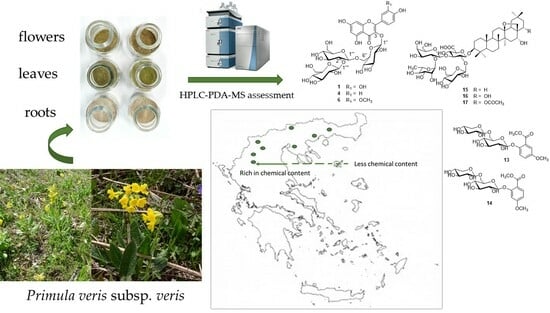
Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Samples
2.3. Sample Preparation for HPLC Analysis
2.4. HPLC Analysis
2.4.1. HPLC-PDA-MS Analysis Instrumentation
2.4.2. Identification and Quantitative Determination of Flavonoids in Flowers and Leaves
2.4.3. Identification and Quantitative Determination of Roots’ Phenolic Glycosides and Saponins
3. Results
3.1. Identification and Quantitative Determination of Flavonols in Cowslip Flowers
3.2. Flavonols’ Identification and Flavonoid Quantitative Determination in Leaves
3.3. Identification and Quantitative Determination of Roots’ Phenolic Glycosides and Saponins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Plants of the World Online. Kew Botanical Gardens. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:702751-1 (accessed on 1 September 2023).
- Brys, R.; Jacquemyn, H. Biological Flora of the British Isles: Primula veris L. J. Ecol. 2009, 97, 581–600. [Google Scholar] [CrossRef]
- Zhao, Z.; Luo, Z.; Yuan, S.; Mei, L.; Zhang, D. Global transcriptome and gene co-expression network analyses on the development of distyly in Primula oreodoxa. Heredity 2019, 123, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Gilmartin, P.M. On the origins of observations of heterostyly in Primula. New Phytol. 2015, 208, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-H.; Zhang, S.-Y.; Guo, Y.; Tang, Z. Conservation status of Primulaceae, a plant family with high endemism, in China. Biol. Conser. 2020, 248, 108675. [Google Scholar] [CrossRef]
- Aavik, T.; Reitalu, T.; Kaldra, M.; Reinula, I.; Hool, K.; Zobel, M.; Pärtel, M.; Uuemaa, E.; Kmoch, A.; Moges, D.M.; et al.; (Institute of Ecology and Earth Sciences, University of Tartu, Tartu, Estonia) Personal communication, 2023.
- Looking for Cowslips. Available online: https://cowslip.science/en (accessed on 30 August 2023).
- EMA/HMPC/113577/2012. Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Radix. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-primula-veris-l/primula-elatior-l-hill-radix_en.pdf (accessed on 25 August 2023).
- Wichtl, M. Primulae radix. In Herbal Drugs and Phytopharmaceuticals; Wichtl, M., Ed.; Medpharm Scientific Publishers: Stuttgart, Germany, 2004; pp. 472–474. [Google Scholar]
- EMA/HMPC/136583/2012. EMA Assessment Report on Primula veris L. and/or Primula elatior (L.) Hill, Flos. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-primula-veris-l/primula-elatior-l-hill-flos_en.pdf (accessed on 25 August 2023).
- Eliopoulos, A.G.; Angelis, A.; Liakakou, A.; Skaltsounis, L.A. In vitro anti-influenza virus activity of non-polar Primula veris subsp. veris Extract. Pharmaceuticals 2022, 15, 1513. [Google Scholar] [CrossRef] [PubMed]
- Latypova, G.M.; Bychenkova, M.A.; Katayev, V.A.; Perfilova, V.N.; Tyurenkov, I.N.; Mokrousov, I.S.; Prokofiev, I.I.; Salikhov, S.h.M.; Iksanova, G.R. Composition and cardioprotective effects of Primula veris L. solid herbal extract in experimental chronic heart failure. Phytomedicine 2018, 54, 17–26. [Google Scholar] [CrossRef]
- Prokofiev, I.I.; Kustova, M.V.; Nesterova, A.A.; Perfilova, V.N.; Khusainova, G.H.; Borodkina, L.E.; Tivon, Y.V.; Tyurenkov, I.N.; Kataev, V.A.; Latypova, G.M. Solid herbal extract of Primula veris L. improves morphofunctional condition of rats’ myocardium in chronic alcohol intoxication. J. Tradit. Complement. Med. 2023, 13, 306–314. [Google Scholar] [CrossRef]
- Ghédira, K.; Goetz, P. Primevère officinale Primula veris L. (Primulaceae). Phytothérapie 2017, 15, 240–244. [Google Scholar] [CrossRef]
- Kahraman, C.; Sari, S.; Küpeli Akkol, E.; Tatli Cankaya, I. Bioactive Saponins of Primula vulgaris Huds. promote wound healing through inhibition of collagenase and elastase enzymes: In vivo, in vitro and in silico evaluations. Chem Biodivers. 2022, 19, e202200582. [Google Scholar] [CrossRef]
- Cosmetic Ingredients. SpecialChem, The Material Selection Platform. Available online: https://cosmetics.specialchem.com/inci/primula-veris-extract (accessed on 31 August 2023).
- Jiang, S.; Deng, L.; Luo, H.; Li, X.; Guo, B.; Jiang, M.; Jia, Y.; Ma, J.; Sun, L.; Huang, Z. Effect of fragrant primula flowers on physiology and psychology in female college students: An empirical study. Front. Psychol. 2021, 12, 607876. [Google Scholar] [CrossRef]
- Plantes Sauvages Comestibles. Available online: https://plantes-sauvages-comestibles.com/la-primevere-mangeons-du-coucou/ (accessed on 31 August 2023).
- Primulas for Scent. The Royal Horticultural Society. Available online: https://www.rhs.org.uk/plants/primula/for-scent (accessed on 31 August 2023).
- Hayta, S.; Smedley, M.A.; Li, J.; Harwood, W.A.; Gilmartin, P.M. Plant regeneration from leaf-derived callus cultures of primrose (Primula vulgaris). Hort. Sci. 2016, 51, 558–562. [Google Scholar] [CrossRef]
- Arrest for Illegal Collection of Primroses in Grammos. Available online: https://dasarxeio.com/2023/05/08/125161/ (accessed on 18 August 2023).
- Arrests of Foreigners for Illegal Collection of Primroses. Available online: https://dasarxeio.com/2023/06/01/125781/ (accessed on 18 August 2023).
- Chintiroglou, P.I.; Krigas, N.; Chatzopoulou, P.; Karioti, A. Development and validation of an HPLC method for the analysis of flowers of wild-growing Primula veris from Epirus, Greece. Planta Med. 2021, 87, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- Strid, A. Atlas of the Aegean Flora Part 1 (Text & Plates) & Part 2 (Maps), 1st ed.; Englera 33 (1 & 2); Botanic Garden and Botanical Museum: Berlin, Germany; Freie Universität: Berlin, Germany, 2016. [Google Scholar]
- Müller, A.; Ganzera, M.; Stuppner, H. Analysis of phenolic glycosides and saponins in Primula elatior and Primula veris (primula root) by liquid chromatography, evaporative light scattering detection and mass spectrometry. J. Chromatogr. A 2006, 1112, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Khalfallah, A.; Karioti, A.; Berrehal, D.; Kabouche, A.; Lucci, M.; Bilia, A.R.; Kabouche, Z. A new flavonol triglycoside and other flavonol glycosides from Astragalus armatus Willd. (Fabaceae). Rec. Nat. Prod. 2013, 8, 12–18. [Google Scholar]
- EMA/HMPC/CHMP/CVMP/201116/20051 Rev. 3 Guideline on Quality of Herbal Medicinal Products2/Traditional Herbal Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/final-guideline-quality-herbal-medicinal-products/traditional-herbal-medicinal-products-revision-3_en.pdf (accessed on 5 September 2023).
- Guide for the Elaboration of Monographs on Herbal Drugs and Herbal Drug Preparations European Pharmacopoeia. 2nd Edition. 2023. Available online: https://www.edqm.eu/documents/52006/66555/Guide+for+the+elaboration+of+monographs+on+HERBAL+DRUGS+AND+HERBAL+DRUG+PREPARATIONS.pdf/bef3251e-fafc-581b-93ef-3c1a95e2793c?t=1684932003926 (accessed on 5 September 2023).
- Colombo, P.S.; Flamini, G.; Christodoulou, M.S.; Rodondi, G.; Vitalini, S.; Passarella, D.; Fico, G. Farinose alpine Primula species: Phytochemical and morphological investigations. Phytochemistry 2014, 98, 151–159. [Google Scholar] [CrossRef]
- Bhutia, T.D.; Valant-Vetschera, K.M.; Adlassnig, W.; Brecker, L. Flavonoids in selected Primula spp.: Bridging micromorphology with chemodiversity. Nat. Prod. Commun. 2012, 7, 1469–1473. [Google Scholar] [CrossRef]
- El Morchid, E.M.; Torres Londoño, P.; Papagiannopoulos, M.; Gobbo-Neto, L.; Müller, C. Variation in flavonoid pattern in leaves and flowers of Primula veris of different origin and impact of UV-B. Biochem. Syst. Ecol. 2014, 53, 81–88. [Google Scholar] [CrossRef]
- Apel, L.; Kammerer, D.R.; Stintzing, F.C.; Spring, O. Comparative metabolite profiling of triterpenoid saponins and flavonoids in flower color mutations of Primula veris L. Int. J. Mol. Sci. 2017, 18, 153. [Google Scholar] [CrossRef]
- Graikou, K.; Mpishinioti, A.; Tsafantakis, N.; Maloupa, E.; Grigoriadou, K.; Chinou, I. Comparative phytochemical analyses of flowers from Primula veris subsp. veris growing wild and from ex situ cultivation in Greece. Foods 2023, 12, 2623. [Google Scholar] [CrossRef]
- Michel, P.; Granica, S.; Rosińska, K.; Glige, M.; Rojek, J.; Poraj, Ł.; Olszewska, M.A. The effect of standardised leaf extracts of Gaultheria procumbens on multiple oxidants, inflammation-related enzymes, and pro-oxidant and pro-inflammatory functions of human neutrophils. Molecules 2022, 27, 3357. [Google Scholar] [CrossRef]
- Çaliş, I.; Yürüker, A.; Rüegger, H.; Wright, A.D.; Sticher, O. Triterpene saponins from Primula veris subsp. macrocalyx and Primula elatior subsp. meyeri. J. Nat. Prod. 1992, 55, 1299–1306. [Google Scholar] [CrossRef]
- Climatic Atlas of Greece. Available online: http://climatlas.hnms.gr/sdi/ (accessed on 7 September 2023).
- Tarapatskyy, M.; Gumienna, A.; Sowa, P.; Kapusta, I.; Puchalski, C. Bioactive phenolic compounds from Primula veris L.: Influence of the extraction conditions and purification. Molecules 2021, 26, 997. [Google Scholar] [CrossRef]
- Holzbach, B.; Reuter, V.; Bacher, M.; Schinnerl, J.; Brecker, L.; Rosenau, T.; Valant-Vetschera, K. Flavonoid diversification in different leaf compartments of Primula auricula (Primulaceae). Biochem. Syst. Ecol. 2021, 98, 104310. [Google Scholar] [CrossRef]
- Włodarczyk, M.; Pasikowski, P.; Osiewała, K.; Frankiewicz, A.; Dryś, A.; Gleńsk, M. In Search of High- Yielding and Single-Compound-Yielding Plants: New Sources of Pharmaceutically Important Saponins from the Primulaceae Family. Biomolecules 2020, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Bączek, K.; Przybyl, J.L.; Mirgos, M.; Kosakowska, O.; Szymborska-Sandhu, I.; Węglarz, Z. Phenolics in Primula veris L. and P. elatior (L.) Hill raw materials. Int. J. Anal. Chem. 2017, 2017, e2871579. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Elkady, W.M.; Gonaid, M.H.; Yousif, M.F.; El-Sayed, M.; Omar, H.A.N. Impact of altitudinal variation on the phytochemical profile, anthelmintic and antimicrobial activity of two Pinus species. Molecules 2021, 26, 3170. [Google Scholar] [CrossRef]



| Abbreviation/Code | Area and Locality | Collection Date | Altitude (m) | Latitude (North) | Longitude (East) | Plant Part Examined |
|---|---|---|---|---|---|---|
| Mets-1/PV-7 | 1 Metsovo, Aoos springs | 22.5.2021 * | 1360 | 39.81573 | 21.09162 | R |
| Mets-2/PV-8 | Metsovo, Aoos springs | 22.5.2021 * | 1365 | 39.84365 | 21.09584 | R |
| Verm-1/PV-9 | Mt Vermio | 23.5.2021 | 1840 | 40.640112 | 21.956557 | F, L, R |
| Verm-2/PV-10 | Mt Vermio, Tsanaktsi | 23.5.2021 | 1826 | 40.571870 | 21.965445 | F, L, R |
| Vasi-1/PV-11 | Mt Vasilitsa, Smixi | 21.5.2021 | 1343 | 40.059212 | 21.115840 | F, L, R |
| Vasi-2/PV-12 | 2 Mt Vasilitsa, summit area | 21.5.2021 | 1850 | 40.064187 | 21.078943 | F, L, R |
| Chor-1/PV-13 | Mt Chortiatis, north | 8.4.2021 | 900 | 40.597500 | 23.103083 | F, L, R |
| Chor-2/PV-14 | Mt Chortiatis, east | 8.4.2021 | 965 | 40.592083 | 23.121972 | F, L, R |
| Chor-3/PV-21 | 3 Mt Chortiatis, south | 18.4.2022 * | 915 | 40.593028 | 23.102278 | L, R |
| Bell/ PV-15 | Mt Belles (Kerkini) | 23.05.2021 | 1272 | 41.312985 | 23.021752 | F, L, R |
| Karp/PV-16 | Mt Karpouzi | 13.6.2021 | 870 | 41.118306 | 24.752167 | F, L, R |
| Pang/PV-17 | Mt Pangaio, Akrovouni | 13.6.2021 | 1540 | 40.916667 | 24.166667 | F, L, R |
| Piso/PV-22 | 4 Mt Varnous, Pisoderi | 4.6.2022 * | 1495 | 40.783917 | 21.261972 | L, R |
| Flowers | Leaves | |||||
|---|---|---|---|---|---|---|
| Νο. | Rt (min) Zorbax® | Rt (min) Luna® | UV (nm) | m/z (-) Negative Mode | Identification | References |
| 1 | 10.63 | 5.35 | 256, 268 sh, 357 | 300.8 [A-H]-, 787.2 [Μ-H]- | quercetin-3-O-β-glucopyranosyl-(1→2)-β-glucopyranosyl-(1→6)-β-glucopyranoside (Q3G) | [23] * |
| 2 | 12.68 | 6.72 | 255, 266 sh, 356 | 300.3 [A-H]-, 755.1 [Μ-H]- | quercetin-3-O-dirhamnosylhexoside | [29] |
| 3 | 13.55 | - | 255, 266 sh, 356 | 301.0 [A-H]-, 625.0 [Μ-H]- | quercetin-3-O-β-glucopyranosyl-(1→6)-β-glucopyranoside | [23] * |
| 4 | 15.05 | 8.33 | 268, 352 | 285.0 [A-H]-, 771.1 [Μ-H]- | kaempferol-3-O-β-glucopyranosyl-(1→2)-β-glucopyranosyl-(1→6)-β-glucopyranoside (K3G) | [23] * |
| 5 | 15.09 | 9.39 | 268, 352 | 285.0 [A-H]-, 739.1 [Μ-H]- | kaempferol-3-O-dirhamnosylhexoside (admixture with 4) | [29,33] |
| 6 | 16.38 | - | 254, 268 sh, 357 | 314.9 [A-H]-, 801.1 [Μ-H]- | isorhamnetin-3-O-β-glucopyranosyl-(1→2)-β-glucopyranosyl-(1→6)-β-glucopyranoside (I3G) | [23] * |
| 7 | 16.38 | - | 254, 268 sh, 357 | 314.9 [A-H]-, 769.1 [Μ-H]- | isorhamnetin-3-O-dirhamnosylhexoside (admixture with 6) | [29,33] |
| 8 | 17.16 | 12.87 | 255, 265 sh, 354 | 301.0 [A-H]-, 609.0 [Μ-H]- | rutin (quercetin-3-O-rutinoside) | std |
| 9 | 18.28 | - | 254, 270 sh, 356 | 314.9 [A-H]-, 639.0 [Μ-H]- | isorhamnetin-3-O-β-glucopyranosyl-(1→6)-β-glucopyranoside | [23] * |
| 10 | 21.12 | 14.92 | 265, 350 | 285.1 [A-H]-, 592.9 [Μ-H]- | nicotiflorin (kaempferol-3-O-rutinoside) | [26] |
| 11 | 22.38 | - | 254, 265 sh, 356 | 314.9 [A-H]-, 622.9 [Μ-H]- | narcissin (isorhamnetin-3-O-rutinoside) | [26] |
| 12 | - | 9.83 | 265, 348 | 284.0 [A-H]-, 739.3 [Μ-H]- | kaempferol-dirhamnosylhexoside | [29,33] |
| I3G * | Q3G * | K3G * | Q-Dirha-Hex * | Mixture K-Dirha-Hex * | Total | Total | |||
|---|---|---|---|---|---|---|---|---|---|
| Sample | Flowers | Flowers | Leaves | Flowers | Leaves | Leaves | Leaves | Flowers | Leaves |
| Verm-1 | 1.63 ± 0.05 (2.74) | 0.59 ± 0.02 (2.55) | 0.63 ± 0.01 (0.67) | 0.30 ± 0.01 (1.16) | 1.37 ± 0.01 (0.69) | 1.23 ± 0.01 (0.82) | 2.99 ± 0.01 (0.13) | 2.53 ± 0.06 (2.17) | 6.23 ± 0.02 (0.26) |
| Verm-2 | 1.79 ± 0.07 (4.00) | 0.58 ± 0.01 (1.86) | 0.59 ± 0.01 (1.55) | 0.40 ± 0.01 (3.49) | 1.21 ± 0.03 (2.82) | 1.17 ± 0.02 (2.01) | 3.26 ± 0.08 (2.56) | 2.78 ± 0.07 (2.47) | 6.23 ± 0.15 (2.33) |
| Vasi-1 | 1.25 ± 0.04 (2.99) | 0.53 ± 0.02 (3.10) | 0.73 ± 0.02 (2.44) | 0.28 ± 0.01 (2.62) | 2.86 ± 0.02 (0.66) | 0.20 ± 0.01 (3.60) | 0.93 ± 0.01 (0.93) | 2.05 ± 0.06 (2.85) | 4.72 ± 0.02 (0.52) |
| Vasi-2 | 1.78 ± 0.05 (2.71) | 0.93 ± 0.02 (1.76) | 1.22 ± 0.01 (1.06) | 0.48 ± 0.01 (1.60) | 2.77 ± 0.03 (0.91) | 0.33 ± 0.01 (1.69) | 1.04 ± 0.02 (1.64) | 3.19 ± 0.07 (2.09) | 5.36 ± 0.02 (0.44) |
| Chor-1 | 0.81 ± 0.03 (3.94) | 0.27 ± 0.01 (1.30) | 0.03 ± 0.01 (2.74) | 0.22 ± 0.01 (2.11) | 1.37 ± 0.03 (1.83) | − | − | 1.31 ± 0.03 (2.36) | 1.40 ± 0.03 (1.85) |
| Chor-2 | 0.67 ± 0.01 (2.14) | 0.19 ± 0.01 (3.12) | 0.18 ± 0.01 (3.27) | 0.07 ± 0.01 (1.93) | 0.06 ± 0.01 (3.58) | 1.07 ± 0.02 (1.89) | 0.96 ± 0.01 (1.09) | 0.93 ± 0.02 (2.04) | 2.26 ± 0.03 (1.51) |
| Chor-3 | - | - | 0.42 ± 0.01 (2.23) | - | 0.21 ± 0.01 (1.70) | 1.12 ± 0.02 (2.00) | 0.91 ± 0.02 (1.66) | - | 2.66 ± 0.04 (1.33) |
| Bell | 0.70 ± 0.02 (2.78) | 0.22 ± 0.01 (2.85) | 0.14 ± 0.01 (2.80) | 0.18 ± 0.01 (3.18) | 2.57 ± 0.05 (2.04) | nq | 0.81 ± 0.01 (1.25) | 1.10 ± 0.03 (2.68) | 3.51 ± 0.06 (1.83) |
| Karp | 0.35 ± 0.01 (2.29) | 0.06 ± 0.01 (4.20) | 0.18 ± 0.01 (0.46) | 0.05 ± 0.01 (2.36) | 0.16 ± 0.01 (2.32) | 0.81 ± 0.02 (1.93) | 0.73 ± 0.01 (0.78) | 0.45 ± 0.01 (2.11) | 1.87 ± 0.03 (1.36) |
| Pang | 0.46 ± 0.02 (3.59) | 0.26 ± 0.01 (3.37) | 0.80 ± 0.02 (2.75) | 0.10 ± 0.01 (1.54) | 2.05 ± 0.03 (1.29) | 0.28 ± 0.01 (3.09) | 0.60 ± 0.01 (1.10) | 0.82 ± 0.03 (3.17) | 3.74 ± 0.05 (1.37) |
| Piso | - | - | 0.66 ± 0.01 (1.92) | - | 4.30 ± 0.04 (0.85) | 0.21 ± 0.01 (3.39) | 1.18 ± 0.01 (0.53) | - | 6.35 ± 0.06 (0.96) |
| Sample | Primulasaponin Ι | Priverosaponin B | Priverosaponin B 22-Acetate | Primeverin | Primulaverin |
|---|---|---|---|---|---|
| Mets-1 | 1.60 ± 0.07 (4.43) | nq | nq | 1.74 ± 0.01 (0.56) | 0.22 ± 0.01 (3.35) |
| Mets-2 | 1.24 ± 0.06 (4.82) | 0.09 ± 0.01 (1.81) | nq | 1.57 ± 0.02 (1.16) | 0.29 ± 0.01 (3.72) |
| Verm-1 | 1.38 ± 0.01 (0.20) | 0.23 ± 0.01 (2.19) | nq | 0.96 ± 0.03 (3.60) | 0.28 ± 0.01 (3.70) |
| Verm-2 | 1.52 ± 0.03 (2.20) | nq | nq | 1.27 ± 0.01 (0.19) | 0.51 ± 0.01 (1.33) |
| Vasi-1 | 0.99 ± 0.04 (4.24) | nq | nq | 0.78 ± 0.03 (3.52) | 0.16 ± 0.01 (3.53) |
| Vasi-2 | 0.80 ± 0.03 (3.89) | nq | nq | 1.17 ± 0.03 (2.97) | 0.16 ± 0.01 (2.64) |
| Chor-1 | 1.08 ± 0.05 (4.81) | nq | nq | − | 0.16 ± 0.01 (2.86) |
| Chor-2 | 1.29 ± 0.02 (1.38) | 0.83 ± 0.04 (4.53) | nq | − | 0.95 ± 0.02 (2.13) |
| Chor-3 | 1.17 ± 0.04 (3.78) | 0.37 ± 0.01 (1.27) | nq | − | 1.31 ± 0.03 (1.91) |
| Bell | 0.79 ± 0.03 (3.58) | 0.13 ± 0.01 (3.39) | nq | − | 0.32 ± 0.01 (3.10) |
| Karp | 1.07 ± 0.01 (1.24) | nq | nq | 0.15 ± 0.07 (4.53) | 1.01 ± 0.04 (4.17) |
| Pang | 0.27 ± 0.01 (3.77) | nq | nq | 0.97 ± 0.02 (2.48) | 0.56 ± 0.02 (4.43) |
| Piso | 1.15 ± 0.05 (4.17) | 0.10 ± 0.01 (1.45) | nq | 0.35 ± 0.02 (4.63) | 0.62 ± 0.03 (4.37) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanis, I.; Chatzopoulou, P.; Krigas, N.; Karioti, A. Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation. Horticulturae 2023, 9, 1120. https://doi.org/10.3390/horticulturae9101120
Stefanis I, Chatzopoulou P, Krigas N, Karioti A. Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation. Horticulturae. 2023; 9(10):1120. https://doi.org/10.3390/horticulturae9101120
Chicago/Turabian StyleStefanis, Ilias, Paschalina Chatzopoulou, Nikos Krigas, and Anastasia Karioti. 2023. "Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation" Horticulturae 9, no. 10: 1120. https://doi.org/10.3390/horticulturae9101120
APA StyleStefanis, I., Chatzopoulou, P., Krigas, N., & Karioti, A. (2023). Exploring the Chemical Content of Primula veris L. subsp. veris Wild-Growing Populations along a Climate Gradient: An HPLC-PDA-MS Quality Assessment of Flowers, Leaves and Roots for Sustainable Exploitation. Horticulturae, 9(10), 1120. https://doi.org/10.3390/horticulturae9101120