Enhancing the Nutritional Profile of Crataegus monogyna Fruits by Optimizing the Extraction Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Fruit Collection and Preparation
2.3. Extraction Procedure
2.4. Response Surface Methodology (RSM) Optimization of Extraction and Experiment Design
2.5. Analyses of Extracts
2.5.1. Determination of Total Polyphenol Content (TPC)
2.5.2. Determination of Total Anthocyanin Content (TAC)
2.5.3. Ascorbic Acid Content (AAC)
2.5.4. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.5. Radical Scavenging Activity (AAR, DPPH Assay)
2.6. HPLC-Based Analysis of the Polyphenolic Compounds
2.7. Statistical Analysis
3. Results and Discussion
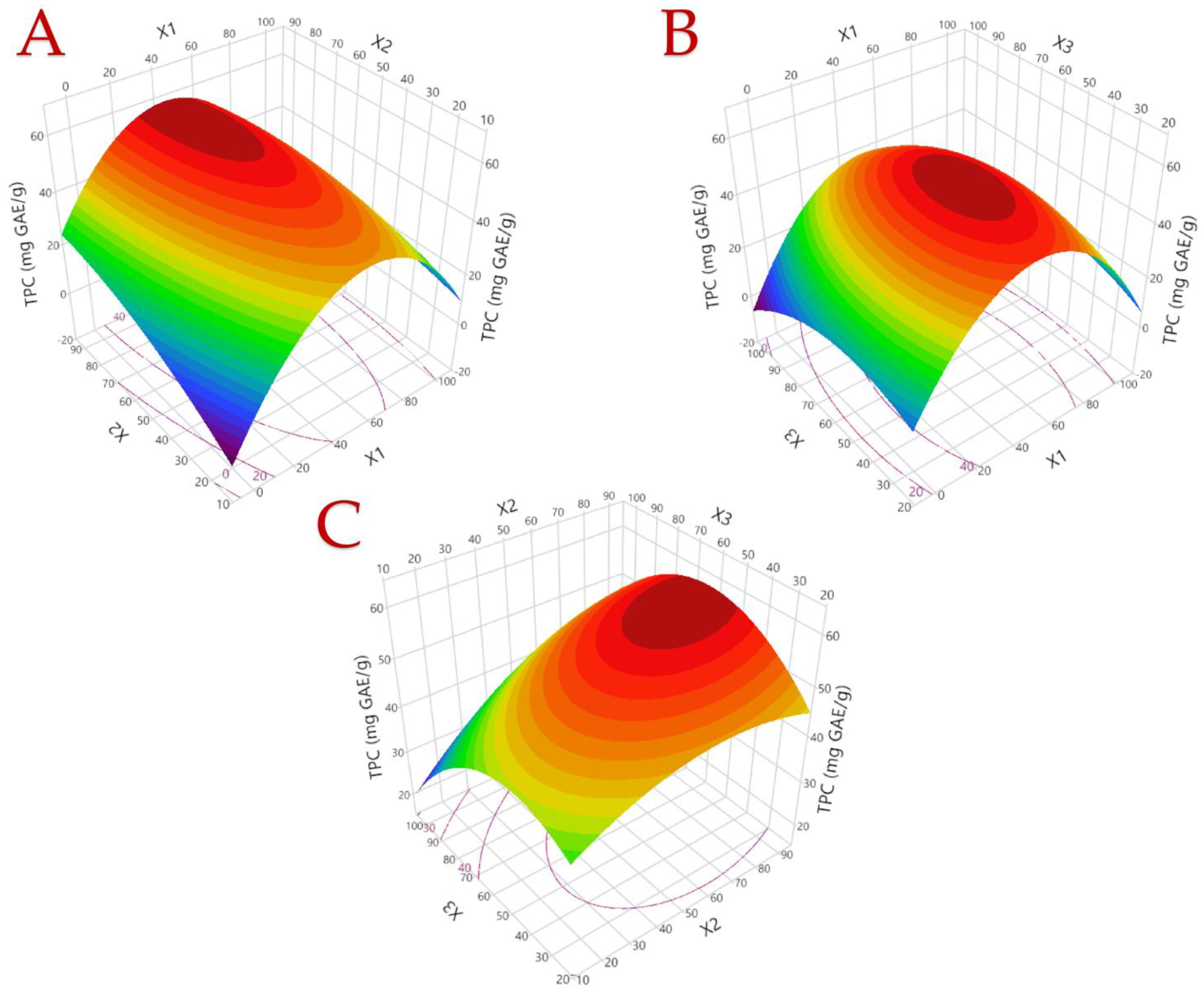
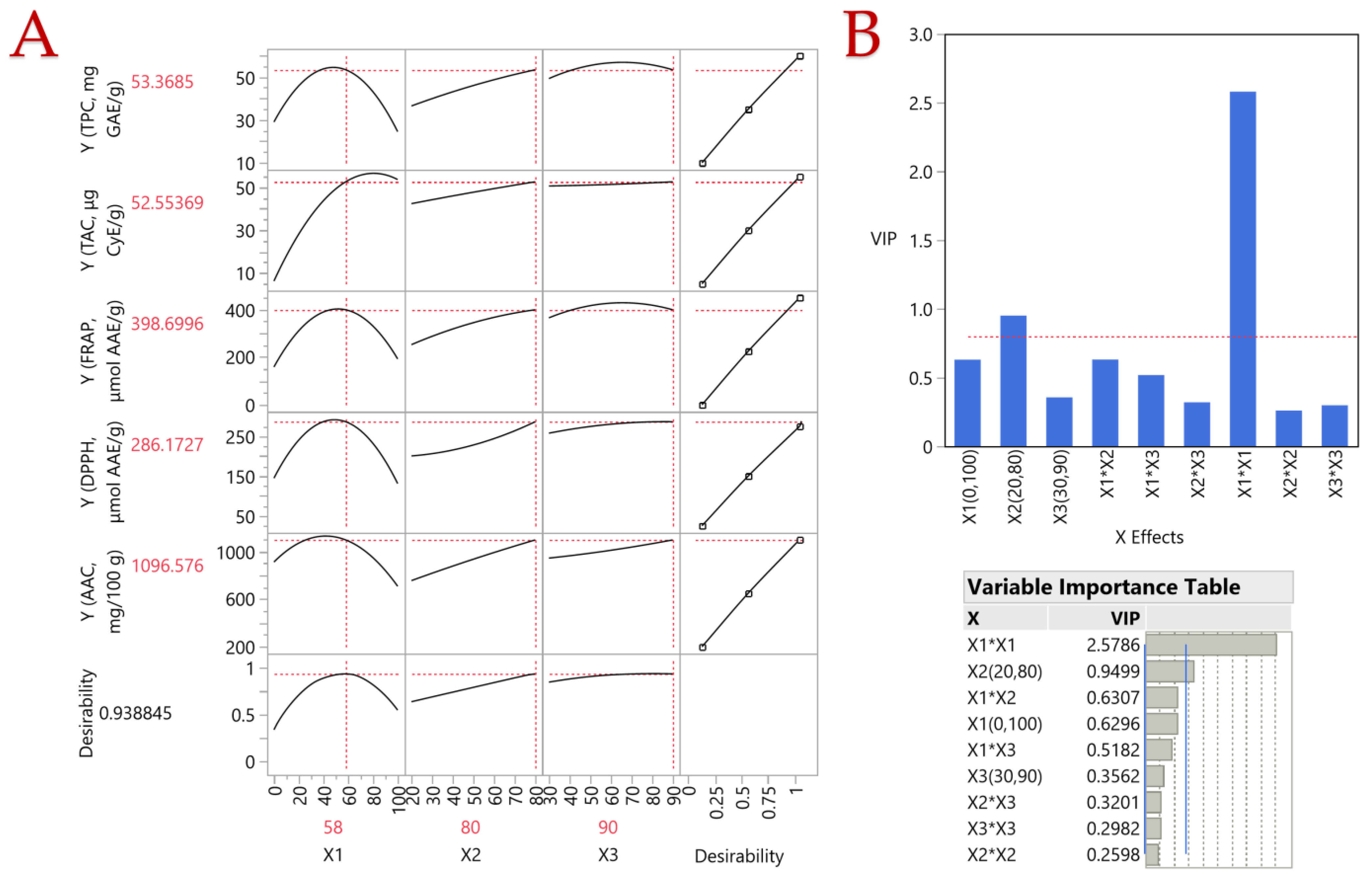
3.1. Extraction Optimization
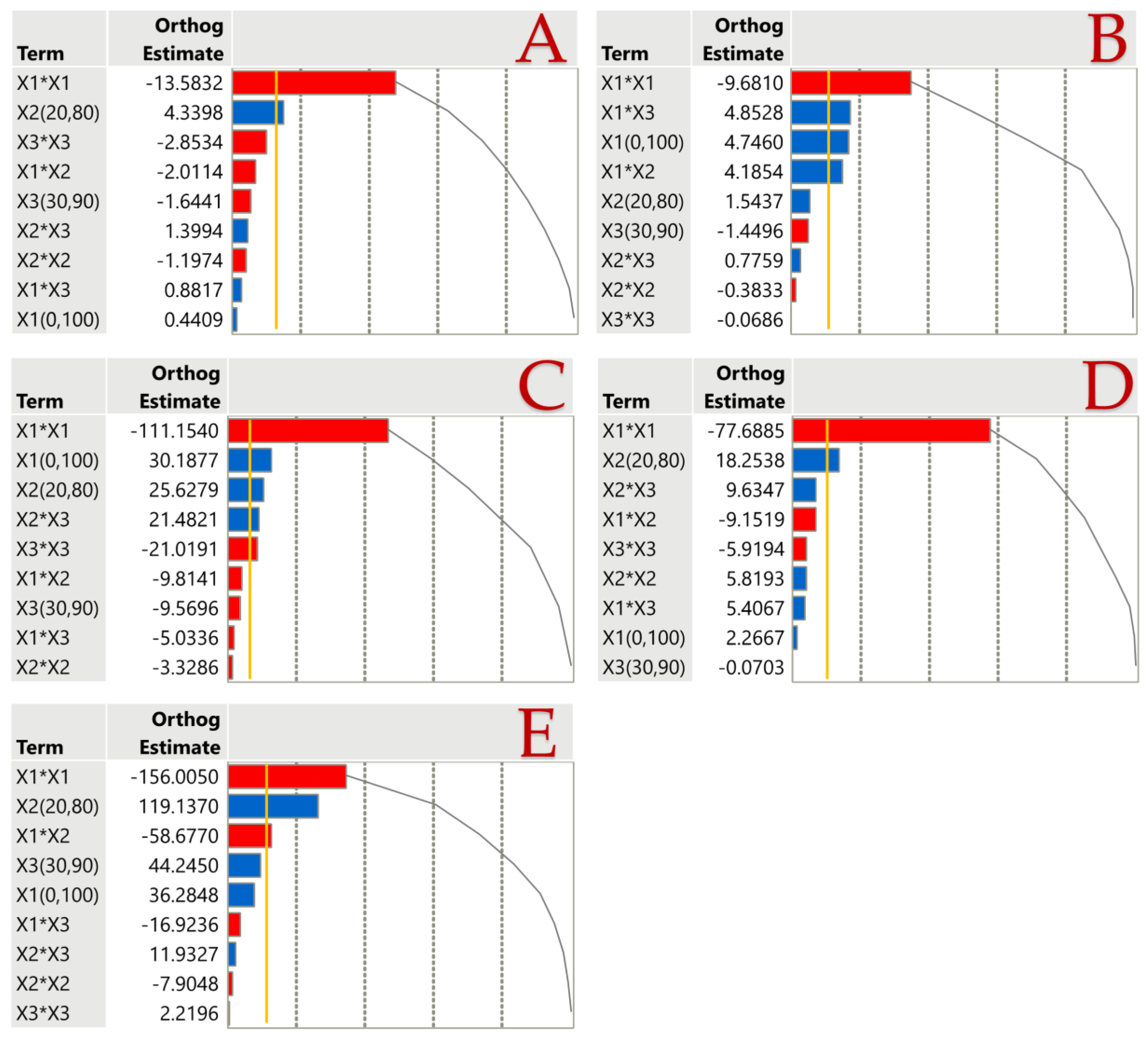
3.2. Impact of Extraction Parameters to Assays through Pareto Plot Analysis
3.3. Analysis of the Extracts
3.3.1. TPC and TAC of the Extracts
3.3.2. Antioxidant Properties of the Extracts
3.3.3. AAC of the Extracts
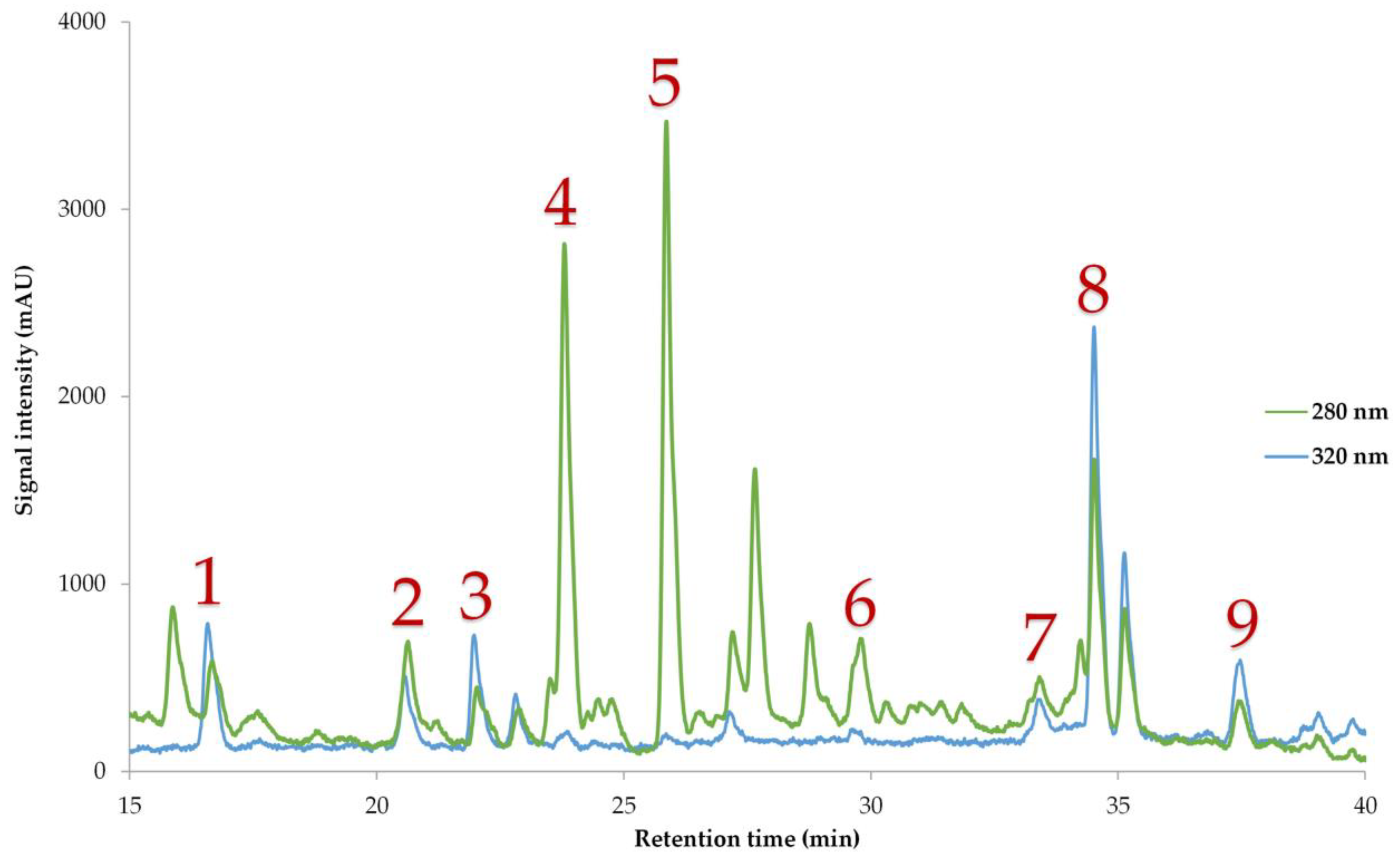
3.3.4. Polyphenolic Compounds of the Optimum Extract
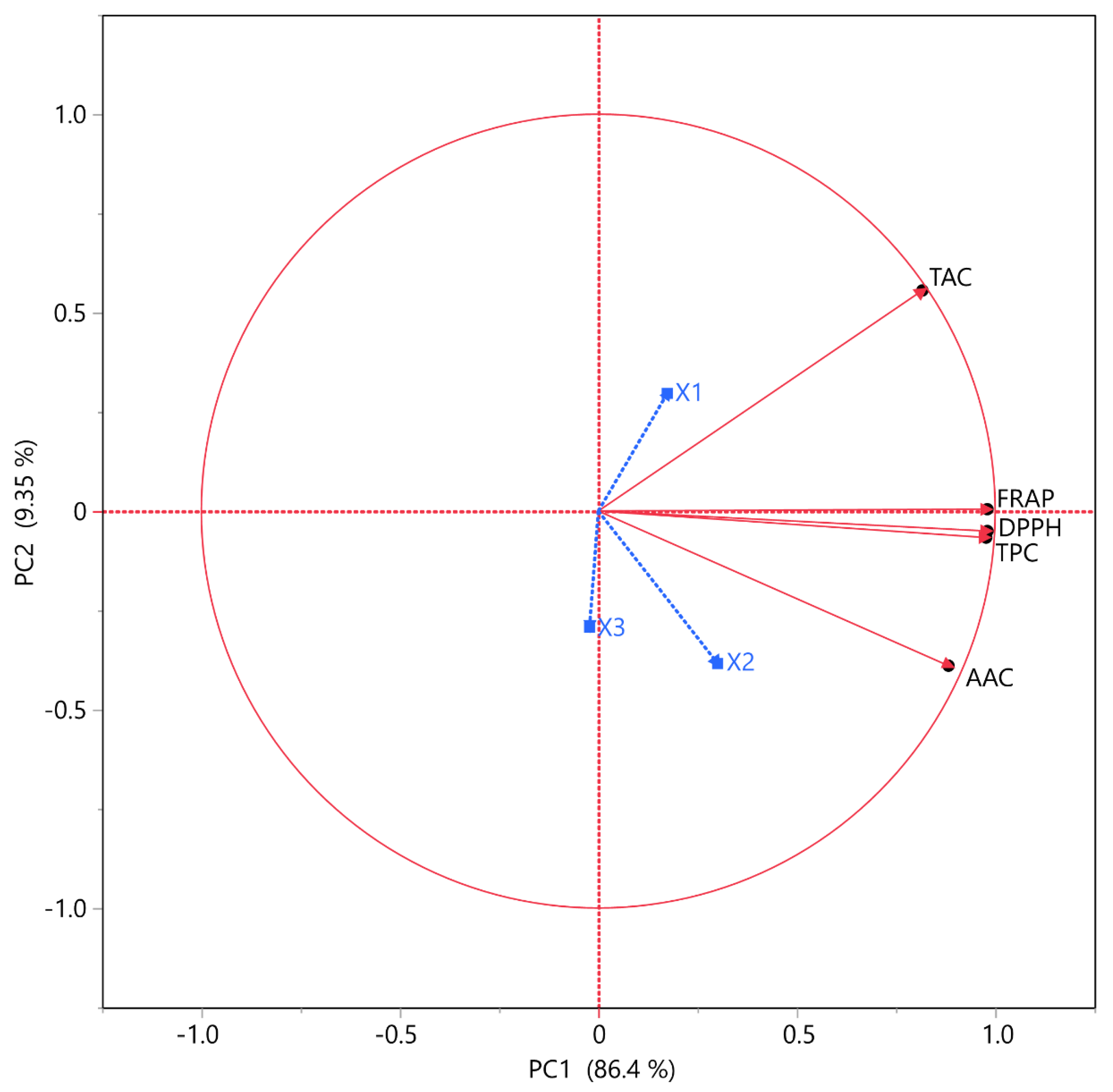
3.4. Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
3.5. Partial Least Squares (PLS) Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gundogdu, M.; Ozrenk, K.; Ercisli, S.; Kan, T.; Kodad, O.; Hegedus, A. Organic Acids, Sugars, Vitamin C Content and Some Pomological Characteristics of Eleven Hawthorn Species (Crataegus Spp.) from Turkey. Biol. Res. 2014, 47, 21. [Google Scholar] [CrossRef] [PubMed]
- Attard, E.; Attard, H. Hawthorn: Crataegus Oxyacantha, Crataegus Monogyna and Related Species. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–293. ISBN 978-0-12-812491-8. [Google Scholar]
- Martinelli, F.; Perrone, A.; Yousefi, S.; Papini, A.; Castiglione, S.; Guarino, F.; Cicatelli, A.; Aelaei, M.; Arad, N.; Gholami, M.; et al. Botanical, Phytochemical, Anti-Microbial and Pharmaceutical Characteristics of Hawthorn (Crataegus monogyna Jacq.), Rosaceae. Molecules 2021, 26, 7266. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, C.P.; Arya, V.; Thakur, N. Ethnomedicinal and Phytopharmacological Potential of Crataegus Oxyacantha Linn—A Review. Asian Pac. J. Trop. Biomed. 2012, 2, 1194–1199. [Google Scholar] [CrossRef]
- Benabderrahmane, W.; Lores, M.; Benaissa, O.; Lamas, J.P.; de Miguel, T.; Amrani, A.; Benayache, F.; Benayache, S. Polyphenolic Content and Bioactivities of Crataegus Oxyacantha L. (Rosaceae). Nat. Prod. Res. 2021, 35, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.I. Revision of Crataegus Sect. Crataegus and Nothosect. Crataeguineae (Rosaceae-Maloideae) in the Old World. Syst. Bot. Monogr. 1992, 35, 1. [Google Scholar] [CrossRef]
- Orhan, I.E. Phytochemical and Pharmacological Activity Profile of Crataegus Oxyacantha L. (Hawthorn)—A Cardiotonic Herb. Curr. Med. Chem. 2019, 25, 4854–4865. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, M.; Rebai, O.; Dhaouadi, K.; Congiu, F.; Tuberoso, C.I.G.; Amri, M.; Fattouch, S. Comparative Analysis of Tunisian Wild Crataegus Azarolus (Yellow Azarole) and Crataegus Monogyna (Red Azarole) Leaf, Fruit, and Traditionally Derived Syrup: Phenolic Profiles and Antioxidant and Antimicrobial Activities of the Aqueous-Acetone Extracts. J. Agric. Food Chem. 2013, 61, 130926133925000. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Comparing the Composition and Bioactivity of Crataegus Monogyna Flowers and Fruits Used in Folk Medicine. Phytochem. Anal. 2011, 22, 181–188. [Google Scholar] [CrossRef]
- Elsadig Karar, M.G.; Kuhnert, N. UPLC-ESI-Q-TOF-MS/MS Characterization of Phenolics from Crataegus Monogyna and Crataegus Laevigata (Hawthorn) Leaves, Fruits and Their Herbal Derived Drops (Crataegutt Tropfen). J. Chem. Biol. Ther. 2016, 1, 2572-0406. [Google Scholar] [CrossRef]
- Cui, T.; Li, J.-Z.; Kayahara, H.; Ma, L.; Wu, L.-X.; Nakamura, K. Quantification of the Polyphenols and Triterpene Acids in Chinese Hawthorn Fruit by High-Performance Liquid Chromatography. J. Agric. Food Chem. 2006, 54, 4574–4581. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Calhelha, R.C.; Barreira, J.C.M.; Dueñas, M.; Carvalho, A.M.; Abreu, R.M.V.; Santos-Buelga, C.; Ferreira, I.C.F.R. Crataegus Monogyna Buds and Fruits Phenolic Extracts: Growth Inhibitory Activity on Human Tumor Cell Lines and Chemical Characterization by HPLC–DAD–ESI/MS. Food Res. Int. 2012, 49, 516–523. [Google Scholar] [CrossRef]
- Ljubuncic, P.; Portnaya, I.; Cogan, U.; Azaizeh, H.; Bomzon, A. Antioxidant Activity of Crataegus Aronia Aqueous Extract Used in Traditional Arab Medicine in Israel. J. Ethnopharmacol. 2005, 101, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Akila, M.; Devaraj, H. Synergistic Effect of Tincture of Crataegus and Mangifera Indica L. Extract on Hyperlipidemic and Antioxidant Status in Atherogenic Rats. Vascul. Pharmacol. 2008, 49, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Kumar, S.P.; Rupesh, D.; Nitin, K. Effects of Methanolic Extract of Crataegus Oxyacantha on Blood Homeostasis in Rat. J. Chem. Pharm. Res. 2011, 3, 675–684. [Google Scholar]
- Tadić, V.M.; Dobrić, S.; Marković, G.M.; Dordević, S.M.; Arsić, I.A.; Menković, N.R.; Stević, T. Anti-Inflammatory, Gastroprotective, Free-Radical-Scavenging, and Antimicrobial Activities of Hawthorn Berries Ethanol Extract. J. Agric. Food Chem. 2008, 56, 7700–7709. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Gillespie, B.; Aaronson, K.D. The Effect of Crataegus Oxycantha Special Extract WS 1442 on Clinical Progression in Patients with Mild to Moderate Symptoms of Heart Failure. Eur. J. Heart Fail. 2008, 10, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Degenring, F.H.; Suter, A.; Weber, M.; Saller, R. A Randomised Double Blind Placebo Controlled Clinical Trial of a Standardised Extract of Fresh Crataegus Berries (Crataegisan) in the Treatment of Patients with Congestive Heart Failure NYHA II. Phytomedicine 2003, 10, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Hanus, M.; Lafon, J.; Mathieu, M. Double-Blind, Randomised, Placebo-Controlled Study to Evaluate the Efficacy and Safety of a Fixed Combination Containing Two Plant Extracts (Crataegus Oxyacantha and Eschscholtzia Californica) and Magnesium in Mild-to-Moderate Anxiety Disorders. Curr. Med. Res. Opin. 2004, 20, 63–71. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Thirupurasundari, C.J.; Devaraj, S.N. Pretreatment with Alcoholic Extract of Crataegus Oxycantha (AEC) Activates Mitochondrial Protection during Isoproterenol—Induced Myocardial Infarction in Rats. Mol. Cell. Biochem. 2006, 292, 59–67. [Google Scholar] [CrossRef]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn Extract for Treating Chronic Heart Failure: Meta-Analysis of Randomized Trials. Am. J. Med. 2003, 114, 665–674. [Google Scholar] [CrossRef]
- Mecheri, A.; Benabderrahmane, W.; Amrani, A.; Boubekri, N.; Benayache, F.; Benayache, S.; Zama, D. Hepatoprotective Effects of Algerian Crataegus Oxyacantha Leaves. Recent Pat. Food. Nutr. Agric. 2019, 10, 70–75. [Google Scholar] [CrossRef]
- Jouad, H.; Lemhadri, A.; Maghrani, M.; Burcelin, R.; Eddouks, M. Hawthorn Evokes a Potent Anti-Hyperglycemic Capacity in Streptozotocin-Induced Diabetic Rats. J. Herb. Pharmacother. 2003, 3, 19–29. [Google Scholar] [CrossRef]
- Keser, S.; Celik, S.; Turkoglu, S.; Yilmaz, Ö.; Turkoglu, I. The Investigation of Some Bioactive Compounds and Antioxidant Properties of Hawthorn (Crataegus Monogyna Subsp. Monogyna Jacq). J. Intercult. Ethnopharmacol. 2014, 3, 51–55. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Masteikova, R.; Majiene, D.; Savickas, A.; Kevelaitis, E.; Bernatoniene, R.; Dvořáčkovâ, K.; Civinskiene, G.; Lekas, R.; Vitkevičius, K.; et al. Free Radical-Scavenging Activities of Crataegus Monogyna Extracts. Medicina (B. Aires) 2008, 44, 706–712. [Google Scholar] [CrossRef]
- Lasseigne, F.T.; Blazich, F.A. Crataegus L. In The Woody Plant Seed Manual; Agriculture Handbook 727; Bonner, F.T., Karrfalt, R.P., Eds.; Forest Service: Washington, DC, USA, 2008; pp. 447–456. [Google Scholar]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K. Optimization of Extraction Parameters for Enhanced Recovery of Bioactive Compounds from Quince Peels Using Response. Foods 2023, 12, 2099. [Google Scholar] [CrossRef]
- Lakka, A.; Grigorakis, S.; Kaltsa, O.; Karageorgou, I.; Batra, G.; Bozinou, E.; Lalas, S.; Makris, D.P. The Effect of Ultrasonication Pretreatment on the Production of Polyphenol-Enriched Extracts from Moringa Oleifera L. (Drumstick Tree) Using a Novel Bio-Based Deep Eutectic Solvent. Appl. Sci. 2020, 10, 220. [Google Scholar] [CrossRef]
- Kourelatou, A.; Chatzimitakos, T.; Athanasiadis, V.; Kotsou, K.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Seeking Optimal Extraction Method for Augmenting Hibiscus Sabdariffa Bioactive Compounds and Antioxidant Activity. Processes 2024, 12, 581. [Google Scholar] [CrossRef]
- Jagota, S.K.; Dani, H.M. A New Colorimetric Technique for the Estimation of Vitamin C Using Folin Phenol Reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef]
- Paleologou, I.; Vasiliou, A.; Grigorakis, S.; Makris, D.P. Optimisation of a Green Ultrasound-Assisted Extraction Process for Potato Peel (Solanum Tuberosum) Polyphenols Using Bio-Solvents and Response Surface Methodology. Biomass Convers. Biorefin. 2016, 6, 289–299. [Google Scholar] [CrossRef]
- Pham, T.N.; Lam, T.D.; Nguyen, M.T.; Le, X.T.; Vo, D.V.N.; Toan, T.Q.; Vo, T.S. Effect of Various Factors on Extraction Efficiency of Total Anthocyanins from Butterfly Pea (Clitoria Ternatea L. Flowers) in Southern Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2019, 544, 012013. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Bozinou, E.; Kotsou, K.; Palaiogiannis, D.; Lalas, S.I. Maximizing the Extraction of Bioactive Compounds from Diospyros Kaki Peel through the Use of a Pulsed Electric Field and Ultrasound Extraction. Biomass 2023, 3, 422–440. [Google Scholar] [CrossRef]
- Wardy, W.; Saalia, F.K.; Steiner-Asiedu, M.; Budu, A.S.; Sefa-Dedeh, S. A Comparison of Some Physical, Chemical and Sensory Attributes of Three Pineapple (Ananas Comosus) Varieties Grown in Ghana. Afr. J. Food Sci. 2009, 3, 22–025. [Google Scholar]
- Usman, I.; Hussain, M.; Imran, A.; Afzaal, M.; Saeed, F.; Javed, M.; Afzal, A.; Ashfaq, I.; Al Jbawi, E.; A. Saewan, S. Traditional and Innovative Approaches for the Extraction of Bioactive Compounds. Int. J. Food Prop. 2022, 25, 1215–1233. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Khaneghah, A.M.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus Spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef]
- Kostić, D.A.; Velicković, J.M.; Mitić, S.S.; Mitic, M.N.; Randelović, S.S. Phenolic Content, and Antioxidant and Antimicrobial Activities of Crataegus oxyacantha L (Rosaceae) Fruit Extract from Southeast Serbia. Trop. J. Pharm. Res. 2012, 11, 117–124. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive Compounds and Antioxidant Activity in Different Types of Berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef]
- Tahirović, A.; Bašić, N. Phenolic Content and Antioxidant Activity of Crataegus monogyna L. Fruit Extracts. Rad. Šumarskog Fak. Univ. Sarajev. 2014, 44, 29–40. [Google Scholar] [CrossRef]
- Yalçın Dokumacı, K.; Uslu, N.; Hacıseferoğulları, H.; Örnek, M.N. Determination of Some Physical and Chemical Properties of Common Hawthorn (Crataegus monogyna Jacq. Var. Monogyna). Erwerbs-Obstbau 2021, 63, 99–106. [Google Scholar] [CrossRef]
- Tamayo-Vives, C.; García-Herrera, P.; Sánchez-Mata, M.C.; Cámara-Hurtado, R.M.; Pérez-Rodríguez, M.L.; Aceituno, L.; Pardo-de-Santayana, M.; Días, M.I.; Barros, L.; Morales, P. Wild Fruits of Crataegus monogyna Jacq. and Sorbus aria (L.) Crantz: From Traditional Foods to Innovative Sources of Pigments and Antioxidant Ingredients for Food Products. Foods 2023, 12, 2427. [Google Scholar] [CrossRef]
- Singh, D.; Kumari, K.; Ahmed, S. Chapter 17—Natural Herbal Products for Cancer Therapy; Jain, B., Pandey, S.B.T.-U.C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 257–268. ISBN 978-0-323-99883-3. [Google Scholar]
- Tahirović, A.; Bašić, N.; Hubijar, I.; Šito, S.; Čabaravdić, A. Comparison of Polyphenol Content and Antioxidant Activity of Extracts from Fruits of Two Crataegus Species. Rad. Šumarskog Fak. Univ. Sarajev. 2015, 45, 38–51. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and Vegetable Intake and the Risk of Cardiovascular Disease, Total Cancer and All-Cause Mortality—A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary Intake and Blood Concentrations of Antioxidants and the Risk of Cardiovascular Disease, Total Cancer, and All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Zhang, J.; Chai, X.; Zhao, F.; Hou, G.; Meng, Q. Food Applications and Potential Health Benefits of Hawthorn. Foods 2022, 11, 2861. [Google Scholar] [CrossRef]
- Tahirović, A.; Čopra–Janićijević, A.; Bašić, N.; Klepo, L.; Subašić, M. Determination of Vitamin C in Some Bosnian Crataegus L. Species. Rad. Šumarskog Fak. Univ. Sarajev. 2012, 42, 43–55. [Google Scholar] [CrossRef]
- Muradoğlu, F.; Gürsoy, S.; Yıldız, K. Quantification Analysis of Biochemical and Phenolic Composition in Hawthorn (Crataegus Spp.) Fruits. Erwerbs-Obstbau 2019, 61, 189–194. [Google Scholar] [CrossRef]
- Yang, B.; Liu, P. Composition and Health Effects of Phenolic Compounds in Hawthorn (Crataegus Spp.) of Different Origins. J. Sci. Food Agric. 2012, 92, 1578–1590. [Google Scholar] [CrossRef]
- Bekbolatova, E.; Kukula-Koch, W.; Baj, T.; Stasiak, N.; Ibadullayeva, G.; Koch, W.; Głowniak, K.; Tulemissov, S.; Sakipova, Z.; Boylan, F. Phenolic Composition and Antioxidant Potential of Different Organs of Kazakh Crataegus Almaatensis Pojark: A Comparison with the European Crataegus Oxyacantha L. Flowers. Open Chem. 2018, 16, 415–426. [Google Scholar] [CrossRef]
- Wu, X.; Song, M.; Qiu, P.; Li, F.; Wang, M.; Zheng, J.; Wang, Q.; Xu, F.; Xiao, H. A Metabolite of Nobiletin, 4′-Demethylnobiletin and Atorvastatin Synergistically Inhibits Human Colon Cancer Cell Growth by Inducing G0/G1 Cell Cycle Arrest and Apoptosis. Food Funct. 2018, 9, 87–95. [Google Scholar] [CrossRef]
- Lou, X.; Yuan, B.; Wang, L.; Xu, H.; Hanna, M.; Yuan, L. Evaluation of Physicochemical Characteristics, Nutritional Composition and Antioxidant Capacity of Chinese Organic Hawthorn Berry (Crataegus Pinnatifida). Int. J. Food Sci. Technol. 2020, 55, 1679–1688. [Google Scholar] [CrossRef]
- Issaadi, O.; Fibiani, M.; Picchi, V.; Scalzo, R.L.; Madani, K. Phenolic Composition and Antioxidant Capacity of Hawthorn (Crataegus oxyacantha L.) Flowers and Fruits Grown in Algeria. J. Complement. Integr. Med. 2021, 17, 20180125. [Google Scholar] [CrossRef]
- Zhu, R.; Zhang, X.; Wang, Y.; Zhang, L.; Wang, C.; Hu, F.; Ning, C.; Chen, G. Pectin Oligosaccharides from Hawthorn (Crataegus Pinnatifida Bunge. Var. Major): Molecular Characterization and Potential Antiglycation Activities. Food Chem. 2019, 286, 129–135. [Google Scholar] [CrossRef]
- Rocchetti, G.; Senizza, B.; Zengin, G.; Mahomodally, M.F.; Senkardes, I.; Lobine, D.; Lucini, L. Untargeted Metabolomic Profiling of Three Crataegus Species (Hawthorn) and Their in Vitro Biological Activities. J. Sci. Food Agric. 2020, 100, 1998–2006. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Oszmiański, J.; Wojdyło, A. Antioxidant Activity of the Phenolic Compounds of Hawthorn, Pine and Skullcap. Food Chem. 2007, 103, 853–859. [Google Scholar] [CrossRef]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic Uses of Epicatechin in Diabetes and Cancer. Vet. World 2017, 10, 869–872. [Google Scholar] [CrossRef]
- Horie, N.; Hirabayashi, N.; Takahashi, Y.; Miyauchi, Y.; Taguchi, H.; Takeishi, K. Synergistic Effect of Green Tea Catechins on Cell Growth and Apoptosis Induction in Gastric Carcinoma Cells. Biol. Pharm. Bull. 2005, 28, 574–579. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Haider, K.; Haider, M.R.; Neha, K.; Yar, M.S. Free Radical Scavengers: An Overview on Heterocyclic Advances and Medicinal Prospects. Eur. J. Med. Chem. 2020, 204, 112607. [Google Scholar] [CrossRef] [PubMed]
- Sainz, R.M.; Lombo, F.; Mayo, J.C. Radical Decisions in Cancer: Redox Control of Cell Growth and Death. Cancers 2012, 4, 442–474. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Q.; Wang, W.; Peng, M.; Zhang, X.-Z. Free Radicals for Cancer Theranostics. Biomaterials 2021, 266, 120474. [Google Scholar] [CrossRef]
| Independent Variables | Coded Units | Coded Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| C (%, v/v) | X1 | 0 | 50 | 100 |
| T (°C) | X2 | 20 | 50 | 80 |
| t (min) | X3 | 30 | 60 | 90 |
| Design Point | Independent Variables | Responses | ||||||
|---|---|---|---|---|---|---|---|---|
| X1 (C, %) | X2 (T, °C) | X3 (t, min) | TPC 1 | TAC 2 | FRAP 3 | DPPH 4 | AAC 5 | |
| 1 | 0 (50) | 0 (50) | 0 (60) | 55.59 | 49.00 | 415.95 | 235.59 | 854.73 |
| 2 | 0 (50) | 0 (50) | 0 (60) | 55.26 | 46.79 | 383.68 | 254.80 | 859.77 |
| 3 | 0 (50) | −1 (20) | −1 (30) | 48.53 | 45.91 | 389.45 | 264.50 | 735.72 |
| 4 | −1 (0) | 0 (50) | −1 (30) | 24.82 | 37.30 | 90.56 | 78.79 | 378.74 |
| 5 | 1 (100) | −1 (20) | 0 (60) | 18.40 | 24.17 | 168.75 | 76.24 | 493.46 |
| 6 | 1 (100) | 0 (50) | −1 (30) | 24.12 | 30.74 | 193.90 | 77.29 | 590.51 |
| 7 | 0 (50) | 1 (80) | −1 (30) | 47.43 | 46.08 | 337.08 | 241.53 | 942.35 |
| 8 | 0 (50) | 0 (50) | 0 (60) | 54.36 | 49.07 | 401.13 | 259.24 | 932.88 |
| 9 | −1 (0) | 0 (50) | 1 (90) | 15.42 | 7.82 | 88.29 | 66.05 | 621.91 |
| 10 | 1 (100) | 1 (80) | 0 (60) | 30.06 | 45.66 | 240.28 | 126.43 | 665.90 |
| 11 | 0 (50) | −1 (20) | 1 (90) | 40.09 | 45.65 | 275.60 | 218.60 | 754.22 |
| 12 | 1 (100) | 0 (50) | 1 (90) | 21.55 | 38.85 | 152.64 | 106.43 | 702.59 |
| 13 | 0 (50) | 1 (80) | 1 (90) | 49.83 | 51.83 | 389.63 | 270.26 | 1053.28 |
| 14 | −1 (0) | −1 (20) | 0 (60) | 10.91 | 26.62 | 49.24 | 47.82 | 213.69 |
| 15 | −1 (0) | 1 (80) | 0 (60) | 38.15 | 15.69 | 196.79 | 168.90 | 840.64 |
| Responses | Second-Order Polynomial Equations (Models) | R2 Predicted | R2 Adjusted | p-Value | Eq. |
|---|---|---|---|---|---|
| TPC | Y = −3.74 + 1.19X1 + 0.46X2 + 0.48X3 − 0.01X12 − 0.003X22 − 0.006X32 − 0.003X1X2 + 0.001X1X3 + 0.003X2X3 | 0.9641 | 0.8996 | 0.0041 | (7) |
| TAC | Y = 57.35 + 0.26X1 − 0.21X2 − 0.44X3 − 0.008X12 − 0.001X22 − 0.001X32 + 0.005X1X2 + 0.006X1X3 + 0.002X2X3 | 0.9618 | 0.8930 | 0.0048 | (8) |
| FRAP | Y = −9.5 + 10.91X1 + 0.14X2 + 3.23X3 − 0.09X12 − 0.01X22 − 0.05X32 − 0.01X1X2 − 0.007X1X3 + 0.05X2X3 | 0.9840 | 0.9553 | 0.0006 | (9) |
| DPPH | Y = 85.31 + 6.47X1 − 1.02X2 + 0.2X3 − 0.06X12 + 0.01X22 − 0.01X32 − 0.01X1X2 + 0.01X1X3 + 0.02X2X3 | 0.9675 | 0.9090 | 0.0033 | (10) |
| AAC | Y = −76.96 + 18.63X1 + 9.41X2 + 1.23X3 − 0.13X12 − 0.02X22 + 0.005X32 − 0.08X1X2 − 0.02X1X3 + 0.03X2X3 | 0.9643 | 0.9002 | 0.0041 | (11) |
| Responses | Optimal Conditions | |||
|---|---|---|---|---|
| Maximum Predicted Response | C (%, v/v) | T (°C) | t (min) | |
| TPC (mg GAE/g dw) | 58.21 ± 8.07 | 45 | 80 | 60 |
| TAC (μg CyE/g dw) | 55.47 ± 9.28 | 75 | 80 | 85 |
| FRAP (μmol AAE/g dw) | 430.19 ± 44.46 | 50 | 80 | 70 |
| DPPH (μmol AAE/g dw) | 292.72 ± 46.39 | 50 | 80 | 80 |
| AAC (mg/100 g dw) | 1115.15 ± 148.12 | 40 | 80 | 87 |
| Polyphenolic Compound | Optimal Extract (mg/g dw) |
|---|---|
| Neochlorogenic acid | 1.41 ± 0.04 |
| Catechin | 1.21 ± 0.04 |
| Chlorogenic acid | 1.35 ± 0.07 |
| Vanillic acid | 7.19 ± 0.47 |
| Epicatechin | 11.68 ± 0.34 |
| p-Coumaric acid | 0.73 ± 0.04 |
| Ferulic acid | 0.4 ± 0.01 |
| Quercetin 3-D-galactoside | 6.24 ± 0.38 |
| Kaempferol-3-glucoside | 0.77 ± 0.03 |
| Total identified | 30.98 ± 1.42 |
| Polyphenolic Compound | Retention Time (min) | Absorbance Maximum (nm) | Equation | R2 |
|---|---|---|---|---|
| Neochlorogenic acid | 16.576 | 324 | y = 28,213.51x + 551.72 | 0.9987 |
| Catechin | 20.977 | 278 | y = 11,920.79x − 128.19 | 0.9973 |
| Chlorogenic acid | 21.965 | 325 | y = 50,320.40x − 23,038.36 | 0.9943 |
| Vanillic acid | 24.041 | 270 | y = 20,000x + 1224 | 0.9939 |
| Epicatechin | 25.921 | 278 | y = 142,099x + 4705.94 | 0.9999 |
| p-Coumaric acid | 30.002 | 309 | y = 120,568.59x + 1059.043 | 0.9998 |
| Ferulic acid | 33.931 | 322 | y = 108,553.73x − 25,916.43 | 0.9992 |
| Quercetin-3-D-galactoside | 34.998 | 257 | y = 41,489.69x − 35,577.55 | 0.9934 |
| Kaempferol-3-glucoside | 38.468 | 265 | y = 50,916.85x − 42,398.83 | 0.9962 |
| Responses | TPC | TAC | FRAP | DPPH | AAC |
|---|---|---|---|---|---|
| TPC | - | 0.7374 | 0.9612 | 0.9718 | 0.8468 |
| TAC | - | 0.7842 | 0.7548 | 0.5452 | |
| FRAP | - | 0.9544 | 0.8370 | ||
| DPPH | - | 0.8529 | |||
| AAC | - |
| Variables | PLS Model Values | Experimental Values |
|---|---|---|
| TPC (mg GAE/g dw) | 53.37 | 45.9 ± 1.04 |
| TAC (μg CyE/g dw) | 52.55 | 53.62 ± 6.8 |
| FRAP (μmol AAE/g dw) | 398.7 | 360.7 ± 14.71 |
| DPPH (μmol AAE/g dw) | 286.17 | 291.67 ± 10.81 |
| AAC (mg/100 g dw) | 1096.58 | 912.65 ± 8.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotsou, K.; Magopoulou, D.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Sfougaris, A.I.; Lalas, S.I. Enhancing the Nutritional Profile of Crataegus monogyna Fruits by Optimizing the Extraction Conditions. Horticulturae 2024, 10, 564. https://doi.org/10.3390/horticulturae10060564
Kotsou K, Magopoulou D, Chatzimitakos T, Athanasiadis V, Bozinou E, Sfougaris AI, Lalas SI. Enhancing the Nutritional Profile of Crataegus monogyna Fruits by Optimizing the Extraction Conditions. Horticulturae. 2024; 10(6):564. https://doi.org/10.3390/horticulturae10060564
Chicago/Turabian StyleKotsou, Konstantina, Dimitra Magopoulou, Theodoros Chatzimitakos, Vassilis Athanasiadis, Eleni Bozinou, Athanassios I. Sfougaris, and Stavros I. Lalas. 2024. "Enhancing the Nutritional Profile of Crataegus monogyna Fruits by Optimizing the Extraction Conditions" Horticulturae 10, no. 6: 564. https://doi.org/10.3390/horticulturae10060564
APA StyleKotsou, K., Magopoulou, D., Chatzimitakos, T., Athanasiadis, V., Bozinou, E., Sfougaris, A. I., & Lalas, S. I. (2024). Enhancing the Nutritional Profile of Crataegus monogyna Fruits by Optimizing the Extraction Conditions. Horticulturae, 10(6), 564. https://doi.org/10.3390/horticulturae10060564










