Abstract
In recent years, a significant impediment to the advancement of China’s agricultural sector is the noteworthy challenge posed by diminished crop yields and quality due to ongoing continuous cropping obstacles. Numerous studies have consistently showcased the potential of plant growth-promoting rhizobacteria (PGPR) and biochar in augmenting the alleviation of continuous cropping barriers. Nevertheless, the potential of PGPR and biochar to remediate and improve continuous cropping peppers in the karst yellow soil area remains unclear. A 2-year field experiment was implemented to examine the impact of PGPR and biochar, when applied alone or in combination, on the production potential of continuous cropping peppers. The results revealed that PGPR and biochar significantly elevated the yield of fresh and dry pepper compared with TF treatment. The utilization of PGPR and biochar resulted in an augmentation of free amino acids, soluble sugar, and vitamin C content in pepper fruits, but a reduction in the nitrate content, which proved advantageous in enhancing the overall quality of peppers. Furthermore, the use of PGPR and biochar demonstrated significant benefits in enhancing NPK accumulation, fertilizer utilization, and economic efficiency. Nevertheless, the co-application of PGPR and biochar yielded significantly better results compared to their individual application. In conclusion, the utilization of PGPR and biochar demonstrated a favorable impact on the productivity and economic benefits of continuous cropping peppers. The simultaneous application of PGPR and biochar represents a promising approach to enhancing yield and improving the quality of peppers in the karst yellow soil region of Southwest China.
1. Introduction
Modern agriculture is confronting novel challenges, and the escalating demand for agricultural goods strains crop production and compels agricultural practices to mitigate their adverse ecological effects [1,2]. Thus, enhancing the sustainable productivity of existing farmlands becomes pivotal to fulfilling forthcoming global crop needs while minimizing environmental detriments. The intensified cultivation of monoculture cash crops has led to complications involving continuous cropping and the emergence of replanting-related diseases, profoundly impacting the viability of cultivable terrain. This situation underscores a substantial peril to both regional food security and environmental integrity [3,4,5]. Numerous researchers have substantiated that the dysregulation of soil microbial diversity stands as the central factor driving challenges in continuous cropping obstacles [6,7,8,9]. In recent years, researchers have increasingly embraced the utilization of beneficial microorganisms and organic amendments to rehabilitate compromised soil in agricultural contexts, and this approach aims to enhance crop yield and quality while improving the soil microenvironment [10,11,12].
Plant growth-promoting rhizobacteria (PGPR) are beneficial microorganisms that reside in the soil microflora of the plant rhizosphere. Specifically, PGPR possess numerous functions, including biological nitrogen fixation, phosphorus and potassium solubilization, antibiotic secretion, and hormone synthesis [13,14]. It has been demonstrated that PGPR can colonize plant roots and enhance the solubilization of insoluble nutrients by releasing organic acids. This colonization and nutrient solubilization contribute to promoting plant growth and improving the host’s uptake and utilization of mineral nutrients [15]. Notably, studies have revealed that PGPR can produce antibiotics, antimicrobial proteins, pathogen cell wall hydrolases, and other biologically active components throughout their growth and reproduction, which can effectively inhibit or eradicate plant pathogenic bacteria [16,17]. Moreover, PGPR has been shown to enhance plant root growth and development by producing growth hormones and cytokinins. It also improves nutrient uptake and helps maintain balanced nutrition in crops [18,19]. Consequently, the utilization of PGPR has been widely recognized as the most effective approach for mitigating the challenges associated with continuous cropping and boosting crop productivity. Similar studies have been conducted using biochar. Biochar can improve soil fertility and reduce the occurrence of diseases and pests by improving the soil microbial environment [20,21,22,23,24,25]. At present, PGPR and biochar are extensively utilized as vital soil amendment materials in agricultural production [26,27].
China stands as a global leader in pepper production. Nonetheless, the challenge of continuous cropping hurdles has pervaded pepper cultivation regions due to the adoption of unsustainable farming and management methods. The long-term continuous cultivation of pepper has fostered an escalation in pest and disease occurrences. As a consequence, both pepper yield and quality have experienced a consistent decline, exerting an adverse effect on economic returns. This predicament significantly hampers the sound and sustainable progression of pepper agriculture [28]. While numerous experiments have underscored the positive influence of individual PGPR or biochar applications, boosting crop yield and enhancing soil quality, it is worth noting that the majority of these experiments were carried out in controlled laboratory or greenhouse settings. Consequently, research encompassing field experiments remains relatively scarce. In this research, we hypothesized that the application of PGPR and biochar would have an ameliorative effect on continues cropping peppers, so a 2-year field experiment was implemented to investigate the potential ameliorative effect of applying PGPR and biochar either alone or in combination on pepper fields that have been continuously planted for 5 years, with the following aims: (1) to observe the effects of PGPR and biochar on fresh yield, dry yield, and the quality of the peppers, (2) to examine the impacts of PGPR and biochar on NPK accumulation and fertilizer use efficiency, and (3) to calculate the effects of applying PGPR and biochar on improving the economic output of peppers.
2. Materials and Methods
2.1. Site Description
The field experiment was implemented from 2021 to 2022 in Guizhou Province of China. Prior to the experiment, the field was cultivated with peppers for a period of five years, spanning 2016–2020. The experimental region is characterized by the presence of yellow soil, which is extensively found in the karst mountains of Southwest China. The yellow soil type at this region was classified as Acrisol in the World Reference Base for Soil Resources (WRB), and was developed from the Triassic limestone and sand shale efflorescence. Table 1 presents the fundamental physicochemical properties of the soil in the experimental region.

Table 1.
The basic physicochemical properties of soil and biochar.
2.2. Experimental Materials
The experimental pepper variety was ‘Zunla 9’, a locally cultivated pod pepper variety. The chemical fertilizer used in the experiment is the best-selling compound fertilizer in the local area, with a total nutrient content of 42% (N content of 18%, P2O5 content of 6%, K2O content of 18%). PGPR solution was from Shandong Lvlong Biotechnology Co., Ltd. (Zhucheng, China), and biochar was from Guizhou Jinyefeng Ecological Agricultural Technology Co., Ltd. (Weining, China). The PGPR solid was produced using a hybrid strain comprising Bacillus amyloliquefaciens and Paenibacillus polymyxa. The effective viable bacterial count exceeded 15 × 108 CFU·g−1. The raw material for producing biochar was distillers’ grains processed at 550 °C. Table 1 lists the fundamental physicochemical properties of biochar.
2.3. Experimental Design
Two pepper planting seasons were included in the field experiments conducted from 2021 to 2022. The study included six treatments, each with three replicates. The detailed treatments were shown in Table 2. It should be noted that the application rate of chemical fertilizers was provided by the local agricultural department. In the TFP, TFB, and TFPB1 treatments, the application rates of PGPR (15.00 kg·hm−2) and biochar (1500.00 kg·hm−2) were also determined based on the recommended application rates of organic remediation materials by the local agricultural department. In the TFPB2 treatment, PGPR and biochar were applied twice as much as in the TFPB treatment, with the aim of exploring whether increasing the application rate of both has a greater potential for improvement when farmers’ economic conditions allow.

Table 2.
The application rates of chemical fertilizer, PGPR, and biochar in different treatments.
In the experiment, prior to transplanting pepper plants, chemical fertilizer and biochar were utilized in the soil as a basal fertilizer. Afterward, a rotary tiller was utilized to blend the fertilizers with the soil thoroughly. Fifteen days after ridging, pepper seedlings were transplanted and then covered with a plastic film. PGPR was applied 20 days after transplanting the pepper seedlings. For the TFP and TFPB1 treatments, PGPR was applied by dissolving 1 kg of PGPR in 300 L of water. The solution was then allowed to stand for 6 h before being poured onto the roots of the peppers. The watering volume was 100 mL·plant−1. For the TFPB2 treatment, 2 kg of PGPR was dissolved in 300 L of water, keeping the other operational steps consistent with the abovementioned method. Furthermore, the PGPR solution was applied through irrigation in the TFP, TFPB1, and TFPB2 treatments. Similarly, the roots of peppers in the CK, TF, and TFB treatments were irrigated with water using the same method, with a watering volume of 100 mL plant−1. This method was employed to mitigate variations in pepper growth stemming from disparities in water supply. Referring to the local recommended planting density for pod peppers, it was 4.5 × 104 plants·ha−1. Each treatment was randomly assigned to a block for the experiment, and carried out in a plot area measuring 40.50 m2. Moreover, consistent field managements were employed to guarantee the precision of the test outcomes.
2.4. Soil Sampling and Analysis
Prior to the application of fertilizers, soil samples were collected from 15 randomly selected sites utilizing a soil auger, at a depth of 0–20 cm. We prepared soil samples according to the method of Ning et al. [29], and determined the physicochemical properties of the soil.
2.5. Plant Sampling and Analysis
In the pod pepper’s maturation phases, six plants were gathered from each plot. We prepared plant samples according to the method of Zhang et al. [28], followed by digestion using a mixture of concentrated H2SO4 and H2O2 to analyze the concentrations of N, P, and K [28]. Additionally, the fresh samples were harvested from each plot to analyze the levels of reducing sugars, VC, free amino acids, and nitrates [28].
2.6. Pepper Yield
The quantity of newly harvested pod peppers in each plot was evaluated according to the ripeness of the pods. The ultimate yield of fresh peppers was ascertained by gauging the combined weight across multiple harvests. Additionally, the moisture content of freshly collected pod peppers was established by subjecting them to laboratory drying. Following this, the yield of dehydrated pod peppers was computed.
2.7. Calculations
We calculated the NPK nutrient accumulation, fertilizer utilization efficiency, and economic benefits using the previous method [28]. It should be noted that the price per kilogram of dried pod pepper was CNY 20.00 in assessing economic benefits, and the cost of chemical fertilizer and biochar was 3350 and 2000 CNY·t−1, respectively. The PGPR was 20.00 CNY·kg−1.
2.8. Statistical Analysis
Single- and multiple-factor analysis of variance (ANOVA) were performed using the SPSS 20.0 software (SPSS Inc., Chicago, IL, USA). Duncan’s method was utilized for multiple comparisons at p < 0.05. The figures were created using Origin 8.0 software (OriginLab Corporation, Northampton, MA, USA).
3. Results
3.1. The Impact of Applying PGPR and Biochar on the Yield
The application of PGPR and biochar positively affected the pepper yield, as illustrated in Figure 1. When compared to TF treatment, the utilization of PGPR and biochar, either separately or in combination (TFP, TFB, TFPB1, and TFPB2), resulted in an increase in the yield of fresh peppers by 11.52–49.68% (2021) and 20.99–66.86% (2022). The TFPB2 treatment yielded the highest amount of fresh peppers over the span of two years, particularly in 2022, where it reached a maximum of 16,315 kg·ha−1. Furthermore, the findings indicated a similarity in the changes observed in both dry pepper yield and fresh pepper yield. In comparison to TF treatment, the applying PGPR and biochar alone or in combination resulted in a significant increase in dry pepper yield by 2.09–53.08% (2021) and 34.43–75.44% (2022), respectively. In both 2021 and 2022, the TFPB2 treatment demonstrated the highest yield of dry peppers.
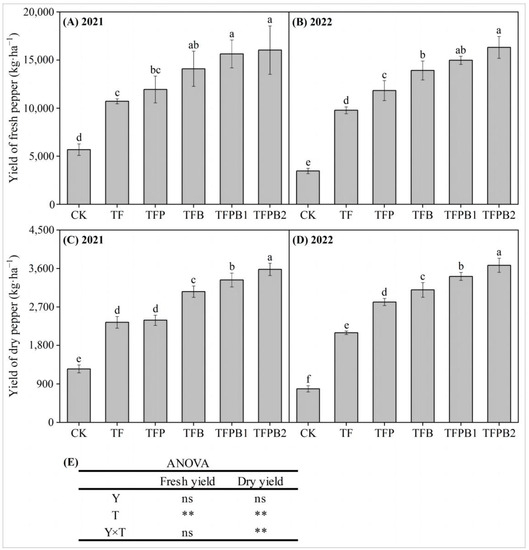
Figure 1.
The yield of fresh (A,B) and dry (C,D) peppers, and ANOVA (E) in different treatments. In (A–D), the different lowercase letters indicate significant differences among different treatments at p < 0.05 in the same year. In (E), Y represents year, T represents different treatments, and Y×T represents the interaction between year and treatment. ns represents no difference, ** represents statistical significance at p < 0.01. The same applies below.
3.2. The Impact of Applying PGPR and Biochar on the Quality of Fresh Pepper Fruits
The quality of fresh pepper fruits was improved by applying PGPR and biochar (Table 3). The results exhibited no significant difference in the content of free amino acids among all treatments in 2021. However, in 2022, the TFPB2 treatment exhibited a significantly higher free amino acid content than the TF treatment. In comparison to TF treatment, the applying PGPR and biochar alone or in combination led to a significant increase in reducing sugar content, with an increase of 24.90–50.87% in 2021 and 37.63–70.82% in 2022. The application of PGPR and biochar in combination (TFPB1 and TFPB2) resulted in an increase of 7.56–20.79% in reducing sugar content in 2021 and 7.42–24.12% in 2022, as compared to the PGPR and biochar treatment alone (TFP and TFB). In the meantime, the utilization of PGPR and biochar alone or in combination resulted in an increase in VC content of 8.42–32.63% in 2021 and 30.59–56.47% in 2022. Furthermore, the application of either PGPR or biochar alone, or their combination, resulted in a notable decrease in the nitrate content of fresh fruits by 16.24–21.61% in 2021 and 21.02–41.56% in 2022, compared to TF treatment. Notably, the combination of PGPR and biochar exhibited the most significant impact.

Table 3.
The fruit quality of fresh pepper in different treatments.
3.3. The Impact of Applying PGPR and Biochar on the NPK Accumulation
The application of PGPR and biochar exhibited a positive impact on the accumulation of NPK nutrients (Figure 2). Relative to the TF treatment, the application of PGPR and biochar, either individually or combined, resulted in an increase of 14.10–70.88%, 4.13–55.08%, and 12.20–49.23% in N, P, and K accumulation in 2021. Moreover, in 2022, the increase reached 42.82–125.60%, 40.54–143.95%, and 41.30–96.94% for the same nutrients. In 2021, the co-application of PGPR and biochar treatments (TFPB1 and TFPB2) resulted in an increase of N, P, and K accumulation by 13.23–49.77%, 16.66–48.93%, and 11.21–33.01%, respectively, compared to application alone (TFB and TFV). Similarly, in 2022, these combined treatments showed an increase of 13.70–57.95%, 15.12–73.58%, and 13.55–39.38% for N, P, and K accumulation, respectively.
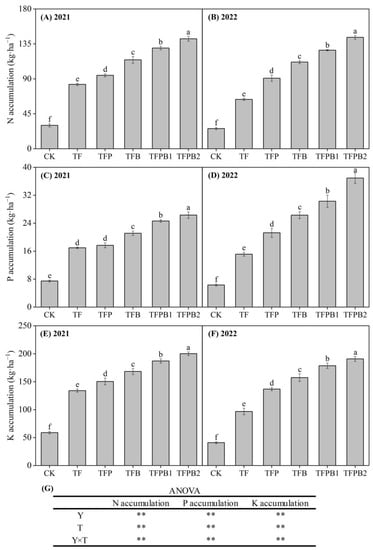
Figure 2.
The N accumulation (A,B), P accumulation (C,D), K accumulation (E,F) in different treatments, and ANOVA (G). In (A–F), the different lowercase letters indicate significant differences among different treatments at p < 0.05 in the same year. In (G), ** represents statistical significance at p < 0.01.
3.4. The Impact of Applying PGPR and Biochar on Agronomic and Recovery Efficiency of NPK
Table 4 demonstrates that the utilization of PGPR and biochar resulted in a significant enhancement in fertilizer efficiency. Compared to the TF treatment, the application of PGPR and biochar alone or in combination resulted in an increase of AEN, AEP, and AEK by 4.46–113.86%, 4.46–113.86%, and 4.46–113.86% in 2021. In 2022, the increase was even higher, ranging from 55.05–120.62%, 55.09–120.77%, and 55.05–120.62%, respectively. In 2021, the REN, REP, and REK values following PGPR and biochar application either alone or in combination were 1.22–2.11 times, 1.07–1.98 times, and 1.22–1.88 times higher than those of the TF treatment in 2021. In 2022, these values were 1.72–3.12 times, 1.70–3.48 times, and 1.71–2.68 times higher than those of the TF treatment, respectively. The co-application of PGPR and biochar treatments (TFPB1 and TFPB2) resulted in an average increase of 7.55% in REN, 8.83% in REP, and 13.32% in REK when compared to their individual application (TFP and TFB).

Table 4.
The fertilizer utilization of different treatments.
3.5. The Impact of Applying PGPR and Biochar on Output Value and Net Income
In comparison with TF treatment (Table 5), the application of PGPR and biochar, either alone or in combination, resulted in an increase in the OV of dry peppers by 2.09–53.08% (2021) and 34.43–75.44% (2022), respectively. Furthermore, compared to TF treatment, the net income of PGPR and biochar, either alone or in combination, increased by 1.63–43.90% in 2021 and 38.55–68.24% in 2022. The co-application of PGPR and biochar (TFPB1 and TFPB2) resulted in a 9.77–41.58% increase in net income in 2021 and an 11.20–21.43% increase in 2022, as compared to the application of PGPR or biochar alone (TFP and TFB). Among all treatments, the TFPB2 treatment exhibited the highest net income for dry peppers over the 2-year period, reaching 59,788 CNY·ha−1 in 2021 and 61,664 CNY·ha−1 in 2022.

Table 5.
The economic benefits of different treatments.
4. Discussion
The study findings demonstrated that PGPR and biochar application, individually or in combination, positively impacted the yield of continuous cropping peppers. Relative to the TF treatment, the yield of fresh peppers increased by 11.52–49.68% in 2021 and 20.99–66.86% in 2022. Meanwhile, the yield of dry peppers increased by 2.09–53.08% in 2021 and 34.43–75.44% in 2022. Studies have shown that PGPR has a significant impact on improving the microbial community structure of rhizosphere soil and enhancing the functional diversity of microorganisms, and such function promotes the growth of beneficial bacteria while inhibiting the growth of pathogenic bacteria [16,30]. Simultaneously, PGPR can create an optimal micro-ecological environment for the plant root system to augment water and fertilizer absorption capacity, thereby facilitating plant growth [31]. Moreover, the beneficial impacts of biochar on crop yields can be ascribed to various factors. Studies have demonstrated that the application of biochar can enhance soil quality. Specifically, biochar can not only addresses the issue of soil compaction caused by excessive fertilizer usage, but also promotes higher crop yields [32,33,34,35,36]. Significantly, utilizing biochar can heighten soil microorganism activity and enhance the microbiological setting, leading to improved conditions for crop root development and yield [37,38,39].
Interestingly, the co-application of PGPR and biochar in this study brought about more significant increases in yield compared to individual applications alone, demonstrating a synergistic effect of the co-application of PGPR and biochar. Previous studies also supported our conclusion. These studies highlighted that the combination of PGPR and biochar could be effective in enhancing the nutrient status and microbial environment of continuous crop barrier soils. The inhibition of harmful pathogens was identified as a significant mechanism [40,41]. This phenomenon can be attributed to the dual stimulating effects of PGPR and biochar [42,43]. On the one hand, PGPR can fulfill important functions, such as solubilizing phosphorus and potassium, fixing nitrogen, and decomposing effective nutrients in the soil. Additionally, they facilitate the release and sequestration of trace elements, thereby enhancing soil nutrient cycling [44,45]. On the other hand, the porous structure of biochar creates an ideal growth environment for PGPR, facilitating their propagation in the rhizosphere soil. This, in turn, leads to significant alterations in soil microbial function and community diversity [46,47].
The quality of fruit serves as a crucial criterion for evaluating the overall quality of agricultural products. The results showed the application of PGPR and biochar increased the levels of fresh pepper quality (Table 3). These results uncovered that the implementation of effective improvement measures positively influenced the quality enhancement of continuous cropping peppers. It has been found that PGPR can induce plant resistance by producing a variety of phytohormones, such as organic acids, gibberellins, and cytokinins [48]. They not only stimulate the accumulation of soluble proteins, proline, and other substances to counteract external environmental stress, but also enhance crop quality [49,50]. Moreover, the use of biochar encourages the harmonization and equilibrium of nutrient metabolism within crops due to its stable fertilization and continuous nutrient release, resulting in improved fruit quality [51,52]. Additionally, the enhancement in fruit quality may be attributed to the utilization of PGPR or biochar, which has been shown to increase the photosynthetic rate of leaves and facilitate the translocation of photosynthetic products to the fruit [27,53]. This process has also been found to have a positive impact on fruit quality. Notably, this study also observed the synergistic benefits of PGPR and biochar in enhancing the quality of continuous cropping peppers, which could be attributed to their complementary effects [47,54]; however this requires further research.
In this research, the application of PGPR and biochar, either alone or in combination, led to a significant increase in the accumulation of NPK (Figure 2) and a significant improvement in fertilizer utilization efficiency (Table 4). Research has shown that PGPR can expedite the decomposition of organic matter in the soil, facilitate the dissolution of insoluble nutrients, and subsequently release available nutrients to enhance the efficacy of soil nutrients. This process is also beneficial for the absorption and accumulation of mineral nutrients in plants [55,56]. In addition, research has indicated that the utilization of biochar enhances the soil carbon-to-nitrogen ratio and suppresses nitrogen conversion and denitrification through soil microorganisms, thereby facilitating the retention of NH4+ and NO3- in the soil [57,58]. Moreover, biochar can serve as a substitute for conventional phosphorus fertilizers due to its naturally high phosphorus content, which can also modify the dynamics and effectiveness of phosphorus in soils through processes like phosphorus adsorption and desorption, as well as by regulating the composition of the soil microbial community [59,60,61]. Furthermore, aside from directly contributing to potassium levels, biochar has the potential to enhance soil potassium content and boost the efficiency of potassium fertilizer utilization through the promotion of microbial activity [62,63]. Notably, the co-application of PGPR and biochar results in a further enhancement of nutrient accumulation and utilization by the plants, as compared to their individual application. This can be attributed to the enhanced colonization ability and survival rate of PGPR in rhizosphere soil through the addition of biochar [64,65]. Furthermore, the synergistic interaction between biochar and PGPR amplifies the capability of functional microorganisms to regulate nutrient transformations and cycling in the rhizosphere soil. This, in turn, promotes the accumulation of nutrients by plants and improves nutrient utilization efficiency [54,66]. We hypothesize that the synergistic mechanism of PGPR and biochar is mainly realized through the improvement of the soil microbial environment (including soil microorganisms and soil enzyme activities, etc.), and although the soil was not evaluated in this study, this will be the focus of our later research.
Importantly, it should be highlighted that the TFP treatment exhibited comparatively suboptimal results concerning both biological and economic aspects in the context of continuous pepper cropping, implying that relying solely on the application of PGPR was not an ideal approach. This could be attributed to the adherence of PGPR to the soil particles’ surface, which hindered the target microorganisms from colonizing the deep soil layers [67,68]. Consequently, this significantly impaired their ability to colonize the root surface [16,69]. Furthermore, the colonization of PGPR is influenced by various soil environmental factors, including temperature, oxygen, and moisture, as well as competition from native microorganisms [54,70]. Microbial immobilization technology is one approach to addressing the aforementioned issues, with biochar being recognized as an exceptional carrier for microorganism immobilization [71,72]. Hence, future studies should prioritize strengthening research on biochar-immobilized PGPR composites to enhance the soil remediation function of biochar and PGPR.
5. Conclusions
The study findings demonstrated that applying PGPR and biochar, either alone or in combination, resulted in increased productivity, improved fruit quality, enhanced fertilizer utilization, and enhanced economic benefits of continuous cropping peppers in the karst region of southwest China. In the current conditions, the synergistic use of PGPR and biochar emerged as the most effective strategy for boosting productivity and economic advantages in the continuous cropping peppers. Thus, the synergistic use of PGPR and biochar (TFPB1) is recommended to alleviate the issues of continuous cropping peppers grown in the karst yellow soil region of Southwest China. In addition, higher PGPR and biochar (TFPB2) can be applied to increase the production potential of continuous chili peppers under better economic conditions.
Author Contributions
M.Z. and J.G. designed the experiments and conducted the research; Y.L., Q.W., X.G., L.L. and M.W. performed most of the experiments and analyzed the data; M.Z. wrote the article. J.G. and M.W. provided recommendations for experiment conduct. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Guizhou Provincial Basic Research Program (Natural Science) (QKHJC-ZK[2023]ZD022) and the Science and Technology Innovation Special Project of Guizhou Academy of Agricultural Sciences (No.[2023]13).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
References
- Walia, S.S.; Kaur, T.; Gupta, R.K.; Siddiqui, M.H.; Rahman, M.A. Long-term impact of the continuous use of organic manures on crop and soil productivity under maize-potato-onion cropping systems. Sustainability 2023, 15, 8254. [Google Scholar] [CrossRef]
- Yang, W.; Zhao, Y.; Yang, Y.; Zhang, M.; Mao, X.; Guo, Y.; Li, X.; Tao, B.; Qi, Y.; Ma, L.; et al. Co-application of biochar and microbial inoculants increases soil phosphorus and potassium fertility and improves soil health and tomato growth. J. Soils Sediments 2023, 23, 947–957. [Google Scholar] [CrossRef]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Liu, Y.; Chen, H.; Hu, Y. Response of bacterial and fungal soil communities to Chinese Fir (Cunninghamia lanceolate) long-term monoculture plantations. Front. Microbiol. 2020, 11, 181. [Google Scholar] [CrossRef]
- Wang, B.; Lu, Y.; Li, W.; He, S.; Lin, R.; Qu, P.; Chen, H.; Zhang, F.; Zhao, M.; Shi, X.; et al. Effects of the continuous cropping of Amomum villosum on rhizosphere soil physicochemical properties, enzyme activities, and microbial communities. Agronomy 2022, 12, 2548. [Google Scholar] [CrossRef]
- Fan, Y.; Liu, J.; Liu, Z.; Hu, X.; Yu, Z.; Li, Y.; Chen, X.; Li, L.; Jin, J.; Wang, G. Chitin amendments eliminate the negative impacts of continuous cropping obstacles on soil properties and microbial assemblage. Front. Plant Sci. 2022, 13, 1067618. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xiong, X.; Tan, L.; Deng, Y.; Du, X.; Yang, X.; Hu, Q. Soil microbial community assembly and stability are associated with potato (Solanum tuberosum L.) fitness under continuous cropping regime. Front. Plant Sci. 2022, 13, 1000045. [Google Scholar] [CrossRef]
- Li, Z.; Alami, M.M.; Tang, H.; Zhao, J.; Nie, Z.; Hu, J.; Shu, S.; Zhu, D.; Yang, T. Applications of Streptomyces jingyangensis T. and Bacillus mucilaginosus A.improve soil health and mitigate the continuous cropping obstacles for Pinellia ternata (Thunb.) Breit. Ind. Crops Prod. 2022, 180, 114691. [Google Scholar] [CrossRef]
- Li, M.; Wang, J.; Zhou, Q.; Yasen, M. Effects of continuous melon cropping on rhizospheric fungal communities. Rhizosphere 2023, 27, 100726. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Biodegradation of soil-applied pesticides by selected strains of plant growth-promoting rhizobacteria (PGPR) and their effects on bacterial growth. Biodegradation 2011, 23, 297–310. [Google Scholar] [CrossRef]
- Hashem, A.; Tabassum, B.; Fathi Abd_Allah, E. Bacillus subtilis: A plant-growth promoting rhizobacterium that also impacts biotic stress. Saudi J. Biol. Sci. 2019, 26, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Kannan, P.; Paramasivan, M.; Marimuthu, S.; Swaminathan, C.; Bose, J. Applying both biochar and phosphobacteria enhances Vigna mungo L. growth and yield in acid soils by increasing soil pH, moisture content, microbial growth and P availability. Agric. Ecosyst. Environ. 2021, 308, 107258. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, H. Modification of rhizosphere microbial communities: A possible mechanism of plant growth promoting rhizobacteria enhancing plant growth and fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of plant growth promoting rhizobacteria in sustainable production of vegetables: Current perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Beheshti, M.; Etesami, H.; Alikhani, H.A. Interaction study of biochar with phosphate-solubilizing bacterium on phosphorus availability in calcareous soil. Arch. Agron. Soil Sci. 2017, 63, 1572–1581. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Ijaz, M.; Tahir, M.; Shahid, M.; Ul-Allah, S.; Sattar, A.; Sher, A.; Mahmood, K.; Hussain, M. Combined application of biochar and PGPR consortia for sustainable production of wheat under semiarid conditions with a reduced dose of synthetic fertilizer. Braz. J. Microbiol. 2019, 50, 449–458. [Google Scholar] [CrossRef]
- Malik, L.; Sanaullah, M.; Mahmood, F.; Hussain, S.; Siddique, M.H.; Anwar, F.; Shahzad, T. Unlocking the potential of co-applied biochar and plant growth-promoting rhizobacteria (PGPR) for sustainable agriculture under stress conditions. Chem. Biol. Technol. Agric. 2022, 9, 58. [Google Scholar] [CrossRef]
- Singh, I. Plant growth promoting rhizobacteria (PGPR) and their various mechanisms for plant growth enhancement in stressful conditions: A review. Eur. J. Biol. Res. 2018, 8, 191–213. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- He, M.; Xiong, X.; Wang, L.; Hou, D.; Bolan, N.S.; Ok, Y.S.; Tsang, D.C.W. A critical review on performance indicators for evaluating soil biota and soil health of biochar-amended soils. J. Hazard. Mater. 2021, 414, 125378. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, B.; Shen, J.; Zhu, X.; Yi, W.; Li, Y.; Wu, J. Contrasting effects of straw and straw-derived biochar applications on soil carbon accumulation and nitrogen use efficiency in double-rice cropping systems. Agric. Ecosyst. Environ. 2021, 311, 107286. [Google Scholar] [CrossRef]
- Yang, X.; Tsibart, A.; Nam, H.; Hur, J.; El-Naggar, A.; Tack, F.M.G.; Wang, C.H.; Lee, Y.H.; Tsang, D.C.W.; Ok, Y.S. Effect of gasification biochar application on soil quality: Trace metal behavior, microbial community, and soil dissolved organic matter. J. Hazard. Mater. 2019, 365, 684–694. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gu, X.; Liu, L.; Gou, J. Biochar application ameliorated the nutrient content and fungal community structure in different yellow soil depths in the karst area of Southwest China. Front. Plant Sci. 2022, 13, 1020832. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Gou, J. Effects of short-term application of Moutai lees biochar on nutrients and fungal community structure in yellow soil of Guizhou. Environ. Sci. Pollut. Res. 2021, 28, 67404–67413. [Google Scholar] [CrossRef]
- Phares, C.A.; Akaba, S. Co-application of compost or inorganic NPK fertilizer with biochar influences soil quality, grain yield and net income of rice. J. Integr. Agric. 2022, 21, 2600–3610. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Zhu, M.; Wan, H.; Chen, Z.; Yang, N.; Duan, J.; Wei, Z.; Hu, T.; Liu, F. Effects of plant growth promoting rhizobacteria (PGPR) strain bacillus licheniformis with biochar amendment on potato growth and water use efficiency under reduced irrigation regime. Agronomy 2022, 12, 1031. [Google Scholar] [CrossRef]
- Zhang, M.; Gou, J.; Wei, Q.; Liu, Y.; Qin, S. Effects of different biochar-based fertilizers on the biological properties and economic benefits of pod pepper (Capsicum annuum var. frutescens L.). Appl. Ecol. Environ. Res. 2021, 19, 2829–2841. [Google Scholar] [CrossRef]
- Ning, C.; Liu, R.; Kuang, X.; Chen, H.; Tian, J.; Cai, K. Nitrogen fertilizer reduction combined with biochar application maintain the yield and nitrogen supply of rice but improve the nitrogen use efficiency. Agronomy 2022, 12, 3039. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, X.; Shen, Z.; Zhu, C.; Jiao, Z.; Li, R.; Shen, Q. Pre-colonization of PGPR triggers rhizosphere microbiota succession associated with crop yield enhancement. Plant Soil 2019, 1–2, 553–567. [Google Scholar] [CrossRef]
- Anbuganesan, V.; Vishnupradeep, R.; Varshini, V.S.; Archana, A.S.; Soundarya, S.; Bruno, L.B.; Rajkumar, M. Effect of plant growth-promoting rhizobacteria and biochar on Ricinus communis growth, physiology, nutrient uptake and soil enzyme activities. Appl. Ecol. Environ. Sci. 2022, 10, 640–651. [Google Scholar] [CrossRef]
- Phares, C.A.; Amoakwah, E.; Danquah, A.; Afrifa, A.; Beyaw, L.R.; Frimpong, K.A. Biochar and NPK fertilizer co-applied with plant growth promoting bacteria (PGPB) enhanced maize grain yield and nutrient use efficiency of inorganic fertilizer. J. Agric. Food Res. 2022, 10, 100434. [Google Scholar] [CrossRef]
- Wang, S.; Gao, P.; Zhang, Q.; Shi, Y.; Guo, X.; Lv, Q.; Wu, W.; Zhang, X.; Li, M.; Meng, Q. Biochar improves soil quality and wheat yield in saline-alkali soils beyond organic fertilizer in a 3-year field trial. Environ. Sci. Pollut. Res. 2023, 30, 19097–19110. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhang, S.; Yan, P.; Aurangzeib, M. Effect of biochar application method and amount on the soil quality and maize yield in Mollisols of Northeast China. Biochar 2022, 4, 56. [Google Scholar] [CrossRef]
- Li, C.; Zhao, C.; Zhao, X.; Wang, Y.; Lv, X.; Zhu, X.; Song, X. Beneficial effects of biochar application with nitrogen fertilizer on soil nitrogen retention, absorption and utilization in maize production. Agronomy 2023, 13, 113. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, L.; Guo, X.; Liu, J.; Zou, B.; Guo, Y.; Zhang, Y.; Li, H.; Zheng, G.; Guo, Y.; et al. Effect of biochar on soil physiochemical properties and bacterial diversity in dry direct-seeded rice paddy fields. Agronomy 2023, 13, 4. [Google Scholar] [CrossRef]
- Wan, H.; Liu, X.; Shi, Q.; Chen, Y.; Jiang, M.; Zhang, J.; Cui, B.; Hou, J.; Wei, Z.; Hossain, M.A.; et al. Biochar amendment alters root morphology of maize plant: Its implications in enhancing nutrient uptake and shoot growth under reduced irrigation regimes. Front. Plant Sci. 2023, 14, 112742. [Google Scholar] [CrossRef]
- Manzoor; Ma, L.; Ni, K.; Ruan, J. Effect of integrated use of rapeseed cake, biochar and chemical fertilizers on root growth, nutrients use efficiency and productivity of tea. Agronomy 2022, 12, 1823. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Xiu, L.; Sun, Y.; Gu, W.; Wang, Y.; Zhang, H.; Chen, W. Soybean yield response of biochar-regulated soil properties and root growth strategy. Agronomy 2022, 12, 1412. [Google Scholar] [CrossRef]
- Hosseini, E.; Zarei, M.; Sepehri, M.; Safarzadeh, S. Do bagasse biochar and microbial inoculants positively affect barley grain yield and nutrients, and microbial activity? J. Plant Nutr. 2021, 45, 522–539. [Google Scholar] [CrossRef]
- Saxena, J.; Rana, G.; Pandey, M. Impact of addition of biochar along with Bacillus sp. on growth and yield of French beans. Sci. Hortic. 2013, 162, 351–356. [Google Scholar] [CrossRef]
- Medeiros, E.V.D.; Silva, L.F.D.; Silva, J.S.A.D.; Costa, D.P.D.; Souza, C.A.F.D.; Berger, L.R.R.; Lima, J.R.D.S.; Hammecker, C. Biochar and Trichoderma spp. in management of plant diseases caused by soilborne fungal pathogens: A review and perspective. Res. Soc. Dev. 2021, 10, e296101522465. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, W.; Sun, Y.; Peng, Y.; Niu, J.; Tan, J.; Wei, M. Biochar and its coupling with microbial inoculants for suppressing plant diseases: A review. Appl. Soil Ecol. 2023, 190, 105025. [Google Scholar] [CrossRef]
- Gorovtsov, A.V.; Minkina, T.M.; Mandzhieva, S.S.; Perelomov, L.V.; Soja, G.; Zamulina, I.V.; Rajput, V.D.; Sushkova, S.N.; Mohan, D.; Yao, J. The mechanisms of biochar interactions with microorganisms in soil. Environ. Geochem. Health 2020, 42, 2495–2518. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, Y.; Wang, Y.; Rong, X.; Peng, J.; Feia, J.; Luo, G. Biochar amendments combined with organic fertilizer improve maize productivity and mitigate nutrient loss by regulating the C-N-P stoichiometry of soil, microbiome, and enzymes. Chemosphere 2023, 324, 138293. [Google Scholar] [CrossRef]
- Bolan, S.; Hou, D.; Wang, L.; Hale, L.; Egamberdieva, D.; Tammeorg, P.; Li, R.; Wang, B.; Xu, J.; Wang, T.; et al. The potential of biochar as a microbial carrier for agricultural and environmental applications. Sci. Total Environ. 2023, 886, 163968. [Google Scholar] [CrossRef]
- Tripti; Kumar, A.; Usmani, Z.; Kumar, V.; Anshumali. Biochar and flyash inoculated with plant growth promoting rhizobacteria act as potential biofertilizer for luxuriant growth and yield of tomato plant. J. Environ. Manag. 2017, 190, 20–27. [Google Scholar] [CrossRef]
- Hale, L.; Luth, M.; Kenney, R.; Crowley, D. Evaluation of pinewood biochar as a carrier of bacterial strain Enterobacter cloacae UW5 for soil inoculation. Appl. Soil Ecol. 2014, 84, 192–199. [Google Scholar] [CrossRef]
- Shi, J.W.; Lu, L.X.; Shi, H.M.; Ye, J.R. Effects of plant growth-promoting rhizobacteria on the growth and soil microbial community of Carya illinoinensis. Curr. Microbiol. 2023, 79, 352. [Google Scholar] [CrossRef]
- He, Y.; Wu, Z.; Ye, B.C.; Wang, J.; Guan, X.; Zhang, J. Viability evaluation of alginate-encapsulated Pseudomonas putida Rs-198 under simulated salt-stress conditions and its effect on cotton growth. Eur. J. Soil Biol. 2016, 75, 135–141. [Google Scholar] [CrossRef]
- Almaroai, Y.A.; Eissa, M.A. Effect of biochar on yield and quality of tomato grown on a metal-contaminated soil. Sci. Hortic. 2020, 265, 109210. [Google Scholar] [CrossRef]
- Lévesque, V.; Jeanne, T.; Dorais, M.; Ziadi, N.; Hogue, R.; Antoun, H. Biochars improve tomato and sweet pepper performance and shift bacterial composition in a peat-based growing medium. Appl. Soil Ecol. 2020, 153, 103579. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Du, B.; Li, H. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Gupta, S.; Kaushal, R.; Sood, G. Impact of plant growth-promoting rhizobacteria on vegetable crop production. Int. J. Veg. Sci. 2018, 24, 289–300. [Google Scholar] [CrossRef]
- Ullah, S.; Ali, I.; Yang, M.; Zhao, Q.; Iqbal, A.; Wu, X.; Ahmad, S.; Muhammad, I.; Khan, A.; Adnan, M.; et al. Partial substitution of urea with biochar induced improvements in soil enzymes activity, ammonia-nitrite oxidizers, and nitrogen uptake in the double-cropping rice system. Microorganisms 2023, 11, 527. [Google Scholar] [CrossRef]
- Zhao, P.; Wang, S.; Liu, D.; Li, H.; Han, S.; Li, M. Study on influence mechanism of biochar on soil nitrogen conversion. Environ. Pollut. Bioavailab. 2022, 34, 419–432. [Google Scholar] [CrossRef]
- Yan, H.; Liu, C.; Yu, W.; Zhu, X.; Chen, B. The aggregate distribution of Pseudomonas aeruginosa on biochar facilitates quorum sensing and biofilm formation. Sci. Total Environ. 2023, 856, 159034. [Google Scholar] [CrossRef]
- Kong, F.; Ling, X.; Iqbal, B.; Zhou, Z.; Meng, Y. Soil phosphorus availability and cotton growth affected by biochar addition under two phosphorus fertilizer levels. Arch. Agron. Soil Sci. 2023, 69, 18–31. [Google Scholar] [CrossRef]
- Pokharel, P.; Chang, S.X. Biochar decreases and nitrification inhibitor increases phosphorus limitation for microbial growth in a wheat-canola rotation. Sci. Total Environ. 2023, 858, 159773. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, K.; Shaghaleh, H.; Qi, Z.; Gao, C.; Chang, T.; Zhang, J.; Zia-ur-Rehman, M.; Hamoud, Y.A. Continuous cropping alters soil hydraulic and physicochemical properties in the karst region of southwestern China. Agronomy 2023, 13, 1416. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Liu, B.; Li, Y.; El-Desouki, Z.; Jiang, C. Over two years study: Peanut biochar promoted potassium availability by mediating the relationship between bacterial community and soil properties. Appl. Soil Ecol. 2022, 176, 104485. [Google Scholar] [CrossRef]
- Bertola, M.; Mattarozzi, M.; Sanangelantoni, A.M.; Careri, M.; Visioli, G. PGPB colonizing three-year biochar-amended soil: Towards biochar-mediated biofertilization. J. Soil Sci. Plant Nutr. 2019, 19, 841–850. [Google Scholar] [CrossRef]
- Zheng, B.X.; Ding, K.; Yang, X.R.; Wadaan, M.A.M.; Hozzein, W.N.; Peñuelas, J.; Zhu, Y.G. Straw biochar increases the abundance of inorganic phosphate solubilizing bacterial community for better rape (Brassica napus) growth and phosphate uptake. Sci. Total Environ. 2019, 647, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Huang, B.; Fernández-García, V.; Miesel, J.; Yan, L.; Lv, C. Biochar and rhizobacteria amendments improve several soil properties and bacterial diversity. Microorganisms 2020, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Santoyo, G.; Urtis-Flores, C.A.; Loeza-Lara, P.D.; Orozco-Mosqueda, M.d.C.; Glick, B.R. Rhizosphere colonization determinants by plant growth-promoting rhizobacteria (PGPR). Biology 2021, 10, 475. [Google Scholar] [CrossRef]
- Batista, B.D.; Lacava, P.T.; Ferrari, A.; Teixeira-Silva, N.S.; Bonatelli, M.L.; Tsui, S.; Mondin, M.; Kitajima, E.W.; Pereira, J.O.; Azevedo, J.L.; et al. Screening of tropically derived, multi-trait plant growth- promoting rhizobacteria and evaluation of corn and soybean colonization ability. Microbiol. Res. 2018, 206, 33–42. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Li, S.; Xu, B.; Gong, Y.; Yang, Y.; Sun, H. Isolation and engineering of plant growth promoting rhizobacteria Pseudomonas aeruginosa for enhanced cadmium bioremediation. J. Gen. Appl. Microbiol. 2016, 62, 258–265. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.; Alhewairini, S.S.; Peng, C.; Naz, I. Application of microbial immobilization technology for remediation of Cr(VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Liu, S.; Tang, W.; Yang, F.; Meng, J.; Chen, W.; Li, X. Influence of biochar application on potassium-solubilizing Bacillus mucilaginosus as potential biofertilizer. Prep. Biochem. Biotechnol. 2016, 47, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; An, Q.; Zhou, Y.; Deng, S.; Miao, Y.; Zhao, B.; Yang, L. Highly synergistic effects on ammonium removal by the co-system of Pseudomonas stutzeri XL-2 and modified walnut shell biochar. Bioresour. Technol. 2019, 280, 239–346. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).