The Extension of Vase Life in Cut Gerbera Flowers through Pretreatment with Gibberellin A3 in Combination with Calcium Chloride
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemical Treatment and Evaluation of Vase Life
2.3. Relative Area of Unopened Tubular Florets
2.4. Measurement of Fresh Weight, Water Uptake, Transpiration, and Elongation of the Flower Stems
2.5. Measurement of EC and pH in CaCl2 Solution
2.6. Statistical Analysis
3. Results
3.1. Effect of GA3 Concentrations on the Vase Life of Cut Gerbera ‘Minou’
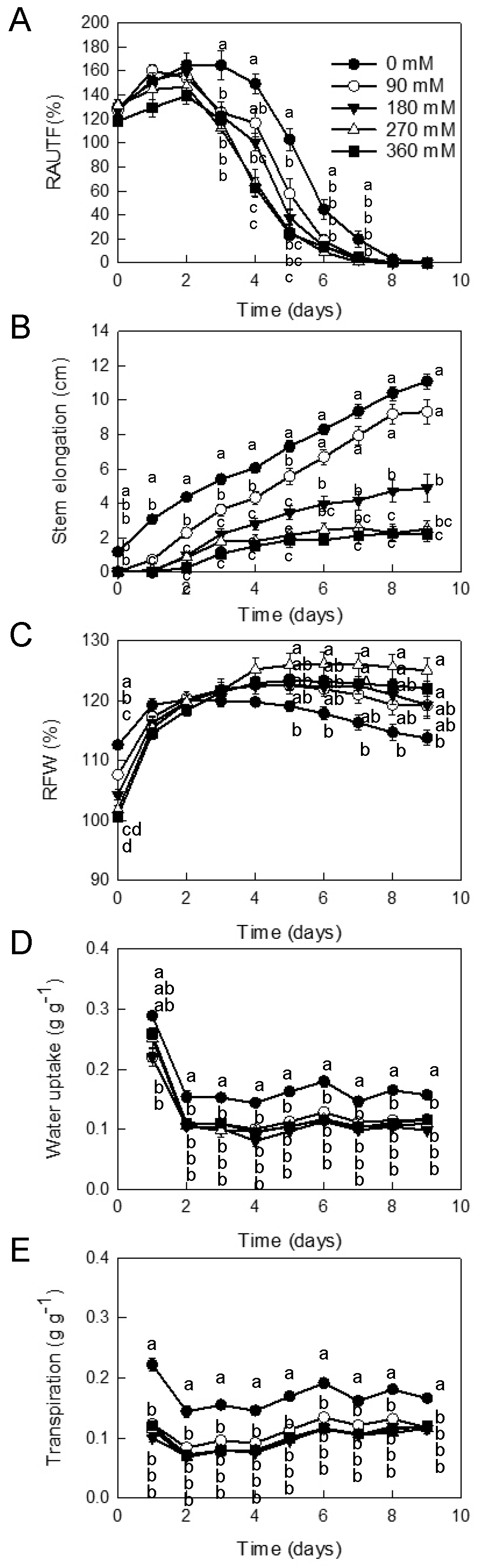
3.2. Effect of CaCl2 Concentrations Combined with GA3 on the Vase Life of Cut Gerbera ‘Minou’
3.3. Effect of GA3, CaCl2, and the Combination on the Vase Life of Gerbera ‘Minou’
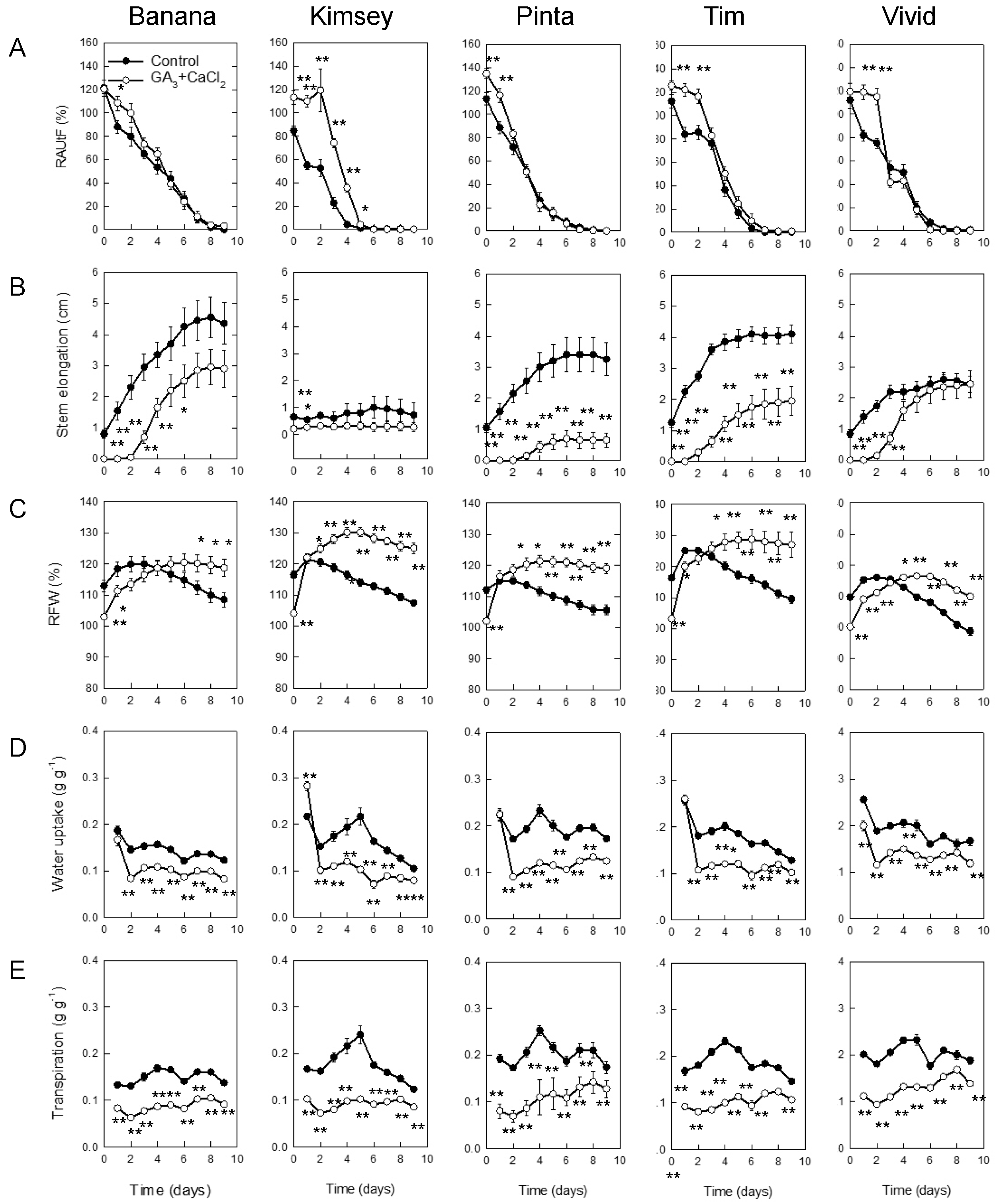
3.4. Effect of Combined Treatment with GA3 and CaCl2 on the Vase Life of the Five Gerbera Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shabanian, S.; Esfahani, M.N.; Karamian, R.; Tran, L.-S.P. Physiological and biochemical modifications by postharvest treatment with sodium nitroprusside extend vase life of cut flowers of two gerbera cultivars. Postharvest Biol. Technol. 2018, 137, 1–8. [Google Scholar] [CrossRef]
- Tonooka, M.; Homma, Y.; Toyoizumi, T.; Ichimura, K. Stem bending in cut gerbera under the condition of suppressing bacterial proliferation is associated with the weakening of the stem strength. Sci. Hortic. 2023, 319, 112153. [Google Scholar] [CrossRef]
- Wernett, H.C.; Sheehan, T.J.; Wilfret, G.J.; Marousky, F.J.; Lyrene, P.M.; Knauft, D.A. Postharvest longevity of cut-flower Gerbera. I. Response to selection for vase life components. J. Am. Soc. Hort. Sci. 1996, 121, 216–221. [Google Scholar]
- Ruiz, L.P.; Atkinson, C.J.; Mansfield, T.A. Calcium in the xylem and its influence on the behavior of stomata. Phil. Trans. R. Soc. Lond. B 1993, 341, 67–74. [Google Scholar]
- van Meeteren, U.; van Gelder, H.; van Ieperen, W. Reconsideration of the use of deionized water as vase water in postharvest experiments on cut flowers. Postharvest Biol. Technol. 1999, 17, 175–187. [Google Scholar] [CrossRef]
- Milani, M.; Pradella, E.M.; Heintze, W.; Schafer, G.; Bender, R.J. The effects of supplemental nitrogen and calcium on the quality and postharvest life of cut gerbera. Ornam. Hortic. 2020, 25, 365–373. [Google Scholar] [CrossRef]
- Gerasopoulos, D.; Chebli, B. Effects of pre-and postharvest calcium applications on the vase life of cut gerberas. J. Hort. Sci. Biotech. 1999, 74, 78–81. [Google Scholar] [CrossRef]
- Geshnizjany, N.; Ramezanian, A.; Khosh-Khui, M. Postharvest life of cut gerbera (Gerbera jamesonii) as affected by nano-silver particles and calcium chloride. Int. J. Hortic. Sci. Technol. 2014, 1, 171–180. [Google Scholar]
- Perik, R.R.J.; Razé, D.; Ferrante, A.; van Doorn, W.G. Stem bending in cut Gerbera jamesonii flowers: Effects of a pulse treatment with sucrose and calcium ions. Postharvest Biol. Technol. 2014, 98, 7–13. [Google Scholar] [CrossRef]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Hicklenton, P.B. GA3 and benzylaminopurine delay leaf yellowing in cut Alstroemeria stems. HortScience 1991, 26, 1198–1199. [Google Scholar] [CrossRef]
- Han, S.S. Growth regulators delay foliar chlorosis of Easter lily leaves. J. Am. Soc. Hortic. Sci. 1995, 120, 254–258. [Google Scholar] [CrossRef]
- Ichimura, K.; Goto, R. Effect of gibberellin A3 on leaf yellowing and vase life of cut Narcissus tazetta var. chinensis flowers. J. Jpn. Soc. Hortic. Sci. 2000, 69, 423–427. [Google Scholar] [CrossRef]
- Goszczynska, D.M.; Zieslin, N.; Mor, Y.; Halevy, A.H. Improvement of postharvest keeping quality of Mercedes roses by gibberellin. Plant Growth Regul. 1990, 9, 293–303. [Google Scholar] [CrossRef]
- Sabehat, A.; Zieslin, N. Promotion of postharvest increase in weight of rose (Rosa hybrida cv. Mercedes) petals by gibberellin. J. Plant Physiol. 1995, 145, 296–298. [Google Scholar] [CrossRef]
- Steinz, B.; Cohen, A. Gibberellic acid promotes flower bud opening on detached flower stalks of statice (Limonium sinuatum L.). HortScience 1982, 17, 903–904. [Google Scholar] [CrossRef]
- Saks, Y.; van Staden, J.; Smith, M.T. Effect of gibberellic acid on carnation flower senescence: Evidence that the delay of carnation flower senescence by gibberellic acid depends on the stage of flower development. Plant Growth Regul. 1992, 11, 45–51. [Google Scholar] [CrossRef]
- Emongor, V.E. Effects of gibberellic acid on postharvest quality and vase life of gerbera cut flower (Gerbera jamesonii). J. Agron. 2004, 3, 191–195. [Google Scholar] [CrossRef]
- Ichimura, K.; Shimizu-Yumoto, H.; Shibuya, K.; Mochizuki, H. Investigation of the vase life of cut flowers in different seasons. Bull. Natl. Inst. Flor. Sci. 2011, 11, 49–65, (In Japanese with English Abstract). [Google Scholar]
- Kato, M.; Kanda, M.; Ichimura, K. Effects of pulse treatments with sucrose, silver thiosulfate and calcium chloride on the vase life and soluble carbohydrate and aurone levels in cut snapdragon flowers. Hortic. J. 2022, 91, 112–121. [Google Scholar] [CrossRef]
- Umeda, S.; Tonooka, M. Influence of season, cultivar, and age on the yield components of Gerbera jamesonii over two years. Bull. Shizuoka Res. Inst. Agric. For. 2020, 13, 1–6, (In Japanese with English Abstract). [Google Scholar]
- Okubo, H.; Uemoto, S. Changes in endogenous gibberellin and auxin activities during first internode elongation in tulip flower stalk. Plant Cell Physiol. 1985, 26, 709–719. [Google Scholar] [CrossRef]
- van Doorn, W.G.; Perik, R.R.J.; Abadie, P.; Harkema, H. A treatment to improve the vase life of cut tulips: Effects on tepal senescence, tepal abscission, leaf yellowing and stem elongation. Postharvest Biol. Technol. 2011, 61, 56–63. [Google Scholar] [CrossRef]
- Cowling, R.J.; Harberd, N.P. Gibberellins control Arabidopsis hypocotyl growth via regulation of cellular elongation. J. Exp. Bot. 1999, 50, 1351–1357. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, J. Effect of gibberellin and uniconazole on mesocotyl elongation of dark-grown maize under different seeding depths. Plant Prod. Sci. 2008, 11, 423–429. [Google Scholar] [CrossRef]
- Tong, H.; Xiao, Y.; Liu, D.; Gao, S.; Liu, L.; Yin, Y.; Jin, Y.; Qian, Q.; Chua, C. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell 2014, 26, 4376–4393. [Google Scholar] [CrossRef]
- van den Heuvel, K.J.P.T.; Barendse, G.W.M.; Wullems, G.J. Effect of gibberellic acid on cell division and cell elongation in anthers of the gibberellin deficient gib-1 mutant of tomato. Plant Biol. 2001, 3, 124–131. [Google Scholar] [CrossRef]
- Chen, J.-J.; Sun, Y.-W.; Sheen, T.-F. Use of cold water for irrigation reduces stem elongation of plug-grown tomato and cabbage seedlings. HortScience 1999, 34, 852–854. [Google Scholar] [CrossRef]
- Litvin, A.G.; van Iersel, M.W.; Malladi, A. Drought stress reduces stem elongation and alters gibberellin-related gene expression during vegetative growth of tomato. J. Am. Soc. Hortic. Sci. 2016, 141, 591–597. [Google Scholar] [CrossRef]
- Mutasa-Göttgens, E.; Hedden, P. Gibberellin as a factor in floral regulatory networks. J. Exp. Bot. 2009, 60, 1979–1989. [Google Scholar] [CrossRef]
- Weiss, D.; Halevy, A.H. Stamens and gibberellin in the regulation of corolla pigmentation and growth in Petunia hybrida. Planta 1989, 179, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, T. Effect of gibberellin biosynthesis inhibitor on prevention of precocious bolting and flowering in Japanese radish (Raphanus sativus L.). JARQ 2003, 37, 175–181. [Google Scholar] [CrossRef][Green Version]
- van Doorn, W.G.; de Witte, Y. Effect of bacteria on scape bending in cut Gerbera jamesonii flowers. J. Am. Soc. Hortic. Sci. 1994, 119, 568–571. [Google Scholar] [CrossRef]
- Tonooka, M.; Homma, Y.; Nukui, H.; Ichimura, K. The effects of bacteria in vase water on the vase life of cut gerbera cultivars. Hortic. Res. 2019, 18, 167–172, (In Japanese with English Abstract). [Google Scholar] [CrossRef][Green Version]

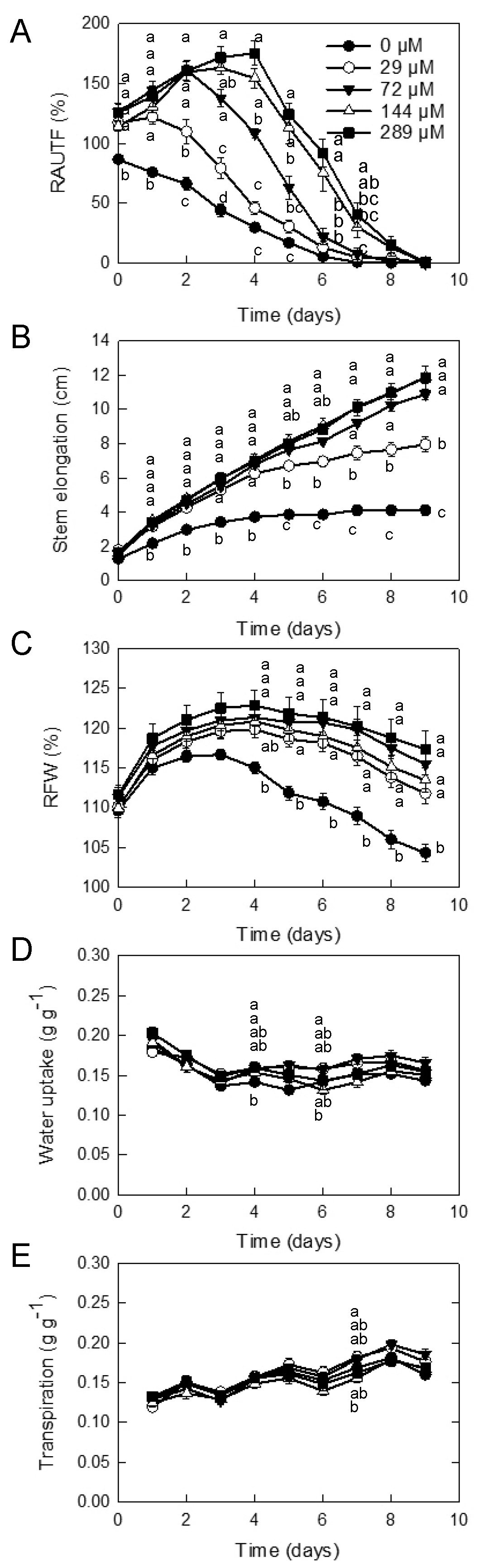


| GA3 | Solution Uptake | GA3 Uptake | Vase Life | Ornamental Value Loss Symptoms (%) | Vase Life of Flowers Showing Symptoms Other than Bending | ||||
|---|---|---|---|---|---|---|---|---|---|
| (µM) | (g g−1) | (μg g−1) | (Days) | Bending | Other | (Days) | |||
| 0 | 0.20 | 0 | a | 16.1 | a | 0 | 100 | 16.1 | b |
| 29 | 0.21 | 2.1 | a | 15.4 | a | 80 | 20 | 16.5 | b |
| 72 | 0.22 | 5.4 | b | 12.5 | b | 70 | 30 | 19.7 | a |
| 144 | 0.20 | 10.1 | c | 9.1 | b | 100 | 0 | ||
| 289 | 0.22 | 22.5 | d | 9.4 | b | 100 | 0 | ||
| CaCl2 | EC | pH | Solution Uptake | CaCl2 Uptake | Vase Life | Ornamental Value Loss Symptoms (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (mM) | (S m−1) | (g g−1) | (mg g−1) | (Days) | Bending | Other | ||||
| 0 | 0.00 | 4.55 | 0.29 | a | 0 | e | 7.7 | b | 100 | 0 |
| 90 | 1.75 | 4.68 | 0.20 | b | 2.0 | d | 10.9 | b | 100 | 0 |
| 180 | 3.21 | 5.19 | 0.16 | c | 3.1 | c | 17.6 | a | 50 | 50 |
| 270 | 4.63 | 6.38 | 0.13 | c | 4.0 | b | 19.3 | a | 10 | 90 |
| 360 | 5.80 | 7.28 | 0.13 | c | 5.1 | a | 19.2 | a | 10 | 90 |
| CaCl2 | GA3 | Solution Uptake | CaCl2 Uptake | GA3 Uptake | Vase Life | Ornamental Value Loss Symptoms (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (mM) | (µM) | (g g−1) | (mg g−1) | (μg g−1) | (Days) | Bending | Other | ||||
| 0 | 0 | 0.29 | a | 0 | b | 0 | c | 15.8 | b | 0 | 100 |
| 270 | 0 | 0.14 | b | 4.2 | a | 0 | c | 14.8 | b | 0 | 100 |
| 0 | 144 | 0.32 | a | 0 | b | 16.0 | a | 9.0 | c | 90 | 10 |
| 270 | 144 | 0.15 | b | 4.5 | a | 7.5 | b | 18.6 | a | 30 | 70 |
| Cultivar | Treatment | Solution Uptake | CaCl2 Uptake | GA3 Uptake | Vase Life | Ornamental Value Loss Symptoms (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g g−1) | (mg g−1) | (μ g−1) | (Days) | Bending | Other | ||||||
| Banana | Control | 0.26 | 0 | 0 | 14.0 | 0 | 100 | ||||
| GA3 + CaCl2 | 0.13 | ** 1 | 3.8 | ** | 6.3 | ** | 17.3 | ** | 0 | 100 | |
| Kimsey | Control | 0.33 | 0 | 0 | 15.5 | 0 | 100 | ||||
| GA3 + CaCl2 | 0.14 | ** | 4.3 | ** | 7.1 | ** | 19.7 | ** | 0 | 100 | |
| Pinta | Control | 0.28 | 0 | 0 | 15.9 | 0 | 100 | ||||
| GA3 + CaCl2 | 0.12 | ** | 3.5 | ** | 5.8 | ** | 20.3 | ** | 0 | 100 | |
| Tim | Control | 0.33 | 0 | 0 | 13.9 | 0 | 100 | ||||
| GA3 + CaCl2 | 0.14 | ** | 4.2 | ** | 7.0 | ** | 16.7 | ** | 0 | 100 | |
| Vivid | Control | 0.29 | 0 | 0 | 9.9 | 0 | 100 | ||||
| GA3 + CaCl2 | 0.14 | ** | 4.0 | ** | 6.8 | ** | 14.3 | ** | 10 | 90 | |
| Two-way | Cultivar (C) | ** | ** | ** | ** | ||||||
| ANOVA 2 | Treatment (T) | ** | ** | ** | ** | ||||||
| C × T | * | ** | ** | NS | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonooka, M.; Homma, Y.; Nukui, H.; Ichimura, K. The Extension of Vase Life in Cut Gerbera Flowers through Pretreatment with Gibberellin A3 in Combination with Calcium Chloride. Horticulturae 2023, 9, 1106. https://doi.org/10.3390/horticulturae9101106
Tonooka M, Homma Y, Nukui H, Ichimura K. The Extension of Vase Life in Cut Gerbera Flowers through Pretreatment with Gibberellin A3 in Combination with Calcium Chloride. Horticulturae. 2023; 9(10):1106. https://doi.org/10.3390/horticulturae9101106
Chicago/Turabian StyleTonooka, Makoto, Yoshiyuki Homma, Hideki Nukui, and Kazuo Ichimura. 2023. "The Extension of Vase Life in Cut Gerbera Flowers through Pretreatment with Gibberellin A3 in Combination with Calcium Chloride" Horticulturae 9, no. 10: 1106. https://doi.org/10.3390/horticulturae9101106
APA StyleTonooka, M., Homma, Y., Nukui, H., & Ichimura, K. (2023). The Extension of Vase Life in Cut Gerbera Flowers through Pretreatment with Gibberellin A3 in Combination with Calcium Chloride. Horticulturae, 9(10), 1106. https://doi.org/10.3390/horticulturae9101106






