Molecular Variation in Some Taxa of Genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Taxon Sampling
2.2. DNA Extraction
2.3. PCR Assay, Purification, and Sequencing
2.4. Phylogenetic Tree Development
2.5. Scoring and Statistical Analysis
3. Results
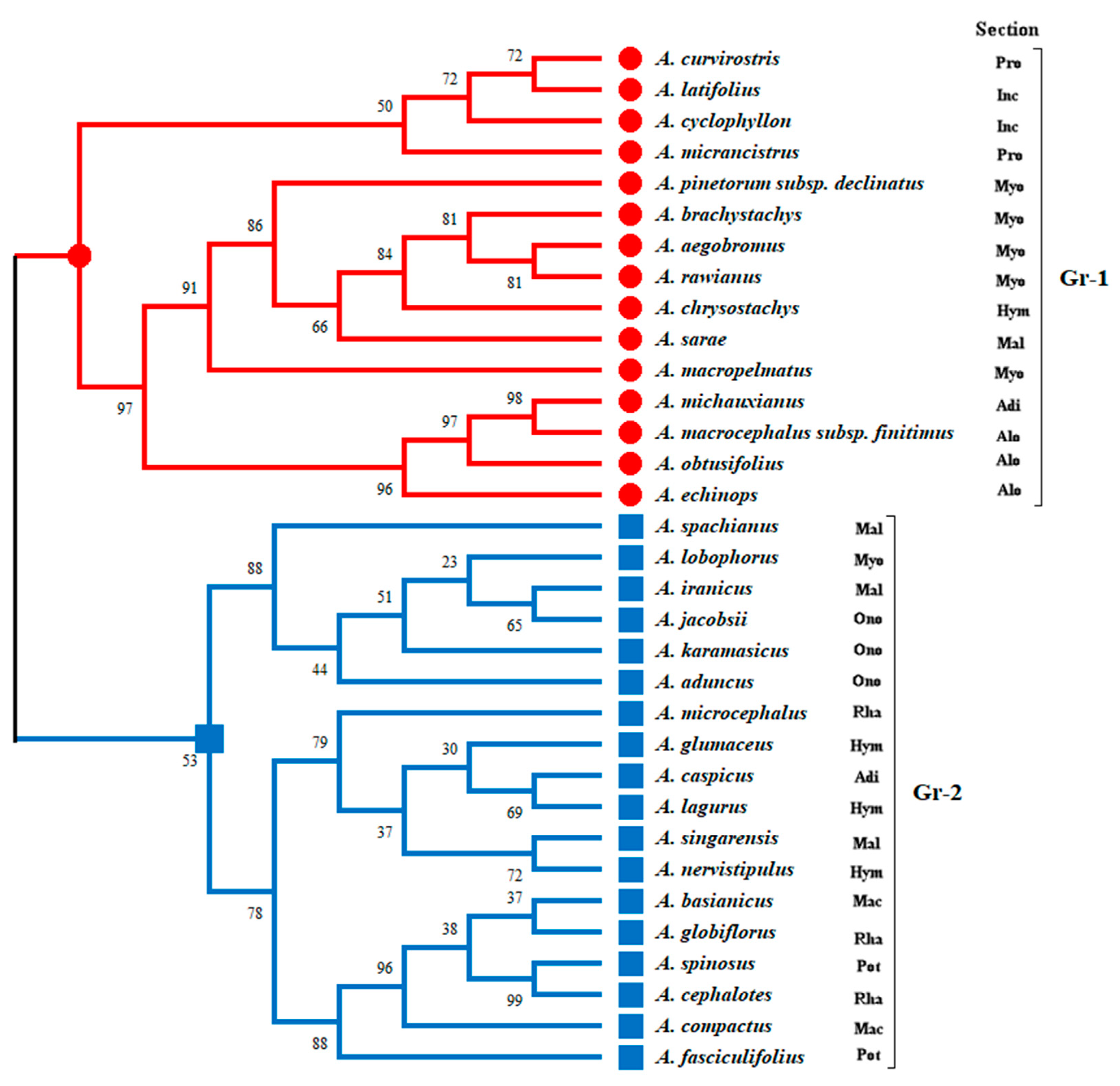
3.1. Alignment of Sequences and Phylogenetic Reconstruction of the Genus Astragalus L.
3.2. Importance of Using ISSR Markers in Detecting Genetic Diversity
3.3. Polymorphism Parameters of CDDP Markers
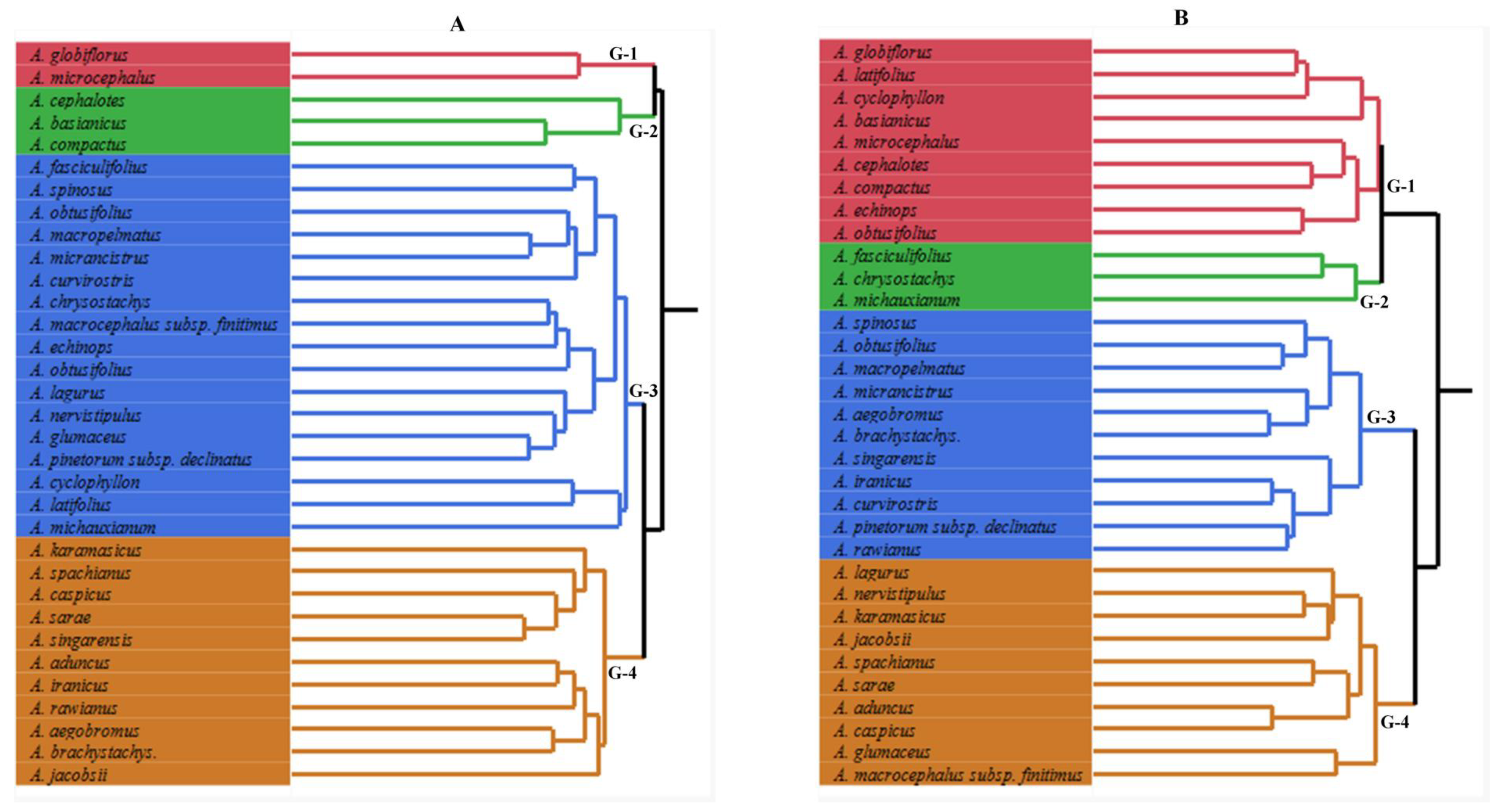
3.4. Cluster Analysis of Astragalus L. Taxa
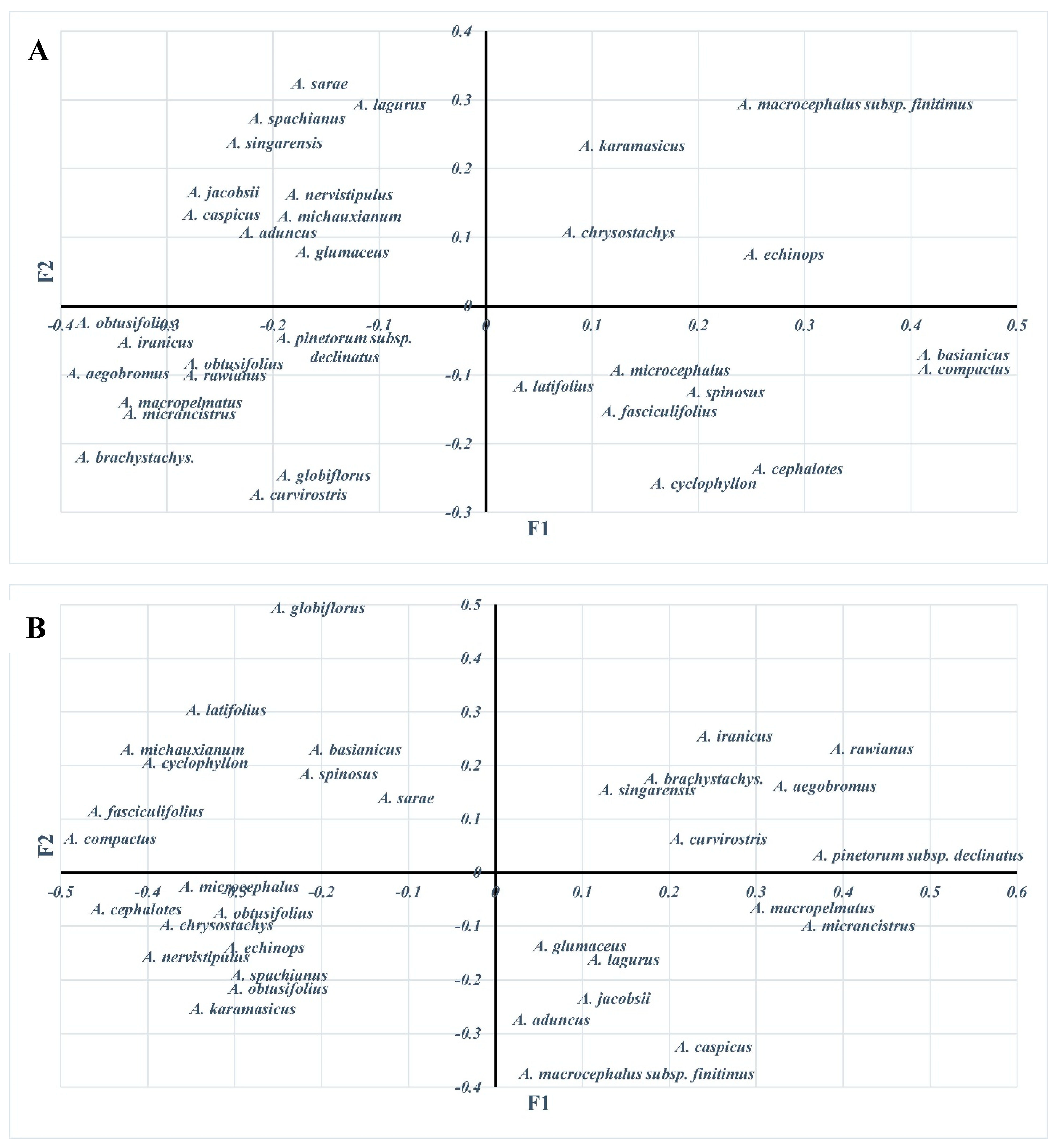
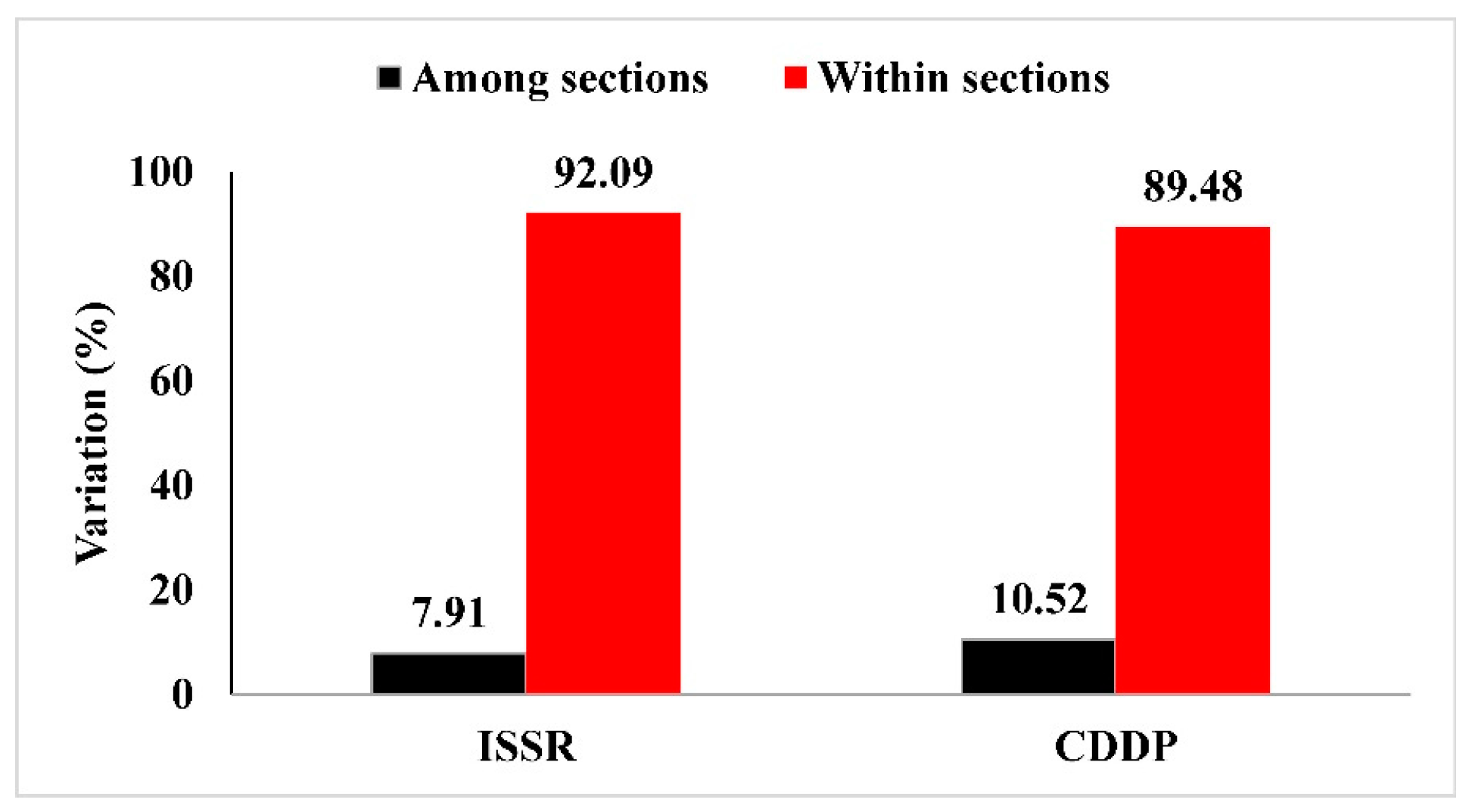
3.5. Molecular Variation Analysis of Astragalus L. Taxa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A review of recent research progress on the astragalus genus. Molecules 2014, 19, 18850–18880. [Google Scholar]
- Zarre, S.; Azani, N. Perspectives in taxonomy and phylogeny of the genus Astragalus (Fabaceae): A review. Prog. Biol. Sci. 2013, 3, 1–6. [Google Scholar]
- Khal, L.; Al-Anbari, A.; AL-Hadeethi, M.; Abdulrazaq, R. Phylogenetic diversity of Trifolium L. species in Iraq. J. Plant Prod. 2020, 11, 1041–1044. [Google Scholar] [CrossRef]
- Lymberakis, P.; Poulakakis, N. Three continents claiming an Archipelago: The evolution of Aegean’s Herpetofaunal diversity. Diversity 2010, 2, 233–255. [Google Scholar] [CrossRef]
- Su, C.; Duan, L.; Liu, P.; Liu, J.; Chang, Z.; Wen, J. Chloroplast phylogenomics and character evolution of eastern Asian Astragalus (Leguminosae): Tackling the phylogenetic structure of the largest genus of flowering plants in Asia. Mol. Phylogenet. Evol. 2021, 156, 107025. [Google Scholar]
- Aguilar, R.; Cristóbal-Pérez, E.J.; Balvino-Olvera, F.J.; de Jesús Aguilar-Aguilar, M.; Aguirre-Acosta, N.; Ashworth, L.; Lobo, J.A.; Martén-Rodríguez, S.; Fuchs, E.J.; Sanchez-Montoya, G.; et al. Habitat fragmentation reduces plant progeny quality: A global synthesis. Ecol. Lett. 2019, 22, 1163–1173. [Google Scholar]
- Raven, P.H.; Wagner, D.L. Agricultural intensification and climate change are rapidly decreasing insect biodiversity. Proc. Natl Acad. Sci. USA 2021, 118, e2002548117. [Google Scholar]
- Dash, S.S.; Mao, A.A. Status, Issues and Challenges of Biodiversity: Higher Plants. In Biodiversity in India; Kaur, S., Ed.; Springer: Singapore, 2022; pp. 25–44. ISBN 978-981-16-9776-0. [Google Scholar]
- Zhang, D.; Jiang, B. Species identification in complex groups of medicinal plants based on DNA barcoding: A case study on Astragalus spp. (Fabaceae) from southwest China. Conser. Genet. Res. 2020, 12, 469–478. [Google Scholar]
- Scherson, R.A.; Vidal, R.; Sanderson, M.J. Phylogeny, biogeography, and rates of diversification of New World Astragalus (Leguminosae) with an emphasis on South American radiations. Am. J. Bot. 2008, 95, 1030–1039. [Google Scholar]
- Azani, N.; Bruneau, A.; Wojciechowski, M.F.; Zarre, S. Miocene climate change as a driving force for multiple origins of annual species in Astragalus (Fabaceae, Papilionoideae). Mol. Phylogenet. Evol. 2019, 137, 210–221. [Google Scholar]
- Yao, H.-B.; Gao, M.-R.; Yu, S.-H. Small organic molecule templating synthesis of organic-inorganic hybrid materials: Their nanostructures and properties. Nanoscale 2010, 2, 323–334. [Google Scholar]
- Monsefi, M.; Abedian, M.; Azarbahram, Z.; Ashraf, M.J. Salvia officinalis L. induces alveolar bud growing in adult female rat mammary glands. Avicenna J. Phytomed. 2015, 5, 560–567. [Google Scholar] [PubMed]
- Khal, L. Molecular taxonomy of some species of genus Salvia L. (Lamiaceae) in Kurdistan region, Iraq. J. Plant Prod. 2020, 11, 967–974. [Google Scholar]
- Essadki, M.; Ouazzani, N.; Lumaret, R.; Moumni, M. ISSR variation in olive-tree cultivars from Morocco and other western countries of the mediterranean Basin. Genet. Resour. Crop Evol. 2006, 53, 475–482. [Google Scholar]
- Aliabadi, F.; Bagheri, A.; Abbasi, S.; Saeidi, H.; Blattner, F.R. High genetic diversity in an endemic and vulnerable species: Evidence from Astragalus cyclophyllon (Fabaceae) in Iran. Genet. Resour. Crop Evol. 2023, 70, 1999–2008. [Google Scholar]
- Bagheri, A.; Abbasi, S.; Mahmoodi, M.; Roofigar, A.A.; Blattner, F.R. Genetic structure and conservation status of Astragalus subrecognitus (Fabaceae): A very rare and narrow endemic species. Plant Ecol. Evol. 2020, 153, 101–107. [Google Scholar]
- Ahmed, A.A.; Qadir, S.A.; Tahir, N.A. Genetic Variation and Structure Analysis of Iraqi Valonia Oak (Quercus aegilops L.) Populations Using Conserved DNA-Derived Polymorphism and Inter-Simple Sequence Repeats Markers. Plant Mol. Biol. Rep. 2022, 41, 1–14. [Google Scholar]
- Aziz, R.R.; Tahir, N.A. Genetic diversity and structure analysis of melon (Cucumis melo L.) genotypes using URP, SRAP, and CDDP markers. Genet. Resour. Crop Evol. 2022, 70, 799–813. [Google Scholar]
- Rasul, K.S.; Grundler, F.M.W.; Abdul-razzak Tahir, N. Genetic diversity and population structure assessment of Iraqi tomato accessions using fruit characteristics and molecular markers. Hortic. Environ. Biotechnol. 2022, 63, 523–538. [Google Scholar]
- Gedik, O.; kürşad, M.; Kiran, Y. Karyological studies on nine Astragalus L. taxa in Turkey. KUS J. Agric. Nat. 2019, 22, 35–44. [Google Scholar]
- Gül, E.; Dölarslan, M. Distribution and importance of some endemic Astragalus L. species in semi-arid environmentally sensitive areas: A case study from northern Turkey. Cerne 2021, 27, e-102559. [Google Scholar]
- Karaman Erkul, S.; Duman, H.; Ateş, M.A. Astragalus oksutdagensis (Fabaceae), a new species from Turkey. Nord. J. Bot. 2022, 2022, 27. [Google Scholar]
- Akhavan Roofigar, A.; Jalili, A. Chromosome numbers report of five endemic Astragalus L. (Fabaceae) species of Iran. Iranian J. Bot. 2021, 27, 177–181. [Google Scholar]
- Rechinger, K.H. Flora Iranica: Flora des Iranischen Hochlandes und der Umrahmenden Gebirge: Persien, Afghanistan, Teile von West-Pakistan, Nord-Iraq, Azerbaidjan, Turkmenistan; Akademische Druck- u. Verlagsanstalt: Graz, Austria, 1963. [Google Scholar]
- Townsend, C.C.; Guest, E. Flora of Iraq; Ministry of Agriculture of the Republic of Iraq: Baghdad, Iraq, 1966.
- Hsiao, C.; Chatterton, N.J.; Asay, K.H.; Jensen, K.B. Molecular phylogeny of the Pooideae (Poaceae) based on nuclear rDNA (ITS) sequences. Theor. Appl. Genet. 1995, 90, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Lateef, D.; Rasul, K.; Rahim, D.; Mustafa, K.; Sleman, S.; Mirza, A.; Aziz, R. Assessment of genetic variation and population structure in Iraqi barley accessions using ISSR, CDDP, and SCoT markers. Czech J. Genet. Plant Breed. 2023, 59, 148–159. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Gharib, S.J.; Abdullah, S.K.; Richardson, M.D. Auxarthron alboluteum related to non-dermatophytic toenail infection in Kurdistan region, Iraq: A case report. Med. Mycol. Case Rep. 2019, 26, 53–56. [Google Scholar] [CrossRef]
- Lopez-Alvarado, J.; Farris, E. Ecology and Evolution of Plants in the Mediterranean Basin: Perspectives and Challenges. Plants 2022, 11, 1584. [Google Scholar] [CrossRef]
- Alnuaimi, A.D.; Wiesenfeld, D.; O’Brien-Simpson, N.M.; Reynolds, E.C.; Peng, B.; McCullough, M.J. The development and validation of a rapid genetic method for species identification and genotyping of medically important fungal pathogens using high-resolution melting curve analysis. Mol. Oral Microbiol. 2014, 29, 117–130. [Google Scholar]
- Yang, M.; Abdalrahman, H.; Sonia, U.; Mohammed, A.I.; Vestine, U.; Wang, M.; Ebadi, A.G.; Toughani, M. The application of DNA molecular markers in the study of Codonopsis species genetic variation, a review. Cell. Mol. Biol. 2020, 66, 23–30. [Google Scholar] [CrossRef]
- Dizkirici, A.; Ekici, M.; Kaya, Z. Comparative molecular phylogenetics of Astragalus L. sections from Turkey with New World Astragalus species using nrDNA ITS sequences. Plant Syst. Evol. 2014, 300, 163–175. [Google Scholar]
- Sheikh Akbari Mehr, R.; Saidi, A.; Kazempour Osaloo, S.; Maassoumi, A.A. Phylogeny of Astragalus section Dissitiflori based on nrDNA ITS and morphological data in Iran. Iran J. Bot. 2012, 18, 1–9. [Google Scholar]
- Sanderson, M.J.; Wojciechowski, M.F. Improved bootstrap confidence limits in large-scale phylogenies, with an example from Neo-Astragalus (Leguminosae). Syst. Biol. 2000, 49, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowski, M.F. Astragalus (Fabaceae): A molecular phylogenetic perspective. Brittonia 2005, 57, 382–396. [Google Scholar] [CrossRef]
- Akhavan Roofigar, A.; Maassoumi, A.A. A new species of Astragalus section Dissitiflori (Fabaceae) from Iran; evidence from morphological and molecular data. Iranian J. Bot. 2020, 26, 6–15. [Google Scholar] [CrossRef]
- Bidarlord, M.; Ghahremaninejad, F.; Maassoumi, A.A. A new species of the genus Astragalus (Leguminosae) from Northwest Iran. Phytotaxa 2016, 252, 280. [Google Scholar] [CrossRef]
- Osaloo, S.K.; Maassoumi, A.A.; Murakami, N. Molecular systematics of the Old World Astragalus (Fabaceae) as inferred from nrDNA ITS sequence data. Brittonia 2005, 57, 367–381. [Google Scholar] [CrossRef]
- Ranjbar, M.; Mahmoudian, B. An overview on cytogenetics of the genus Astragalus subgenus Hypoglottis (Fabaceae). Caryologia 2015, 68, 109–124. [Google Scholar] [CrossRef][Green Version]
- Milligan, B.; Leebens-Mack, J.; Strand, A. Conservation genetics: Beyond the maintenance of marker diversity. Mol. Ecol. 1994, 3, 423–435. [Google Scholar] [CrossRef]
- Kushanov, F.N.; Turaev, O.S.; Ernazarova, D.K.; Gapparov, B.M.; Oripova, B.B.; Kudratova, M.K.; Rafieva, F.U.; Khalikov, K.K.; Erjigitov, D.S.; Khidirov, M.T.; et al. Genetic diversity, QTL mapping, and marker-assisted selection technology in cotton (Gossypium spp.). Front. Plant Sci. 2021, 12, 779386. [Google Scholar]
- Abd El-Ghani, M.M.; El-Sayed, A.S.A.; Moubarak, A.; Rashad, R.; Nosier, H.; Khattab, A. Biosystematic study on some Egyptian species of Astragalus L. (Fabaceae). Agriculture 2021, 11, 125. [Google Scholar] [CrossRef]
- Amom, T.; Nongdam, P. The use of molecular marker methods in plants: A review. Int. J. Curr. Res. Rev. 2017, 9, 1–7. [Google Scholar]
- Lateef, D.D. DNA marker technologies in plants and applications for crop improvements. J. Biosci. Med. 2015, 3, 7–18. [Google Scholar] [CrossRef]
- Oluyinka Christopher, A. Comparative analyses of diversity and similarity indices of west bank forest and block a forest of the International Institute of Tropical Agriculture (IITA) Ibadan, Oyo State, Nigeria. Int. J. For. Res. 2020, 2020, 4865845. [Google Scholar] [CrossRef]
- Szabo, K.; Pamfil, D.; Bădărău, A.S.; Hârţa, M. Assessment of genetic diversity and population structure of the endangered astragalus exscapus subsp. Transsilvanicus through DNA-based molecular markers. Plants 2021, 10, 2732. [Google Scholar] [CrossRef]
- Eltaher, S.; Sallam, A.; Belamkar, V.; Emara, H.A.; Nower, A.A.; Salem, K.F.M.; Poland, J.; Baenziger, P.S. Genetic diversity and population structure of F3: 6 Nebraska winter wheat genotypes using genotyping-by-sequencing. Front. Genet. 2018, 9, 76. [Google Scholar] [CrossRef]
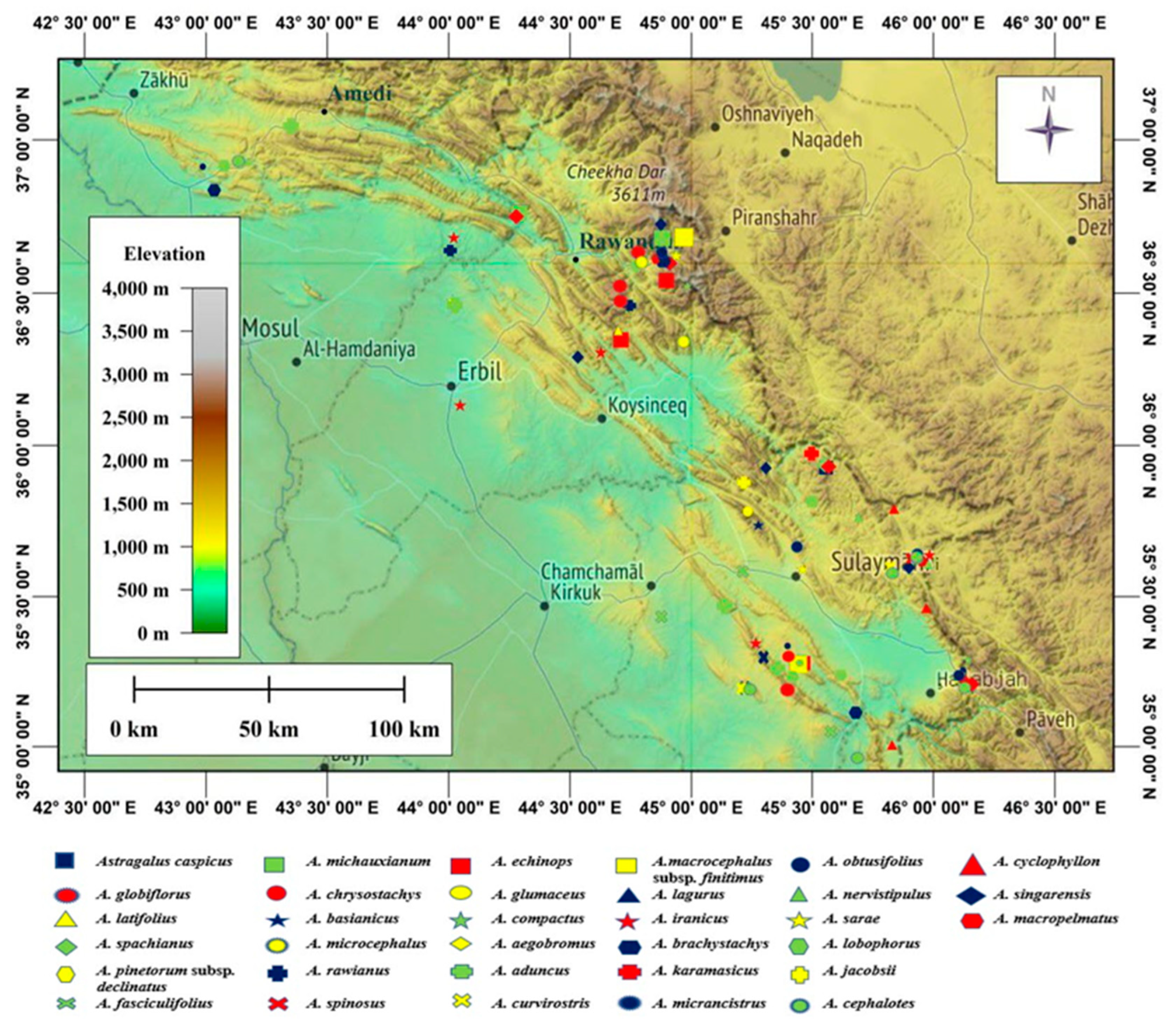
| Section Name | Taxa Name | Voucher Number | Location | Altitude (m) | Accession Number |
|---|---|---|---|---|---|
| Adiaspastus (Sect-1) | Astragalus caspicus M. Bieb. | AL-1 | MSU | 1570 | OP947045 |
| A. michauxianum Boiss. | AL-2 | MSU, MRO, and MAM | 1296–2070 | OP964729 | |
| Alopecias (Sect-2) | A. echinops Auch. ex Boiss. | AL-3 | MSU and MRO | 860–1380 | OP947536 |
| A. macrocephalus subsp. Finitimus Bunge. | AL-4 | MSU and MRO | 1210–1400 | OP948301 | |
| A. obtusifolius DC. | AL-5 | MSU, MRO, and MAM | 160–340 | OP950215 | |
| A. chrysostachys Boiss. | AL-6 | MSU | 1350–1790 | OP947139 | |
| Hymenostegis (Sect-3) | A. glumaceus Boiss. | AL-7 | MSU | 1735 | OP948166 |
| A. lagurus Willd. | AL-8 | MSU and MAM | 1330–1680 | OP959921 | |
| A. nervistipulus Boiss. & Hausskn. | AL-9 | MSU | 1360–1750 | OP948699 | |
| Incai (Sect-4) | A. cyclophyllon Beck. | AL-10 | MSU | 1090 | OP947523 |
| A. latifolius Lam. | AL-11 | MSU | 1247 | OP948257 | |
| Macrophyllium (Sect-5) | A. basianicus Boiss. & Hausskn. | AL-12 | MSU and MRO | 870–1490 | OP964679 |
| A. compactus Lam. | AL-13 | MSU | 1387 | OP947498 | |
| Malacothrix (Sect-6) | A. iranicus Bunge. | AL-14 | MSU, MRO, FAR, and MAM | 900–1970 | OP948645 |
| A. sarae Eig. | AL-15 | MSU and MAM | 1100–1450 | OP964723 | |
| A. singarensis Boiss. | AL-16 | MSU, MRO and MAM | 800–1725 | OP964726 | |
| A. spachianus Boiss. & Buhse. | AL-17 | MSU | 1495 | OP984381 | |
| Myobroma (Sect-7) | A. aegobromus Boiss. | AL-18 | MSU and MRO | 1300–2450 | OP942443 |
| A. brachystachys DC. | AL-19 | MSU, MRO, and MAM | 250–1200 | OP964725 | |
| A. lobophorus Boiss. | AL-20 | MSU, MRO and MAM | 1000–1680 | OP948282 | |
| A. macropelmatus Bunge. | AL-21 | MSU and MRO | 1360–1840 | OP948644 | |
| A. pinetorum subsp. Declinatus (Kuntze.) Podlech. | AL-22 | MSU and FAR | 1980–2795 | OP950280 | |
| A. rawianus C.C. Towns. | AL-23 | MSU and MAM | 1480–2055 | OP964724 | |
| Onobrychium (Sect-8) | A. aduncus Willd. | AL-24 | MSU and MAM | 870 | OP942435 |
| A. karamasicus Boiss. & Balansa. | AL-25 | MSU | 1200 | OP948228 | |
| A. jacobsii Podlech. | AL-26 | MSU | 997 | OP948225 | |
| Poterion (Sect-9) | A. fasciculifolius Boiss. | AL-27 | MSU and FKI | 250–470 | OP957370 |
| A. spinosus (Forssk.) Muschl. | AL-28 | MSU | 435–660 | OP984382 | |
| Proseliu (Sect-10) | A. curvirostris Boiss. | AL-29 | MSU | 1160–1400 | OP984362 |
| A. micrancistrus Boiss. & Hausskn. | AL-30 | MSU and MRO | 1420–1870 | OP950200 | |
| Rhacophorus (Sect-11) | A. cephalotes Banks & Sol. | AL-31 | MSU, MRO, and MAM | 720–1280 | OP947089 |
| A. globiflorus Boiss. | AL-32 | MSU | 1400–1680 | OP948150 | |
| A. microcephalus Willd. | AL-33 | MSU and MRO | 1480–2370 | OP950203 |
| Markers | Annealing Temperature (°C) | Sequence (5′–3′) |
|---|---|---|
| ISSR | ||
| ISSR-6 | 50 | GCCTCCTCCTCCTCCTCC |
| ISSR-9 | 50 | CACACACACACACACATG |
| ISSR-11 | 50 | ACACACACACACACACGG |
| ISSR-12 | 50 | AGAGAGAGAGAGAGAGCT |
| UBC-810 | 50 | GAGAGAGAGAGAGAGAT |
| UBC-814 | 50 | CTCTCTCTCTCTCTCTA |
| UBC-815 | 50 | CTCTCTCTCTCTCTCTG |
| UBC-818 | 50 | CACACACACACACACAG |
| UBC-822 | 50 | TCTCTCTCTCTCTCTCA |
| UBC-825 | 50 | ACACACACACACACACT |
| UBC-826 | 50 | ACACACACACACACACC |
| UBC-834 | 50 | AGAGAGAGAGAGAGAGGT |
| UBC-841 | 50 | GAGAGAGAGAGAGAGACTC |
| UBC-847 | 50 | CACACACACACACACAGC |
| CDDP | ||
| ABP-1 | 50 | ACSCCSATCCACCGC |
| ERF=1 | 50 | CACTACCCCGGSCTSCG |
| ERF-2 | 50 | GCSGAGATCCGSGACCC |
| KNOX-2 | 50 | CACTGGTGGGAGCTSCAC |
| KNOX-3 | 50 | AAGCGSCACTGGAAGCC |
| MYB-1 | 50 | GGCAAGGGCTGCCGC |
| MYB-2 | 50 | GGCAAGGGCTGCCGG |
| WRKY-R1 | 50 | GTGGTTGTGTCTTGCC |
| WRKY-R3 | 50 | GCASGTGTGCTCGCC |
| WRKY-R3B | 50 | CCGCTCGTGTGSACG |
| ISSR Markers | NPB | PIC | Na | Ne | I | He | uHe |
|---|---|---|---|---|---|---|---|
| ISSR-6 | 24.00 | 0.33 | 0.99 | 1.28 | 0.23 | 0.16 | 0.19 |
| ISSR-9 | 28.00 | 0.30 | 0.83 | 1.22 | 0.20 | 0.13 | 0.16 |
| ISSR-11 | 28.00 | 0.32 | 0.97 | 1.27 | 0.24 | 0.16 | 0.20 |
| ISSR-12 | 22.00 | 0.37 | 1.06 | 1.30 | 0.27 | 0.18 | 0.22 |
| UBC-810 | 21.00 | 0.37 | 1.03 | 1.28 | 0.26 | 0.17 | 0.21 |
| UBC-818 | 20.00 | 0.37 | 1.07 | 1.30 | 0.27 | 0.18 | 0.22 |
| UBC-814 | 23.00 | 0.40 | 1.26 | 1.35 | 0.31 | 0.21 | 0.26 |
| UBC-815 | 25.00 | 0.38 | 1.21 | 1.34 | 0.31 | 0.21 | 0.25 |
| UBC-822 | 24.00 | 0.37 | 1.05 | 1.30 | 0.26 | 0.17 | 0.21 |
| UBC-825 | 20.00 | 0.36 | 0.93 | 1.26 | 0.23 | 0.15 | 0.19 |
| UBC-826 | 25.00 | 0.32 | 0.89 | 1.25 | 0.22 | 0.15 | 0.18 |
| UBC-834 | 18.00 | 0.31 | 0.89 | 1.27 | 0.23 | 0.16 | 0.19 |
| UBC-841 | 23.00 | 0.33 | 0.98 | 1.29 | 0.25 | 0.17 | 0.21 |
| UBC-847 | 17.00 | 0.31 | 0.99 | 1.27 | 0.23 | 0.16 | 0.19 |
| Total | 318.00 | 4.84 | 14.15 | 17.96 | 3.51 | 2.36 | 2.89 |
| Mean | 22.71 | 0.35 | 1.01 | 1.28 | 0.25 | 0.17 | 0.21 |
| CDDP Markers | NPB | PIC | Na | Ne | I | He | uHe |
|---|---|---|---|---|---|---|---|
| ABP-1 | 15.00 | 0.41 | 1.15 | 1.31 | 0.27 | 0.18 | 0.22 |
| ERF-1 | 15.00 | 0.33 | 0.98 | 1.28 | 0.24 | 0.16 | 0.20 |
| ERF-2 | 14.00 | 0.40 | 1.32 | 1.42 | 0.35 | 0.24 | 0.30 |
| KNOX-2 | 16.00 | 0.37 | 0.99 | 1.29 | 0.24 | 0.16 | 0.20 |
| KNOX-3 | 12.00 | 0.31 | 0.92 | 1.23 | 0.19 | 0.13 | 0.16 |
| MYB-1 | 12.00 | 0.37 | 1.17 | 1.35 | 0.29 | 0.20 | 0.25 |
| MYB-2 | 13.00 | 0.40 | 1.07 | 1.33 | 0.28 | 0.19 | 0.23 |
| WRKY-R1 | 13.00 | 0.44 | 1.25 | 1.40 | 0.34 | 0.23 | 0.28 |
| WRKY-R3 | 10.00 | 0.29 | 0.85 | 1.24 | 0.20 | 0.14 | 0.17 |
| WRKY-R3B | 12.00 | 0.40 | 1.29 | 1.38 | 0.31 | 0.22 | 0.26 |
| Total | 132.00 | 3.72 | 10.98 | 13.22 | 2.72 | 1.85 | 2.26 |
| Mean | 13.20 | 0.37 | 1.10 | 1.32 | 0.27 | 0.19 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khal, L.H.; Tahir, N.A.-r.; Abdul-Razaq, R.T. Molecular Variation in Some Taxa of Genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region. Horticulturae 2023, 9, 1110. https://doi.org/10.3390/horticulturae9101110
Khal LH, Tahir NA-r, Abdul-Razaq RT. Molecular Variation in Some Taxa of Genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region. Horticulturae. 2023; 9(10):1110. https://doi.org/10.3390/horticulturae9101110
Chicago/Turabian StyleKhal, Lanja Hewa, Nawroz Abdul-razzak Tahir, and Rupak Tofiq Abdul-Razaq. 2023. "Molecular Variation in Some Taxa of Genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region" Horticulturae 9, no. 10: 1110. https://doi.org/10.3390/horticulturae9101110
APA StyleKhal, L. H., Tahir, N. A.-r., & Abdul-Razaq, R. T. (2023). Molecular Variation in Some Taxa of Genus Astragalus L. (Fabaceae) in the Iraqi Kurdistan Region. Horticulturae, 9(10), 1110. https://doi.org/10.3390/horticulturae9101110







