In Silico Analysis of the MAPK Gene Family in Cabbage and Its Expression during Development and Stress Response
Abstract
:1. Introduction
2. Materials and Methods
2.1. Identification and In Silico Analysis of MAPK Family Members in Cabbage
2.2. Multiple Sequence Alignment and Construction of Phylogenetic Tree
2.3. Chromosomal Localization and Synteny Analysis of BoMAPK Genes
2.4. Gene Structure and Motif Analysis of BoMAPK Genes
2.5. Cis-Acting Elements Analysis in BoMAPK Genes Promoter
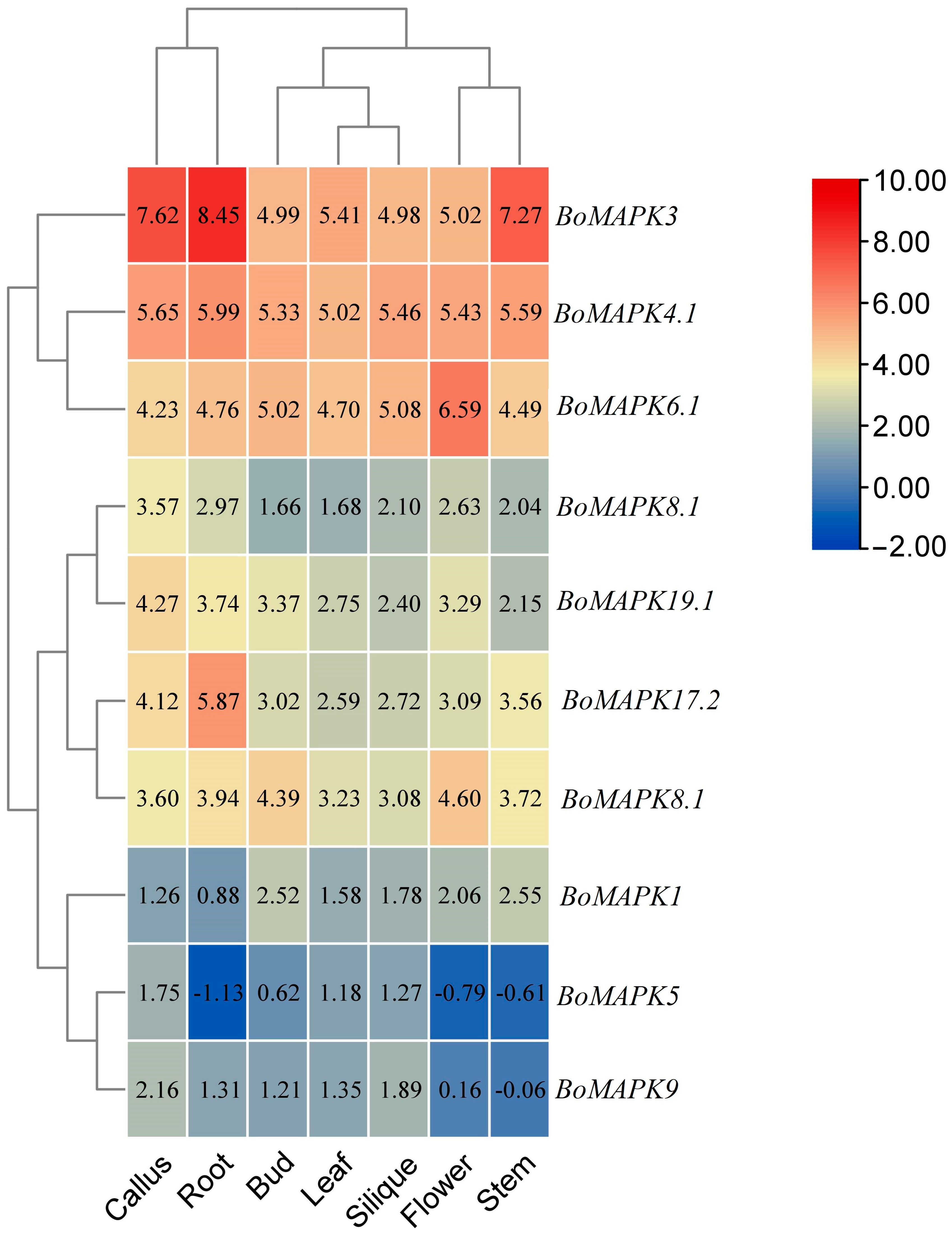
2.6. Expression Profiles of BoMAPK Genes in Different Tissues
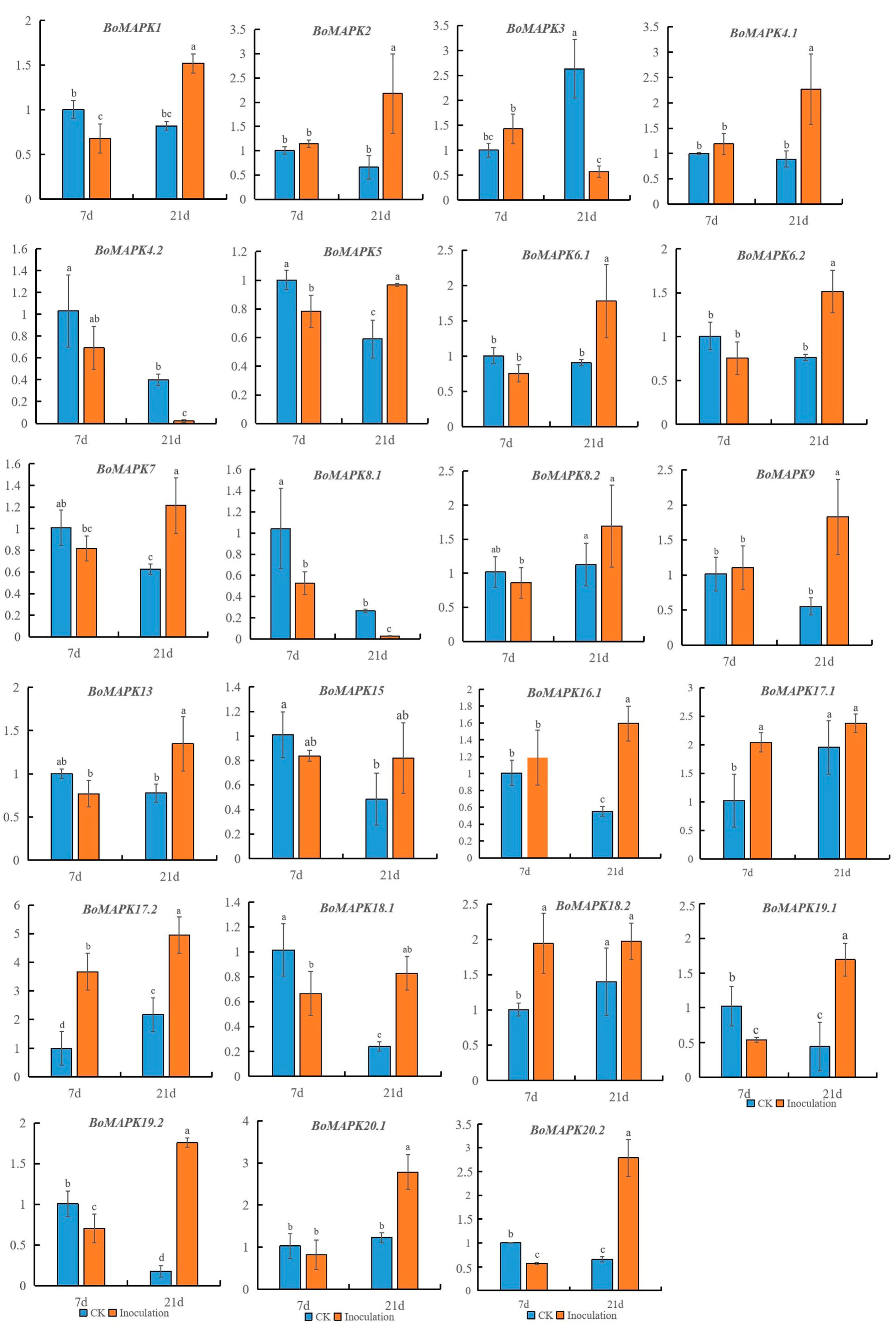
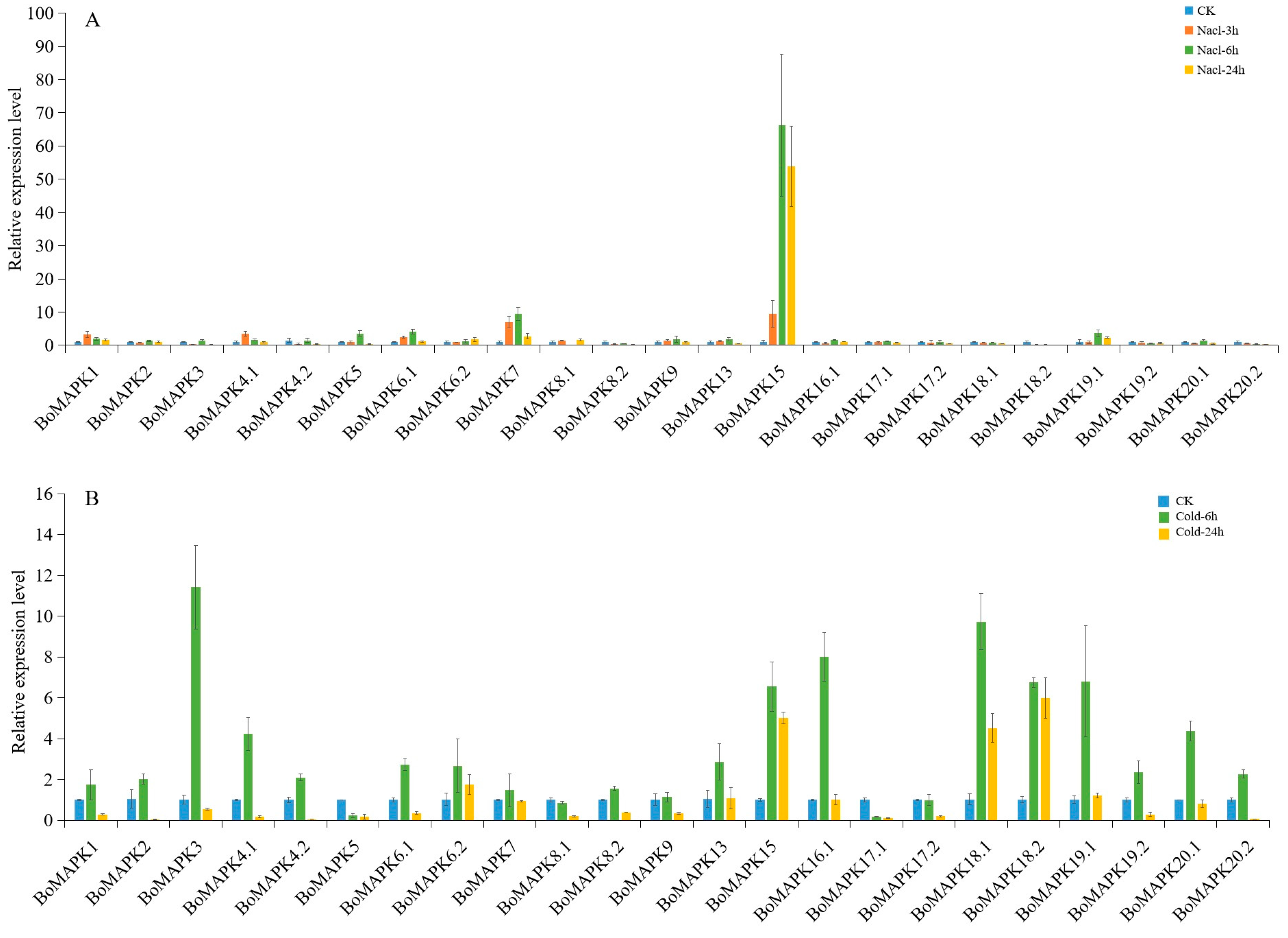
2.7. Plant Materials and Treatment Methods
2.8. RNA Extraction and RT-qPCR
2.9. Protein–protein Interaction Network Prediction
3. Results
3.1. Identification of MAPK Gene Family Members in Cabbage
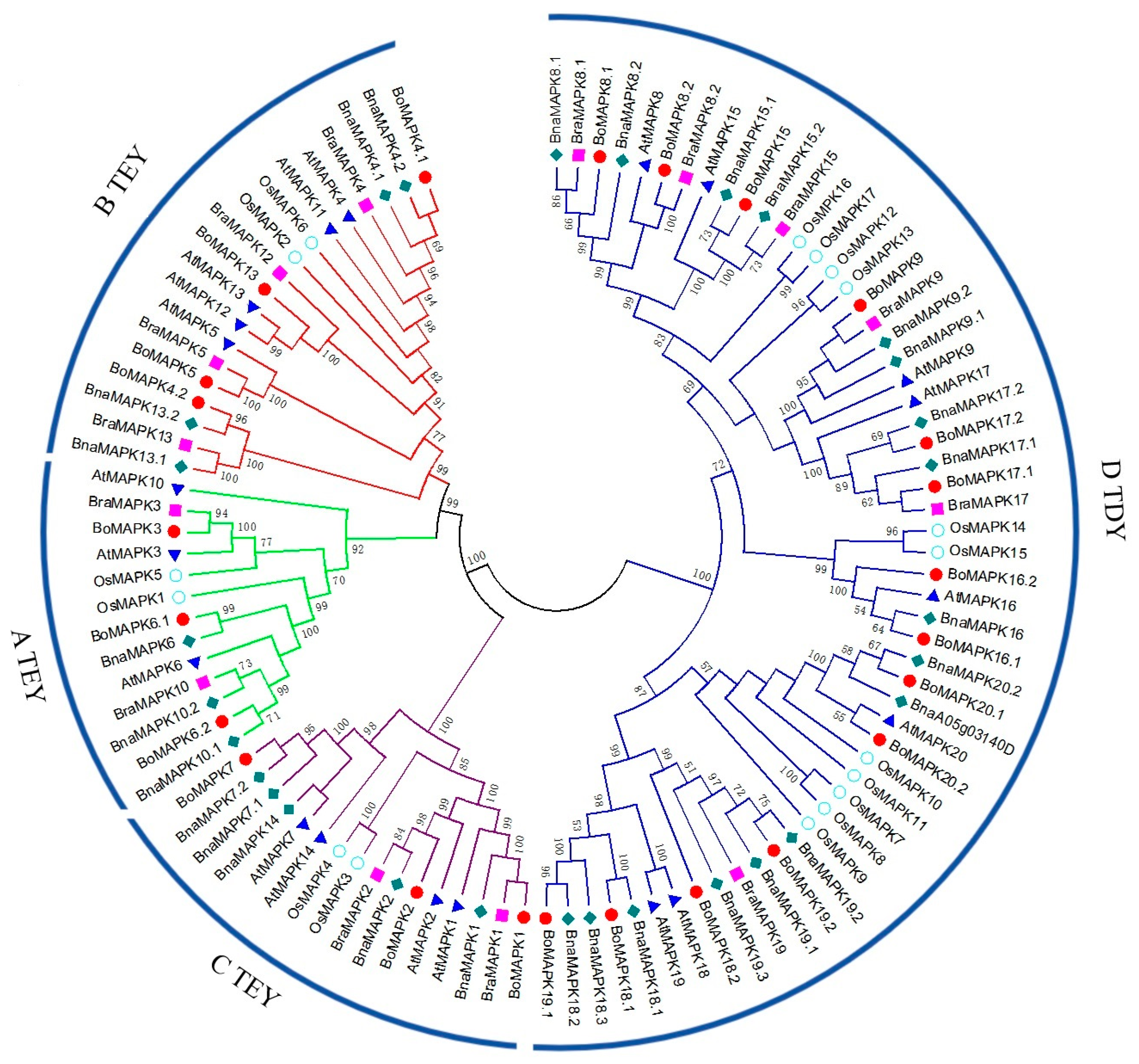
3.2. Phylogenetic Analysis of BoMAPK Gene Family
3.3. Chromosome Location and Synteny Analysis of BoMAPK Genes
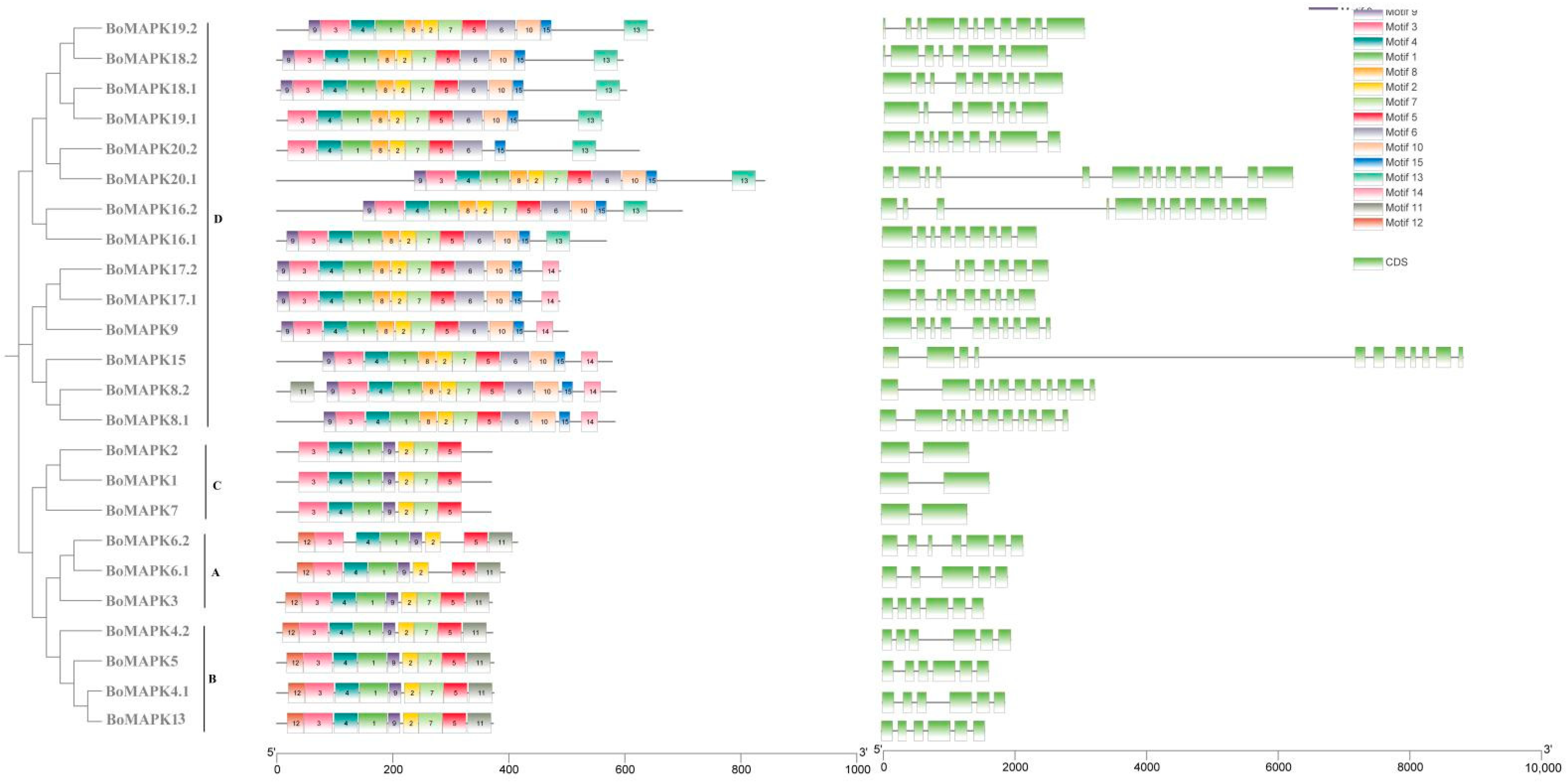
3.4. Conserved Motifs and Gene Structure Analysis of BoMAPK Gene Family
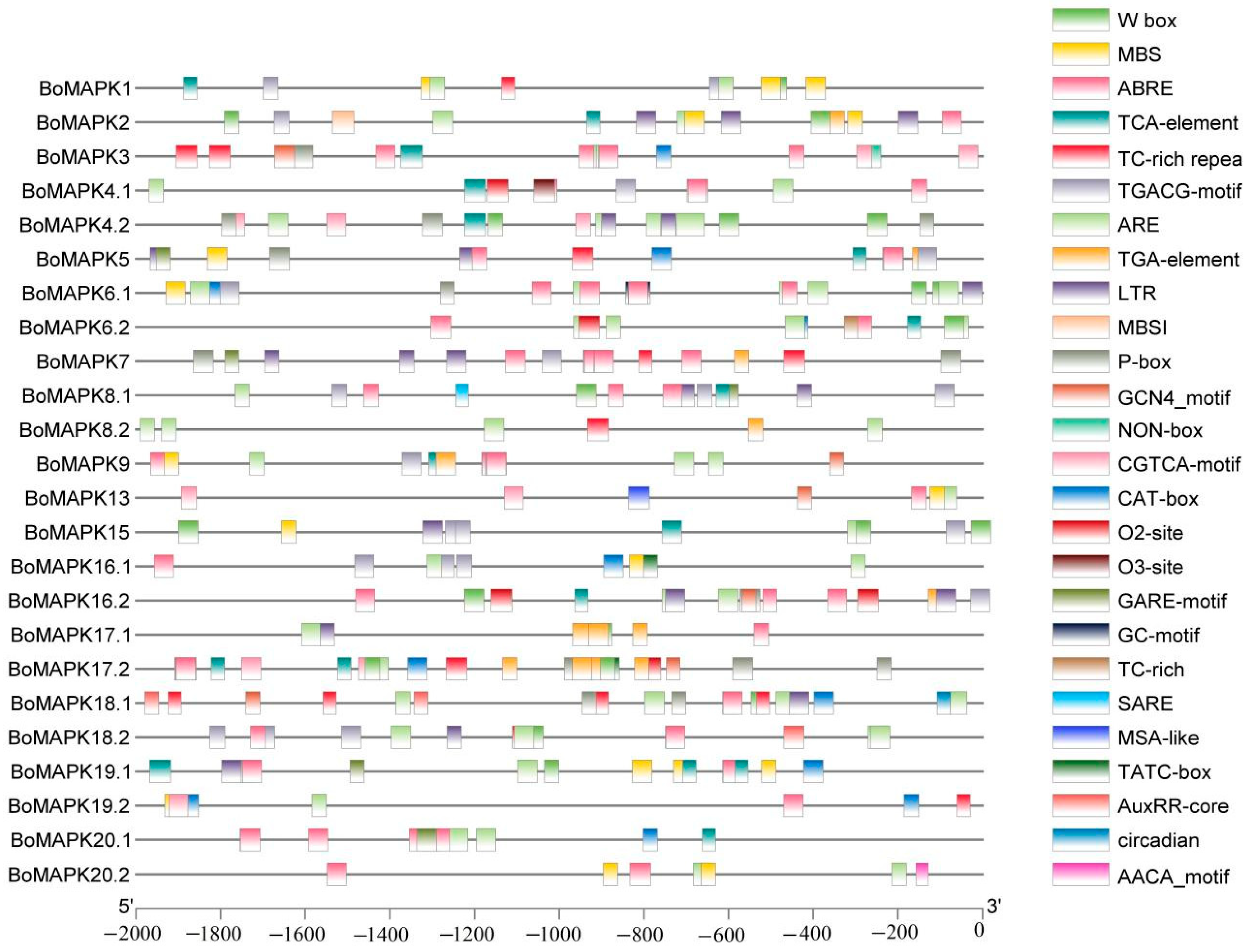
3.5. Number and Types of Cis-Acting Elements of BoMAPK Genes Promoter
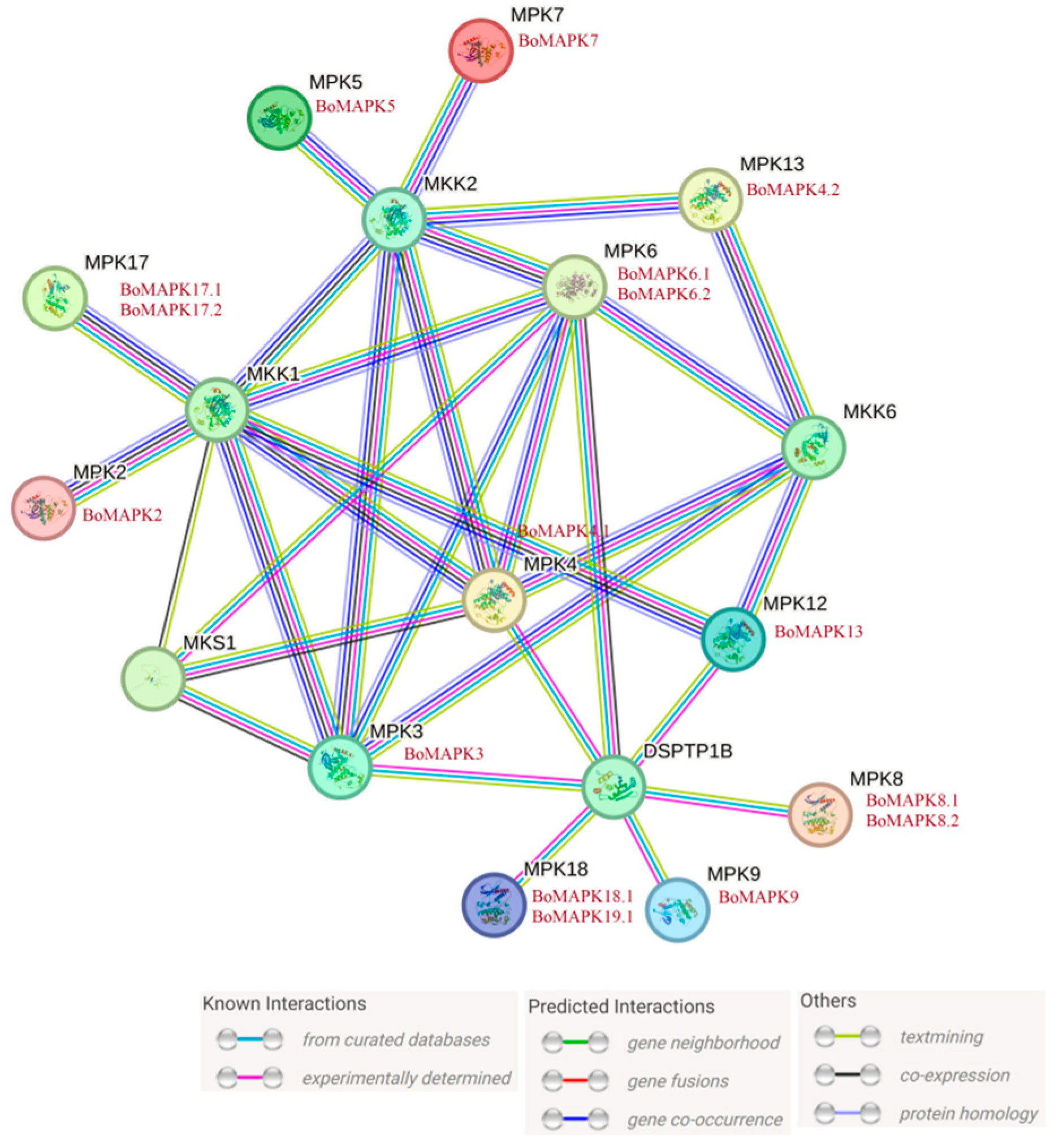
3.6. Prediction and Analysis of Protein–protein Interaction Network
3.7. Expression Analysis of BoMAPKs in Different Tissues and Development
3.8. Expression Pattern of BoMAPKs under Clubroot Disease Infection
3.9. Expression Profiles of BoMAPKs under Cold and Salt Stress Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C.; et al. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.C.; Petersen, M.; Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu. Rev. Plant Biol. 2010, 61, 621–649. [Google Scholar] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian MAP kinase signaling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, T.; Adachi, M.; Moriguchi, T.; Nishida, E. A conserved docking motif in MAP kinases common to substrates activators and regulators. Nat. Cell Biol. 2000, 2, 110–116. [Google Scholar] [CrossRef]
- Jonak, C.; Okrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Chen, J.; Wang, L.; Yuan, M. Update on the roles of rice MAPK cascades. Int. J. Mol. Sci. 2021, 22, 1679. [Google Scholar] [CrossRef]
- Liu, Y.K.; Zhang, D.; Wang, L.; Li, D.Q. Genome-Wide Analysis of Mitogen-Activated Protein Kinase Gene Family in Maize. Plant Mol. Biol. Rep. 2013, 31, 1446–1460. [Google Scholar] [CrossRef]
- Cui, L.; Yang, G.; Yan, J.; Pan, Y.; Nie, X. Genome-wide identifcation expression profles and regulatory network of MAPK cascade gene family in barley. BMC Genom. 2019, 20, 750. [Google Scholar] [CrossRef]
- Zhou, H.; Ren, S.; Han, Y.; Zhang, Q.; Qin, L.; Xing, Y. Identifcation and analysis of mitogen-activated protein kinase (MAPK) cascades in Fragaria vesca. Int. J. Mol. Sci. 2017, 18, 1766. [Google Scholar] [CrossRef]
- Meng, X.; Wang, H.; He, Y.; Liu, Y.; Walker, J.C.; Torii, K.U.; Zhang, S. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 2012, 24, 4948–4960. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Yu, X.; Xu, X.; Li, Y.; Yuan, W.; Xu, Y.; Mao, C.; Zhang, S.; Xu, J. The YDA-MKK4/MKK5-MPK3/MPK6 Cascade Functions Downstream of the RGF1-RGI Ligand-Receptor Pair in Regulating Mitotic Activity in Root Apical Meristem. Mol. Plant 2020, 13, 1608–1623. [Google Scholar] [CrossRef] [PubMed]
- Hord, C.L.; Sun, Y.J.; Pillitteri, L.J.; Torii, K.U.; Wang, H.; Zhang, S.; Ma, H. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases MPK3 and MPK6 and the ERECTA and related receptor-like kinases. Mol. Plant 2008, 1, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Brodersen, P.; Naested, H.; Andreasson, E.; Lindhart, U.; Johansen, B.; Nielsen, H.B.; Lacy, M.; Austin, M.J.; Parker, J.E.; et al. Arabidopsis map kinase 4 negatively regulates systemic acquired resistance. Cell 2000, 103, 1111–1120. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Liu, Y.; Yang, M.; Wang, L.; Liu, X.; Zhang, Y.; Chen, Q.; Li, M.; Lin, Y.; et al. MAPK5 and MAPK10 overexpression influences strawberry fruit ripening, antioxidant capacity and resistance to Botrytis cinerea. Planta 2021, 255, 19. [Google Scholar] [CrossRef]
- Mao, W.; Han, Y.; Chen, Y.; Sun, M.; Feng, Q.; Li, L.; Liu, L.; Zhang, K.; Wei, L.; Han, Z.; et al. Low temperature inhibits anthocyanin accumulation in strawberry fruit by activating FvMAPK3-induced phosphorylation of FvMYB10 and degradation of Chalcone Synthase 1. Plant Cell 2022, 34, 1226–1249. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, H.; Sun, L.; Wu, W.; Li, C.; Wu, Q. Genome-wide identifcation of MAPK gene family members in Fagopyrum tataricum and their expression during development and stress responses. BMC Genom. 2022, 23, 96. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Li, F.; Liu, H.; Yang, W.; Chong, K.; Xu, Y. OsMAPK3 Phosphorylates OsbHLH002/OsICE1 and Inhibits Its Ubiquitination to Activate OsTPP1 and Enhances Rice Chilling Tolerance. Dev. Cell 2017, 43, 731–743. [Google Scholar] [CrossRef]
- Jia, M.; Luo, N.; Meng, X.; Song, X.; Jing, Y.; Kou, L.; Liu, G.; Huang, X.; Wang, Y.; Li, J.; et al. OsMPK4 promotes phosphorylation and degradation of IPA1 in response to salt stress to confer salt tolerance in rice. J. Genet. Genom. 2022, 49, 766–775. [Google Scholar] [CrossRef]
- Teige, M.; Scheikl, E.; Eulgem, T.; Dóczi, R.; Ichimura, K.; Shinozaki, K.; Dangl, J.L.; Hirt, H. The MKK2 pathway mediates cold and salt stress signaling in Arabidopsis. Mol. Cell 2004, 15, 141–152. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Si, T.; Hsu, C.C.; Wang, L.; Zayed, O.; Yu, Z.; Zhu, Y.; Dong, J.; Tao, W.A.; et al. MAP kinase cascades regulate the cold response by modulating ICE1 protein stability. Dev. Cell 2017, 43, 618–629.e5. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liu, J.; Bi, D.; Zhang, Z.; Cheng, F.; Chen, S.; Zhang, Y. MEKK1 MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res. 2008, 18, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Qu, N.; Gao, M.; Zhang, Z.; Ding, X.; Yang, F.; Li, Y.; Dong, O.X.; Chen, S.; Li, X.; et al. The MEKK1-MKK1/MKK2-MPK4 kinase cascade negatively regulates immunity mediated by a mitogen-activated protein kinase kinase kinase in Arabidopsis. Plant Cell 2012, 24, 2225–2236. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez-Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Liu, Y.; Yang, K.Y.; Han, L.; Mao, G.; Glazebrook, J.; Zhang, S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 5638–5643. [Google Scholar] [CrossRef]
- Wang, H.; Ngwenyama, N.; Liu, Y.; Walker, J.C.; Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 2007, 19, 63–73. [Google Scholar] [CrossRef]
- Xu, R.; Duan, P.G.; Yu, H.Y.; Zhou, Z.K.; Zhang, B.L.; Wang, R.C.; Li, J.; Zhang, G.Z.; Zhuang, S.S.; Lyu, J.; et al. Control of Grain Size and Weight by the OsMKKK10-OsMKK4-OsMAPK6 Signaling Pathway in Rice. Mol. Plant 2018, 11, 860–873. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Chen, K.; Dong, N.Q.; Shi, C.L.; Ye, W.W.; Gao, J.P.; Shan, J.X.; Lin, H.X. GRAIN SIZE AND NUMBER1 Negatively Regulates the OsMKKK10-OsMKK4-OsMPK6 Cascade to Coordinate the Trade-off between Grain Number per Panicle and Grain Size in Rice. Plant Cell 2018, 30, 871–888. [Google Scholar] [CrossRef]
- Javed, M.A.; Schwelm, A.; Zamani-Noor, N.; Salih, R.; Silvestre Vañó, M.; Wu, J.; González García, M.; Heick, T.M.; Luo, C.; Prakash, P.; et al. The clubroot pathogen Plasmodiophora brassicae: A profile update. Mol. Plant Pathol. 2023, 24, 89–106. [Google Scholar] [CrossRef]
- Chen, H.; Wang, T.; He, X.; Cai, X.; Lin, R.; Liang, J.; Wu, J.; King, G.; Wang, X. BRAD V3.0: An upgraded Brassicaceae database. Nucleic Acids Res. 2022, 50, D1432–D1441. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Wu, J.; Liang, J.; Lin, R.; Zhang, K.; Cheng, F.; Wang, X. Improved Brassica oleracea JZS assembly reveals significant changing of LTR-RT dynamics in different morphotypes. Theor. Appl. Genet. 2022, 133, 3187–3199. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated Profile HMM Searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [PubMed]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An Important Software for Molecular Biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Li, H.; Wen, X.; Wei, M.; Huang, X.; Dai, S.; Ruan, L.; Yu, Y. Genome-Wide Identification, Characterization, and Expression Pattern of MYB Gene Family in Melastoma candidum. Horticulturae 2023, 9, 708. [Google Scholar] [CrossRef]
- Yu, J.; Tehrim, S.; Zhang, F.; Tong, C.; Huang, J.; Cheng, X.; Dong, C.; Zhou, Y.; Qin, R.; Hua, W.; et al. Genome-wide comparative analysis of NBS-encoding genes between Brassica species and Arabidopsis thaliana. BMC Genom. 2014, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, Y.; Yang, X.; Tong, C.; Edwards, D.; Parkin, I.A.; Zhao, M.; Ma, J.; Yu, J.; Huang, S.; et al. The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat. Commun. 2014, 5, 3930. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.; Liu, Y.; Cao, X.; Liu, Z.; Li, M. MAPK Gene Family in Lactuca sativa: Genome-Wide Identification, Regulatory Network, and Expression Patterns in Stem Development and Stress Responses. Horticulturae 2022, 8, 1087. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Yu, F.; Tang, J.; Shan, X.; Bao, K.; Yu, L.; Wang, H.; Fei, Z.; Li, J. Genome-wide characterization and expression profiling of SWEET genes in cabbage (Brassica oleracea var. capitata L.) reveal their roles in chilling and clubroot disease responses. BMC Genom. 2019, 20, 93. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wan, Y.; Meng, X.; Zhang, X.; Yao, M.; Miu, W.; Zhu, D.; Yuan, D.; Lu, K.; Li, J.; et al. Genome-Wide Identification and Analysis of MKK and MAPK Gene Families in Brassica Species and Response to Stress in Brassica napus. Int. J. Mol. Sci. 2021, 22, 544. [Google Scholar] [CrossRef] [PubMed]
- Cannon, S.B.; Mitra, A.; Baumgarten, A.; Young, N.D.; May, G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004, 4, 10. [Google Scholar] [CrossRef]
- Zhang, J.Z. Evolution by gene duplication: An update. Trends Ecol. Evol. 2003, 18, 292–298. [Google Scholar] [CrossRef]
- Guan, Y.; Meng, X.; Khanna, R.; LaMontagne, E.; Liu, Y.; Zhang, S. Phosphorylation of a WRKY transcription factor by MAPKs is required for pollen development and function in Arabidopsis. PLoS Genet. 2014, 10, e1004384. [Google Scholar] [CrossRef]
- Zheng, Y.; Deng, X.; Qu, A.; Zhang, M.; Tao, Y.; Yang, L.; Liu, Y.; Xu, J.; Zhang, S. Regulation of pollen lipid body biogenesis by MAP kinases and downstream WRKY transcription factors in Arabidopsis. PLoS Genet. 2018, 14, e1007880. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, R.; He, J.; Zhou, Z.; Wang, J.; Xiong, Y.; Xu, T. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc. Natl. Acad. Sci. USA 2019, 116, 21285–21290. [Google Scholar] [CrossRef]
- Lee, H.; Jun, Y.S.; Cha, O.K.; Sheen, J. Mitogen-activated protein kinases MPK3 and MPK6 are required for stem cell maintenance in the Arabidopsis shoot apical meristem. Plant Cell Rep. 2019, 38, 311–319. [Google Scholar] [CrossRef]
- Meng, X.; Xu, J.; He, Y.; Yang, K.Y.; Mordorski, B.; Liu, Y.; Zhang, S. Phosphorylation of an ERF transcription factor by Arabidopsis MPK3/MPK6 regulates plant defense gene induction and fungal resistance. Plant Cell 2013, 25, 1126–1142. [Google Scholar] [CrossRef]
- Wang, Z.; Mao, H.; Dong, C.; Ji, R.; Cai, L.; Fu, H.; Liu, S. Overexpression of Brassica napus MPK4 enhances resistance to Sclerotinia sclerotiorum in oilseed rape. Mol. Plant Microbe Interac. 2009, 22, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3- and MPK6-Mediated ICE1 Phosphorylation Negatively Regulates ICE1 Stability and Freezing Tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e4. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, J.; Wang, F.; Xie, C.; Lv, B.; Yu, Z.; Dai, S.; Liu, X.; Xia, G.; Tian, H.; et al. MPK3/6-induced degradation of ARR1/10/12 promotes salt tolerance in Arabidopsis. EMBO Rep. 2021, 22, e52457. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Gene ID | Number of AA | MW (Da) | pI | AI | GRAVY | Localization Prediction |
|---|---|---|---|---|---|---|---|
| BoMAPK1 | BolC05g008000.2J | 369 | 42,485.25 | 6.67 | 98.27 | −0.248 | Cytoplasm |
| BoMAPK2 | BolC01g035020.2J | 370 | 42,488.03 | 6.23 | 98.78 | −0.223 | Cytoplasm |
| BoMAPK3 | BolC03g065400.2J | 370 | 42,592.77 | 5.70 | 92.00 | −0.312 | Cytoskeleton |
| BoMAPK4.1 | BolC03g035260.2J | 373 | 42,513.48 | 5.85 | 89.14 | −0.332 | Cytoplasm |
| BoMAPK4.2 | BolC08g057380.2J | 371 | 43,151.41 | 5.10 | 89.60 | −0.371 | Cytoplasm |
| BoMAPK5 | BolC09g032170.2J | 373 | 42,809.88 | 5.57 | 91.98 | −0.302 | Cytoplasm |
| BoMAPK6.1 | BolC04g005090.2J | 392 | 44,850.29 | 5.27 | 91.35 | −0.299 | Nucleus |
| BoMAPK6.2 | BolC03g026650.2J | 414 | 47,076.99 | 5.35 | 93.12 | −0.220 | Chloroplast |
| BoMAPK7 | BolC09g012900.2J | 368 | 42,234.85 | 7.18 | 94.29 | −0.238 | Cytoplasm |
| BoMAPK8.1 | BolC08g051030.2J | 582 | 65,637.18 | 6.57 | 77.42 | −0.554 | Cytoplasm |
| BoMAPK8.2 | BolC05g015790.2J | 584 | 65,863.30 | 6.08 | 78.51 | −0.527 | Chloroplast |
| BoMAPK9 | BolC05g047830.2J | 501 | 57,381.46 | 8.12 | 80.06 | −0.479 | Cytoplasm |
| BoMAPK13 | BolC04g067890.2J | 372 | 42,589.85 | 8.03 | 88.79 | −0.305 | Cytoplasm |
| BoMAPK15 | BolC06g032120.2J | 577 | 65,327.03 | 7.29 | 80.12 | −0.571 | Cytoplasm |
| BoMAPK16.1 | BolC09g053590.2J | 567 | 64,784.07 | 8.81 | 77.23 | −0.517 | Mitochondrion |
| BoMAPK16.2 | BolC03g009910.2J | 698 | 79,579.79 | 9.27 | 76.98 | −0.549 | Chloroplast |
| BoMAPK17.1 | BolC07g031210.2J | 487 | 55,627.59 | 6.51 | 81.25 | −0.419 | Cytoplasm |
| BoMAPK17.2 | BolC02g049250.2J | 488 | 55,755.70 | 6.64 | 80.68 | −0.434 | Cytoplasm |
| BoMAPK18.1 | BolC06g008540.2J | 602 | 67,838.36 | 9.20 | 76.64 | −0.446 | Nucleus |
| BoMAPK18.2 | BolC05g051570.2J | 596 | 67,131.79 | 9.26 | 78.05 | −0.476 | Nucleus |
| BoMAPK19.1 | BolC06g014820.2J | 561 | 63,486.84 | 9.27 | 83.28 | −0.377 | Chloroplast |
| BoMAPK19.2 | BolC01g048030.2J | 648 | 72,685.31 | 9.48 | 78.12 | −0.465 | Chloroplast |
| BoMAPK20.1 | BolC04g004430.2J | 840 | 95,445.32 | 9.00 | 76.42 | −0.460 | Nucleus |
| BoMAPK20.2 | BolC03g026050.2J | 624 | 70,463.18 | 8.58 | 81.97 | −0.450 | Cytoplasm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Chen, J.; Zhu, X.; Tai, X.; Bo, T. In Silico Analysis of the MAPK Gene Family in Cabbage and Its Expression during Development and Stress Response. Horticulturae 2023, 9, 1119. https://doi.org/10.3390/horticulturae9101119
Wang M, Chen J, Zhu X, Tai X, Bo T. In Silico Analysis of the MAPK Gene Family in Cabbage and Its Expression during Development and Stress Response. Horticulturae. 2023; 9(10):1119. https://doi.org/10.3390/horticulturae9101119
Chicago/Turabian StyleWang, Min, Jinxiu Chen, Xiaowei Zhu, Xiang Tai, and Tianyue Bo. 2023. "In Silico Analysis of the MAPK Gene Family in Cabbage and Its Expression during Development and Stress Response" Horticulturae 9, no. 10: 1119. https://doi.org/10.3390/horticulturae9101119
APA StyleWang, M., Chen, J., Zhu, X., Tai, X., & Bo, T. (2023). In Silico Analysis of the MAPK Gene Family in Cabbage and Its Expression during Development and Stress Response. Horticulturae, 9(10), 1119. https://doi.org/10.3390/horticulturae9101119





