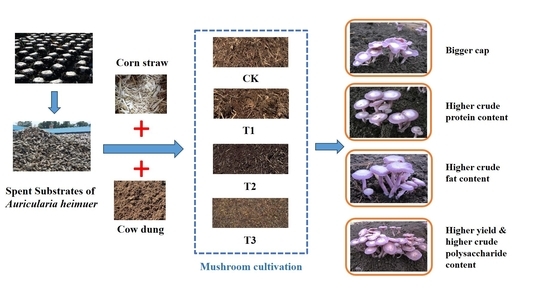
Turn Waste into Treasure: Spent Substrates of Auricularia heimuer Can Be Used as the Substrate for Lepista sordida Cultivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lepista Sordida Strain
2.2. Substrate Preparation
2.2.1. Preparation of SSA and Corn Straw
SSA Preparation
Corn Straw Preparation
2.2.2. Analysis of the Proximate Components of SSA
2.3. Substrate Formula
2.4. The Specific Manner of Fermentation
2.5. Key Points of Cultivation
2.6. Investigation of Agronomic Traits
2.7. Chemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Components of SSA
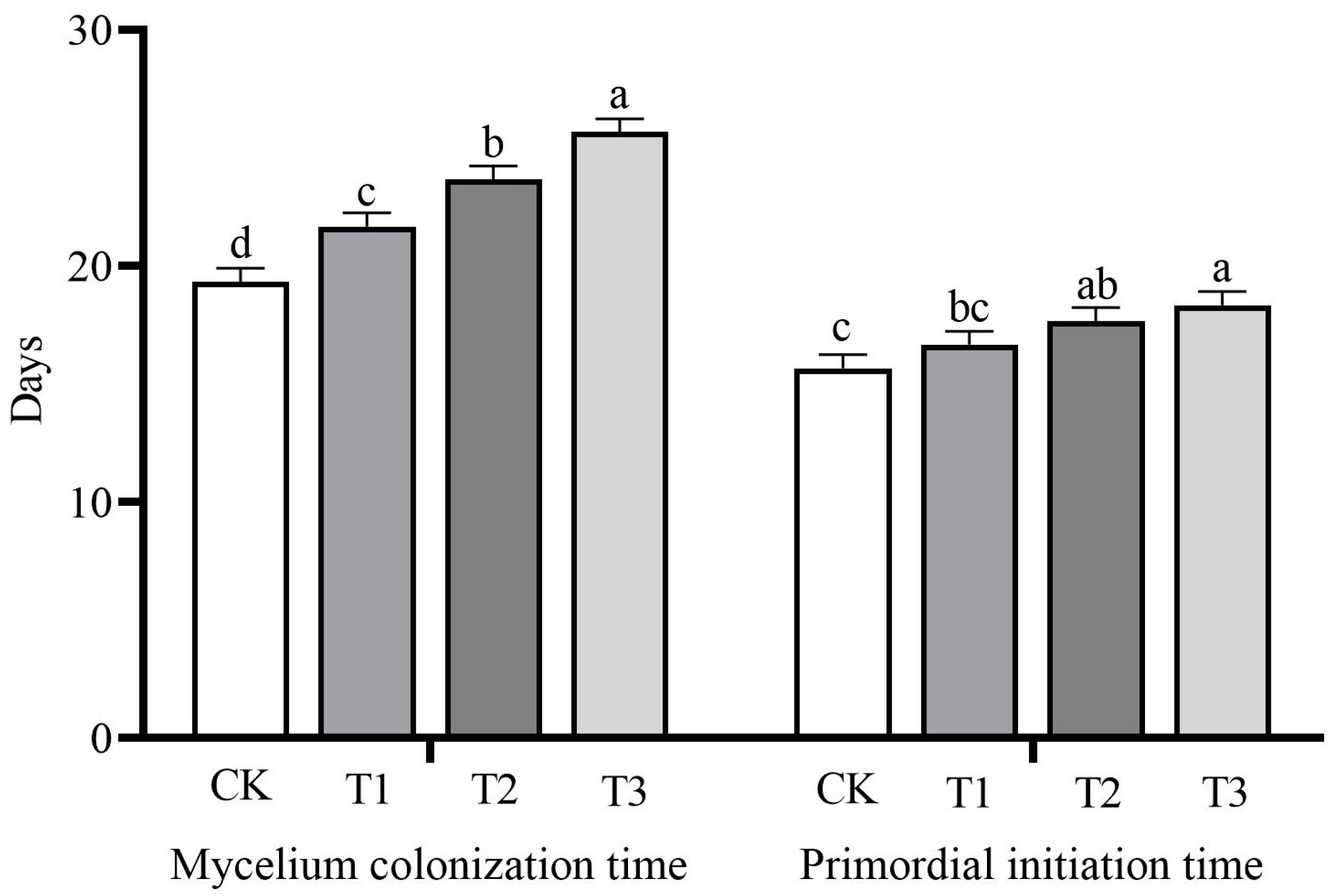
3.2. Mycelia Growth and Primordial Initiation
3.3. Fruiting Body Morphology
3.4. Yield and Biological Efficiency
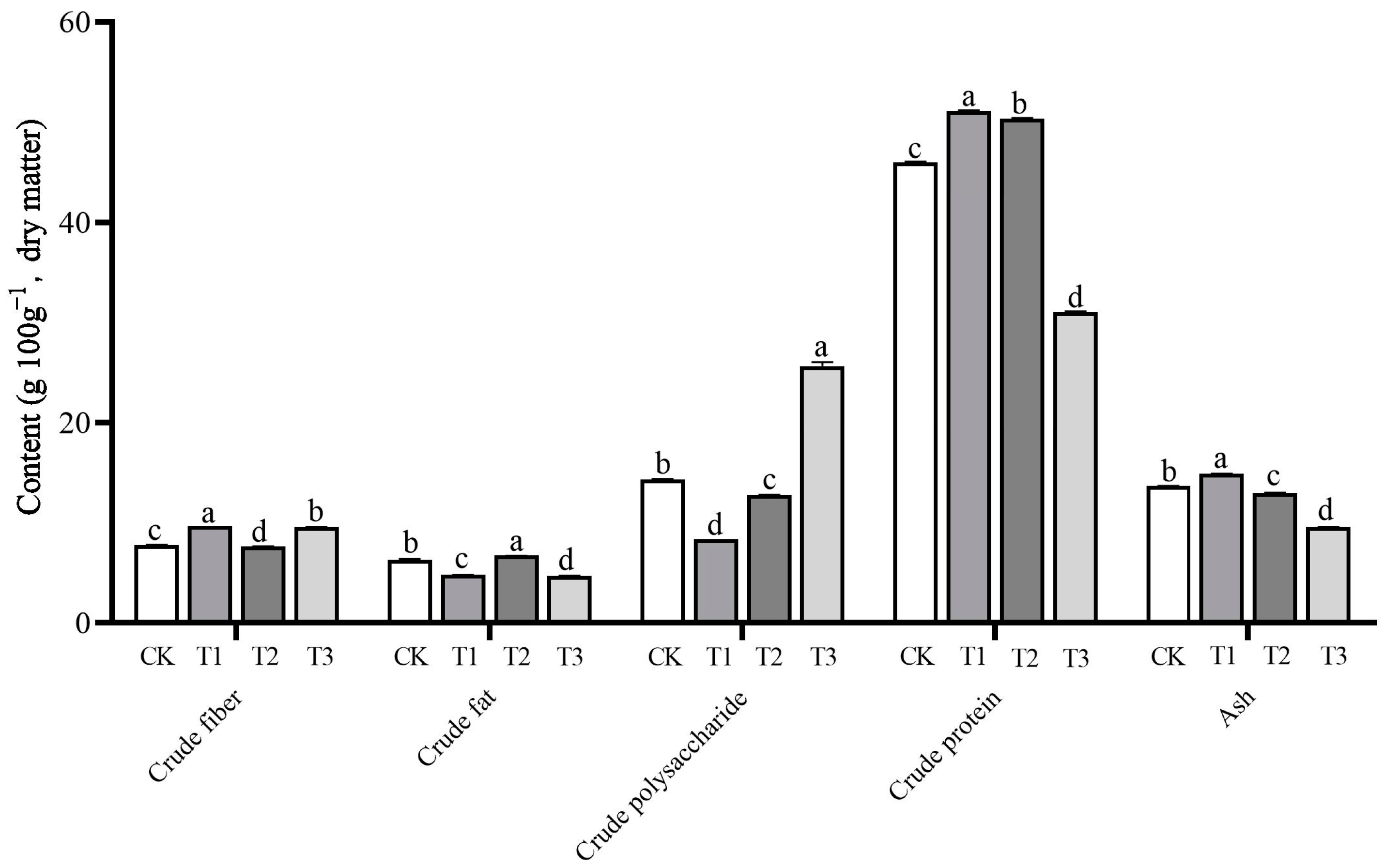
3.5. Nutritional Value
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thongbai, B.; Wittstein, K.; Richter, C.; Steven, L.M.; Kevin, D.H.; Naritsada, T.; Namphung, K.; Ekachai, C.; Marc, S. Successful cultivation of a valuable wild strain of Lepista sordida from Thailand. Mycol. Prog. 2017, 16, 311–323. [Google Scholar] [CrossRef]
- Li, Y.; Li, T.H.; Yang, Z.L.; Bau, T.; Dai, Y.C. Atlas of Chinese Macrofungal Resources, 1st ed.; Zhongyuan Farmers Publishing House: Zhengzhou, China, 2015; pp. 907–908. [Google Scholar]
- Lu, C.Y.; Zhong, Y.J.; Rao, L.Q. The nutrient constituents analyses on three species of wild edible mushrooms domesticated. Jishou Univ. (Nat. Sci. Ed.) 1993, 14, 38–41. [Google Scholar]
- Luo, X.Y.; Hong, J.; Zhang, Y.M. Study of trace elements in Lepista sordida. Edible Fungi China 2003, 22, 43–44. [Google Scholar]
- Luo, X.Y.; Hong, J.; Zhang, Y.M. Study on amino acids in Lepista sordida. Amino Acids Biot. Resour. 2003, 25, 14–15. [Google Scholar]
- Luo, Q.; Yan, L.; Xu, P.; Xiong, C.; Yang, Z.R.; Hu, P.; Hu, H.D.; Hong, R. Discovery of a polysaccharide from the fruiting bodies of Lepista sordida as potent inhibitors of indoleamine 2,3-dioxygenase (IDO) in HepG2 cells via blocking of STAT1-mediated JAK-PKC-δ signaling pathways. Carbohyd. Polym. 2018, 197, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Ji, S.A.; Park, S.H.; Kim, J.P. Lepistatins A–C, chlorinated sesquiterpenes from the cultured basidiomycete Lepista sordida. Phytochemistry 2017, 143, 111–114. [Google Scholar] [CrossRef]
- Miao, S.S.; Mao, X.H.; Pei, R.; Miao, S.P.; Xiang, C.; Lv, Y.J.; Yang, X.G.; Sun, J.; Jia, S.S.; Liu, Y.P. Lepista sordida polysaccharide induces apoptosis of Hep-2 cancer cells via mitochondrial pathway. Int. J. Biol. Macromol. 2013, 61, 97–101. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, Q.; Wu, L.S.; Yang, Z.R. Structural characterization of an immunoregulatory polysaccharide from the fruiting bodies of Lepista sordida. Carbohyd. Polym. 2012, 88, 820–824. [Google Scholar] [CrossRef]
- Zhong, W.Q.; Liu, N.; Xie, Y.G. Antioxidant and anti-aging activities of mycelial polysaccharides from Lepista sordida. Int. J. Biol. Macromol. 2013, 60, 355–359. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Li, Y.H.; Lu, Y.X.; Feng, X.B.; Tian, G.T.; Liu, Q.H. Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia. Food Sci. Hum. Well. 2021, 10, 536–544. [Google Scholar] [CrossRef]
- Tian, G.T.; Yang, Q.F.; Xu, X.Z. A study on the domestication cultivation of lepista sordida. Acta Edulis Fungi 2003, 10, 52–56. [Google Scholar]
- Lun, Z.M. Artificial Cultivation and Polysaccharide Structure Analysis of Lepista sordida. Ph.D. Thesis, Northeast Forestry University, Harbin, China, 2014. [Google Scholar]
- Li, W.S.; Lue, Y.S.; Wu, T.Y.; Tsai, S.J.; Chen, M.H. Domestication of native Lepista sordida (Fr.) Singer in Taiwan. J. Taiwan Agric. Res. 2014, 63, 216–224. Available online: https://www.researchgate.net/publication/269991501 (accessed on 2 September 2021).
- Zhou, H.M.; Zhang, Y.Z.; Chai, H.M.; Zhang, X.L.; Luo, W.D.; Liu, D.Q.; Ji, L.J. Biological characteristics and cultivation of a wild-type Lepista sordida Strain. Acta Edulis Fungi 2017, 24, 39–44. [Google Scholar] [CrossRef]
- Xu, D.; Zhao, H.; Li, B.; Sun, C.Q.; Wang, Z.D.; Ma, J. Artificial cultivation of a novel wild strain of Lepista sordida (sowerby) pat from south west China. Bangladesh J. Bot. 2021, 50, 813–818. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Malysheva, V.F.; Du, P.; Dai, Y.C. Species clarification of the most important and cultivated Auricularia mushroom “Heimuer”: Evidence from morphological and molecular data. Phytotaxa 2014, 186, 241–253. [Google Scholar] [CrossRef]
- Leong, Y.K.; Ma, T.W.; Chang, J.S.; Yang, F.C. Recent advances and future directions on the valorization of spent mushroom substrate (SMS): A review. Bioresour. Technol. 2022, 344, 126–157. [Google Scholar] [CrossRef]
- Wang, S.X.; Xu, F.; Li, Z.M.; Zhao, S.; Song, S.; Rong, C.B.; Geng, X.L.; Liu, Y. The spent mushroom substrates of Hypsizigus marmoreus can be an effective component for growing the oyster mushroom Pleurotus ostreatus. Sci. Hortic.-Amsterdam. 2015, 186, 217–222. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, Z.; Xia, X.; Lu, Y.; Zhang, G. Cultivation of Coprinus comatus using spent mushroom substrates and spent grain from a winery. Acta Edulis Fungi 2012, 19, 44–46. [Google Scholar] [CrossRef]
- Lu, L.X.; Yao, F.J.; Zhang, Y.M. New technology on cultivation of Agaricus bisporus by using of Auricularia auricular residue. North. Hortic. 2014, 8, 130–132. [Google Scholar]
- Wang, Z.B.; Zou, L.; Nima, P.Z.; Wei, X.A.; Tan, Y.; Wen, X. Fungus chaff study on cultivation technology of Hohenbuehelia serotina. Chin. Agric. Sci. Bulletin. 2012, 28, 255–259. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J. Collaborative study of acid-detergent fiber and lignin. J. Assoc. Off. Anal. Chem. 1973, 56, 781–784. [Google Scholar] [CrossRef]
- Huang, S.Z. Biological characteristics and key cultivation techniques of Lepista sordida mushroom. Edible Fungi 2010, 4, 53–54. [Google Scholar]
- Zou, Y.J.; Du, F.; Zhang, H.J.; Hu, Q.X. Evaluation of Korshinsk Peashrub (Caragana korshinskii Kom.) as a substrate for the cultivation of Pleurotus eryngii. Waste Biomass Valori. 2019, 10, 2879–2885. [Google Scholar] [CrossRef]
- Estrada, A.E.R.; del Mar Jimenez-Gasco, M.; Royse, D.J. Improvement of yield of Pleurotus eryngii var. eryngii by substrate supplementation and use of a casing overlay. Bioresour. Technol. 2009, 100, 5270–5276. [Google Scholar] [CrossRef]
- Cunha Zied, D.; S´anchez, J.E.; Noble, R.; Pardo-Gim´enez, A. Use of spent mushroom substrate in new mushroom crops to promote the transition towards a circular economy. Agronomy 2020, 10, 1239. [Google Scholar] [CrossRef]
- Gern, R.M.M.; Libardi Junior, N.; Patrício, G.N.; Wisbeck, E.; Chaves, M.B.; Furlan, S.A. Cultivation of Agaricus blazei on Pleurotus spp. spent substrate. Braz. Arch. Biol. Technol. 2010, 53, 939–944. [Google Scholar] [CrossRef]
- Li, Z.; Yu, C.; Huang, J.; Bao, D.; Li, Y.; Zhou, F. Cultivation of Volvariella volvacea using spent mushroom substrates. Acta Edulis Fungi 2016, 23, 27–30. [Google Scholar]
- Obodai, M.; Cleland-Okine, J.; Vowotor, K.A. Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. J. Ind. Microbiol. Biotechnol. 2003, 30, 146–149. [Google Scholar] [CrossRef]
- Noonsong, V.; Puttakun, N.; Tinsirisuk, M.; Seephueak, P. Recycling of spent Pleurotus compost for production of the Agrocybe cylindracea. Mycosphere 2016, 7, 36–43. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of lignocellulosic residues by Agrocybe cylindracea and Pleurotus ostreatus mushroom fungi—Assessment of their effect on the final product and spent substrate properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Atila, F. Evaluation of suitability of various agro-wastes for productivity of Pleurotus djamor, Pleurotus citrinopileatus and Pleurotus eryngii mushrooms. J. Exp. Agric. Int. 2017, 17, 1–11. Available online: http://creativecommons.org/licenses/by/4.0 (accessed on 6 July 2021). [CrossRef]
- Wu, C.Y.; Liang, C.H.; Liang, Z.C. Evaluation of using spent mushroom sawdust wastes for cultivation of Auricularia polytricha. Agronomy 2020, 10, 1892. [Google Scholar] [CrossRef]
- Liang, Z.C.; Wu, C.Y.; Shieh, Z.L.; Cheng, S.L. Utilization of grass plants for cultivation of Pleurotus citrinopileatus. Int. Biodeterior. Biodegrad. 2009, 63, 509–514. [Google Scholar] [CrossRef]
- Rizki, M.; Tamai, Y. Effects of different nitrogen rich substrates and their combination to the yield performance of oyster mushroom (Pleurotus ostreatus). World J. Microbiol. Biotechnol. 2011, 27, 1695–1702. [Google Scholar] [CrossRef]
- Yang, W.J.; Guo, F.L.; Wan, Z.J. Yield and size of oyster mushroom grown on rice/wheat straw basal substrate supplemented with cotton seed hull. Saudi J. Biol. Sci. 2013, 20, 333–338. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Fu, Y.P.; Li, D.; Sun, X.Z.; Li, C.T.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Xu, F.; Li, Z.M.; Liu, Y.; Rong, C.B.; Wang, S.X. Evaluation of edible mushroom Oudemansiella canarii cultivation on different lignocellulosic substrates. Saudi J. Biol. Sci. 2016, 23, 607–613. [Google Scholar] [CrossRef]
- Skalicka-Woźniak, K.; Szypowski, J.; Łoś, R.; Siwulski, M.; Sobieralski, K.; Głowniak, K.; Malm, A. Evaluation of polysaccharides content in fruit bodies and their antimicrobial activity of four Ganoderma lucidum (W Curt.: Fr.) P. Karst. strains cultivated on different wood type substrates. Acta Soc. Bot. 2012, 81, 17–21. [Google Scholar] [CrossRef]


| Material | Treatment Group | |||
|---|---|---|---|---|
| CK [25] | T1 | T2 | T3 | |
| Corn straw | 73 | 33 | 0 | 0 |
| SSA | 0 | 40 | 73 | 98 |
| Cow dung | 25 | 25 | 25 | 0 |
| Gypsum | 1 | 1 | 1 | 1 |
| Lime | 1 | 1 | 1 | 1 |
| Treatment Group | Fresh Weight of the Mushrooms by Flushes (kg m−2) | BE (%) | |||
|---|---|---|---|---|---|
| First Flush | Second Flush | Third Flush | Total Fresh Weight (kg m−2) | ||
| CK | 2.09 ± 0.02 ab | 1.17 ± 0.13 a | 0.64 ± 0.13 c | 3.90 ± 0.74 b | 30.00 ± 0.77 b |
| T1 | 2.18 ± 0.03 ab | 1.22 ± 0.05 a | 0.66 ± 0.09 bc | 4.06 ± 0.77 b | 31.18 ± 0.62 b |
| T2 | 2.05 ± 0.16 b | 1.10 ± 0.07 a | 0.88 ± 0.11 ab | 4.03 ± 0.62 b | 30.97 ± 1.79 b |
| T3 | 2.24 ± 0.09 a | 1.24 ± 0.10 a | 1.03 ± 0.15 a | 4.51 ± 0.65 a | 34.69 ± 1.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, C.; Pan, C.; Wang, Y.; Ma, Y.; Wang, F.; Shi, L.; Wang, S.; Wang, J.; Liu, S.; Zhang, P.; et al. Turn Waste into Treasure: Spent Substrates of Auricularia heimuer Can Be Used as the Substrate for Lepista sordida Cultivation. Horticulturae 2023, 9, 1074. https://doi.org/10.3390/horticulturae9101074
Sheng C, Pan C, Wang Y, Ma Y, Wang F, Shi L, Wang S, Wang J, Liu S, Zhang P, et al. Turn Waste into Treasure: Spent Substrates of Auricularia heimuer Can Be Used as the Substrate for Lepista sordida Cultivation. Horticulturae. 2023; 9(10):1074. https://doi.org/10.3390/horticulturae9101074
Chicago/Turabian StyleSheng, Chunge, Chunlei Pan, Yanfeng Wang, Yinpeng Ma, Fei Wang, Lei Shi, Shurong Wang, Jinhe Wang, Shuqin Liu, Peng Zhang, and et al. 2023. "Turn Waste into Treasure: Spent Substrates of Auricularia heimuer Can Be Used as the Substrate for Lepista sordida Cultivation" Horticulturae 9, no. 10: 1074. https://doi.org/10.3390/horticulturae9101074
APA StyleSheng, C., Pan, C., Wang, Y., Ma, Y., Wang, F., Shi, L., Wang, S., Wang, J., Liu, S., Zhang, P., Liu, Z., Yu, H., & Zhao, J. (2023). Turn Waste into Treasure: Spent Substrates of Auricularia heimuer Can Be Used as the Substrate for Lepista sordida Cultivation. Horticulturae, 9(10), 1074. https://doi.org/10.3390/horticulturae9101074