stress reduced root and shoot development of boysenberry plants grown in vitro. The addition of AgNPs significantly alleviated the adverse effect of drought stress and increased the plant growth parameters. Antioxidant activity of superoxide dismutase (SOD) and catalase (CAT) enzymes increased in boysenberry leaves when treated with AgNPs under drought conditions, while the amount of malondialdehyde (MDA) decreased. As a result of the study, 0.1 mg L−1 AgNPs is recommended as the most effective dose to promote the growth and development of in vitro boysenberry plants under drought stress.
Keywords:
blackberry; berries; boysenberry; functional food; nanosilver; nanoparticles; water stress 1. Introduction
The cultivation of berries is the subject of wide-ranging studies due to the fact that they have very tasty fruits and that they are recommended to be consumed at increasing rates in the daily diet [1,2]. Rubus fructorious, in the genus Rubus, is widely grown in temperate climatic regions of the world due to increasing consumer demand [3,4] and is one of the most popular horticultural berry fruit species. Blackberries are used in many sectors such as food, cosmetics, and health, and can be consumed fresh and processed. Therefore, due to the high antioxidant properties and valuable nutritional content of the fruits, it is recommended by the authorities as a healthy food [1,4,5]. Blackberries are also described as functional food in connection with their organoleptic properties and high content of polyphenol compounds [2,4,6]. Compared to many other fruit species, blackberry has advantages such as ease of cultivation and marketing, providing raw materials to the industry, being suitable for family business, high income from small areas, and low production costs [1,2,3,6]. Boysenberry is described as hybrid Rubus berry of Rubus baileyanus and Rubus loganobaccus [7]. This fruit is especially effective in reducing oxidative stress and is rich in anthocyanins, ellagic acid and ellagitannins, which are important for human health [8,9]. The cultivation of Boysenberry, which is also preferred by the food industry, is increasing rapidly all over the world due to its suitability for fresh consumption and processing and its higher fruit quality compared to other blackberry varieties [10,11]. Unfortunately, the production of ‘Boysenberry’, whose large fruit, resistance to many diseases and pests, and thornless varieties have been bred, has not yet reached the level it deserves [12,13]. The reasons for this situation include problems such as decreasing agricultural areas, loss of production due to diseases and pests, climate change and changing soil conditions [10,14].
This plant, which can be propagated by vegetative methods by hard or softwood cuttings, one-year-old suckers, and layering or root cuttings, can also be propagated under in vitro conditions quickly and highly efficiently in a relatively short time with limited space and without seasonal variation [3]. One of the most important problems in the cultivation of this plant is the increasing drought day by day.
Considering the stress-affected rates of usable areas around the world, drought has the highest rate [15,16]. Drought stress causes physiological, biochemical and molecular responses in plants and negatively affects growth and yield [17,18,19,20]. Drought stress occurs when the amount of water lost by the plant through transpiration is more than it takes. Under these conditions, competition begins between plant organs for water, the water potential gradient between different parts of the plant is disrupted, and the plant is under water stress [17,19,20]. The thornless cultivars of Rubus L. species have soft and juicy fruits and a root system that does not reach the deep soil profile [21,22]. For this reason, the plant needs frequent watering and the vegetative growth and development of this plant are significantly affected by drought [23,24,25]. Water stress, which has the negative effects mentioned in all plants, is one of the factors limiting the production in blackberry cultivation and restricting the morphological, enzymatic and physiological activities of the plant. Researchers report that drought stress can decrease shoot, leaf and root development, and leaf number and disrupt water use efficiency, thus causing physiological and biochemical problems in blackberry plants [25,26,27]. In addition, under drought stress conditions, the total leaf area, leaf relative water content (RWC) and thus the photosynthesis rate decreases [28]. The loss of intracellular water causes dehydration due to the increase in concentration, and in this case, the collapse of the plasma membrane leads to ruptures and autolysis of the cytoplasm [26,27,28]. Protein structure and membrane stability in the plant may be adversely affected by this situation. Photosynthetic ability is determined by the total leaf area in that plant and the photosynthetic activity of each leaf. With drought stress, the total leaf area decreases, and therefore, photosynthesis decreases [25,27,28,29]. In this case, changes in the rate of photosynthesis can reduce vegetative growth and severely delay the development of boysenberry [23] and blackberry [27,29]. The first response of plants to drought stress is stomatal closure, which causes oxidative stress. In order to protect against oxidative stress in plant cells, there is a highly efficient antioxidant defense system including superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) enzymes [24,30].
Changing climatic conditions have increased the pressure of abiotic stress factors on agricultural production. More studies are needed to increase the drought tolerance of the plant for economical and sustainable blackberry cultivation. The development of new varieties resistant to stress conditions takes a long time and costs are high, especially in fruit growing. Nanoparticles (NPs) can be used in many different ways due to their physico-chemical properties such as high surface area, high reactivity, variable pore size and particle morphology. In recent years, NPs, which are environmentally friendly, have been used to alleviate the effects of abiotic stresses, including drought [31,32,33,34,35]. Metallic nanoparticles have an extremely large surface area/volume ratio and the ability to regulate electron exchange [36]. Silver nanoparticles, a metallic, antimicrobial/antibacterial nanomaterial, increase the chlorophyll content and photosynthesis rate, thereby promoting plant growth and development [36,37,38,39,40,41]. Researchers have reported that NP application contributes to the regulation of antioxidant enzyme activity and cellular water balance [31]. Also, some researchers stated that AgNPs have the potential to develop resistance against drought stress in different plants such as wheat [42,43], eggplant [44], thymus [45], lettuce [46] and lentil [47].
In light of all this information, it is clearly seen that the sustainability of agricultural production is at the mercy of the climate system [48]. However, in order to overcome this situation, it is necessary to carry out research to combat agricultural drought. When previous reports on drought are examined, it is thought that NP applications can be an effective alternative to alleviate the negative effects of drought. As a matter of fact, different NP applications have shown positive results in reducing the effects of drought in various plants [31,32,42,43,44,46,47], but no research reporting the effects of AgNPs on boysenberry has been found. Research to reduce yield loss due to drought is important for the future of this plant, which has important benefits for human health and high economic value. Therefore, this research aimed to determine the effects of different concentrations of AgNP treatments on drought tolerance of blackberries grown in vitro.
2. Materials and Methods
2.1. Plant Material
In the study, Boysenberry (Rubus ursinus Chamisso and Schlenhtendal) is used as plant material which is a hybrid Rubus berry derived from a cross between loganberry (Rubus loganobaccus Bailey) and trailing blackberry (Rubus fruticosus L.) [10]. Stock plant materials, used for micropropagation were obtained from plants grown in Akdeniz University Research and Application Area, Turkey.
2.2. AgNPs Suspension Preparation
AgNPs with an average particle size of <30 nm, surface area > 15 m2 g−1 and purity > 99% were used as nanoparticles (Nanografi Chemical Company, Ankara, Turkey). The morphological study of AgNPs was conducted by a scanning electron microscope (SEM) (ZEİSS-LEO 1430 ZEİSS, Oberkochen, Germany) (Figure 1). PVP-coated AgNPs were purchased as a dry powder and suspended in deionized water to prepare solutions at a ratio of 0.1 and 0.2 mg L−1 AgNPs (resistance > 18 MΩ cm; suspension pH = 5.8). The stock suspension was dissolved by sonicating for 30 min using a probe-type sonicator (JL-360, Shanghai, USA at 40 kHz) [49]. These stock solutions were used for preparing the MS culture media at the multiplication stage.

Figure 1.
Scanning electron microscopy (SEM) image of AgNPs.
2.3. İn Vitro Establishment of Cultures
Shoot tips and nodal cuttings (3–4 cm) of 3-year-old Boysenberry plants were washed thoroughly under running tap water for 2 h, then surface sterilized with 70% for 30 s and further sterilized by 30% bleach solution (5.25~NaOCl) with 0.2 mL L−1 of Tween 20 for 20 min followed by explants were washed three times with sterile distilled water under a laminar airflow cabinet [12].
Murashige and Skoog (MS) [50] culture medium containing 3% sucrose and 0.7% agar and 0.44% Gelrite was prepared by dissolving in distilled water and pH was adjusted to 5.8 before autoclaving at 121 °C for 20 min. Single bud explants were cut into 0.7–1 cm species and cultured two in each baby jar (330 mL). Explants were incubated at 24 ± 1 °C temperature, 16 h photoperiod, 40 μmol m−2 s−1 irradiance intensity and 70–80% relative humidity in the growth room [12]. After 5 weeks young shoots were used as an explant experiment.
The treatments consisted of drought stress at three levels (0, 4, 8%) of polyethylene glycol (PEG 6000) and three levels (0, 0.1, 0.2 mg L−1) AgNPs. Mixtures containing AgNPs were added to the media prepared for the subculturing of plants. The addition of PEG 6000 was added to the medium before pH measurement; 135 young shoots [3 repetition × (5 explants × 3 PEG doses × 3 AgNPs doses)] were transferred to the MS media supplemented with 0.5 mL BAP + 0.5 mL IBA and 30 g L−1 sucrose and cultured in the growth room same conditions as described above [12]. Shoot response was recorded 50 days after cultivation.
2.4. Growth Parameters
In order to determine the effect of applications on the vegetative development of plantlets, at the end of the experiment, shoot fresh weight (SFW) (g), shoot dry weight (SDW) (g), shoot length (SL) (mm), stem diameter (SD) (mm), root fresh weight (RFW) (g), root dry weight (RDW) (g), root length (RL) (mm), number of leaves (LN) (per plant), leaf width (LW) (mm), leaf length (LL) (mm) were determined. After measuring the fresh weights of the shoots and roots, they were placed in paper bags and kept in an oven at 70 °C for 48 h. Dry weight was determined by weighing the dried shoots.
2.5. Physiological Parameters
Measurement of the relative water contents (RWC%) was performed according to Sanchez et al. [51]. FW, TW and DW in the equation represent the fresh, turgid and dry weights of the sample, respectively. After the FW of the leaves was measured on a precision scale, the leaves were placed in Petri dishes containing distilled water and kept in the dark at 4 °C for 24 h to measure the TW value. The DW value was determined after the leaves were dried in the oven at 70 °C for 4 days.
RWC was calculated using the following equation:
RWC = [(FW − DW)/(TW − DW)] × 100
The SPAD index was measured using a chlorophyll content meter (SPAD-502, Konica Minolta Sensing, Inc., Tokyo, Japan).
2.6. Biochemical Parameters
Fresh leaves (0.5 g) were homogenized in 5 mL of 50 mM potassium phosphate buffer (pH 7.6 + 0.1 mM EDTA + 2% insoluble polyvinylpolypyrrolidone) in an ice bath to perform SOD, CAT and MDA analyses for each treatment. The supernatant formed by centrifuging the homogenate at 15,000× g for 15 min at 4 °C was stored at −20 °C for enzyme measurements.
Leaf superoxide dismutase (SOD) activity was measured according to the method of reduction of NBT (nitro blue tetrazolium chloride) by O2-under light [52]. The absorbance of leaf extract was spectrophotometrically measured at 560 nm wavelength. Catalase (CAT) activity is determined by following the consumption of H2O2 (E = 39.4 mM cm−1) at 240 nm [52]. Heath and Packer’s [53] method was used to measure the content of Malondialdehyde (MDA). The absorbance of the leaf extracts was read at 532 and 600 nm by spectrophotometer.
All photochemical quantifications were conducted on a Thermo Scientific Multiskan GO spectrophotometer (Multiskan™ GO Microplate Spectrophotometer; Thermo Fisher Scientific, Waltham, MA, USA) at a temperature of 4 °C.
2.7. Statistical Analysis
All measurements in this work were replicated three times. Data of morphological and physiological indices were subjected to an analysis of variance (ANOVA) using the software SPSS 22.0. Duncan’s multiple range test (p < 0.05) was used to determine the significance among all means between different treatments. Error bars in graphs represent ± standard error.
3. Results
3.1. Growth Parameters
The results showed that PEG-induced water stress and AgNPs had a significant effect on growth parameters. As seen in Table 1 addition of AgNPs significantly alleviated the adverse effect of drought stress and increased the SFW, SDW, SL, SD, RFW, RDW and RL. SFW in the presence of 0.1 mg L−1 (0.72 g) and 0.2 mg L−1 (0.74 g) AgNPs was significantly higher compared to the control (0.39 g) (Table 1). In addition, the SWF value increased approximately twice with the addition of 0.1 mg and 0.2 mg L−1 AgNPs in plants where 4% and 8% PEG were applied to induce drought stress. The highest SDW (0.126 g) was determined in the control group in the application of 0.1 mg L−1 AgNPs. SDW values of in vitro plants decreased at 4% and 8% PEG levels, where drought stress conditions were created. However, with the addition of 0.1 and 0.2 mg L−1 AgNPs in 4% PEG application, the negative effects of drought on SDW were eliminated. As a matter of fact, Table 1 shows that SDW values obtained from 4% PEG + 0.1 mg L−1 AgNPs (0.056 g) and 4% PEG + 0.2 mg L−1 AgNPs (0.058 g) applications are in the same statistical group as 0% PEG + 0 mg L−1 AgNPs (0.078 g) application without drought stress. The mean SL values of the in vitro plants obtained from different applications are also similar to the SDW results. In Table 1, where the effects of PEG applied at different doses are shown, it is seen that the highest mean SL values were obtained from 0.1 mg L−1 AgNPs applications. As with all other vegetative growth criteria, SD values were higher in the control group, where PEG was not applied. Unlike SFW, SDW and SL criteria, it is seen that the average highest SD value in 4% PEG application is obtained with the addition of 0.2 mg L−1 AgNPs (2.13 mm). The effect of AgNPs on root growth of plants grown in vitro under drought stress was investigated in the study. Similar results in shoot growth of plants were also recorded for root growth (Table 1). It was determined that the highest RFW values were obtained with the addition of 0.1 mg L−1 AgNPs in all applications. The best RDW value was seen in the control group (0% PEG + 0.1 mg L−1 AgNPS; 0.227 g). Meanwhile, the highest RL (29.43 mm) was achieved on 0% PEG + 0.2 mg L−1 AgNPs which differed significantly from the control treatment (15.93 mm). In short, if we evaluate the effect of the applications on the growth criteria in Table 1, we can say the following; drought stress induced by PEG, reduced root and shoot development of boysenberry plants grown in vitro. AgNP application was successful in eliminating the negative effects of drought. In 0%, 4% and 8% PEG applications, better results were obtained with the addition of 0.1 mg L−1 AgNPs compared to the addition of 0.2 mg L−1 AgNPs.

Table 1.
The effect of different AgNP doses on in vitro shoot growth of boysenberry under drought conditions.
The effect of PEG and AgNPs applied at different doses on the leaf growth of plants is given in Figure 2. It is clearly seen in Figure 1a–c that the addition of AgNPs under drought or normal conditions has a positive effect on the leaf growth of in vitro boysenberry plants. In 4% and 8% PEG applications, with the addition of 0.1 or 0.2 mg L−1 AgNPs, similar values were obtained with the control conditions in terms of LN, LW and LL. These results show that the use of AgNPs is successful in preventing the effects of drought stress in terms of leaf growth.
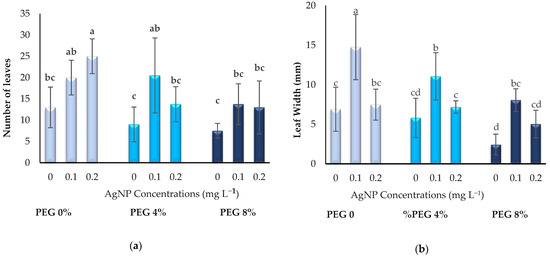
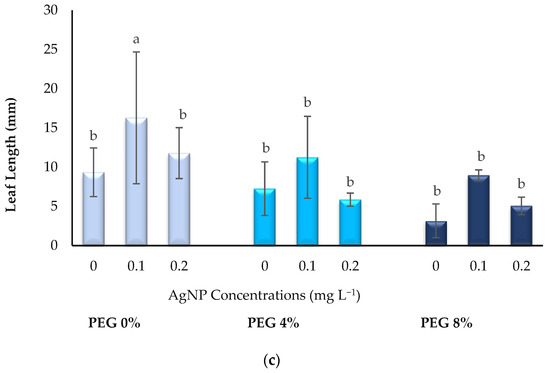
Figure 2.
Influence of PEG and AgNPs treatment in in vitro boysenberry leaves: (a) Number of leaves; (b) Leaf width (mm); (c) Leaf length (mm). Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
3.2. Physiological Parameters
According to the results of the study, the effect of PEG and AgNPs application on SPAD index and RWC values of boysenberry leaves grown in vitro was found to be statistically significant (p < 0.05) (Figure 3). The highest SPAD index values were recorded in the control group plants. The mean value obtained from the application of 4% PEG + 0.1 mg L−1 AgNPs (64.28) was found to be statistically similar to the control group. The lowest SPAD index averages were observed in the 8% PEG group in which the highest dose was applied. Among the different treatments, the highest RWC values were reached in plants without drought stress and without AgNPs application (61.59%), and also in plants where 4% PEG + 0.1 mg L−1 AgNPs (62.51%) were applied. In this case, it can be said that the addition of AgNPs under 4% PEG-induced drought stress is effective in maintaining the SPAD index and RWC content of in vitro plants. However, in the 8% PEG application, the RWC content and SPAD index values of the plants were lower than the control group plants, despite the addition of AgNPs (Figure 3).
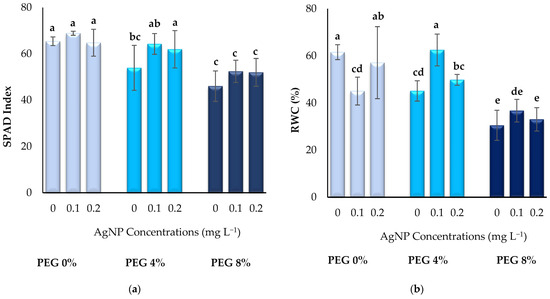
Figure 3.
Effect of PEG and AgNPs treatment on SPAD index (a) and RWC (b) in vitro boysenberry leaves. Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
3.3. Biochemical Parameters
As seen in Figure 4a–c, in vitro plants showed an increasing trend in SOD and CAT activities due to drought stress caused by PEG and AgNPs application. The highest SOD (281 U g−1 fw) and CAT (14.74 U mg−1 fw) activity values were determined in the group in which 8% PEG + 0.2 mg L−1 AgNPs were administered. MDA content decreased with the addition of AgNPs in all treatments. While the maximum MDA contents were found in the presence of 8% PEG (4.36 nmol g−1 FW), the lowest value was found in the application of 0% PEG + 0.2 mg L−1 AgNPs (1.87 nmol g−1 FW).
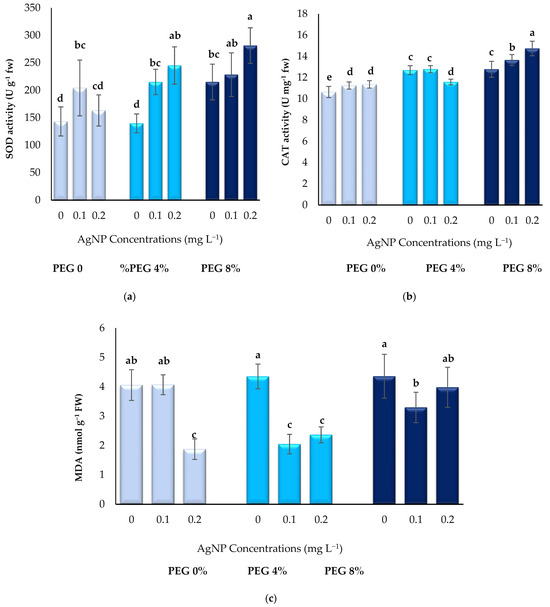
Figure 4.
Effect of PEG and AgNPs treatment on SOD activities (a), CAT activities (b) and MDA content (c) of boysenberry leaves under in vitro conditions. Values are the mean ± S.D. of three replicates (n = 3). Common letters are not significant (p < 0.05).
4. Discussion
The negative effects of drought stress on the growth of plants in vitro and ex vitro are known [54,55,56,57,58].
According to the data obtained from our study, shoot and root development decreased in in vitro boysenberry plants under drought-stress conditions. Many researchers have reported similar results in berries under drought conditions in vitro [32,58,59,60]. Sharma et al. [61] similarly reported that drought stress caused by PEG application at different doses (5%, 10% and 15%) significantly decreased the germination percentage and shoot-root lengths at the seedling stage of fifteen wheat genotypes. Due to the physical and chemical properties of different nanoparticles, their positive effects on plant growth and their ability to eliminate abiotic stress factors are among the current research topics [62,63,64,65,66]. According to the results obtained, it can be said that AgNPs, whose effectiveness against drought stress was evaluated within the scope of this study, were effective in the production of boysenberry under in vitro conditions. Silver nanoparticles are one of the materials that have become widespread in agricultural production in recent years and have attracted attention due to their positive effects on plant growth and development [66,67,68,69]. There is also a report on the effect of AgNPs on shoot and root growth promotion in strawberries by Tung et al. [70]. It is thought that this effect of AgNPs, especially on root growth, may be related to their ability to block ethylene signals [68,71]. Ethylene production, which disrupts auxin translocation, causes hyperhydricity and causes tissue death, can be inhibited by low concentrations of AgNO3 [72,73]. Ag+ interferes with the binding of the ethylene receptor site and helps reduce ethylene production with the promotion of polyamine biosynthesis [74]. Salama et al. reported that small concentrations of silver nanoparticles had a stimulating effect on the growth of common bean and corn plantlets, while increased concentrations had an inhibitory effect [38]. In addition, it is known that silver particles, one of the most used materials after carbon nanotubes among different nanoparticles, are a wonderful plant-growth stimulator [75,76]. Additionally, many researchers have reported that silver nitrate is effective for enhancing plant growth and development under in vitro conditions [68,72,77,78,79].
Although, in a large number of studies, it is mentioned that AgNPs have a positive effect on plant growth and development [37,38,70,71,75,80,81,82], also stated that these particles can cause toxicity in some studies [83,84,85]. It has been reported that nanosilver preparations can affect the metabolism, respiration and reproduction of microorganisms, and therefore, may cause toxicity [86]. In our study, no inhibitory effect of this material was found. The effect of nanosilver particles, which have more surface area in contact with outer space due to their small size, and thus increase the amount and efficiency of adhesion to the cell surface [87], may vary according to the dose of use and the growing medium. Some researchers have stated that when metallic ions such as silver or heavy metals accumulate in high concentrations in plant tissue, they cause the polymerization of phenol with the peroxidase enzyme that chelates heavy metals, and this leads to toxicity [81,88,89]. As a result of the study, it was determined that AgNPs had a significant effect on the RWC and SPAD index of boysenberry plants under drought stress in vitro growing conditions. The effects of AgNPs on RWC and SPAD index are similar to the reports that Fenugreek seeds had higher shoot and root growth with the addition of AgNPs under in vitro conditions [69,75]. This may be related to the increased water and nutrient uptake of seeds treated with AgNPs. Mubashir et al. [90] investigating the efficacy of nano-nutrient solutions (NNS) on growth and biochemical attributes of tomatoes under drought stress, reported that NNS administration resulted in more osmolyte production such as sugars and free amino acids, which may have contributed significantly to the maintenance of tissue water content. Osmolites, which have the ability to maintain the water potential gradient, have a significant effect on plant growth and productivity and have a protective effect in drought conditions [91,92]. Some researchers state that AgNPs play an important role in photosynthetic activity and improve plant growth and development criteria by promoting the production of Indole acetic acid (IAA) [93,94]. Also, the increase in the SPAD index is thought to be related to the inhibition of ethylene. As a matter of fact, it is reported that ethylene causes tissue death and reduces chlorophyll content [73].
Antioxidant activity of SOD and CAT enzymes increased in boysenberry leaves when treated with AgNPs under drought conditions. Some researchers similarly report that AgNP application increases SOD and CAT enzymes [95,96]. Drought stress causes an increase in ROS accumulation and causes significant oxidative damage through lipid peroxidation [97]. Reactive oxygen species (ROS) accumulated in leaves due to stress cause oxidative damage to cell organelles and membrane elements [98]. ROS causes lipid peroxidation by interacting with phospholipids and fatty acids, thereby increasing the amount of MDA. For this reason, lipid peroxidation is usually measured by MDA content [99,100]. The results obtained from the study revealed that AgNP application decreased the amount of MDA, and therefore, had a protective effect against oxidative stress. It is stated that there may be increases in the secondary metabolite content of plants exposed to nanoparticles [101,102,103]. It has been reported that AgNPs increase oxidative stress and increase phenolic and flavonoid levels in rice [95] and potato [104]. Phenols and flavonoids are known to have the ability to protrude into the lipid bilayer, preventing ROS-mediated lipid peroxidation and maintaining membrane fluidity and function [105,106]. Increased phenol and flavonoid synthesis in plant tissues can improve the antioxidant system under control and drought conditions [107,108,109].
5. Conclusions
The shoot and root development of plants decreased gradually with increasing PEG dose applications; however, the addition of AgNPs managed to prevent this decrease. Especially, the 0.1 mg L−1 AgNPs dose added to the medium, even in 4% and 8% PEG applications, was instrumental in obtaining results close to the shoot and root growth values obtained under normal (non-stress) conditions. When the results obtained at the end of the study were evaluated, it was determined that the antioxidant system of boysenberry plants in which AgNPs applied in vitro improved further under control and drought conditions. These results show that AgNP addition at appropriate doses is an active ingredient to be applied to in vitro boysenberry plants under drought stress.
Author Contributions
Conceptualization, S.Ş. and H.S.; methodology, S.Ş. and H.S.; data curation, S.Ş. and H.S.; writing—original draft preparation, S.Ş. and H.S.; writing—review and editing, S.Ş.; supervision, S.Ş.; project administration, S.Ş. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Kamile Ulukapı and Ayşe Gül Nasırcılar for allowing the use of their laboratories for the realization of the analyses and measurements planned within the scope of the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kolarević, T.; Milinčić, D.D.; Vujović, T.; Gašić, U.M.; Prokić, L.; Kostić, A.Ž.; Cerović, R.; Stanojevic, S.P.; Tešić, Ž.L.; Pešić, M.B. Phenolic Compounds and Antioxidant Properties of Field-Grown and In vitro Leaves, and Calluses in Blackberry and Blueberry. Horticulturae 2021, 7, 420. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Iuhas, C.I.; Ayvaz, H.; Rugină, D.; Stanilă, A.; Dulf, F.; Bunea, A.; Socaci, S.A.; Socaciu, C.; Pintea, A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants 2019, 8, 540. [Google Scholar] [CrossRef] [PubMed]
- Dewir, Y.H.; Al-Ali, A.M.; Rihan, H.Z.; Alshahrani, T.; Alwahibi, M.S.; Almutairi, K.F.; Naidoo, Y.; Fuller, M.P. Effects of Artificial Light Spectra and Sucrose on the Leaf Pigments, Growth, and Rooting of Blackberry (Rubus fruticosus) Microshoots. Agronomy 2023, 13, 89. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Gościniak, A.; Szymanowska, D.; Szwajgier, D.; Baranowska-Wójcik, E.; Szulc, P.; Dreczka, D.; Simon, M.; Cielecka-Piontek, J. Blackberry Leaves as New Functional Food? Screening Antioxidant, Anti-Inflammatory and Microbiological Activities in Correlation with Phytochemical Analysis. Antioxidants 2021, 10, 1945. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.; da Silva, T.L.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Lamari, F.N. Berry leaves: An alternative source of bioactive natural products of nutritional and medicinal value. Antioxidants 2016, 5, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.R.; Stafne, E.T.; Hall, H.K.; Finn, C.E. Blackberry breeding and genetics. Plant Breed. Rev. 2007, 29, 19. [Google Scholar]
- Mineo, S.; Noguchi, A.; Nagakura, Y.; Kobori, K.; Ohta, T.; Sakaguchi, E.; Ichiyanagi, T. Boysenberry Polyphenols Suppressed Elevation of Plasma Triglyceride Levels in Rats. J. Nutr. Sci. Vitaminol. 2015, 61, 306–312. [Google Scholar] [CrossRef][Green Version]
- Ryu, J.; Kwon, S.-J.; Jo, Y.D.; Choi, H.-I.; Kang, K.-Y.; Nam, B.M.; Kim, D.-G.; Jin, C.-H.; Kim, J.-B.; Kim, E.-Y.; et al. Fruit quality and chemical contents of hybrid boysenberry (Rubus ursinus) lines developed by hybridization and gamma irradiation. Plant Breed. Biotechnol. 2007, 5, 228–236. [Google Scholar] [CrossRef]
- Wood, G.A.; Andersen, M.T.; Forster, R.L.S.; Braithwaite, M.; Hall, H.K. History of Boysenberry and Youngberry in New Zealand in relation to their problems with Boysenberry decline, the association of a fungal pathogen, and possibly a phytoplasma, with this disease. N. Zldn. J. Crop Hortic. Sci. 1999, 27, 281–295. [Google Scholar] [CrossRef]
- Ipek, A.; Barut, E.; Gulen, H.; Ipek, M. Genetic diversity among some blackberry cultivars and their relationship with boysenberry assessed by AFLP markers. Afr. J. Biotechnol. 2009, 8, 4830–4834. [Google Scholar]
- Şener, S.; Kurt, Z. The effects of Benzyl Amino Purine (BAP) and Indole Butyric Acid (IBA) on in vitro shoot proliferation and rooting of Boysenberry. COMU J. Agric. Fac. 2022, 10, 161–168. [Google Scholar]
- Hall, H.K.; Langford, G. The ‘Boysenberry’: Development of the cultivar and industries in California, Oregon and New Zealand. Acta Hortic. 2008, 777, 103–108. [Google Scholar] [CrossRef]
- Walter, M.; Harris-Virgin, P.; Thomas, W.; Tate, G.; Waipara, N.W.; Langford, G. Agrochemicals suitable for downy mildew control in New Zealand boysenberry production. Crop Prot. 2004, 23, 327–333. [Google Scholar] [CrossRef]
- Kalefetoğlu, T.; Ekmekçi, Y. The effects of drought on plants and tolerance mechanisms. Gazi Univ. J. Sci. 2005, 18, 723–740. [Google Scholar]
- Sade, B.; Soylu, S.; Yetim, E. Drought and oxidative stress. Afr. J. Biotechnol. 2011, 10, 11102–11109. [Google Scholar]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought stress tolerance in plants: Interplay of molecular, biochemical and physiological responses in important development stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed Priming: A Feasible Strategy to Enhance Drought Tolerance in Crop Plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef]
- Gelaw, T.A.; Sanan-Mishra, N. Non-coding RNAs in response to drought stress. Int. J. Mol. Sci. 2021, 22, 12519. [Google Scholar] [CrossRef]
- Pritts, M.P.; Langhans, R.W.; Whitlow, T.H.; Kelly, M.J.; Roberts, A. Winter raspberry production in greenhouses. HortTechnology 1999, 9, 13–15. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hall, H.K.; Ainge, G.D.; Mowat, A.D. Breeding Rubus cultivars for high anthocyanin content and high antioxidant capacity. Acta Hortic. 2002, 585, 495–499. [Google Scholar] [CrossRef]
- Şener, S.; Duran, C.N. The effect of mycorrhiza applications and different irrigation regimes on growth and development characteristics of blackberry cuttings. Turk. J. Agric. Food Sci. Technol. 2020, 8, 638–642. [Google Scholar]
- Yang, H.; Liu, H.; Wu, W.; Li, W.; Lyu, L. Drought-induced oxidative damage and antioxidant responses in blackberry cultivar ‘Hull Thornless’. Pak. J. Agric. Res. 2019, 33, 643–651. [Google Scholar] [CrossRef]
- Orlikowska, T.; Kucharska, D.; Horbowicz, M. The reaction of raspberry and blackberry cultivars to drought stress simulated in vitro by polyethylene glycol (PEG) 6000. Acta Hortic. 2009, 839, 337–342. [Google Scholar] [CrossRef]
- Dascălu, M.; Istrate, M.; Grădinariu, G.; Zlati, C.; Bernardis, R.; Prodan, N.D.; Sfichi-Duke, L. Soil Moisture Study and Its Influences on Blackberry Culture for North East Moldova County. Lucrări Științifice, Universitatea de Științe Agricole Și Medicină Veterinară” Ion Ionescu de la Brad” Iași, Seria Horticultură, Iași, Romania, 2013. [Google Scholar]
- Zhang, C.; Yang, H.; Wu, W.; Li, W. Effect of drought stress on physiological changes and leaf surface morphology in the blackberry. Braz. J. Bot. 2017, 40, 625–634. [Google Scholar] [CrossRef]
- Yang, H.Y.; Zhang, C.H.; Wu, W.L.; Lyu, L.F. Influence of drought stress on the leaf morphology and physiological characteristics in blackberry (Rubus L.) seedlings. In Proceedings of the XXX International Horticultural Congress IHC2018: III International Berry Fruit Symposium, İstanbul, Turkey, 12–16 August 2018. [Google Scholar]
- Yang, H.; Zhang, C.; Wu, W.; Li, W.; Wei, Y.; Dong, S. Physiological responses of blackberry cultivar ‘Ningzhi 1′ to drought stress. Russ. J. Plant Physiol. 2015, 62, 472–479. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, L.; Guo, H.; Wang, Y.; Yuan, H.; Yan, D.; Zheng, B. Spermidine induces physiological and biochemical changes in southern highbush blueberry under drought stress. Braz. J. Bot. 2017, 40, 841–851. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Daneshvar Hakimi Meybodi, N.; Peijnenburg, W. Mitigation of the Effect of Drought on Growth and Yield of Pomegranates by Foliar Spraying of Different Sizes of Selenium Nanoparticles. J. Sci. Food Agric. 2021, 101, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, A.A.; Havas, F.; Ghaderi, N. Application of iron nanoparticles and salicylic acid in in vitro culture of strawberries (Fragaria × ananassa Duch.) to cope with drought stress. Plant. Cell Tissue Organ. Cult. (PCTOC) 2018, 132, 511–523. [Google Scholar] [CrossRef]
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 2015, 5, 436–467. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zafar, S.; Javaid, A.; Perveen, S.; Hasnain, Z.; Ihtisham, M.; Abbas, A.; Usman, M.; El-Sappah, A.H.; Abbas, M. Zinc Nanoparticles (ZnNPs): High-Fidelity Amelioration in Turnip (Brassica rapa L.) Production under Drought Stress. Sustainability 2023, 15, 6512. [Google Scholar] [CrossRef]
- Abbas, M.; Yan, K.; Li, J.; Zafar, S.; Hasnain, Z.; Aslam, N.; Iqbal, N.; Hussain, S.S.; Usman, M.; Abbas, M.; et al. Agri-Nanotechnology and Tree Nanobionics: Augmentation in Crop Yield, Biosafety, and Biomass Accumulation. Front. Bioeng. Biotechnol. 2022, 10, 853045. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotech. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Sengottaiyan, A.; Mythili, R.; Selvankumar, T.; Aravinthan, A.; Kamala-Kannan, S.; Manoharan, K.; Thiyagarajan, P.; Govarthanan, M.; Kim, J.-H. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res. Chem. Intermed. 2016, 42, 3095–3103. [Google Scholar] [CrossRef]
- Salama, H.M. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana Gene Expression in Response to Silver Nanoparticles and Silver Ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE 2013, 8, 68–75. [Google Scholar] [CrossRef]
- Hatami, M.; Naghdi Badi, H.; Ghorbanpour, M. Nano-elicitation of secondary pharmaceutical metabolites in plant cells: A review. J. Med. Plants 2019, 18, 6–36. [Google Scholar] [CrossRef]
- Ahmed, F.; Javed, B.; Razzaq, A.; Mashwani, Z.U.R. Applications of copper and silver nanoparticles on wheat plants to induce drought tolerance and increase yield. IET Nanobiotechnol. 2021, 15, 68–78. [Google Scholar] [CrossRef]
- Sarwar, M.; Saleem, M.F.; Ullah, N.; Khan, M.J.; Maqsood, H.; Ahmad, H.; Tanveer, A.; Shahid, M. Silver nanoparticles protect tillering in drought-stressed wheat by improving leaf water relations and physiological functioning. Funct. Plant Biol. 2023. [Google Scholar] [CrossRef]
- Alabdallah, N.M.; Hasan, M.M.; Salih, A.M.; Roushdy, S.S.; Al-Shammari, A.S.; Alsanie, S.I.; El-Zaidy, M. Silver nanoparticles improve growth and protect against oxidative damage in eggplant seedlings under drought stress. Plant Soil Environ. 2021, 67, 617–624. [Google Scholar] [CrossRef]
- Ghavam, M. Effect of silver nanoparticles on tolerance to drought stress in Thymus daenensis Celak and Thymus vulgaris L. in germination and early growth stages. Environ. Stress. Crop Sci. 2019, 12, 555–566. [Google Scholar]
- Akhoundnejad, Y.; Karakas, O.; Demirci, O. Response of Lettuce to Silver Nanoparticles Under Drought Conditions. Iran. J. Sci. Technol. Trans. A Sci. 2022, 46, 111–120. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Ganjali, A. The effect of silver nanoparticle on lentil seed germination under drought stress. Int. J. Farm. Allied Sci. 2016, 5, 208–212. [Google Scholar]
- Grayson, M. Agriculture and drought. Nat. Outlook 2013, 501, S1. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sanchez, F.J.; de Andrés, E.F.; Tenorio, J.L.; Ayerbe, L. Growth of epicotyls, turgor maintenance and osmotic adjustment in pea plants (Pisum sativum L.) subjected to water stress. Field Crops Res. 2004, 86, 81–90. [Google Scholar] [CrossRef]
- Cakmak, I. Activity of ascorbate-dependent H2O2-scavenging enzymes and leaf chlorosis are enhanced in magnesium-and potassium-deficient leaves, but not in phosphorus-deficient leaves. J. Exp. Bot. 1994, 45, 1259–1266. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Oğuz, İ.; Oğuz, H.İ.; Kafkas, N.E. Strawberry cultivation techniques. In Recent Studies on Strawberries, 1st ed.; Kafkas, N.E., Ed.; IntechOpen: London, UK, 2022; pp. 3–23. [Google Scholar]
- Şener, S. Abiotic stress factors and strawberry cultivation. In Impact of Climate Change on Agriculture, 1st ed.; Akçal, A., Çamoğlu, G., Tan, S., Eds.; Holistence Publications: Çanakkale, Turkey, 2021; pp. 111–125. [Google Scholar]
- Debnath, S.C.; Goyali, J.C. In Vitro Propagation and Variation of Antioxidant Properties in Micropropagated Vaccinium Berry Plants—A Review. Molecules 2020, 25, 788. [Google Scholar] [CrossRef] [PubMed]
- Sayğı, H. Adverse effects of climate Change on agriculture: An evaluation of fruit and honey bee farming. Asian J. Agric. Rural Dev. 2020, 10, 504–514. [Google Scholar] [CrossRef]
- Molnar, S.; Clapa, D.; Mitre, V. Response of the Five Highbush Blueberry Cultivars to In Vitro Induced Drought Stress by Polyethylene Glycol. Agronomy 2022, 12, 732. [Google Scholar] [CrossRef]
- Mazurek, M.; Siekierzyńska, A.; Jacek, B.; Litwińczuk, W. Differences in response to drought stress among highbush blueberry plants propagated conventionally and by tissue culture. Plant Biosyst. 2021, 155, 172–178. [Google Scholar] [CrossRef]
- Hussein, E.A.; El-Kerdany, A.Y.; Afifi, M.K. Effect of drought and salinity stresses on two strawberry cultivars during their regeneration in vitro. Int. J. Innov. Sci. Eng. Technol. 2017, 4, 83–93. [Google Scholar]
- Sharma, V.; Kumar, A.; Chaudhary, A.; Mishra, A.; Rawat, S.; Shami, V.; Kaushik, P. Response of Wheat Genotypes to Drought Stress Stimulated by PEG. Stresses 2022, 2, 26–51. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Kumari, A.; Harish; Singh, V.K.; Verma, K.K.; Mandzhieva, S.; Sushkova, S.; Srivastava, S.; Keswani, C. Coping with the challenges of abiotic stress in plants: New dimensions in the field application of nanoparticles. Plants 2021, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tiwari, S.; Pandey, J.; Lata, C.; Singh, I.K. Role of nanoparticles in crop improvement and abiotic stress management. J. Biotechnol. 2021, 337, 57–70. [Google Scholar] [CrossRef]
- Faizan, M.; Yu, F.; Chen, C.; Faraz, A.; Hayat, S. Zinc oxide nanoparticles help to enhance plant growth and alleviate abiotic stress: A review. Curr. Protein Pept. Sci. 2020, 22, 362–375. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; Ha, C.V. Copper Nanoparticle Application Enhances Plant Growth and Grain Yield in Maize under Drought Stress Conditions. Plant Biol. 2020, 41, 364–375. [Google Scholar]
- Alabdallah, N.M.; Hasan, M.; Hammami, I.; Alghamdi, A.I.; Alshehri, D.; Alatawi, H.A. Green synthesized metal oxide nanoparticles mediate growth regulation and physiology of crop plants under drought stress. Plants 2021, 10, 1730. [Google Scholar] [CrossRef] [PubMed]
- Amna; Ali, B.; Azeem, M.A.; Qayyum, A.; Mustafa, G.; Ahmad, M.A.; Javed, M.T.; Chaudhary, H.J. Bio-Fabricated Silver Nanoparticles: A Sustainable Approach for Augmentation of Plant Growth and Pathogen Control. In Sustainable Agriculture Reviews 53; Springer: Berlin/Heidelberg, Germany, 2021; pp. 345–371. [Google Scholar]
- Mahendran, D.; Geetha, N.; Venkatachalam, P. Role of silver nitrate and silver nano-particles on tissue culture medium and enhanced the plant growth and development. In In Vitro Plant Breeding towards Novel Agronomic Traits: Biotic and Abiotic Stress Tolerance; Springer: Singapore, 2019; pp. 59–74. ISBN 9789813298248. [Google Scholar]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Tung, H.T.; Thuong, T.T.; Cuong, D.M.; Luan, V.Q.; Hien, V.T.; Hieu, T.; Nam, N.B.; Phuong, H.T.N.; Van The Vinh, B.; Khai, H.D.; et al. Silver nanoparticles improved explant disinfection, in vitro growth, runner formation and limited ethylene accumulation during micropropagation of strawberry (Fragaria × ananassa). Plant Cell Tissue Organ Cult. 2021, 145, 393–403. [Google Scholar] [CrossRef]
- Rezvani, N.; Sorooshzadeh, A.; Farhadi, N. Effect of nano-silver on growth of saffron in flooding stress. Int. J. Agric. Biol. Eng. 2012, 6, 11–16. [Google Scholar]
- Qin, Y.; Zhang, S.; Zhang, L.X.; Zhu, D.; Asghar, S. Response of strawberry cv. Toyonokain vitro to silver nitrate (AgNO3). Hortscience 2005, 40, 747–751. [Google Scholar] [CrossRef]
- Lentini, Z.; Mussell, H.; Mutschler, M.A.; Earle, E.D. Ethylene generation and reversal of ethylene effects during development in vitro of rapid-cycling Brassica campestris L. Plant Sci. 1988, 54, 75–81. [Google Scholar] [CrossRef]
- Roustan, J.P.; Latché, A.; Fallot, J. Inhibition of ethylene production and stimulation of carrot somatic embryogenesis by salicylic acid. Biol. Plant 1990, 32, 273–276. [Google Scholar] [CrossRef]
- Hojjat, S.S.; Hojjat, H. Effect of Nano Silver on Seed Germination and Seedling Growth in Fenugreek Seed. ETP Int. J. Food Eng. 2015, 1, 106–110. [Google Scholar] [CrossRef]
- Sharon, M.; Choudhary, A.K.; Kumar, R. Nanotechnology in a Gricultural Diseases and food safety. J. Phytol. 2010, 2, 83–92. [Google Scholar]
- Zarei, S.; Ehsanpour, A.A. Ethylene inhibition with silver nitrate (AgNO3) and pyrazinamide (PZA) ameliorates in vitro salt tolerance of tomato (Lycopersicon esculentum L) plantlets. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 154, 239–247. [Google Scholar] [CrossRef]
- Shah, S.H.; Ali, S.; Jan, S.A.; Din, J.; Ali, G.M. Assessment of Silver Nitrate on Callus Induction and in Vitro Shoot Regeneration in Tomato (Solanum lycopersicum Mill.). Pak. J. Bot. 2014, 46, 2163–2172. [Google Scholar]
- Mohiuddin, A.; Chowdhury, M.; Abdullah, Z.C.; Napis, S. Influence of silver nitrate (ethylene inhibitor) on cucumber in vitro shoot regeneration. Plant Cell Tissue Organ Cult. 1997, 51, 75–78. [Google Scholar] [CrossRef]
- Wasaya, A.; Yasir, T.A.; Sarwar, N.; Farooq, O.; Sheikh, G.R.; Baloch, A.W. Improving growth and yield of mungbean (Vigna radiata L.) through foliar application of silver and zinc nanoparticles. Pure Appl. Biol. 2020, 9, 790–797. [Google Scholar] [CrossRef]
- Nejatzadeh-Barandozi, F.; Darvishzadeh, F.; Aminkhani, A. Effect of nano silver and silver nitrate on seed yield of (Ocimum basilicum L.). Org. Med. Chem. Lett. 2014, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Li, M.; Chang, F.-Y.; Li, W.; Yin, L.-Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef]
- Gruyer, N.; Dorais, M.; Bastien, C.; Dassylva, N.; Triffault-Bouchet, G. Interaction between silver nanoparticles and plant growth. Acta Hortic. 2014, 1037, 795–800. [Google Scholar] [CrossRef]
- Syu, Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant. Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Lok, C.N.; Ho, C.M.; Chen, R.; He, Q.Y.; Yu, W.Y.; Sun, H.; Tam, P.K.H.; Chiu, J.F.; Che, C.M. Silver nanoparticles: Partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 2007, 12, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Elzaawely, A.A.; Xuan, T.D.; Tawata, S. Changes in essential oil, kava pyrones and total phenolics of Alpinia zerumbet (Pers.) BL Burtt. & RM Sm. leaves exposed to copper sulphate. Environ. Exper. Bot. 2007, 59, 347–353. [Google Scholar]
- Lavid, N.; Schwartz, A.; Yarden, O.; Tel-Or, E. The involvement of polyphenols and peroxidase activities in heavy-metal accumulation by epidermal glands of the waterlily (Nymphaeaceae). Planta 2001, 212, 323–331. [Google Scholar] [CrossRef]
- Mubashir, A.; Nisa, Z.U.; Shah, A.A.; Kiran, M.; Hussain, I.; Ali, N.; Zhang, L.; Madnay, M.; Alsiary, W.A.; Korany, S.M.; et al. Effect of foliar application of nano-nutrients solution on growth and biochemical attributes of tomato (Solanum lycopersicum) under drought stress. Front. Plant Sci. 2022, 13, 1066790. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Tyagi, S.R.; Wani, M.R.; Ahmad, P. Drought Tolerance: Role of Organic Osmolytes, Growth Regulators, and Mineral Nutrients. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Springer: New York, NY, USA, 2014; pp. 25–55. [Google Scholar]
- Ahanger, M.A.; Agarwal, R.M. Potassium up-regulates antioxidant metabolism and alleviates growth inhibition under water and osmotic stress in wheat (Triticum aestivum L.). Protoplasma 2017, 254, 1471–1486. [Google Scholar] [CrossRef]
- Najafi, S.; Jamei, R. Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of Mung bean (Vigna radiata). J. Stress Physiol. Biochem. 2014, 10, 316–325. [Google Scholar]
- Razzaq, A.; Ammara, R.; Jhanzab, H.M.; Mahmood, T.; Hafeez, A.; Hussain, S. A novel nanomaterial to enhance growth and yield of wheat. J. Nanosci. Technol. 2016, 2, 55–58. [Google Scholar]
- Gupta, S.D.; Agarwal, A.; Pradhan, S. Phytostimulatory effect of silver nanoparticles (AgNPs) on rice seedling growth: An insight from antioxidative enzyme activities and gene expression patterns. Ecotoxicol. Environ. Saf. 2018, 161, 624–633. [Google Scholar] [CrossRef]
- Karami Mehrian, S.; Heidari, R.; Rahmani, F. Effect of silver nanoparticles on free amino acids content and antioxidant defense system of tomato plants. Indian J. Plant Physiol. 2015, 20, 257–263. [Google Scholar] [CrossRef]
- Nxele, X.; Klein, A.; Ndimba, B. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 108, 261–266. [Google Scholar] [CrossRef]
- Zhao, Z.; Xu, L.; Wang, Y.; Li, B.; Zhang, W.; Li, X. Toxicity mechanism of silver nanoparticles to Chlamydomonas reinhardtii: Photosynthesis, oxidative stress, membrane permeability, and ultrastructure analysis. Environ. Sci. Pollut. Res. 2020, 28, 15032–15042. [Google Scholar] [CrossRef]
- Ebrahimian, E.; Bybordi, A. Effect of salinity, salicylic acid, silicium and ascorbic acid on lipid peroxidation, antioxidant enzyme activity and fatty acid content of sunflower. Afr. J. Agric. Res. 2012, 7, 3685–3694. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.; Zulfiqar, F.; Alam, M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A review of recent experimental evidence. Int. J. Mol. Sci. 2020, 22, 8695. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, G.; Yao, L.; Huang, L.; Wang, J.; Gao, W. Effects of metal nanoparticles and other preparative materials in the environment on plants: From the perspective of improving secondary metabolites. J. Agric. Food. Chem. 2022, 70, 916–933. [Google Scholar] [CrossRef] [PubMed]
- Genady, E.A.; Qaid, E.A.; Fahmy, A.H. Copper sulfate nanoparticales in vitro applications on Verbena bipinnatifida Nutt. Stimulating growth and total phenolic content increasments. Int. J. Pharm. Res. Allied Sci. 2016, 5, 196–202. [Google Scholar]
- Ghanati, F.; Bakhtiarian, S. Effect of Methyl Jasmonate and Silver Nanoparticles on Production of Secondary Metabolites by Calendula officinalis L. (Asteraceae). Trop. J. Pharm. Res. 2014, 13, 1783–1789. [Google Scholar] [CrossRef]
- Bagherzadeh Homaee, M.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving Growth and Photosynthetic Performance of Drought Stressed Tomato by Application of Nano-Organic Fertilizer Involves up-Regulation of Nitrogen, Antioxidant and Osmolyte Metabolism. Ecotoxicol. Environ. Saf. 2021, 216, 112195. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Erlejman, A.G.; Verstraeten, S.V.; Keen, C.L.; Fraga, C.G. Flavonoid-membrane interactions: A protective role of flavonoids at the membrane surface? Clin. Dev. Immunol. 2005, 12, 19–25. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Liu, M.; Li, X.; Liu, Y.; Cao, B. Regulation of flavanone 3-hydroxylase gene involved in the flavonoid biosynthesis pathway in response to UV-B radiation and drought stress in the desert plant, Reaumuria soongorica. Plant Physiol. Biochem. 2013, 73, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Diao, J.; Ji, J.; Wang, G.; Guan, C.; Jin, C.; Wang, Y. Molecular cloning and identification of a flavanone 3-hydroxylase gene from Lycium chinense, and its overexpression enhances drought stress in tobacco. Plant Physiol. Biochem. 2016, 98, 89–100. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).