Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Sampling and Peel Extraction
2.3. Preliminary Fractionation
2.4. Determination of Total Phenolic Compounds Content
2.5. DPPH Radical Scavenging Activity Assay
2.6. Mixture Design and Optimization of the Extraction Process Using Response Surface Methodology (RSM)
2.7. HPLC Analysis
2.8. Cell Viability
2.9. Antimicrobial Activity Evaluation
2.10. Mixture Design Statistical Study
2.11. Statistical Analysis
3. Results
3.1. Preliminary Fractionation
3.2. Mixture Optimization
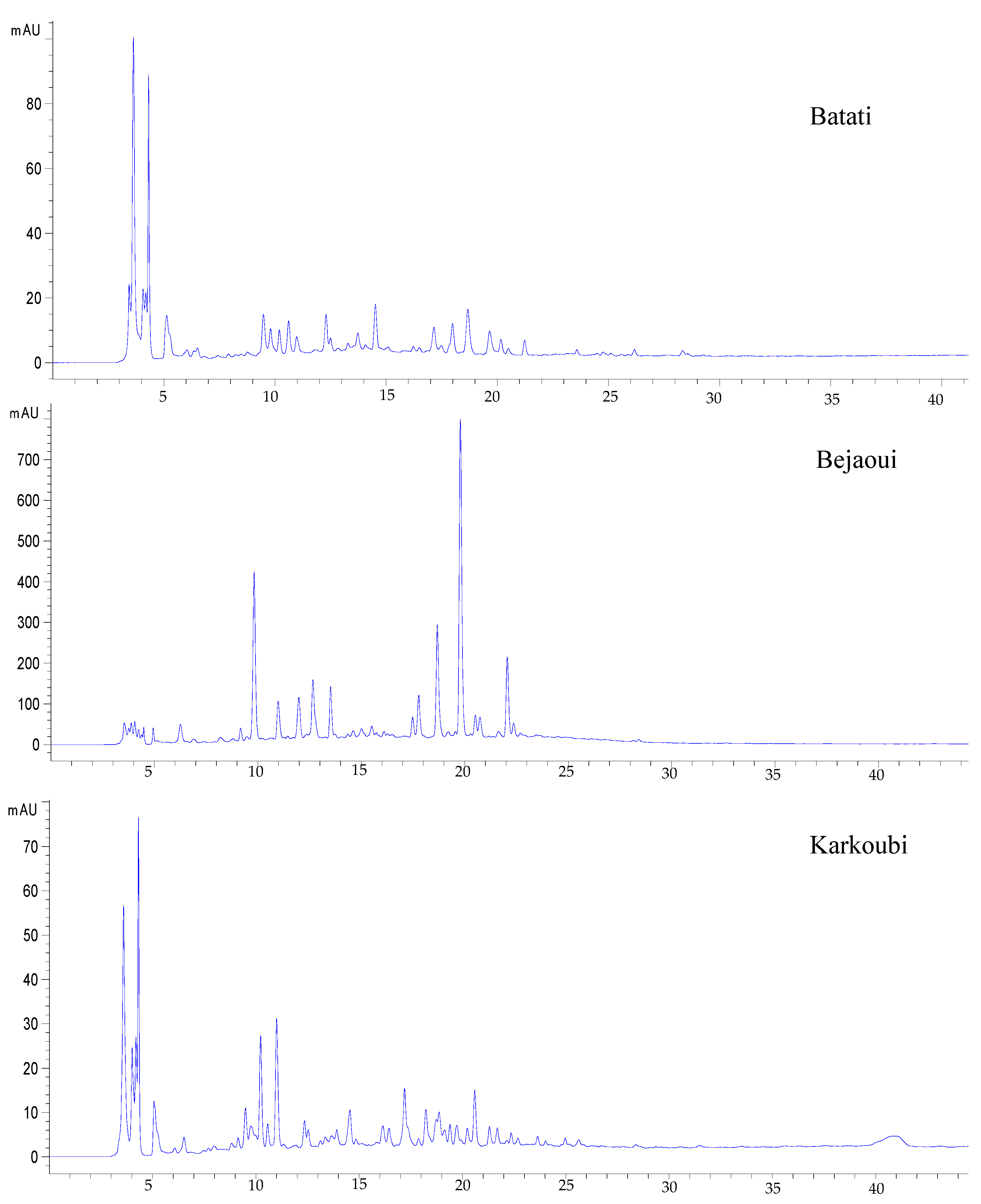
3.3. HPLC Analysis of Peel-Refined Extracts
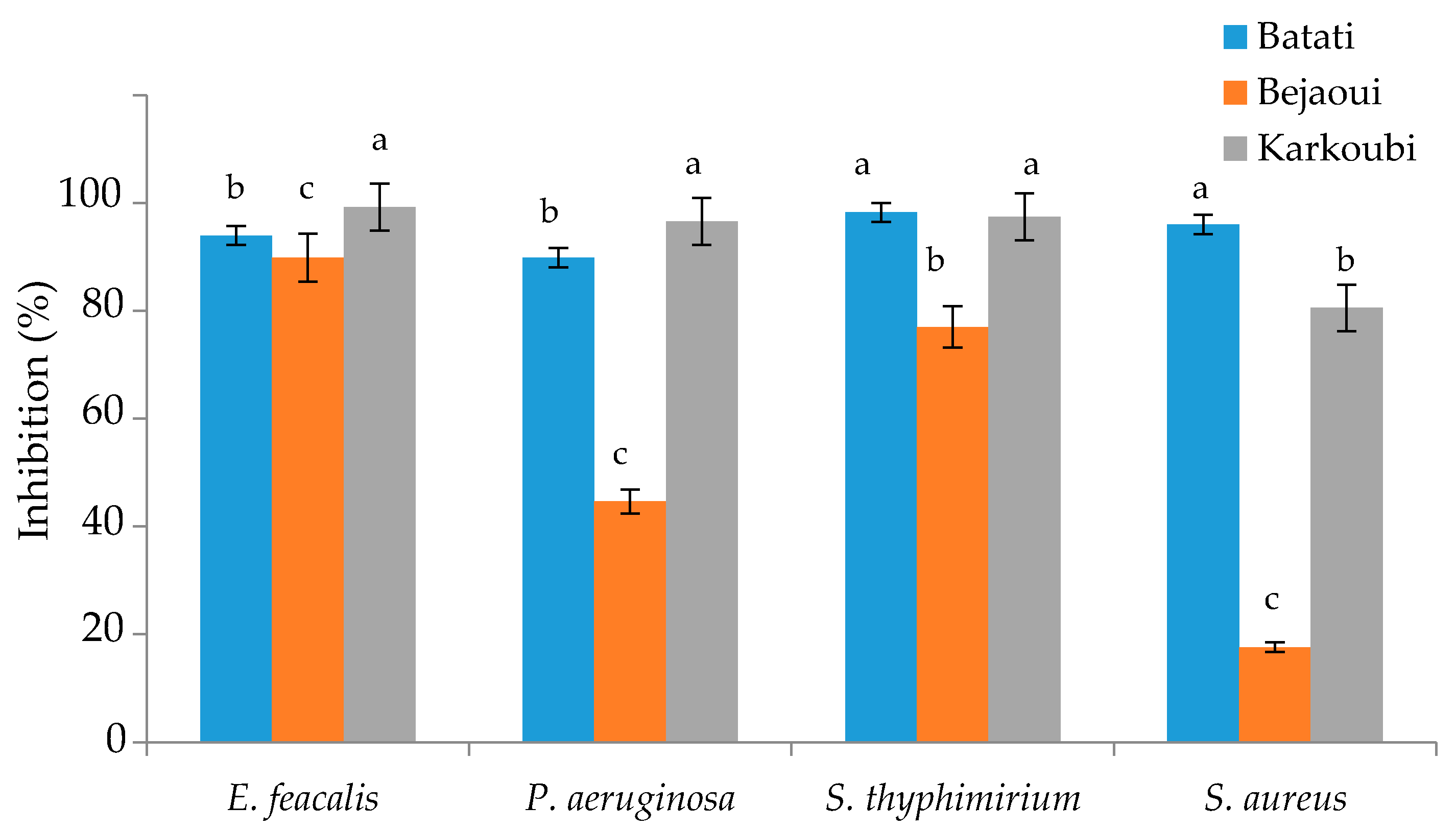
3.4. Antibacterial Activity of Peel-Refined Extracts
3.5. Cell Viability of Peel-Refined Extracts
4. Discussion
4.1. HPLC Analysis of Peel-Refined Extracts
4.2. Antibacterial Activity of Peel-Refined Extracts
4.3. Cell Viability of Peel-Refined Extracts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V.; Vangnai, A.S.; Sharma, N.; Kaur, K.; Chakraborty, P.; Umesh, M.; Singhal, B.; Utreja, D.; Carrasco, E.U.; Andler, R.; et al. Bioengineering of Biowaste to Recover Bioproducts and Bioenergy: A Circular Economy Approach towards Sustainable Zero-Waste Environment. Chemosphere 2023, 319, 138005. [Google Scholar] [CrossRef] [PubMed]
- Gungor, K.K.; Torun, M. Pumpkin Peel Valorization Using Green Extraction Technology to Obtain β-Carotene Fortified Mayonnaise. Waste Biomass Valorization 2022, 13, 4375–4388. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Shetty, A.A.; Rana, R.; Buckseth, T.; Preetham, S.P. Waste Utilization in Cucurbits: A Review. Waste Biomass Valorization 2012, 3, 363–368. [Google Scholar] [CrossRef]
- Jayesree, N.; Hang, P.K.; Priyangaa, A.; Krishnamurthy, N.P.; Ramanan, R.N.; Turki, M.S.A.; Charis, M.G.; Ooi, C.W. Valorisation of Carrot Peel Waste by Water-Induced Hydrocolloidal Complexation for Extraction of Carote and Pectin. Chemosphere 2021, 272, 129919. [Google Scholar] [CrossRef] [PubMed]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Yakovleva, N.; Camacho-Ferre, F. Analysis of the Circular Economic Production Models and Their Approach in Agriculture and Agricultural Waste Biomass Management. Int. J. Environ. Res. Public Health 2020, 17, 9549. [Google Scholar] [CrossRef] [PubMed]
- Forde, C.G.; de Graaf, K. Influence of Sensory Properties in Moderating Eating Behaviors and Food Intake. Front. Nutr. 2022, 9, 841444. [Google Scholar] [CrossRef]
- Adelina, N.M.; Wang, H.; Zhang, L.; Yang, K.; Zhang, L.; Zhao, Y. Evaluation of Roasting Conditions as an Attempt to Improve Bioactive Compounds and Antioxidant Activities of Pine Nut Shell and Skin. Waste Biomass Valorization 2022, 13, 845–861. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A Comparative Review on the Extraction, Antioxidant Content and Antioxidant Potential of Different Parts of Walnut (Juglans regia L.) Fruit and Tree. Molecules 2019, 24, 2133. [Google Scholar] [CrossRef]
- Tripathi, M.; Lal, B.; Syed, A.; Mishra, P.K.; Elgorban, A.M.; Verma, M.; Singh, R.; Mohammad, A.; Srivastava, N. International Journal of Biological Macromolecules Production of Fermentable Glucose from Bioconversion of Cellulose Using Efficient Microbial Cellulases Produced from Water Hyacinth Waste. Int. J. Biol. Macromol. 2023, 252, 126376. [Google Scholar] [CrossRef]
- Hussain, A.; Kausar, T.; Din, A.; Murtaza, M.A.; Jamil, M.A.; Noreen, S.; ur Rehman, H.; Shabbir, H.; Ramzan, M.A. Determination of Total Phenolic, Flavonoid, Carotenoid, and Mineral Contents in Peel, Flesh, and Seeds of Pumpkin (Cucurbita maxima). J. Food Process. Preserv. 2021, 45, e15542. [Google Scholar] [CrossRef]
- Noh, N.A.N.M.; Karim, L.; Omar, S.R. Value-Added Products from Pumpkin Wastes: A Review. Malays. J. Sci. Health Technol. 2022, 8, 77–84. [Google Scholar] [CrossRef]
- Mala, K.S.; Kurian, A.E. Nutritional Composition and Antioxidant Activity of Pumpkin Wastes. Int. J. Pharm. Chem. Biol. Sci. 2016, 6, 336–344. [Google Scholar]
- Akbar, A.; Sadiq, M.B.; Ali, I.; Muhammad, N.; Rehman, Z.; Khan, M.N.; Muhammad, J.; Khan, S.A.; Rehman, F.U.; Anal, A.K. Synthesis and Antimicrobial Activity of Zinc Oxide Nanoparticles against Foodborne Pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 2019, 17, 36–42. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Soteyome, T.; Peters, B.M.; Shirtliff, M.E.; Liu, J.; Harro, J.M. Polymicrobial Interaction and Biofilms between Staphylococcus aureus and Pseudomonas aeruginosa: An Underestimated Concern in Food Safety. Curr. Opin. Food Sci. 2019, 26, 57–64. [Google Scholar] [CrossRef]
- Gundogan, N.; Ataol, O.; Torlak, F.O. Determination of Some Virulence Factors in Staphylococcus aureus, Enterococcus faecalis and Enterococcus faecium Isolated from Meat and Milk Products. J. Food Saf. 2013, 33, 387–393. [Google Scholar] [CrossRef]
- Martino, G.P.; Espariz, M.; Gallina Nizo, G.; Esteban, L.; Blancato, V.S.; Magni, C. Safety Assessment and Functional Properties of Four Enterococci Strains Isolated from Regional Argentinean Cheese. Int. J. Food Microbiol. 2018, 277, 1–9. [Google Scholar] [CrossRef]
- Wu, M.C.; Li, H.c.; Wu, P.H.; Huang, P.H.; Wang, Y.T. Assessment of Oligogalacturonide from Citrus Pectin as a Potential Antibacterial Agent against Foodborne Pathogens. J. Food Sci. 2014, 79, M1541–M1544. [Google Scholar] [CrossRef]
- Román, S.; Sánchez-Siles, L.M.; Siegrist, M. The Importance of Food Naturalness for Consumers: Results of a Systematic Review. Trends Food Sci. Technol. 2017, 67, 44–57. [Google Scholar] [CrossRef]
- Ben Mansour, R.; Falleh, H.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. The Use of Response Surface Methodology to Optimize Assisted Extraction of Bioactive Compounds from Cucurbita maxima Fruit By-Products. Processes 2023, 11, 1726. [Google Scholar] [CrossRef]
- Abudureheman, B.; Liu, H.; Zhang, D.; Guan, K. Identification of Physical Dormancy and Dormancy Release Patterns in Several Species (Fabaceae) of the Cold Desert, North-West China. Seed Sci. Res. 2014, 24, 133–145. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two New Flavonoids and Other Constituents in Licorice Root: Their Relative Astringency and Radical Scavenging Effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef] [PubMed]
- Zeouk, I.; Ouedrhiri, W.; Jiménez, I.A.; Morales, J.L.; Bazzocchi, I.L.; Bekhti, K. Intra-Combined Antioxidant Activity and Chemical Characterization of Three Fractions from Rhamnus alaternus Extract: Mixture Design. Ind. Crops Prod. 2020, 144, 112054. [Google Scholar] [CrossRef]
- Boulaaba, M.; Medini, F.; Hajlaoui, H.; Mkadmini, K.; Falleh, H.; Ksouri, R.; Isoda, H.; Smaoui, A.; Abdelly, C. Biological Activities and Phytochemical Analysis of Phenolic Extracts from Salsola kali L. Role of Endogenous Factors in the Selection of the Best Plant Extracts. S. Afr. J. Bot. 2019, 123, 193–199. [Google Scholar] [CrossRef]
- Pereira, R.B.; Rahali, F.Z.; Nehme, R.; Falleh, H.; Ben Jemaa, M.; Sellami, I.H.; Ksouri, R.; Bouhallab, S.; Ceciliani, F.; Abdennebi-Najar, L.; et al. Anti-Inflammatory Activity of Essential Oils from Tunisian Aromatic and Medicinal Plants and Their Major Constituents in THP-1 Macrophages. Food Res. Int. 2023, 167, 112678. [Google Scholar] [CrossRef] [PubMed]
- Benjemaa, M.; Snoussi, M.; Falleh, H.; Hessini, K.; Msaada, K.; Flamini, G.; Ksouri, R. Chemical Composition, Antibacterial and Antifungal Activities of Four Essential Oils Collected in the North-East of Tunisia. J. Essent. Oil-Bear. Plants 2022, 25, 338–355. [Google Scholar] [CrossRef]
- Ammar, A.H.; Zagrouba, F.; Romdhane, M. Optimization of Operating Conditions of Tunisian Myrtle (Myrtus communis L.) Essential Oil Extraction by a Hydrodistillation Process Using a 24 Complete Factorial Design. Flavour Fragr. J. 2010, 25, 503–507. [Google Scholar] [CrossRef]
- Dardavila, M.M.; Pappou, S.; Savvidou, M.G.; Louli, V.; Stamatis, H.; Magoulas, K.; Voutsas, E. Extraction of Bioactive Compounds from C. Vulgaris Biomass Using Deep Eutectic Solvents. Molecules 2023, 28, 415. [Google Scholar] [CrossRef]
- Karker, M.; Falleh, H.; Msaada, K.; Smaoui, A.; Abdelly, C.; Legault, J.; Ksouri, R. Antioxidant, Anti-Inflammatory and Anticancer Activities of the Medicinal Halophyte Reaumuria vermiculata. EXCLI J. 2016, 15, 297–307. [Google Scholar] [CrossRef]
- Munhoz, V.M.; Longhini, R.; Souza, J.R.P.; Zequi, J.A.C.; Mello, E.V.S.L.; Lopes, G.C.; Mello, J.C.P. Extraction of Flavonoids from Tagetes Patula: Process Optimization and Screening for Biological Activity. Rev. Bras. Farmacogn. 2014, 24, 576–583. [Google Scholar] [CrossRef]
- Kultys, E.; Kurek, M.A. Green Extraction of Carotenoids from Fruit and Vegetable Byproducts: A Review. Molecules 2022, 27, 518. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Lavecchia, R. Influence of Extraction Conditions on the Recovery of Phenolic Antioxidants from Spent Coffee Grounds. Am. J. Appl. Sci. 2013, 10, 478–486. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of Solvent Type and Extraction Conditions on the Recovery of Phenolic Compounds from Artichoke Waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of Mango Byproducts: Study of the Effect of Extraction Solvent and Temperature on Their Antioxidant Properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Iannone, A.; Lavecchia, R. Water-Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food Waste: A Potential Bioresource for Extraction of Nutraceuticals and Bioactive Compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s New in Biopotential of Fruit and Vegetable by-Products Applied in the Food Processing Industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Vinson, J.A.; Dabbagh, Y.A.; Serry, M.M.; Jang, J. Plant Flavonoids, Especially Tea Flavonols, Are Powerful Antioxidants Using an in Vitro Oxidation Model for Heart Disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar] [CrossRef]
- Trabelsi, N.; Oueslati, S.; Henry-Vitrac, C.; Waffo-Téguo, P.; Medini, F.; Mérillon, J.M.; Abdelly, C.; Ksouri, R. Phenolic Contents and Biological Activities of Limoniastrum guyonianum Fractions Obtained by Centrifugal Partition Chromatography. Ind. Crops Prod. 2013, 49, 740–746. [Google Scholar] [CrossRef]
- Leichtweis, M.G.; Molina, A.K.; Pires, T.C.S.; Dias, M.I.; Calhelha, R.; Bachari, K.; Ziani, B.E.C.; Oliveira, M.B.P.P.; Pereira, C.; Barros, L. Biological Activity of Pumpkin Byproducts: Antimicrobial and Antioxidant Properties. Molecules 2022, 27, 8366. [Google Scholar] [CrossRef]
- Mokhtar, M.; Bouamar, S.; Di Lorenzo, A.; Temporini, C.; Daglia, M.; Riazi, A. The Influence of Ripeness on the Phenolic Content, Antioxidant and Antimicrobial Activities of Pumpkins (Cucurbita moschata Duchesne). Molecules 2021, 26, 3623. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, A.C.; Botezatu, A.V.D.; Furdui, B.; Vinatoru, C.; Maggi, F.; Caprioli, G.; Dinica, R.M. Comparative Study of the Chemical Compositions and Antioxidant Activities of Fresh Juices from Romanian Cucurbitaceae Varieties. Molecules 2020, 25, 5468. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.A.M.; Berenguer, C.V.; Andrade, C.F.P.; Câmara, J.S. Unveiling the Bioactive Potential of Fresh Fruit and Vegetable Waste in Human Health from a Consumer Perspective. Appl. Sci. 2022, 12, 2747. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous Optimization of the Ultrasound-Assisted Extraction for Phenolic Compounds Content and Antioxidant Activity of Lycium ruthenicum Murr. Fruit Using Response Surface Methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, C.; Shi, M.; Hong, S.; Guo, S.; Li, J.; Liang, T.; Song, P.; Xu, R.; Li, N. Kaempferol Inhibits SARS-CoV-2 Invasion by Impairing Heptad Repeats-Mediated Viral Fusion. Phytomedicine 2023, 118, 154942. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The Mechanism of Anticancer Action and Potential Clinical Use of Kaempferol in the Treatment of Breast Cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in avonoid Research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar]
- Chonoko, U.; Rufai, A. Phytochemical Screening and Antibacterial Activity of Cucurbita pepo (Pumpkin) against Staphylococcus aureus and Salmonella typhi. Bayero J. Pure Appl. Sci. 2011, 4, 145–147. [Google Scholar] [CrossRef]
- Asif, M.; Naqvi, S.A.R.; Sherazi, T.A.; Ahmad, M.; Zahoor, A.F.; Shahzad, S.A.; Hussain, Z.; Mahmood, H.; Mahmood, N. Antioxidant, Antibacterial and Antiproliferative Activities of Pumpkin (Cucurbit) Peel and Puree Extracts—An in Vitro. Study. Pak. J. Pharm. Sci. 2017, 30, 1327–1334. [Google Scholar] [PubMed]
- Alabassi, H.M.; Kadri, Z.H.M.; Gathwan, M.A.; Kadern, Y.J.; Kadhim, Z.K. Therapeutic Effect of Pumpkin (Cucurbita pepo L.) on Post Burn Injury in White Mice. Syst. Rev. Pharm. 2020, 11, 616–620. [Google Scholar] [CrossRef]
- Dissanayake, D.M.R.H.; Deraniyagala, S.A.; Hettiarachchi, C.M.; Thiripuranathar, G. The Study of Antioxidant and Antibacterial Properties of Skin, Seeds and Leaves of the Sri Lankan Variety of Pumpkin. IOSR J. Pharm. 2018, 8, 43–48. [Google Scholar]
- Cerda-Opazo, P.; Gotteland, M.; Oyarzun-Ampuero, F.A.; Garcia, L. Design, Development and Evaluation of Nanoemulsion Containing Avocado Peel Extract with Anticancer Potential: A Novel Biological Active Ingredient to Enrich Food. Food Hydrocoll. 2021, 111, 106370. [Google Scholar] [CrossRef]
- Frauches, N.S.; Montenegro, J.; Amaral, T.; Abreu, J.P.; Laiber, G.; Junior, J.; Borguini, R.; Santiago, M.; Pacheco, S.; Nakajima, V.M.; et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and in Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules 2021, 26, 564. [Google Scholar] [CrossRef]
- Elkholy, Y.; Helal, M.; Hamza, A.; Masoud, M.; Badr, S. The Potential Cytotoxicity and Antimicrobial Activities for Rind and Seeds Oil Extracts of Pumpkin (Cucurbita pepo L.). J. Agric. Chem. Biotechnol. 2009, 34, 19–38. [Google Scholar] [CrossRef]
- Rathinavelu, A.; Levy, A.; Sivanesan, D.; Murugan, D.; Jornadal, J.; Quinonez, Y.; Jaffe, M.; Gossell-Williams, M. Cytotoxic Effect of Pumpkin (Curcurbita pepo) Seed Extracts in LNCAP Prostate Cancer Cells Is Mediated through Apoptosis. Curr. Top. Nutraceutical Res. 2013, 11, 137–143. [Google Scholar]
- Piccolella, S.; Bianco, A.; Crescente, G.; Santillo, A.; Baccari, G.C.; Pacifico, S. Recovering Cucurbita Pepo Cv. ‘Lungo Fiorentino’ Wastes: UHPLC-HRMS/MS Metabolic Profile, the Basis for Establishing Their Nutra- And Cosmeceutical Valorisation. Molecules 2019, 24, 1479. [Google Scholar] [CrossRef] [PubMed]
- Gaweł-Bęben, K.; Czech, K.; Strzępek-Gomółka, M.; Czop, M.; Szczepanik, M.; Lichtarska, A.; Kukula-Koch, W. Assessment of Cucurbita spp. Peel Extracts as Potential Sources of Active Substances for Skin Care and Dermatology. Molecules 2022, 27, 7618. [Google Scholar] [CrossRef]
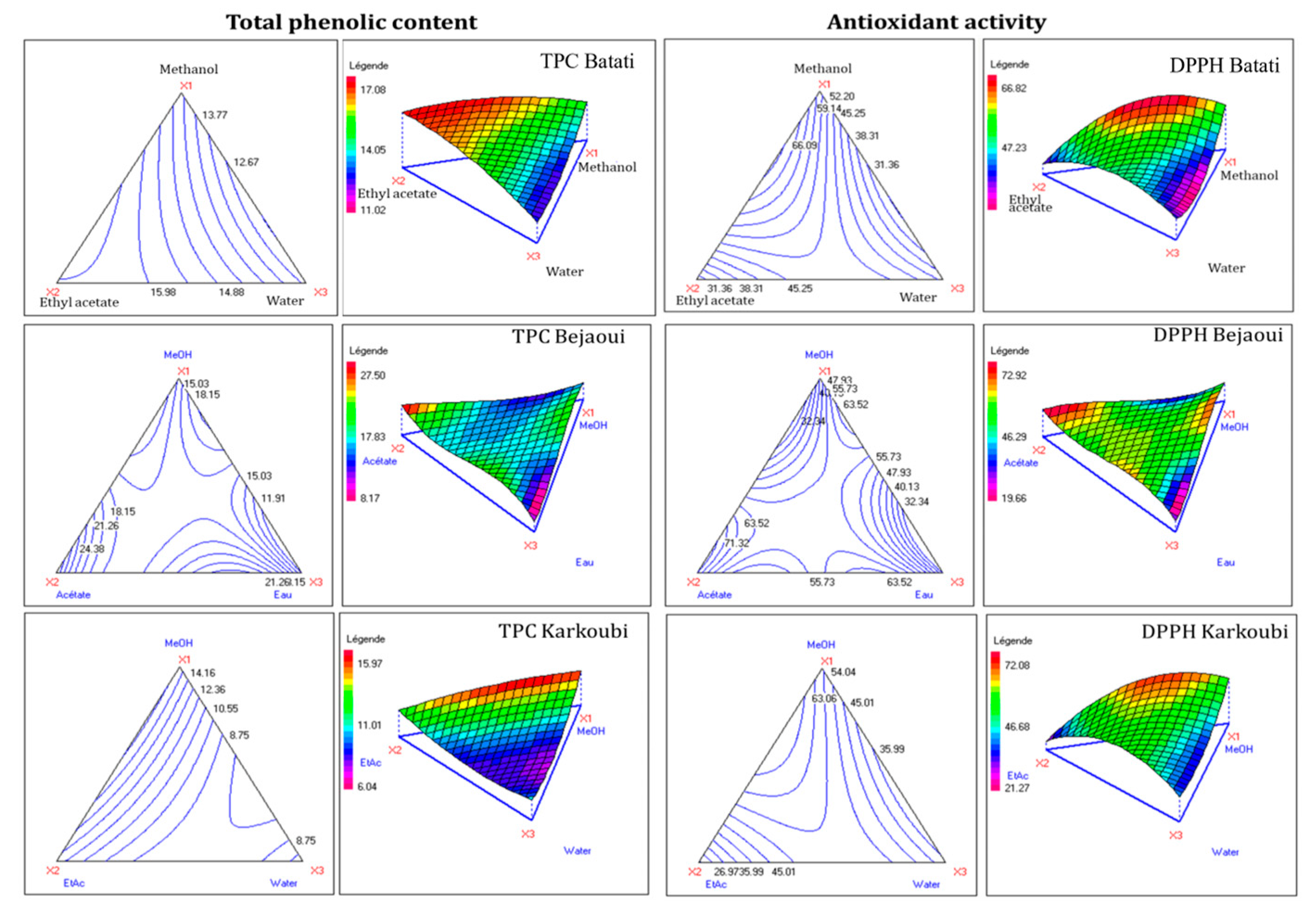

| Experimental Factors | Landraces | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experiment N° | X1 | X2 | X3 | Batati | Bejaoui | Karkoubi | |||
| Y1 | Y2 | Y1 | Y2 | Y1 | Y2 | ||||
| 1 | 1.0000 | 0.0000 | 0.0000 | 14.44 | 53.32 | 14.59 | 56.17 | 15.42 | 48.82 |
| 2 | 0.0000 | 1.0000 | 0.0000 | 17.08 | 29.57 | 12.31 | 22.46 | 27.50 | 71.32 |
| 3 | 0.0000 | 0.0000 | 1.0000 | 12.64 | 37.98 | 10.00 | 34.47 | 10.35 | 28.44 |
| 4 | 0.6667 | 0.3333 | 0.0000 | 15.92 | 66.09 | 15.97 | 72.08 | 12.92 | 32.54 |
| 5 | 0.3333 | 0.6667 | 0.0000 | 16.10 | 55.23 | 14.00 | 50.96 | 19.12 | 63.06 |
| 6 | 0.6667 | 0.0000 | 0.3333 | 12.53 | 27.89 | 9.62 | 30.56 | 18.96 | 58.97 |
| 7 | 0.3333 | 0.3333 | 0.3333 | 15.85 | 49.62 | 9.23 | 51.97 | 16.85 | 59.60 |
| 8 | 0.0000 | 0.6667 | 0.3333 | 15.30 | 44.93 | 8.47 | 48.07 | 16.30 | 51.52 |
| 9 | 0.3333 | 0.0000 | 0.6667 | 11.02 | 31.59 | 6.04 | 36.93 | 10.67 | 30.25 |
| 10 | 0.0000 | 0.3333 | 0.6667 | 14.50 | 47.61 | 7.49 | 48.07 | 23.41 | 65.52 |
| 11 | 0.6667 | 0.1667 | 0.1667 | 15.20 | 58.95 | 11.13 | 55.73 | 15.48 | 55.50 |
| 12 | 0.1667 | 0.6667 | 0.1667 | 16.20 | 50.88 | 10.50 | 52.70 | 15.92 | 54.10 |
| 13 | 0.1667 | 0.1667 | 0.6667 | 14.64 | 35.40 | 10.00 | 53.71 | 16.16 | 46.97 |
| Total Polyphenols Content | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batati Peel | Bejaoui Peel | Karkoubi Peel | |||||||||||||
| Coefficient | F.Inflation | Ecart-Type | t.exp. | Signif. % | Coefficient | F.Inflation | Ecart-Type | t.exp. | Signif. % | Coefficient | F.Inflation | Ecart-Type | t.exp. | Signif. % | |
| b1 | 14.473 | 2.30 | 0.799 | 18.12 | *** | 15.35 | 2.71 | 0.842 | 18.23 | *** | 14.78 | 2.30 | 1.013 | 14.6 | *** |
| b2 | 16.485 | 2.30 | 0.799 | 20.64 | *** | 27.41 | 2.71 | 0.842 | 32.57 | *** | 11.94 | 2.30 | 1.013 | 11.79 | *** |
| b3 | 12.526 | 2.30 | 0.799 | 15.69 | *** | 10.27 | 2.71 | 0.842 | 12.21 | *** | 10.14 | 2.30 | 1.013 | 10.02 | *** |
| b12 | 4.369 | 2.25 | 3.528 | 1.24 | 25.5% | −24.63 | 2.72 | 3.763 | −6.55 | ** | 6.44 | 2.25 | 4.475 | 1.44 | 19.2% |
| b13 | −4.783 | 2.25 | 3.528 | −1.36 | 21.6% | 8.39 | 2.72 | 3.763 | 2.23 | 11.1% | −19.53 | 2.25 | 4.475 | −4.37 | ** |
| b23 | 4.120 | 2.25 | 3.528 | 1.17 | 28.1% | 4.11 | 2.72 | 3.763 | 1.09 | 35.6% | −12.31 | 2.25 | 4.475 | −2.75 | * |
| Antiradical activity | |||||||||||||||
| b1 | 53.91 | 2.30 | 4.022 | 13.4 | *** | 48.409 | 2.71 | 4.185 | 11.57 | *** | 54.77 | 2.30 | 5.39 | 10.15 | *** |
| b2 | 28.97 | 2.30 | 4.022 | 7.2 | *** | 71.30 | 2.71 | 4.185 | 17.04 | *** | 21.27 | 2.30 | 5.39 | 3.94 | ** |
| b3 | 37.4 | 2.30 | 4.022 | 9.3 | *** | 28.32 | 2.71 | 4.185 | 6.77 | ** | 37.14 | 2.30 | 5.39 | 6.88 | *** |
| b12 | 95.07 | 2.25 | 17.770 | 5.35 | ** | −54.62 | 2.72 | 18.706 | −2.92 | 6.0% | 107.64 | 2.25 | 23.84 | 4.51 | ** |
| b13 | −68.16 | 2.25 | 17.770 | −3.84 | ** | 29.09 | 2.72 | 18.706 | 1.56 | 21.7% | −47.89 | 2.25 | 23.84 | −2.01 | 8.3% |
| b23 | 57.22 | 2.25 | 17.770 | 3.22 | * | 34.86 | 2.72 | 18.706 | 1.86 | 15.9% | 93.58 | 2.25 | 23.84 | 3.92 | ** |
| Batati | Bejaoui | Karkoubi | ||||
|---|---|---|---|---|---|---|
| TPC | DPPH Test | TPC | DPPH Test | TPC | DPPH Test | |
| Predicted values | 16.44 ± 1.5 | 65.54 ± 7.2 | 16.06 ± 0.3 | 49.88 ± 10.7 | 15 ± 1.2 | 66.94 ± 2.3 |
| Experimental values | 15.60 ± 1.3 | 64.14 ± 2.1 | 16.17 ± 0.9 | 50.47 ± 3.2 | 16.17 ± 0.5 | 62.24 ± 2.2 |
| Compounds | Retention Time (min) | Content (mg/g E) | ||
|---|---|---|---|---|
| Batati | Bejaoui | Karkoubi | ||
| Vanillic acid | 5.12 | 0.1 ± 0.01 a | 0.04 ± 0.002 b | 0.14 ± 0.02 a |
| Gallic acid | 6.1 | 0.01 ± 0.001 b | 0.43 ± 0.002 a | 0.03 ± 0.001 b |
| Catechin gallate | 7.34 | - | 0.15 ± 0.02 a | - |
| Hydroxytyrosol | 9.15 | 0.25 ± 0.03 b | 0.42 ± 0.04 a | - |
| Epigallocatechin | 10.68 | 0.48 ± 0.02 a | 0.24 ± 0.01 b | 0.18 ± 0.02 c |
| Resorcinol | 11.55 | - | 0.08 ± 0.002 a | - |
| Chlorogenic acid | 11.6 | - | 2.17 ± 0.6 a | - |
| Catechin | 12.18 | 0.50 ± 0.02 b | 8.06 ± 0.5 a | 0.26 ± 0.02 b |
| Catechol | 13.4 | - | 0.07 ± 0.001 a | - |
| Epicatechin | 13.82 | 0.36 ± 0.08 b | 5.00 ± 0.32 a | - |
| Caffeic acid | 14.25 | - | 0.05 ± 0.002 a | 0.07 ± 0.001 a |
| Sinapic acid | 14.47 | 0.12 ± 0.03 a | 0.12 ± 0.03 a | - |
| Myrecitin 3-O-galactoside | 15.37 | - | 0.08 ± 0.001 a | - |
| Rutin | 16.44 | 0.04 ± 0.002 b | 0.16 ± 0.02 a | 0.08 ± 0.002 b |
| Ellagic acid | 17.42 | 0.01 ± 0.001 c | 0.35 ± 0.03 a | 0.14 ± 0.01 b |
| Vanillin | 17.79 | - | 2.00 ± 0.04 a | - |
| p-Coumaric acid | 17.9 | 0.08 ± 0.001 a | - | - |
| Kaempferol | 18.28 | 0.31 ± 0.02 b | 5.01 ± 0.12 a | 0.16 ± 0.01 b |
| Ferulic acid | 18.87 | - | - | 0.07 ± 0.01 a |
| Myrecitin | 22.54 | - | 0.08 ± 0.001 a | - |
| resveratrol | 24.5 | 0.03 ± 0.002 b | 0.07 ± 0.001 a | - |
| Quercetin | 26.16 | 0.04 ± 0.001 a | 0.03 ± 0.001 a | - |
| Apigenin | 28.31 | - | 0.29 ± 0.02 a | - |
| Total | 2.39 b | 24.9 a | 1.13 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, R.B.; Falleh, H.; Nefzi, N.; Dakhlaoui, S.; Selmi, S.; Hammami, M.; Barros, L.; Petropoulos, S.A.; Tarchoun, N.; Ksouri, R. Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach. Horticulturae 2023, 9, 1111. https://doi.org/10.3390/horticulturae9101111
Mansour RB, Falleh H, Nefzi N, Dakhlaoui S, Selmi S, Hammami M, Barros L, Petropoulos SA, Tarchoun N, Ksouri R. Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach. Horticulturae. 2023; 9(10):1111. https://doi.org/10.3390/horticulturae9101111
Chicago/Turabian StyleMansour, Rim Ben, Hanen Falleh, Nermine Nefzi, Sarra Dakhlaoui, Sawssen Selmi, Majdi Hammami, Lillian Barros, Spyridon A. Petropoulos, Neji Tarchoun, and Riadh Ksouri. 2023. "Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach" Horticulturae 9, no. 10: 1111. https://doi.org/10.3390/horticulturae9101111
APA StyleMansour, R. B., Falleh, H., Nefzi, N., Dakhlaoui, S., Selmi, S., Hammami, M., Barros, L., Petropoulos, S. A., Tarchoun, N., & Ksouri, R. (2023). Improved Recovery of Antioxidant Compounds from Refined Pumpkin Peel Extract: A Mixture Design Method Approach. Horticulturae, 9(10), 1111. https://doi.org/10.3390/horticulturae9101111









