The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
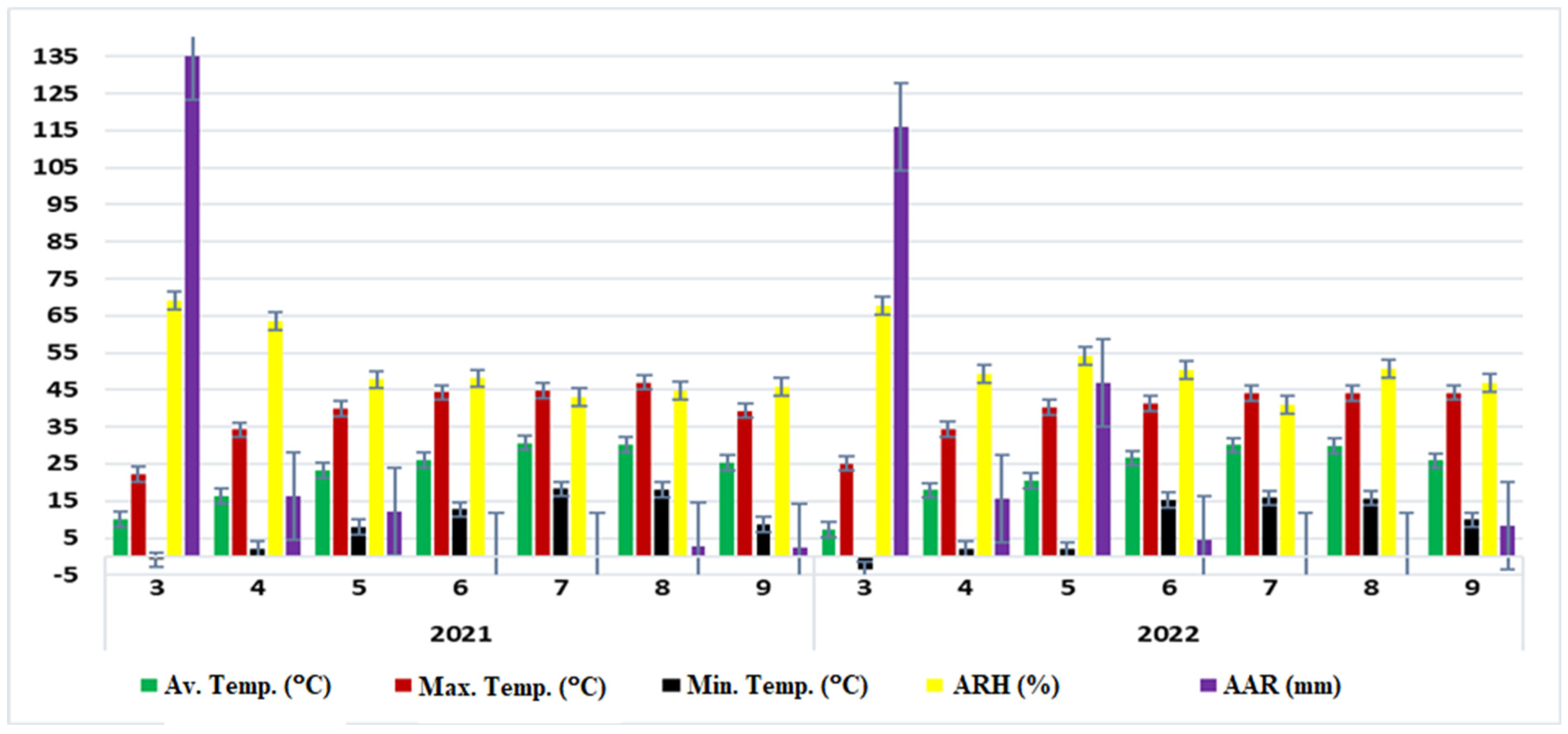
2.1.1. Experimental Site Description
2.1.2. Plant Material
2.2. Method
2.2.1. Pomological Analyzes
2.2.2. Sample Preparation for Biochemical Properties
Total Phenolics
Total Antioxidant Capacity
2.2.3. Oil Content
2.2.4. Protein Content
2.2.5. Analysis of Fatty Acids
2.2.6. Analysis of Sugar Content
2.2.7. Analysis of Organic Acid Contents
2.2.8. Statistical Assessments
3. Results and Discussion
3.1. Fruit Quality Parameters
3.2. Total Phenolics and Total Antioxidant Capacity
3.3. Protein and Oil Contents
3.4. Quantification of Fatty Acids
3.5. Organic Acid Content
3.6. Carbohydrate (Sugars) Content
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, H.; Bai, H.; Jing, Y.; Li, W.; Yin, S.; Zhou, H. A pair of taxifolin-3-O-arabinofuranoside isomers from Juglans regia L. Nat. Prod. Res. 2017, 31, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Demir, B.; Sayıncı, B.; Çetin, N.; Yaman, M.; Çömlek, R.; Aydın, Y.; Sütyemez, M. Elliptic Fourier based analysis and multivariate approaches for size and shape distinctions of walnut (Juglans regia L.) cultivars. Grasas Aceites 2018, 69, e271. [Google Scholar] [CrossRef]
- Ercişli, S.; Sayinci, B.; Kara, M.; Yildiz, C.; Özturk, I. Determination of size and shape features of walnut (Juglans regia L.) cultivars using image processing. Sci. Hortic. 2012, 131, 47–55. [Google Scholar] [CrossRef]
- Nadeem, M.A. Deciphering the genetic diversity and population structure of Turkish bread wheat germplasm using iPBS-retrotransposons markers. Mol. Biol. Rep. 2021, 48, 6739–6748. [Google Scholar] [CrossRef]
- Sümbül, A.; Yildiz, E.; Nadeem, M.A. Elucidating the genetic variations among Turkish grape varieties using morphological and molecular markers. Genet. Resour. Crop Evol. 2023, 70, 1349–1361. [Google Scholar] [CrossRef]
- FAO—Food and Agriculture Organization of the United Nations. Crops Data. 2021. Available online: http://www.fao.org/faostat/en/#data (accessed on 25 August 2023).
- Akbari, V.; Jamei, R.; Heidari, R.; Esfahlan, A.J. Antiradical activity of different parts of Walnut (Juglans regia L.) fruit as a function of genotype. Food Chem. 2012, 135, 2404–2410. [Google Scholar] [CrossRef]
- Khadivi-Khub, A.; Ebrahimi, A.; Sheibani, F.; Esmaeili, A. Phenological and pomological characterization of Persian walnut to select promising trees. Euphytica 2015, 205, 557–567. [Google Scholar] [CrossRef]
- Bayazit, S.; Çalışkan, O.; Kılıç, D. Yükseltinin Chandler Ceviz Çeşidinde Meyve Kalite Özelliklerine Etkisi. Gaziosmanpaşa J. Sci. Res. 2020, 9, 124–132. [Google Scholar]
- Iqbal, J. Morphological, physiological and molecular markers for the adaptation of wheat in drought condition. Asian J. Biotechnol. Genet. Eng. 2019, 2, 1–13. [Google Scholar]
- Bayazit, S.; Sumbul, A. Determination of Fruit Quality and Fatty Acid Composition of Turkish Walnut (Juglans regia) Cultivars and Genotypes Grown in Subtropical Climate of Eastern Mediterranean Region. Int. J. Agric. Biol. 2012, 14, 419–424. [Google Scholar]
- Ünver, H.; Sakar, E.; Sülüşoğlu, M. Determination of pomological and morphological characteristics with fatty acid composition of high kernel ratio walnut genotypes. Erwerbs Obstbau 2016, 58, 11–18. [Google Scholar] [CrossRef]
- Kafkas, E.; Burgut, A.; Ozcan, H.; Ozcan, A.; Sutyemez, M.; Kafkas, S.; Türemis, N. Fatty acid, total phenol and tocopherol profiles of some walnut cultivars: A comparative study. Food Nutr. Sci. 2017, 8, 1074–1084. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Estruch, R.; Salas-Salvadó, J. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Izquierdo-Pulido, M.; Noé, V. Urolithin A causes p21 up-regulation in prostate cancer cells. Eur. J. Nutr. 2016, 55, 1099–1112. [Google Scholar] [CrossRef]
- Poulose, S.M.; Miller, M.G.; Shukitt-Hale, B. Role of walnuts in maintaining brain health with age. J. Nutr. 2014, 144, 561S–566S. [Google Scholar] [CrossRef]
- Pribis, P. Effects of Walnut Consumption on Mood in Young Adults-A Randomized Controlled Trial. Nutrients 2016, 8, 668. [Google Scholar] [CrossRef]
- Cole, J.B.; Florez, J.C. Genetics of diabetes mellitus and diabetes complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Njike, V.Y.; Ayettey, R.; Petraro, P.; Treu, J.A.; Katz, D.L. Walnut ingestion in adults at risk for diabetes: Effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res. Care 2015, 3, e000115. [Google Scholar] [CrossRef]
- Zileli, R.; Şemşek, O.; Ozkamcı, H.; Gürkan Diker, G. The Attendance to Sport of Women Living in Bilecik, Obesıty Prevalence and Risk Factors. Marmara Univ. J. Sports Sci. 2016, 1, 85–98. [Google Scholar]
- Cosmulescu, S.; Trandafir, I.; Nour, V. Chemical Composition and Antioxidant Activity of Walnut Pollen Samples. Not. Bot. Horti Agrobot. 2015, 43, 361–365. [Google Scholar] [CrossRef][Green Version]
- Ürün, I.; Attar, S.H.; Sönmez, D.A.; Gündesli, M.A.; Ercişli, S.; Kafkas, N.E.; Bandić, L.M.; Duralija, B. Comparison of polyphenol, sugar, organic acid, volatile vompounds, and antioxidant capacity of commercially grown strawberry cultivars in Turkey. Plants 2021, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Arcan, Ü.M.; Sütyemez, M.; Bükücü, Ş.B.; Özcan, A.; Gündeşli, M.A.; Kafkas, S.; Kafkas, N.E. Determination of fatty acid and tocopherol contents in Chandler× Kaplan-86 F1 walnut population. Turk. J. Agric. For. 2021, 45, 434–453. [Google Scholar] [CrossRef]
- Okatan, V.; Gündeşli, M.A.; Kafkas, N.E.; Attar, Ş.H.; Kahramanoğlu, İ.; Usanmaz, S.; Aşkın, M.A. Phenolic Compounds, Antioxidant Activity, Fatty Acids and Volatile Profiles of 18 Different Walnut (Juglans regia L.) Cultivars and Genotypes. Erwerbs Obstbau 2022, 64, 247–260. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Kafkas, E.; Attar, S.H.; Gundesli, M.A.; Ozcan, A.; Ergun, M. Phenolic and fatty acid profile, and protein content of different walnut cultivars and genotypes (Juglans regia L.) grown in the USA. Int. J. Fruit Sci. 2020, 20, S1711–S1720. [Google Scholar] [CrossRef]
- Spanos, G.A.; Wrolstad, R.E. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. J. Agric. Food Chem. 1990, 38, 1565–1571. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of Association of Official Analytical Chemists, 18th ed.; AOAC: Washington, DC, USA, 2010. [Google Scholar]
- Kacar, B. Bitki Besleme Uygulama Kılavuzu; Agriculture Faculty Publications No 900, Practice Guides No 214; Ankara University: Ankara, Turkey, 1984; p. 140. [Google Scholar]
- James, G.S. Analytical Chemistry of Foods; Blackie Academic and Professional: London, UK, 1995; pp. 117–120. [Google Scholar]
- Bernardez, M.M.; Miguelez, J.M.; Queijeiro, J.G. HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). J. Food Compos. Anal. 2004, 17, 63–67. [Google Scholar] [CrossRef]
- Koyuncu, M.A.; Ekinci, K.; Savran, E. Cracking Characteristics of Walnut. Biosyst. Eng. 2004, 87, 305–311. [Google Scholar] [CrossRef]
- Muradoglu, F.; Oguz, H.I.; Yildiz, K. Some chemical composition of walnut (Juglans regia L.) selections from Eastern Turkey. Afr. J. Agric. Res. 2010, 5, 2379–2385. [Google Scholar]
- Şimsek, M. Selection of walnut types with high fruit bearing and quality in Sanliurfa population. Int. J. Phys. Sci. 2010, 5, 992–996. [Google Scholar]
- Polat, M.; Okatan, V.; Güclü, F. Determination of some physical and chemical properties of walnut (Juglans regia L.) genotypes grown in the central districy of Bitlis/Turkey. Sci. Pap. B Hortic. 2015, 59, 81–86. [Google Scholar]
- Büyüksolak, Z.; Aşkin, M.; Kahramanoglu, I.; Okatan, V. Effects of Altitude on the Pomological Characteristics and Chemical Properties of ‘Chandler’ Walnuts: A Case Study in Uşak Province. Acta Agrobot. 2020, 73, 7333. [Google Scholar] [CrossRef]
- Balcı, B. Bazı Ceviz (Juglans regia L.) Çeşitlerinde Farklı Ekolojilerin Verim ve Kalite Ögelerine Etkileri Üzerine Araştırmalar. Ph.D. Thesis, Ege University, İzmir, Turkey, 2002. [Google Scholar]
- Yıldız, K.; Akça, Y.; Ünver, H.; Oğuz, H.İ. Seçilmiş Ceviz Genotiplerine Ait Bazı Meyve Özelliklerinin Değerlendirilmesi. J. Agric. Fac. Gaziosmanpaşa Univ. 2017, 34, 164–169. [Google Scholar] [CrossRef]
- Kazankaya, A.; Doğan, A.; Piral, K.; Yaviç, A.; Encü, T. Bitlis Yöresi ümitvar ceviz (Juglans regia L.) tiplerinin belirlenmesi. Yüzüncü Yıl Univ. J. Agric. Sci. 2017, 27, 172–182. [Google Scholar] [CrossRef]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Food Sci. Technol. 2010, 30, 254–259. [Google Scholar] [CrossRef]
- Kornsteiner, M.; Wagner, K.H.; Elmadfa, I. Tocopherols and total phenolics in 10 different nut types. Food Chem. 2006, 98, 381–387. [Google Scholar] [CrossRef]
- Tosun, M.; Çelik, F.; Ercişli, S.O.; Yilmaz, S. Bioactive contents of commercial cultivars and local genotypes of walnut (Juglans regia L.). In Proceedings of the International Conference on Environmental and Agriculture Engineering (IPCBEE), Chengdu, China, 29–31 July 2011; Volume 15, pp. 110–114. [Google Scholar]
- Okatan, V.; Bulduk, I.; Kaki, B.; Gundesli, M.A.; Usanmaz, S.; Alas, T.; Hajizadeh, H.S. Identification and quantification of biochemical composition and antioxidant activity of walnut pollens. Pak. J. Bot. 2021, 53, 1–10. [Google Scholar] [CrossRef]
- Yalçın, B.; Yıldırım, A.; Yıldırım, F.; Çelik, C. Bazı Ceviz Çeşitlerinin Isparta Ekolojisinde Biyokimyasal ve Mineral Madde İçerikleri. Adnan Menderes Üniv. Ziraat Fak. Derg. 2021, 18, 285–291. [Google Scholar] [CrossRef]
- Erdoğan, Ü.; Argin, S.; Turan, M.; Çakmakçı, R.; Olgun, M. Biochemical and bioactive content in fruits of walnut (Juglans regia L.) genotypes from Turkey. Fresenius Environ. Bull. 2021, 30, 6713–6727. [Google Scholar]
- Doğan, M.; Akgul, A. Fatty acid composition of some walnut (Juglans regia L.) cultivars from east Anatolia. Grasas Aceites 2005, 56, 328–331. [Google Scholar] [CrossRef]
- Popa, V.M.; Hadaruga, N.; Gruia, A.; Raba, D.N.; Moldovan, C.; Poiana, A.M. Gas chromatography from the GC-MS analysis for the walnut grown in Romania. J. Agroaliment. Process. Technol. 2011, 17, 261–265. [Google Scholar]
- Özrenk, K.; Javidipour, I.; Yarilgac, T.; Balta, F.; Gündoğdu, M. Fatty acids, tocopherols, selenium and total carotene of pistachios (P. vera L.) from Diyarbakır (Southestern Turkey) and walnuts (J. regia L.) from Erzincan (Eastern Turkey). Food Sci. Technol. Int. 2012, 18, 55–62. [Google Scholar] [CrossRef]
- Bouabdallah, I.; Bouali, I.; Martinez-Force, E.; Albouchi, A.; Perez Camino, M.D.C.; Boukhchina, S. Composition of fatty acids, triacylglycerols and polar compounds of different walnut varieties (Juglans regia L.) from Tunisia. Nat. Prod. Res. 2014, 28, 1826–1833. [Google Scholar] [CrossRef]
- Beyhan, O.; Ozcan, A.; Ozcan, H.; Kafkas, E.; Kafkas, S.; Sutyemez, M.; Ercisli, S. Fat, fatty acids and tocopherolcontent of several walnut genotypes. Not. Bot. Horti Agrobot. 2017, 45, 437–441. [Google Scholar] [CrossRef]
- Ünver, H.; Çelik, M. Ankara Yöresi Cevizlerinin (Juglans regia L.) Seleksiyon Yoluyla Islahı. Bahçe 2005, 34, 83–90. [Google Scholar]
- Rabrenović, B.; Dimić, E.; Maksimović, M.; Šobajić, S.; Gajić-Krstajić, L. Determination of fatty acid and tocopherol compositions and the oxidative stability of walnut (Juglans regia L.) cultivars grown in Serbia. Czech J. Food Sci. 2011, 29, 74–78. [Google Scholar] [CrossRef]
- Yuemei, C.; Junmin, D.; Caihong, Z. The analysis on fat characteristics of walnut varieties in different production areas of Shanxi Province. J. Plant Stud. 2014, 3, 28. [Google Scholar] [CrossRef]
- Fuentealba, C.; Hernández, I.; Saa, S.; Toledo, L.; Burdiles, P.; Chirinos, R.; Campos, D.; Brown, P.; Pedreschi, R. Colour and in vitro quality attributes of walnuts from different growing conditions correlate with key precursors of primary and secondary metabolism. Food Chem. 2017, 232, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Kazankaya, A.; Balta, M.F.; Yörük, I.H.; Balta, F.; Battal, P. Analysis of Sugar Composition in Nut Crops. Asian J. Chem. 2008, 20, 1519–1525. [Google Scholar]
- Gundogdu, M.; Ozrenk, K.; Ercisli, S.; Kan, T.; Kodad, O.; Hegedus, A. Organic acids, sugars, vitamin C content and some pomological characteristics of eleven hawthorn species (Crataegus spp.) from Turkey. Biol. Res. 2014, 47, 21. [Google Scholar] [CrossRef] [PubMed]
- Koc, A.; Keles, H.; Ercisli, S. Some pomological properties of promising seed propagated walnut genotypes from inner Turkey. Not. Bot. Horti Agrobot. 2019, 47, 1094–1099. [Google Scholar] [CrossRef]
- Ae, N.; Arihara, J.; Okada, K.; Yoshihara, T.; Johansen, C. Phosphorus uptake by pigeon pea and its role in cropping systems of the Indian subcontinent. Science 1990, 248, 477–480. [Google Scholar] [CrossRef]
- Rao, G.; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef]
- Mohammadi, S.A.; Prasanna, B. Analysis of genetic diversity in crop plants—Salient statistical tools and considerations. Crop Sci. 2003, 43, 1235–1248. [Google Scholar] [CrossRef]


| Location | Genotypes | Fruit Weight (g) | Kernel Weight (mm) | Kernel Percentage (%) | Fruit Width (mm) | Fruit Length (mm) | Fruit Height (mm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 13.90 ± 0.11 kl | 15.15 ± 1.93 a | 8.00 ± 0.12 g | 8.50 ± 1.45 a | 57.54 ± 0.63 de | 56.03 ± 6.05 a | 43.42 ± 0.65 de | 44.05 ± 4.39 a | 35.07 ± 0.53 de | 34.45 ± 2.01 a | 38.18 ± 0.57 hi | 38.72 ± 4.48 a |
| G-2 | 13.69 ± 0.36 l | 7.25 ± 0.11 h | 52.99 ± 2.12 g–i | 45.40 ± 0.68 c | 34.49 ± 0.0 e–g | 34.85 ± 0.52 l | |||||||
| G-3 | 17.89 ± 0.21 a | 9.10 ± 0.14 c | 50.85 ± 1.34 g–i | 43.05 ± 0.65 e | 35.76 ± 0.52 cd | 40.92 ± 0.61 ef | |||||||
| G-4 | 15.28 ± 0.35 g–i | 8.40 ± 0.13 ef | 54.97 ± 0.73 ef | 48.34 ± 0.73 b | 36.33 ± 0.54 bc | 39.22 ± 0.59 g | |||||||
| G-7 | 17.15 ± 0.23 bc | 9.00 ± 0.13 cd | 52.47 ± 0.50 g–i | 43.37 ± 0.65 de | 36.30 ± 0.54 bc | 40.29 ± 0.60 f | |||||||
| G-10 | 17.34 ± 0.45 ab | 9.90 ± 0.15 b | 57.12 ± 2.20 de | 47.31 ± 0.71 b | 36.65 ± 0.55 bc | 42.18 ± 0.63 d | |||||||
| G-11 | 15.39 ± 0.52 f–h | 11.00 ± 0.16 a | 71.49 ± 2.14 a | 44.46 ± 0.67 cd | 35.80 ± 0.54 cd | 48.68 ± 0.73 a | |||||||
| G-12 | 16.01 ± 0.46 d–f | 9.12 ± 0.14 c | 56.98 ± 0.94 de | 47.47 ± 0.71 b | 38.98 ± 0.58 a | 43.46 ± 0.65 c | |||||||
| G-14 | 15.70 ± 0.46 e–g | 9.90 ± 0.15 b | 63.09 ± 2.73 b | 44.67 ± 0.67 c | 35.97 ± 0.54 cd | 37.92 ± 0.57 ji | |||||||
| G-16 | 14.70 ± 0.33 ij | 8.40 ± 0.13 ef | 57.16 ± 2.16 de | 51.03 ± 0.77 a | 36.45 ± 0.55 bc | 37.85 ± 0.57 ji | |||||||
| Maraş-18 | 16.50 ± 0.53 cd | 8.74 ± 0.25 d | 53.01 ± 2.48 g–i | 42.58 ± 0.64 e | 36.12 ± 0.0 bc | 35.55 ± 0.53 kl | |||||||
| Chandler | 12.14 ± 0.22 m | 6.13 ± 0.51 i | 50.56 ± 4.86 ij | 36.60 ± 0.55 h | 30.87 ± 0.48 mn | 33.34 ± 0.50 m | |||||||
| Franquette | 11.27 ± 0.53 n | 5.64 ± 0.35 j | 50.16 ± 5.15 h–j | 34.30 ± 0.86 i | 30.54 ± 1.04 n | 30.93 ± 0.40 op | |||||||
| High (1200 m) | G-1 | 12.08 ± 0.77 m | 14.05 ± 1.89 b | 6.25 ± 0.09 i | 7.48 ± 1.40 b | 51.89 ± 3.95 g–i | 53.11 ± 6.16 b | 41.42 ± 0.62 fg | 42.10 ± 4.09 b | 34.07 ± 0.51 fg | 33.10 ± 1.81 b | 37.18 ± 0.56 j | 36.59 ± 4.29 b |
| G-2 | 12.08 ± 0.32 m | 6.25 ± 0.09 i | 49.26 ± 0.69 ij | 42.40 ± 0.64 ef | 31.49 ± 0.47 k–m | 31.85 ± 0.48 on | |||||||
| G-3 | 16.11 ± 0.20 de | 8.10 ± 0.12 fg | 50.29 ± 1.35 ij | 41.05 ± 0.62 g | 32.76 ± 0.49 h–j | 38.92 ± 0.58 hg | |||||||
| G-4 | 14.60 ± 0.38 ij | 7.40 ± 0.11 h | 50.68 ± 0.61 ij | 45.34 ± 0.68 c | 32.33 ± 0.48 i–k | 37.22 ± 0.56 j | |||||||
| G-7 | 17.09 ± 0.62 bc | 8.00 ± 0.12 g | 46.84 ± 2.15 j | 41.37 ± 0.62 fg | 31.30 ± 0.47 l–n | 38.29 ± 0.57 hi | |||||||
| G-10 | 14.94 ± 0.48 h–j | 7.90 ± 0.12 g | 52.90 ± 1.26 g–i | 45.31 ± 0.68 c | 33.65 ± 0.50 gh | 40.18 ± 0.60 f | |||||||
| G-11 | 15.29 ± 0.48 g–i | 10.00 ± 0.15 b | 65.43 ± 1.67 b | 42.46 ± 0.64 ef | 34.80 ± 0.52 ef | 45.68 ± 0.69 b | |||||||
| G-12 | 13.74 ± 0.39 l | 8.12 ± 0.12 fg | 59.12 ± 2.59 cd | 45.47 ± 0.68 c | 36.98 ± 0.55 b | 41.46 ± 0.62 de | |||||||
| G-14 | 14.45 ± 0.53 jk | 8.90 ± 0.13 cd | 61.64 ± 3.10 bc | 42.67 ± 0.64 e | 32.97 ± 0.49 hi | 34.92 ± 0.52 l | |||||||
| G-16 | 13.54 ± 0.15 l | 7.40 ± 0.11 h | 54.65 ± 1.03 e–g | 50.03 ± 0.75 a | 34.45 ± 0.52 e–g | 35.85 ± 0.54 k | |||||||
| Maraş-18 | 16.12 ± 0.08 de | 8.70 ± 0.13 de | 53.96 ± 0.68 e–h | 40.58 ± 0.61 g | 34.12 ± 0.51 fg | 31.55 ± 0.47 on | |||||||
| Chandler | 11.35 ± 0.56 n | 4.80 ± 0.07 k | 42.31 ± 1.54 k | 35.60 ± 0.53 h | 31.87 ± 0.46 j–l | 32.34 ± 0.49 n | |||||||
| Franquette | 10.74 ± 0.25 n | 5.52 ± 0.34 j | 51.49± 3.60 f–i | 33.62 ± 0.85 i | 31.17 ± 1.02 l–n | 30.31 ± 0.39 p | |||||||
| LSD0.05 | 0.69 ** | 0.19 ** | 0.30 ** | 0.08 ** | 3.89 ** | 1.08 ** | 1.11 ** | 0.30 ** | 0.94 ** | 0.26 | 0.92 ** | 0.25 ** | |
| Altitude | Genotype | Total Phenolics | DPPH Inhibition | DPPH Radical | |||
|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 412.96 ± 0.58 a | 293.12 ± 53.24 A | 71.63 ± 0.50 cd | 63.43 ± 6.12 B | 64.36 ± 0.50 cd | 56.43 ± 6.12 A |
| G-2 | 237.51 ± 0.91 s | 64.43 ± 0.50 i | 57.43 ± 0.50 i | ||||
| G-3 | 293.86 ± 0.31 i | 56.89 ± 0.18 l | 49.89 ± 0.18 l | ||||
| G-4 | 300.09 ± 0.70 g | 57.85 ± 0.27 j | 50.85 ± 0.27 k | ||||
| G-7 | 384.96 ± 0.28 c | 68.43 ± 0.32 f | 61.43 ± 0.32 f | ||||
| G-10 | 254.60 ± 0.52 p | 64.84 ± 0.39 hi | 57.84 ± 0.39 hi | ||||
| G-11 | 242.06 ± 0.60 r | 65.52 ± 0.40 h | 58.52 ± 0.40 h | ||||
| G-12 | 253.31 ± 0.48 p | 70.94 ± 0.25 d | 63.94 ± 0.25 d | ||||
| G-14 | 295.82 ± 0.78 h | 71.99 ± 0.27 bc | 64.99 ± 0.27 bc | ||||
| G-16 | 335.90 ± 0.89 f | 55.07 ± 0.22 m | 48.07 ± 0.22 m | ||||
| Maraş-18 | 292.03 ± 0.95 j | 64.76 ± 0.19 i | 57.76 ± 0.19 i | ||||
| Chandler | 241.67 ± 0.94 r | 57.57 ± 0.51 kl | 50.57 ± 0.51 kl | ||||
| Franquette | 265.75 ± 0.87 o | 54.65 ± 0.52 m | 47.65 ± 0.52 m | ||||
| High (1200 m) | G-1 | 392.31 ± 0.94 b | 298.70 ± 49.85 B | 75.92 ± 0.53 a | 70.40 ± 3.93 A | 68.92 ± 0.53 a | 63.04 ± 3.68 B |
| G-2 | 290.45 ± 0.60 k | 68.30 ± 0.53 fg | 61.30 ± 0.53 f | ||||
| G-3 | 279.17 ± 0.30 m | 72.62 ± 0.53 b | 65.62 ± 0.53 b | ||||
| G-4 | 285.89 ± 0.59 l | 61.32 ± 0.41 j | 54.32 ± 0.41 j | ||||
| G-7 | 367.31 ± 0.92 d | 72.53 ± 0.28 b | 65.53 ± 0.28 b | ||||
| G-10 | 254.79 ± 0.80 p | 72.09 ± 0.34 bc | 65.09 ± 0.34 bc | ||||
| G-11 | 234.94 ± 0.61 t | 69.45 ± 0.22 e | 62.45 ± 0.22 e | ||||
| G-12 | 246.86 ± 0.74 q | 75.19 ± 0.42 a | 63.94 ± 0.42 d | ||||
| G-14 | 247.80 ± 0.62 q | 72.31 ± 0.25 bc | 64.99 ± 0.25 bc | ||||
| G-16 | 360.88 ± 0.49 e | 71.93 ± 0.27 bc | 64.93 ± 0.27 bc | ||||
| Maraş-18 | 295.16 ± 0.92 hi | 71.17 ± 0.17 d | 64.17 ± 0.17 d | ||||
| Chandler | 242.06 ± 0.64 r | 67.57 ± 0.19 g | 60.57 ± 0.19 g | ||||
| Franquette | 268.51 ± 0.73 n | 64.76 ± 0.18 i | 57.76 ± 0.18 i | ||||
| LSD0.05 | 1.42 ** | 0.38 ** | 0.74 ** | 0.21 ** | 0.72 ** | 0.18 ** | |
| Altitude | Genotype | Protein Content (%) | Oil Content (%) | ||
|---|---|---|---|---|---|
| Low (500 m) | G-1 | 15.41 ± 0.42 lm | 17.63 ± 2.03 A | 55.04 ± 1.49 lm | 63.33 ± 7.68 A |
| G-2 | 15.34 ± 0.37 m | 54.78 ± 1.33 m | |||
| G-3 | 16.35 ± 0.20 jk | 58.42 ± 0.73 jk | |||
| G-4 | 14.96 ± 0.24 m | 53.44 ± 0.87 mn | |||
| G-7 | 19.11 ± 0.18 de | 68.28 ± 0.66 de | |||
| G-10 | 19.36 ± 0.26 cd | 69.14 ± 0.93 cd | |||
| G-11 | 20.22 ± 0.42 b | 72.22 ± 1.48 b | |||
| G-12 | 19.78 ± 0.32 bc | 70.67 ± 1.16 bc | |||
| G-14 | 19.11 ± 0.26 de | 68.25 ± 0.94 de | |||
| G-16 | 21.32 ± 0.34 a | 76.17 ± 1.23 a | |||
| Maraş-18 | 18.10 ± 0.16 f | 64.64 ± 0.58 f | |||
| Chandler | 13.71 ± 0.37 o | 57.57 ± 0.51 k | |||
| Franquette | 16.47 ± 0.15 jk | 54.65 ± 0.52 m | |||
| High (1200 m) | G-1 | 13.56 ± 0.37 o | 15.65 ± 1.92 B | 48.44 ± 1.31 o | 55.43 ± 7.26 B |
| G-2 | 13.49 ± 0.33 o | 48.21 ± 1.17 p | |||
| G-3 | 14.39 ± 0.18 n | 51.41 ± 0.64 no | |||
| G-4 | 13.16 ± 0.21 op | 47.03 ± 0.77 p | |||
| G-7 | 16.82 ± 0.16 ij | 60.08 ± 0.58 ij | |||
| G-10 | 17.03 ± 0.23 hi | 60.84 ± 0.82 hi | |||
| G-11 | 17.79 ± 0.37 fg | 63.56 ± 1.30 fg | |||
| G-12 | 17.41 ± 0.29 gh | 62.19 ± 1.02 gh | |||
| G-14 | 16.81 ± 0.23 ij | 60.06 ± 0.83 ij | |||
| G-16 | 18.76 ± 0.30 e | 67.03 ± 1.08 e | |||
| Maraş-18 | 15.92 ± 0.14 kl | 56.88 ± 0.51 kl | |||
| Chandler | 12.07 ± 0.16 p | 43.11 ± 1.16 p | |||
| Franquette | 14.49 ± 0.13 lm | 51.76 ± 0.47 no | |||
| LSD0.05 | 0.54 ** | 0.14 ** | 1.94 ** | 0.54 ** | |
| Altitude | Genotypes | Palmitic Acid (%) | Stearic Acid (%) | Myristic Acid (%) | Other Fat Acids (%) | Σ Saturated FA | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 6.16 ± 0.12 | 7.24 ± 0.48 | 3.37 ± 0.43 | 3.50 ± 0.29 A | 0.06 ± 0.01 | 0.05 ± 0.01 | 0.50 ± 0.68 | 0.98 ± 0.98 | 10.09 |
| G-2 | 7.39 ± 0.02 | 3.56 ± 0.06 | 0.05 ± 0.01 | 0.25 ± 0.17 | 11.25 | |||||
| G-3 | 7.81 ± 0.05 | 3.13 ± 0.08 | 0.07 ± 0.02 | 0.89 ± 0.47 | 11.90 | |||||
| G-4 | 7.33 ± 0.05 | 3.55 ± 0.05 | 0.05 ± 0.01 | 0.97 ± 1.07 | 11.90 | |||||
| G-7 | 7.75 ± 0.04 | 3.87 ± 0.07 | 0.04 ± 0.01 | 0.18 ± 1.95 | 11.84 | |||||
| G-10 | 6.91 ± 0.16 | 3.86 ± 0.00 | 0.06 ± 0.01 | 0.04 ± 0.58 | 10.84 | |||||
| G-11 | 6.64 ± 0.50 | 3.90 ± 0.03 | 0.06 ± 0.01 | 0.01 ± 1.31 | 10.61 | |||||
| G-12 | 7.19 ± 0.16 | 3.32 ± 0.29 | 0.05 ± 0.01 | 0.68 ± 0.74 | 11.24 | |||||
| G-14 | 6.93 ± 0.13 | 3.23 ± 0.5 | 0.06 ± 0.01 | 0.31 ± 0.33 | 10.53 | |||||
| G-16 | 7.26 ± 0.11 | 3.50 ± 0.03 | 0.03 ± 0.01 | 0.15 ± 0.43 | 10.94 | |||||
| Maraş-18 | 6.83 ± 0.40 | 3.53 ± 0.09 | 0.05 ± 0.01 | 0.07 ± 0.69 | 10.48 | |||||
| Chandler | 7.49 ± 0.10 | 3.50 ± 0.17 | 0.06 ± 0.01 | 1.33 ± 0.67 | 12.38 | |||||
| Franquette | 7.48 ± 0.00 | 3.21 ± 0.02 | 0.05 ± 0.01 | 0.30 ± 0.94 | 11.04 | |||||
| High (1200 m) | G-1 | 6.81 ± 0.55 | 7.24 ± 0.41 | 2.93 ± 0.39 | 2.78 ± 0.24 B | 0.05 ± 0.01 | 0.05 ± 0.01 | 0.01 ± 1.23 | 0.42 ± 1.25 | 9.80 |
| G-2 | 7.37 ± 0.08 | 2.74 ± 0.04 | 0.05 ± 0.01 | 0.78 ± 0.74 | 10.94 | |||||
| G-3 | 7.78 ± 0.14 | 2.41 ± 0.06 | 0.06 ± 0.02 | 0.57 ± 0.60 | 10.82 | |||||
| G-4 | 7.31 ± 0.14 | 2.73 ± 0.04 | 0.04 ± 0.01 | 0.75 ± 0.40 | 10.83 | |||||
| G-7 | 7.73 ± 0.08 | 2.98 ± 0.05 | 0.04 ± 0.01 | 0.01 ± 1.46 | 10.76 | |||||
| G-10 | 6.89 ± 0.11 | 2.97 ± 0.00 | 0.05 ± 0.01 | 1.63 ± 0.77 | 11.54 | |||||
| G-11 | 6.63 ± 0.53 | 3.00 ± 0.02 | 0.06 ± 0.01 | 1.91 ± 0.88 | 11.60 | |||||
| G-12 | 7.17 ± 0.24 | 2.56 ± 0.22 | 0.06 ± 0.01 | 0.48 ± 1.57 | 10.81 | |||||
| G-14 | 6.91 ± 0.07 | 2.85 ± 0.30 | 0.03 ± 0.01 | 1.97 ± 1.46 | 11.76 | |||||
| G-16 | 7.24 ± 0.20 | 2.70 ± 0.07 | 0.06 ± 0.01 | 1.73 ± 0.84 | 11.73 | |||||
| Maraş-18 | 7.18 ± 0.09 | 2.79 ± 0.06 | 0.05 ± 0.01 | 1.90 ± 0.71 | 11.92 | |||||
| Chandler | 7.47 ± 0.07 | 2.94 ± 0.17 | 0.05 ± 0.01 | 0.42 ± 0.07 | 10.48 | |||||
| Franquette | 7.57 ± 0.02 | 2.53 ± 0.06 | 0.05 ± 0.01 | 0.75 ± 1.33 | 10.90 | |||||
| LSD0.05 | N.S. | N.S. | N.S. | 0.08 ** | N.S. | N.S. | N.S. | N.S. | ||
| Altitude | Genotypes | Oleic Acid (%) | Palmitoleic Acid (%) | Total MUFA | Linolenic Acid (%) | Linoleic Acid (%) | Total PUFA | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 18.66 ± 0.65 i–l | 7.24 ± 0.48 | 0.06 ± 0.01 | 0.05 ± 0.01 | 18.72 | 9.03 ± 0.06 f–k | 9.92 ± 0.58 A | 61.12 ± 1.15 b–d | 60.62 ± 1.20 A | 70.15 |
| G-2 | 18.35 ± 0.38 j–m | 0.05 ± 0.01 | 18.40 | 9.47 ± 0.06 d–i | 60.49 ± 0.18 c–f | 69.96 | |||||
| G-3 | 18.48 ± 0.43 j–l | 0.06 ± 0.01 | 18.54 | 8.61 ± 0.35 kl | 60.36 ± 0.15 d–g | 68.97 | |||||
| G-4 | 17.83 ± 0.27 lm | 0.05 ± 0.00 | 17.88 | 9.63 ± 0.34 d–g | 59.77 ± 1.14 e–h | 69.40 | |||||
| G-7 | 19.42 ± 0.68 d–i | 0.04 ± 0.01 | 19.46 | 8.96 ± 0.71 g–k | 58.82 ± 0.81 h–k | 67.78 | |||||
| G-10 | 17.82 ± 0.26 lm | 0.05 ± 0.00 | 17.87 | 9.72 ± 0.32 d–f | 60.97 ± 0.42 b–e | 70.69 | |||||
| G-11 | 17.47 ± 0.33 m | 0.06 ± 0.00 | 17.53 | 8.74 ± 0.43 i–k | 62.44 ± 0.74 a | 71.18 | |||||
| G-12 | 20.27 ± 0.78 b–d | 0.02 ± 0.01 | 20.29 | 7.93 ± 0.43 l | 59.69 ± 0.67 f–h | 67.62 | |||||
| G-14 | 18.81 ± 0.20 h–k | 0.06 ± 0.00 | 18.87 | 9.12 ± 0.15 e–k | 60.96 ± 0.43 b–e | 70.08 | |||||
| G-16 | 17.98 ± 0.19 k–m | 0.03 ± 0.00 | 18.01 | 8.68 ± 0.24 k | 62.00 ± 0.11 ab | 70.68 | |||||
| Maraş-18 | 18.51 ± 0.18 j–l | 0.05 ± 0.00 | 18.56 | 8.66 ± 0.29 kl | 61.67 ± 0.49 a–c | 70.33 | |||||
| Chandler | 18.61 ± 0.20 i–l | 0.05 ± 0.00 | 18.66 | 8.70 ± 0.42 jk | 59.49 ± 0.77 f–i | 68.19 | |||||
| Franquette | 19.17 ± 0.46 e–j | 0.04 ± 0.00 | 19.21 | 8.88 ± 0.27 h–k | 60.33 ± 0.39 d–g | 69.21 | |||||
| High (1200 m) | G-1 | 19.78 ± 0.69 b–f | 18.57 ± 0.89 B | 0.06 ± 0.01 | 0.05 ± 0.01 | 19.84 | 10.02 ± 0.07 b–d | 8.93 ± 0.64 B | 59.38 ± 0.47 f–i | 58.60 ± 0.83 B | 69.40 |
| G-2 | 19.45± 0.40 c–i | 0.05 ± 0.01 | 19.50 | 10.51 ± 0.07 a–c | 58.54 ± 0.45 h–l | 69.05 | |||||
| G-3 | 19.59 ± 0.45 c–h | 0.06 ± 0.01 | 19.65 | 9.56 ± 0.39 d–h | 59.12 ± 0.77 g–k | 68.68 | |||||
| G-4 | 18.89 ± 0.28 f–j | 0.05 ± 0.00 | 18.94 | 10.69 ± 0.38 ab | 58.90 ± 042 h–k | 69.59 | |||||
| G-7 | 20.59 ± 0.72 b | 0.04 ± 0.01 | 20.63 | 9.94 ± 0.79 cd | 58.90 ± 0.55 h–k | 68.84 | |||||
| G-10 | 18.88 ± 0.28 g–j | 0.05 ± 0.00 | 18.93 | 10.79 ± 0.36 a | 58.10 ± 0.49 j–l | 68.89 | |||||
| G-11 | 18.52 ± 0.35 j–l | 0.06 ± 0.00 | 18.58 | 9.70 ± 0.48 d–g | 59.31 ± 0.40 g–j | 69.01 | |||||
| G-12 | 21.48 ± 0.83 a | 0.02 ± 0.01 | 21.50 | 8.80 ± 0.48 i–k | 58.43 ± 1.03 ij | 67.23 | |||||
| G-14 | 19.93 ± 0.21 b–e | 0.05 ± 0.00 | 19.98 | 10.13 ± 0.16 a–d | 57.33 0.91± l | 67.46 | |||||
| G-16 | 19.06 ± 0.21 e–j | 0.03 ± 0.00 | 19.09 | 9.80 ± 0.15 c–e | 58.95 ± 0.41 h–k | 68.75 | |||||
| Maraş-18 | 19.62 ± 0.19 c–h | 0.05 ± 0.00 | 19.67 | 9.99 ± 0.21 b–d | 57.90 ± 0.48 kl | 67.89 | |||||
| Chandler | 19.73 ± 0.21 b–g | 0.05 ± 0.00 | 19.78 | 9.58 ± 0.42 d–h | 59.22 ± 0.10 g–j | 68.80 | |||||
| Franquette | 20.32 ± 0.48 bc | 0.04 ± 0.00 | 20.36 | 9.43 ± 0.37 d–j | 58.70 ± 0.41 h–l | 68.13 | |||||
| LSD0.05 | 0.88 ** | 0.24 ** | N.S. | N.S. | 0.74 ** | 0.20 ** | 1.24 ** | 0.34 ** | |||
| Altitude | Genotypes | Oxalic Acid (%) | Citric Acid (%) | Malic Acid (%) | Succinic Acid (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 0.27 ± 0.02 a | 3.09 ± 0.05 A | 1.59 ± 0.00 b | 1.17 ± 0.45 | 4.99 ± 0.08 c | 4.27 ± 1.48 A | 1.38 ± 0.00 d–f | 2.00 ± 1.17 B |
| G-2 | 0.23 ± 0.02 ab | 1.06 ± 0.11 cd | 3.35 ± 0.11 f–h | 0.78 ± 0.08 f | |||||
| G-3 | 0.22 ± 0.01 bc | 2.08 ± 0.09 a | 7.33 ± 0.04 a | 2.86 ± 0.03 a–c | |||||
| G-4 | 0.19 ± 0.02 b–d | 1.62 ± 0.03 b | 6.57 ± 0.05 b | 2.40 ± 0.04 c–e | |||||
| G-7 | 0.19 ± 0.00 b–d | 1.71 ± 0.02 b | 5.21 ± 0.02 c | 2.75 ± 0.05 b–d | |||||
| G-10 | 0.16 ± 0.00 de | 1.15 ± 0.03 cd | 4.43 ± 0.06 d | 0.93 ± 0.04 f | |||||
| G-11 | 0.09 ± 0.00 f | 0.56 ± 0.05 f | 1.39 ± 0.09 i | 0.43 ± 0.05 f | |||||
| G-12 | 0.10 ± 0.00 f | 0.71 ± 0.04 ef | 4.33 ± 0.16 d | 1.08 ± 0.01 ef | |||||
| G-14 | 0.10 ± 0.00 f | 0.53 ± 0.04 f | 3.28 ± 0.06 gh | 1.03 ± 0.01 ef | |||||
| G-16 | 0.12 ± 0.05 ef | 0.87 ± 0.09 de | 3.51 ± 0.04 fg | 1.00 ± 0.01 f | |||||
| Maras-18 | 0.18 ± 0.01 cd | 1.22 ± 0.02 c | 4.31 ± 0.11 de | 4.03 ± 0.24 ab | |||||
| Chandler | 0.22 ± 0.03 bc | 1.04 ± 0.05 cd | 2.97 ± 0.07 h | 3.03 ± 0.63 a–c | |||||
| Franquette | 0.15 ± 0.02 de | 1.01 ± 0.05 cd | 3.81 ± 0.19 ef | 4.20 ± 0.03 a | |||||
| High (1200 m) | G-1 | 0.22 ± 0.01 b | 2.85 ± 0.07 B | 1.30 ± 0.08 b–d | 0.18 ± 0.39 | 4.20 ± 0.04 b–d | 3.71 ± 1.09 B | 5.97 ± 0.12 a | 3.41 ± 1.61 A |
| G-2 | 0.22 ± 0.01 b | 1.72 ± 0.02 ab | 4.25 ± 0.17 b–d | 0.83 ± 0.08 f | |||||
| G-3 | 0.21 ± 0.01 bc | 1.02 ± 0.03 de | 5.78 ± 0.20 a | 3.81 ± 0.22 c–e | |||||
| G-4 | 0.17 ± 0.01 cd | 1.69 ± 0.00 a–c | 3.07 ± 0.15 ef | 3.70 ± 0.04 de | |||||
| G-7 | 0.21 ± 004 bc | 0.39 ± 0.02 f | 4.70 ± 0.07 ab | 4.92 ± 0.22 b | |||||
| G-10 | 0.33 ± 0.02 a | 1.85 ± 0.01 a | 4.51 ± 0.00 bc | 1.26 ± 0.04 f | |||||
| G-11 | 0.17 ± 0.00 cd | 1.24 ± 0.22 cd | 1.35 ± 0.03 g | 3.06 ± 0.22 e | |||||
| G-12 | 0.15 ± 0.00 d | 1.12 ± 0.05 de | 3.25 ± 0.03 d–f | 4.43 ± 0.04 b–d | |||||
| G-14 | 0.15 ± 0.02 d | 0.90 ± 0.10 de | 3.91 ± 0.12 b–e | 0.89 ± 0.14 f | |||||
| G-16 | 0.00 ± 0.00 e | 1.34 ± 0.04 b–d | 2.27 ± 0.06 fg | 1.25 ± 0.04 f | |||||
| Maras-18 | 0.16 ± 0.00 d | 1.05 ± 0.13 de | 3.70 ± 0.14 b–e | 5.08 ± 0.12 ab | |||||
| Chandler | 0.23 ± 0.02 b | 0.93 ± 0.06 de | 3.41 ± 0.09 c–e | 4.70 ± 0.11 bc | |||||
| Franquette | 0.15 ± 0.05 d | 0.78 ± 0.03 ef | 3.80 ± 0.26 b–e | 4.40 ± 0.21 b–d | |||||
| LSD0.05 | 0.44 ** | 0.01 ** | 0.38 ** | N.S. | 0.84 ** | 0.22 ** | 1.06 ** | 0.28 ** | |
| Altitude | Genotypes | Sucrose (%) | Glucose (%) | Fructose (%) | Total Sugar (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Low (500 m) | G-1 | 3.06 ± 0.02 a–c | 2.38 ± 0.60 A | 0.20 ± 0.02 k | 0.35 ± 0.03 | 0.21 ± 0.03 ij | 0.34 ± 0.10 A | 3.48 ± 0.03 b–d | 3.04 ± 0.03 A |
| G-2 | 2.08 ± 0.01 e–g | 0.22 ± 0.01 i–k | 0.15 ± 0.00 jk | 2.45 ± 0.00 h–k | |||||
| G-3 | 2.08 ± 0.08 e–g | 0.40 ± 0.09 c–e | 0.38 ± 0.04 b–e | 2.78 ± 0.05 g–i | |||||
| G-4 | 3.12 ± 0.00 ab | 0.24 ± 0.02 h–k | 0.14 ± 0.00 k | 3.51 ± 0.02 bc | |||||
| G-7 | 1.42 ± 0.02 jk | 0.49 ± 0.01 a | 0.43 ± 0.02 bc | 2.35 ± 0.05 i–l | |||||
| G-10 | 2.42 ± 0.04 de | 0.37 ± 0.08 d–f | 0.32 ± 0.01 e–g | 3.12 ± 0.13 d–f | |||||
| G-11 | 2.08 ± 0.00 e–g | 0.33 ± 0.00 e–g | 0.28 ± 0.00 gh | 2.70 ± 0.01 g–i | |||||
| G-12 | 2.91 ± 0.01 a–c | 0.48 ± 0.10 ab | 0.40 ± 0.00 b–d | 3.79 ± 0.09 ab | |||||
| G-14 | 1.34 ± 0.01 jk | 0.40 ± 0.00 c–e | 0.33 ± 0.01 e–g | 2.07 ± 0.00 lm | |||||
| G-16 | 3.13 ± 0.01 a | 0.43 ± 0.01 a–d | 0.33 ± 0.01 e–g | 3.89 ± 0.04 a | |||||
| Maras-18 | 2.75 ± 0.08 b–d | 0.40 ± 0.08 b–e | 0.33 ± 0.02 e–g | 3.49 ± 0.02 bc | |||||
| Chandler | 1.91 ± 0.08 gh | 0.35 ± 0.04 e–g | 0.25 ± 0.05 hi | 2.51 ± 0.06 h–k | |||||
| Franquette | 2.71 ± 0.13 cd | 0.29 ± 0.03 g–i | 0.32 ± 0.05 e–g | 3.33 ± 0.06 c–e | |||||
| High (1200 m) | G-1 | 3.03 ± 0.01 a–c | 2.10 ± 0.65 B | 0.21 ± 0.00 jk | 0.35 ± 0.03 | 0.20 ± 0.03 i–k | 0.30 ± 0.07 B | 3.45 ± 0.07 c–e | 2.80 ± 0.00 B |
| G-2 | 1.16 ± 0.08 k | 0.29 ± 0.00 g–j | 0.37 ± 0.01 c–f | 1.83 ± 0.09 m | |||||
| G-3 | 1.90 ± 0.04 gh | 0.40 ± 0.01 c–e | 0.37 ± 0.05 b–f | 2.68 ± 0.00 g–j | |||||
| G-4 | 3.10 ± 0.06 ab | 0.29 ± 0.02 f–h | 0.24 ± 0.08 hi | 3.65 ± 0.03 a–c | |||||
| G-7 | 1.47 ± 0.04 jk | 0.43 ± 0.01 a–d | 0.38 ± 0.03 b–e | 2.29 ± 0.08 kl | |||||
| G-10 | 1.64 ± 0.07 h–j | 0.44 ± 0.01 a–d | 0.43 ± 0.01 b | 2.52 ± 0.07 h–k | |||||
| G-11 | 1.69 ± 0.06 h–j | 0.35 ± 0.02 e–g | 0.29 ± 0.01 gh | 2.33 ± 0.10 j–l | |||||
| G-12 | 2.92 ± 0.05 a–c | 0.33 ± 0.01 e–g | 0.36 ± 0.04 d–f | 3.62 ± 0.02 a–c | |||||
| G-14 | 1.87 ± 0.00 g–i | 0.41 ± 0.00 b–e | 0.38 ± 0.01 b–f | 2.66 ± 0.01 g–j | |||||
| G-16 | 1.53 ± 0.00 ij | 0.46 ± 0.01 a–c | 0.54 ± 0.00 a | 2.54 ± 0.05 h–k | |||||
| Maras-18 | 2.33 ± 0.06 ef | 0.34 ± 0.02 e–g | 0.32 ± 0.01 fg | 2.99 ± 0.08 e–g | |||||
| Chandler | 1.90 ± 0.08 gh | 0.23 ± 0.01 h–k | 0.20 ± 0.01 i–k | 2.34 ± 0.18 j–l | |||||
| Franquette | 2.78 ± 0.11 a–c | 0.34 ± 0.01 e–g | 0.36 ± 0.03 d–f | 3.49 ± 0.15 bc | |||||
| LSD0.05 | 0.36 ** | 0.10 ** | 0.06 ** | N.S. | 0.06 ** | 0.02 ** | 0.34 ** | 0.98 ** | |
| Properties | PCA 1 | PCA 2 | PCA 3 | PCA 4 | PCA 5 | PCA 6 | PCA 7 |
|---|---|---|---|---|---|---|---|
| T.F | −0.048 | −0.055 | 0.190 | 0.211 | −0.121 | 0.493 | 0.399 |
| T.A | −0.029 | 0.124 | 0.284 | 0.232 | −0.335 | −0.315 | 0.047 |
| Protein | 0.350 | −0.057 | −0.021 | −0.019 | −0.043 | 0.053 | 0.202 |
| Oil | 0.356 | −0.036 | −0.010 | −0.039 | −0.040 | 0.078 | 0.166 |
| Oleic A. | −0.002 | −0.391 | −0.028 | −0.016 | 0.228 | 0.181 | −0.303 |
| Linoleic A. | 0.064 | 0.259 | −0.256 | 0.173 | −0.051 | −0.223 | 0.363 |
| Linolenic A. | −0.092 | 0.289 | 0.241 | −0.030 | −0.275 | 0.179 | −0.035 |
| Palmitic A. | −0.105 | −0.270 | 0.267 | −0.167 | −0.094 | −0.317 | 0.052 |
| Palmitoleic A. | −0.160 | 0.328 | −0.055 | −0.137 | 0.268 | −0.136 | 0.179 |
| Stearic A. | 0.089 | 0.228 | 0.030 | −0.151 | −0.138 | 0.534 | 0.090 |
| Miristic A. | −0.160 | 0.330 | −0.038 | −0.135 | 0.272 | −0.108 | 0.199 |
| Sucrose | −0.074 | −0.064 | −0.117 | 0.520 | 0.100 | 0.031 | 0.030 |
| Glucose | 0.272 | −0.178 | 0.201 | −0.157 | 0.040 | −0.055 | 0.241 |
| Fructose | 0.246 | −0.220 | 0.161 | −0.130 | −0.012 | −0.110 | 0.250 |
| T. Sugar | −0.017 | −0.131 | −0.090 | 0.505 | 0.124 | −0.001 | 0.116 |
| Oxalic A. | −0.242 | 0.128 | 0.194 | −0.102 | 0.212 | 0.264 | −0.170 |
| Citric A. | −0.144 | 0.200 | 0.353 | 0.241 | 0.016 | −0.068 | −0.029 |
| Malic A. | −0.143 | −0.084 | 0.418 | 0.065 | 0.282 | −0.084 | 0.015 |
| Succinic A. | −0.188 | −0.193 | −0.032 | 0.084 | 0.332 | 0.117 | 0.392 |
| S.F.W | 0.201 | 0.085 | 0.361 | −0.052 | 0.284 | −0.022 | 0.086 |
| I.F.W | 0.292 | 0.181 | 0.095 | −0.027 | 0.250 | −0.045 | −0.013 |
| I.R | 0.265 | 0.199 | −0.240 | 0.030 | 0.099 | −0.048 | −0.114 |
| F.L | 0.239 | 0.125 | 0.223 | 0.247 | −0.174 | −0.004 | −0.197 |
| F.W | 0.280 | 0.036 | 0.080 | 0.258 | 0.199 | 0.053 | −0.247 |
| F.H | 0.241 | 0.182 | 0.050 | 0.050 | 0.283 | 0.018 | −0.157 |
| Eigenvalue | 7.41 | 4.67 | 3.26 | 3.10 | 2.03 | 1.47 | 1.00 |
| Variations (%) | 29.64 | 18.70 | 13.05 | 12.39 | 8.12 | 5.88 | 3.98 |
| Total variation (%) | 29.64 | 48.34 | 61.39 | 73.78 | 81.90 | 87.78 | 91.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gündeşli, M.A.; Uğur, R.; Yaman, M. The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.). Horticulturae 2023, 9, 1086. https://doi.org/10.3390/horticulturae9101086
Gündeşli MA, Uğur R, Yaman M. The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.). Horticulturae. 2023; 9(10):1086. https://doi.org/10.3390/horticulturae9101086
Chicago/Turabian StyleGündeşli, Muhammet Ali, Remzi Uğur, and Mehmet Yaman. 2023. "The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.)" Horticulturae 9, no. 10: 1086. https://doi.org/10.3390/horticulturae9101086
APA StyleGündeşli, M. A., Uğur, R., & Yaman, M. (2023). The Effects of Altitude on Fruit Characteristics, Nutrient Chemicals, and Biochemical Properties of Walnut Fruits (Juglans regia L.). Horticulturae, 9(10), 1086. https://doi.org/10.3390/horticulturae9101086







