Abstract
Spaceflight is known to produce genetic changes in seeds, usually accelerating aging, though species and varietal differences have been poorly investigated. Comparisons were carried out in terms of yield, biochemical characteristics and mineral composition between mature plants grown from seeds subjected to a one-year spaceflight, belonging to Brassicaceae (Brassica juncea and Eruca sativa), Apiaceae (Anethus graveolens, and Coriandrum sativum), and Asteraceae (Lactuca sativa, six cultivars) families, and non-treated control. Among the studied species, only Brassica juncea and Eruca sativa demonstrated a growth stimulation effect caused by seed spaceflight, while significant growth inhibition was recorded in Apiaceae plants and three cultivars of Lactuca sativa L. No differences in the total antioxidant activity (AOA), polyphenol and ascorbic acid content were detected between ‘space-treated’ and control plants. On the contrary, significant decrease in proline accumulation and increase in malonic dialdehyde and photosynthetic pigments levels were shown by Brassicaceae species. The effect of long-term seed spaceflight on the mineral composition of mature plants was reflected in the inhibition of accumulation of all 24 elements analyzed in Apiaceae plants, except for Se, whose concentration was higher in all ‘space-treated’ plants compared to the control. Spaceflight seed storage increased V levels in lettuce and decreased Na accumulation in all the investigated species. The results reveal species-dependent changes in the accumulation of macro-, micro- and toxic elements in Apiaceae, Brassicacea, and Asteraceae representatives due to spaceflight seed storage. The detected differences in plant elemental composition between ‘space’ treatment and control partly explain the corresponding yield gap and suggest a relationship between mineral status and adaptability. The highest beneficial effect of spaceflight seed storage on yield was recorded in Eruca sativa cultivar, Rusalochka.
1. Introduction
The effects of environmental stresses on plant growth and development have constantly drawn attention of researchers due to the vital role of plants in human survival both on Earth and in long-term spaceflight. In the latter case, cosmic radiation, microgravity, decreased magnetic field, and circadian rhythms disturbances represent the main stress factors affecting both humans and plants [1]. Some studies investigated seed viability in conditions of long-term exposure to the harsh space environment without and with shielding against space radiation [2,3,4]. Previous investigations were conducted with seeds of rice [5], tomato [6,7], Eruca sativa [8], Arabidopsis, tobacco [3], licorice [9], Glycyrrhiza uralensis [10], Robina pseudoacazia [11], and Acer mono maxim [12]. Multiple investigations of seeds of more than 140 species [13,14,15] have allowed researchers to identify more than 200 promising varieties with premium quality, yield and disease resistance, suggesting great species and varietal differences in plant response to spaceflight seed storage [16]. A remarkable increase in germination rate was recorded in space-mutated seeds of wheat, maize, barley, triticale, soybean, sunflower, cucumber, tomato and cotton, while no significant differences were noted in rice, pea, millet, lettuce, sweet pepper and tobacco. Conversely, reduced seed germination rates were recorded in sorghum, watermelon, eggplant, radish and towel gourd [15,17]. Half-year spaceflight storage of lettuce rocket seeds led to reduced ‘space’ seed germination vigor, and a remarkable increase in seed aging-sensitivity [8]. Significant oxidant stress in tomato fruit was recorded due to half a year seed storage in ISS, reflected in high total antioxidant activity, polyphenol and ascorbic acid contents [6]. Investigations of the effect of a 15-day space flight revealed an acceleration of alfalfa seed germination and inhibition of the root growth due to chromosomal damage and abnormal mitosis induced by cosmic radiation [18]. Other results revealed reduced germination, lethality, sterility, and accelerated senescence [19,20,21]. Multiple studies conducted in ISS revealed significant changes in hormonal, amino acid, protein biosynthesis, and redox homeostasis in plants due to short-term spaceflight [5,22,23,24,25]. The latter phenomena suggest that the response variations to spaceflight seed storage are highly species and varietal dependent, and there are great prospects for quick space plant breeding to produce crops with shortened vegetation phases, high yields, and resistance to diseases [26,27,28].
The need to evaluate the patterns of plant protection in these conditions stimulates further investigations to reveal similarities in the response of different species to spaceflight seed storage. In this respect, the present study was aimed at assessing species and varietal differences in biochemical and mineral composition of mature plants belonging to Apiaceae, Asteraceae, and Brassicaceae families, caused by one-year seed spaceflight storage.
2. Material and Methods
2.1. Experimental Protocol and Growing Conditions
Research was carried out in a greenhouse at the Federal Scientific Vegetable Center, to assess yield, biochemical and mineral characteristics of green and spicy flavoring plants, i.e., 6 cultivars of Lactuca sativa L., Asteraceae family (cvs. Moskovsky parnikovy, Petrovich, Synthesis. Picnic, Cavalier and Bouquet), 2 species of Brassicaceae family (Brassica juncea, cv. Sudarushka, and Eruca sativa, cv. Rusalochka) and 2 representatives of Apiaceae family (Anethus graveolens, cv. Kulinar, and Coriandrum sativum, cv. Yubilar) grown from seeds subjected to the International Space Station (ISS) ‘Science’ module in sealed foil bags from 5 October 2021 to 10 October 2022 (Figure 1), in comparison with plants from untreated seeds.
Figure 1.
Seed appearance of the tested species and cultivars.
A randomized complete block design was used for the treatment distribution in the greenhouse, with three replicates.
The air temperature on board during the spaceflight seed storage was 22–23 °C, with 25% humidity inside the seed sealed foil bags. After returning to Earth, the seeds were kept in a refrigerator at +7 °C before sowing. The control seeds were also stored in sealed foil bags at the same temperature and humidity conditions, during the whole experiment at the Federal Scientific Vegetable Center.
Sowing was carried out on 20 April 2023 in plastic containers, 7.5 L each, with 15 plants per pot, and were grown in the greenhouse. The harvests of Lactuca sativa, Brassica juncea and Eruca sativa took place on 11 May 2023, and those of Anethus graveolens and Coriandrum sativum on 15 May 2023 during the phase of commercial usefulness.
2.2. Sample Preparation
After harvesting, plants were cleaned with fresh water to remove any soil and dried with filter paper. Leaf area, plant total weight, and height were measured. The samples were homogenized and a fraction of them was used as fresh homogenates to determine ascorbic acid and nitrates; the other part was dried at 70 °C to constant weight and used to measure polyphenols, proline, malonic dialdehyde content, total antioxidant activity and mineral composition.
2.3. Dry Matter
The dry residue was assessed gravimetrically by drying the samples in an oven for 3 days at 70 °C until constant weight.
2.4. Mineral Composition
The Al, As, B, Ca, Cd, Co, Cr, Cu, Fe, I, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, Se, Si, Sr, V, and Zn contents in dried homogenized samples were assessed using ICP-MS on the quadruple mass-spectrometer Nexion 300D (Perkin Elmer Inc., Shelton, CT, USA), equipped with the seven-port FAST valve and an ESI SC DX4 autosampler (Elemental Scientific Inc., Omaha, NE, USA) at the Biotic Medicine Center (Moscow, Russia). Rhodium 103 Rh was used as an internal standard to eliminate instability during measurements. The Quantitation was performed using external standards (Merck IV, multi-element standard solution); Perkin– Elmer standard solutions for P, Si, and V, and all the standard curves were obtained at five different concentrations. For quality checking purposes, internal controls and reference materials were tested together with the samples daily. Microwave digestion of samples was carried out with sub-boiled HNO3 diluted 1:150 with distilled deionized water (Fluka No. 02, 650 Sigma-Aldrich, Co., Saint Louis, MO, USA) in the Berghof SW-4 DAP-40 microwave system (Berghof Products + Instruments Gmb H, 72, 800 Eningen, Germany). The instrument conditions and acquisition parameters were: plasma power and argon flow, 1500 and 18 L min−1, respectively; aux argon flow, 1.6 L min−1; nebulizer argon flow, 0.98 L min−1; sample introduction system, ESI ST PFA concentric nebulizer and ESI PFA cyclonic spray chamber (Elemental Scientific Inc., Omaha, NE, USA); sampler and slimmer cone material, platinum; injector, ESI Quartz 2.0 mm I.D.; sample flow, 637 L min−1; internal standard flow, 84 L min−1; dwell time and acquisition mode, 10–100 ms and peak hopping for all analytes; sweeps per reading, 1; reading per replicate, 10; replicate number, 3; DRC mode, 0.55 mL min−1 ammonia (294993-Aldrich Sigma-Aldrich, Co., St. Louis, MO 63103, USA) for Ca, K, Na, Fe, Cr, V, optimized individually for RPa and RPq; STD mode, for the remainder of analytes at RPa = 0 and RPq = 0.25.
2.5. Ascorbic Acid
The ascorbic acid content was determined by visual titration of leaf extracts in 3% trichloroacetic acid with sodium 2,6-dichlorophenol indophenolate solution (Tillman’s reagent) [29].
2.6. Total Polyphenols (TP)
Total polyphenols were determined in 70% ethanol extract using the Folin–Ciocalteu colorimetric method as previously described [30]. A half gram of dry homogenates was extracted with 20 mL of 70% ethanol at 80 °C for 1 h. The mixture was cooled down and quantitatively transferred to a volumetric flask, and the volume was adjusted to 25 mL. The mixture was filtered through filter paper, and 1 mL of the resulting solution was transferred to a 25 mL volumetric flask, to which 2.5 mL of saturated Na2CO3 solution and 0.25 mL of diluted (1:1) Folin–Ciocalteu reagent were added. The volume was brought to 25 mL with distilled water. One hour later, the solutions were analyzed through a spectrophotometer (Unico 2804 UV, Suite E Dayton, NJ, USA), and the concentration of polyphenols was calculated according to the absorption of the reaction mixture at 730 nm. As an external standard, 0.02% gallic acid was used. The results were expressed as mg of Gallic Acid Equivalent per g of dry weight (mg GAE g−1 d.w.).
2.7. Antioxidant Activity (AOA)
The antioxidant activity was assessed using a redox titration method [30] via titration of 0.01 N KMnO4 solution with ethanolic extracts of dry samples, as described in the Section 2.6. The reduction of KMnO4 to colorless Mn+2 in this process reflects the quantity of antioxidants dissolvable in 70% ethanol. The values are expressed in mg Gallic Acid Equivalents (mg GAE g−1 d.w.).
2.8. Proline
Proline concentration was determined according to [31], with slight modifications. About 50 mg of dry homogenized plant leaves were ground with 10 mL of 3% sulfur salicylic acid in a mortar. The mixture was filtered and 1 mL of the resulting filtrate, 2 mL of ninhydrin reagent and 2 mL of acetic acid were heated at 95 °C for 1 h. Proline concentration was assessed using the absorption value of the reaction mixture toluene extract at 505 nm, and a calibration curve with 5 different proline (Merck, Rahway, NJ, USA) concentrations.
2.9. Malonic Dialdehyde
Lipid peroxidation was measured by tracing malonic dialdehyde content using thiobarbituric acid method as described by Heath and Parker [32], with a small modification. About 0.1 g of dried samples were mixed with 10 mL of 0.5% thiobarbituric acid solution and heated at 95 °C for half an hour. The mixture was cooled down and the absorption at 232 nm was determined. MDA content was calculated using the extinction value equal to 155 mM cm−1.
2.10. Photosynthetic Pigments
About 50 mg of fresh samples was homogenized in a porcelain mortar with 10 mL of 96% ethanol. The homogenized sample mixture was quantitatively transferred to a volumetric flask, bringing the volume to 25 mL, and then the mixture was filtered through filter paper. In the resulting solution, analyses of chlorophyll-a, chlorophyll-b and carotene were performed through a spectrophotometer (Unico 2804 UV, USA). The calculation of chlorophyll and carotene concentrations was achieved using appropriate equations [33]:
where A = Absorbance, Ch-a = Chlorophyll a, Ch-b = Chlorophyll b and C c = Carotene.
Ch-a = 13.36A664 − 5.19A649;
Ch-b = 27.43A649 − 8.12A664;
C c = (1000A470 − 2.13 Ch-a − 87.63 Ch-b)/209;
2.11. Statistical Analysis
The data were processed by one-way analysis of variance and mean separations were performed through the Duncan’s multiple range test, with reference to a 0.05 probability level, using SPSS software version 28 (IBM, Armonk, NY, USA). Data expressed as a percentage were subjected to angular transformation before processing.
3. Results and Discussion
According to plant classification, the three studied plant families belong to Asterids and Rosits clades and differ in their seed dormancy type (Table 1). Indeed, the physiological dormancy type is predominant in seeds of Brassicaceae and Asteraceae species, whereas the morphological and morpho-physiological type of dormancy is more typical of Apiaceae seeds [34,35,36].

Table 1.
Characteristics of Brassicaceae, Asteraceae and Apiaceae families.
3.1. Yield and Biometrical Characteristics
The results of the present study indicate great differences between the examined families in mature plant response to long-term spaceflight seed storage. A negative effect of long-term spaceflight on the seed quality was recorded in the present work by the fact that seeds of only four Lactuca sativa cultivars germinated, while the seeds of cultivars Cavalier and Bouquet did not produce any seedlings. These results are consistent with the well-known observation that long-term exposure to microgravity inside spaceships is often associated with accelerated aging in humans and plants [37], and oxidation promotion of the most important molecules, such as proteins, lipids, and nucleic acids [38,39].
Nevertheless, the behavior of other plant species and cultivars demonstrated extremely high variability in response to spaceflight seed storage (Table 1). Indeed, the weight and height of control and experimental lettuce plants did not differ significantly for cultivars Petrovich and Picnic, and these parameters were slightly higher for cvs. Moskovsky parnikovy and Synthesis, grown from ‘space’ seeds, while variations in dry matter content were insignificant.
Indian mustard demonstrated dry matter increase due to seed spaceflight exposure, and a 23% weight increase.
The most impactful effects of seed long-term spaceflight storage on plant growth were recorded in the experimental plants of Eruca sativa, whose weight and height were 2.40 and 1.32 times higher than the corresponding parameters of the control plants (Table 1).
Interestingly, a previous investigation by Chandler et al. [8] demonstrated significant inhibition of Eruca sativa seedling growth as a result of one-year seed storage in ISS, contrary to the above results. Taking into account the existence of significant varietal differences in the response to the seed spaceflight storage recorded in lettuce cultivars (Table 1) and other crops [16], this may suggest a similar phenomenon for different Eruca sativa cultivars, though the latter hypothesis needs further investigations. Besides, seed germination and mature plant often demonstrate contradictory response to seeds spaceflight storage [16]. In any case, cultivar Rusalochka of Eruca sativa grown from spaceflight seeds seems the most promising.
Among the representatives of the three families investigated, the ‘space-treated’ plants of Apiaceae showed the most significant growth inhibition resulting in a decrease of 1.26 times in dill and 1.60 times in coriander weight (Table 2; Figure 2). Further investigations are needed to unveil if this phenomenon is associated with the morpho-physiological type of Apiaceae seed dormancy.

Table 2.
Yield and biometrical characteristics of control and ‘space-treated seed’ plants.
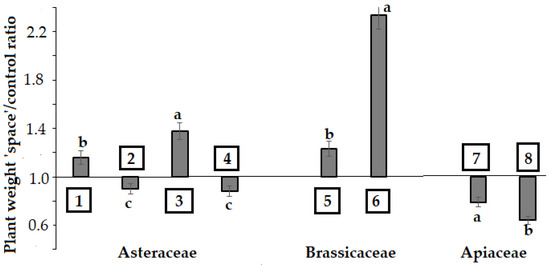
Figure 2.
Differences in plant weight between ‘space-treated’ and control plants. Lactuca sativa: (1) cv. Moskovsky parnikovy; (2) cv. Petrovich; (3) cv. Synthesis; (4) cv. Picnic; Brassica juncea: (5) cv. Sudarushka; Eruca sativa: (6) cv. Rusalochka; Anethum graveolens: (7) cv. Kulinar; Coriandrum sativum: (8) cv. Yubilar. Within each family, values with the same letters (‘a’, ‘b’, ‘c’) do not differ statistically according to Duncan test at p < 0.05; ‘c’ means that weight differences between ‘space-treated’ and control plants are not significant at p > 0.05.
Table 2 data indicate that the Asteraceae species (Lactuca sativa) demonstrated significant varietal differences between the cultivars showing a total growth inhibition (cvs. Bouquet and Cavalier) or a slight growth stimulation, while a negative effect on plant growth was recorded for Apiaceae plants and a positive one for Brassicaceae species (Figure 2). In this respect, leaf area of Brassicaceae ‘space-treated’ plants showed significantly higher values compared to control plants (Figure 3). Conversely, the leaf area of lettuce cultivars (Synthesis, Petrovich and Moskovsky parnikovy, Picnic) did not differ between ‘space-treated’ and control plants (Figure 3).
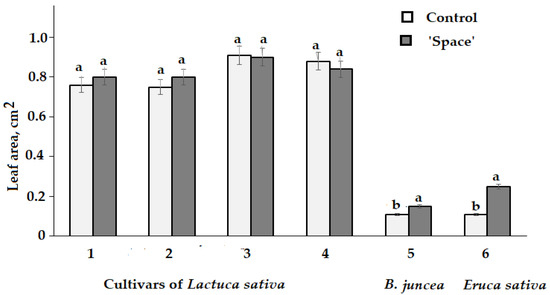
Figure 3.
Leaf area of ‘space-treated’ and control plants. Lactuca sativa: (1) cv. Moskovsky parnikovy, (2) cv. Petrovich, (3) cv. Synthesis, (4) cv. Picnic; Brassica juncea: (5) cv. Sudarushka; Eruca sativa: (6) cv. Rusalochka. Within each species and cultivar, values with the same letters do not differ statistically according to Duncan test at p < 0.05.
3.2. Photosynthetic Pigments
Changes in the intensity of plant growth due to ionized radiation and microgravity are closely related to the intensity of photosynthesis (Table 3). Among Lactuca sativa cultivars, only Picnic showed a significant decrease in the total chlorophyll and carotene content in leaves of ‘space-treated’ plants compared to control ones, while the opposite effect was revealed for Brassicaceae representatives. Indeed, the total chlorophyll and carotene content in leaves of Indian mustard was 1.31–1.33 times higher in plants grown from ‘space-treated’ seeds. These values reached 2.53 and 1.82 times increase in leaves of Eruca sativa, which is in accordance with the recorded growth stimulation of these plants (Table 2). Significant differences in carotene levels between leaves of ‘space-treated’ and the control plants suggest an important role for these substances in plant adaptation as well as disturbances in photopigments accumulation due to spaceflight seed storage. Indeed, carotenoids have an important role in light capture, antioxidant protection, phytohormone biosynthesis [40,41] and stabilization of photosynthetic apparatus [42]. The present results demonstrated a close relationship between chlorophyll and carotene accumulation in lettuce leaves (r = 0.959 at p < 0.001). The significant carotene decrease in ‘space-treated’ Apiaceae plant leaves, compared to leaves of control plants, relates to growth inhibition of Apiaceae crops and a tendency to chlorophyll level decrease.

Table 3.
Leaf photosynthetic pigment levels in ‘space-treated seed’ and control plants (mg g−1 f.w.).
3.3. Antioxidant Status
The data presented in Table 4 indicate that long-term spaceflight did not significantly affect the accumulation of ascorbic acid, total phenolics, and the level of total antioxidant activity (AOA) of mature plants. Low antioxidant status changes have been previously recorded also in tomato grown from half-year spaceflight stored seeds [6]. The stability of antioxidant parameters in the present experiment entails the existence of special adaptation mechanism of plants to unfavorable seed storage conditions.

Table 4.
Leaf antioxidant status of ‘space-treated seed’ and control plants.
Indeed, among the parameters tested, only proline content decreased significantly in Indian mustard and rocket of the Brassicaceae family with the corresponding enhanced accumulation of malonic dialdehyde (MDA). The decrease in the proline content and the increase of MDA accumulation in Brassicaceae plants due to ionizing radiation and microgravity indicate the existence of significant oxidant stress in these plants, causing lipids peroxidation, and suggesting the inability of plants to activate the synthesis of proline known to promote membrane stabilization, maintain cell turgor, and decrease the concentration of reactive oxygen species [43,44].
In this respect, the following paradox may be highlighted: despite the increase in oxidant stress caused by spaceflight storage of seeds and lack of significant changes in antioxidants accumulation, Brassicaceae plants demonstrated enhanced ability both to synthetize photosynthetic pigments (Table 3) and provide high yield (Table 2).
3.4. Mineral Composition
Viability of plants and protection against unfavorable environmental conditions are closely connected also with mineral nutrition and the ability to accumulate essential macro- and micro- elements. According to the present results, spaceflight seed storage did not significantly affect the concentration of nitrates in mature plants. Indeed, nitrate levels in lettuce leaves reached 0.60–0.71%, were in the range of 0.50–0.70% in Brassicaceae plants, and 0.71% and 0.82% in dill and coriander species, respectively.
Ash content showed decreased values in the ‘space-treated’ Brassicaceae and Apiaceae species with a rather controversial effect on lettuce cultivars (Figure 4). Indeed, among the cultivars tested, significantly decreased ash content in plants grown from spaceflight stored seeds was recorded only in cvs. Synthesis and Moskovsky parnikovy, while a tendency to higher ash levels was observed for cvs. Picnic and Petrovich (Figure 4). Generally, decreased ash content in plants relates to malnutrition and, in case of plants subjected to radiation and microgravity, to a possible disruption of macro-elements transport across plants [45]. Extremely scant information regarding the effect of ionizing radiation and microgravity on mineral composition of plants makes the present investigation relevant to elements profile of the tested crops (Table 5, Table 6 and Table 7).
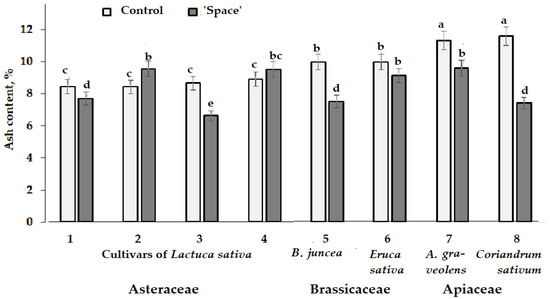
Figure 4.
Effect of seed spaceflight storage on ash content in plants belonging to Asteraceae (Lactuca sativa: 1—cv. Moskovsky parnikovy; 2—cv. Petrovich; 3—cv. Synthesis; 4—cv. Picnic), Brassicaceae (5—Brassica juncea: cv. Sudarushka; 6—Eruca sativa: cv. Rusalochka) and Apiaceae (7—A. graveolens: cv. Kulinar; 8—Coriandrum sativum: cv. Yunilar) families. Values with the same letters do not differ statistically according to Duncan test at p < 0.05.

Table 5.
Mineral composition of Brassicaceae plants grown from spaceflight long-stored and control seeds (mg kg−1 d.w.).

Table 6.
Mineral composition of Apiaceae plants, grown from spaceflight long-stored and control seeds.

Table 7.
Mineral composition of Lactuca sativa plants, grown from spaceflight long-stored and control seeds.
The results of the present research indicate the existence of great changes in macro-, micro- and toxic elements accumulation, due to one-year seed storage in ISS, in ‘space-treated’ and control plants belonging to Brassicaceae, Apiaceae, and Asteraceae families.
Both significant species and varietal differences arose from the comparison between the species belonging to the three families examined, in terms of mineral accumulation (Table 8). In this respect, the remarkable accumulation decrease of most macro- and micro-elements in dill and coriander due to long-term spaceflight seed storage was in agreement with the detected growth inhibition. The most significant increase in plant yield of rocket (more than twice) may be connected with the relative stability of macro-element content and evident decrease in accumulation of toxic elements, such as Al, As, Cr, Pb, Sr and V.

Table 8.
Changes in macro-, micro- and toxic element accumulation in Brassicaceae, Apiaceae and Asteraceae species, as a result of long-term seed storage on ISS.
The 23% yield increase of mustard due to utilization of ‘space’ seeds may reflect the ability of this plant to accumulate toxic elements without growth inhibition [46]. Indeed, besides the decreased contents of K, Mg, and P in ‘space-treated’ plants, Indian mustard showed increased levels of Mn, Se, and Si, known to participate in plant protection against stresses [47]. This plant is known to highly tolerate and accumulate heavy metals and is often used for phytoremediation of heavy metals such as Ni [48] and Pb [49]. Indian mustard demonstrates the ability to avoid metal toxicity via metal binding to the cell wall, or by reducing transport across the cell membrane, by chelation of the metal ions, and compartmentalization into the vacuole [50].
On the other hand, Al, Cr, Ni, b, and V may also have a beneficial effect on plant growth, like the stimulation of root formation elicited by low Cr concentration [51,52]. Moreover, aluminum is toxic to plants in acidic soil but demonstrates a growth stimulation effect and mitigates environmental stresses in neutral soil [53]. At low concentration, Ni is known to activate superoxide dismutase, stimulate respiration, and nitrogen fixation [54].
The growth stimulation effect of V was previously described in several plant species: pepper, maize, basil, and tomato [55]; this element is known to act as a redox catalyzer in electron transportation in photosystems I and II, depending on the environmental conditions [55,56].
Lettuce plants showed significant varietal differences in mineral accumulation: a remarkable decrease of macro-element content (cv. M. parnikovy), lack of significant changes in macro-element concentration (cvs. Picnic and Petrovich) and a decrease of potassium content in the cultivar Synthesis. Each cultivar was characterized by its own specific response: cv. Petrovich and Synthesis increased the accumulation of many important micro-elements (Co, Cu, Fe, I, Se) but also toxic elements (Al, As, Cr, Sr, and V). The cv. Moskovsky parnikovy demonstrated decreased levels of some macro- and micro-elements as well as of the toxic Cd, Pb, and Sr. The cultivar Picnic showed only the increase of As, Cr, and V.
Overall, based on the data presented in Table 8, several peculiarities in Brassicaceae, Apiaceae, and Asteraceae representatives may be highlighted.
3.4.1. Vanadium
Indeed, all Asteraceae species showed a significant increase in V accumulation (Figure 5).
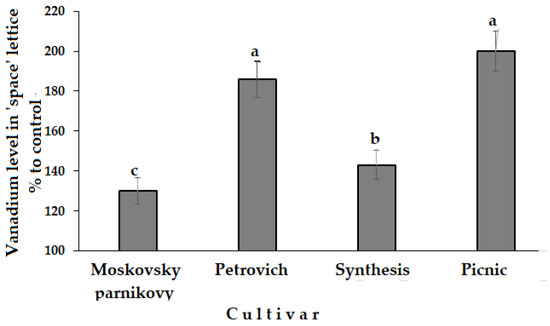
Figure 5.
Effect of seed spaceflight storage on V accumulation in lettuce cultivars. Values with the same letters do not differ statistically according to Duncan test at p < 0.05.
Positive or negative effects of V on plants primarily depend on its concentration [56]. High levels are known to reduce plant growth and disturb physiological status, while low concentrations show growth stimulation [56]. Among the four lettuce cultivars examined, only cv. Synthesis was characterized by significant yield increase (38%) due to spaceflight seed storage, while Petrovich and Picnic demonstrated a tendency to growth inhibition and Moskovsky parnikovy a slight yield improvement (16%) compared to control plants.
3.4.2. Selenium
Furthermore, the plants of the three families examined had an increase in Se levels due to spaceflight seed storage effect (Figure 6). The latter result reflects the ability of Se in plant protection and is of special interest taking into account the protective properties of this element against all forms of oxidant stresses, including heavy metals [57,58,59]. In this respect, the increased accumulation levels of heavy metals in Indian mustard and lettuce cultivars may demonstrate a decreased harmful effect due to high levels of Se accumulation.
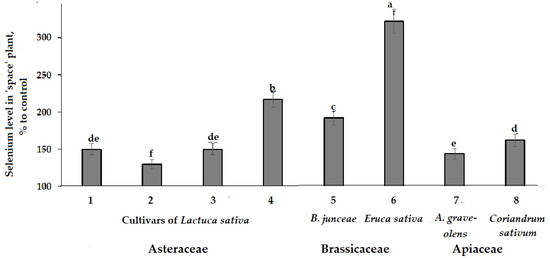
Figure 6.
Effect of seed spaceflight storage on Se accumulation in plants belonging to Asteraceae (Lactuca sativa: 1—cv. Moskovsky parnikovy; 2—cv. Petrovich; 3—cv. Synthesis; 4—cv. Picnic), Brassicaceae (Brassica juncea: 5—cv. Sudarushka; Eruca sativa: 6—cv. Rusalochka) and Apiaceae (A. graveolens: 7—cv. Kulinar; Coriandrum sativum: 8—cv. Yunilar) families. Values with the same letters do not differ statistically according to Duncan test at p < 0.05.
Furthermore, the comparison of nutritional profiles of the tested plant species suggests that the plants with ‘space’ growth stimulation effect (mustard and rocket) are characterized by seeds with the highest levels of protein and the lowest of carbohydrates (Figure 7). In this respect, rocket seeds rank first both in protein accumulation and ability to increase Se content in mature plants due to long-term spaceflight seed storage (Figure 6). Genetic changes in plant seeds subjected to cosmic radiation primarily affect the biosynthesis of amino acids and proteins [8,21], substances capable of replacing sulphur in methionine and cysteine with selenium [57].
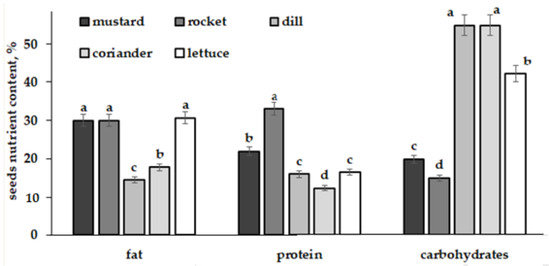
Figure 7.
Nutritional profile of the tested species. Within each quality parameter, values with the same letters do not differ statistically according to Duncan test at p < 0.05.
3.4.3. Sodium
Changes of Na accumulation due to spaceflight seed storage is another valuable peculiarity of ionizing radiation and microgravity effect. Figure 8 data indicate sodium concentration decrease in tissues of most plants with the highest effect on Indian mustard (Figure 8).
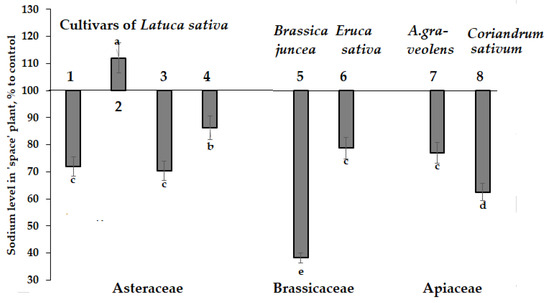
Figure 8.
Effect of seed spaceflight storage on Na accumulation in plants belonging to Asteraceae (Lactuca sativa: 1—cv. Moskovsky parnikovy; 2—cv. Petrovich; 3—cv. Synthesis; 4—cv. Picnic), Brassicaceae (Brassica juncea: 5—cv. Sudarushka; Eruca sativa: 6—cv. Rusalochka) and Apiaceae (A. graveolens: 7—cv. Kulinar; Coriandrum sativum: 8—cv. Yunilar) families. Values with the same letters do not differ statistically according to Duncan test at p < 0.05.
The concentrations of sodium (Na+) and potassium (K+) in plant tissues are important determinants of stress tolerance and adaptability level [60]. In this respect, Indian mustard showed the highest K/Na increase (1.91 times higher than the control values) providing a beneficial growth stimulation effect on plants grown from ‘space-treated’ seeds.
4. Conclusions
The results of the present investigation revealed species and varietal differences in plant response to long-term seed spaceflight storage. Among Apiaceae, Asteraceae, and Brassicaceae families, the first one demonstrated significant plant growth inhibition due to long-term seed spaceflight storage, while the last one showed significant yield increase and stimulation of photosynthetic pigments biosynthesis in ‘space-treated’ plants. Dramatic decrease of macro- and micro-elements, except Se, was recorded in Apiaceae plants. The remarkable increase and decrease of Se and Na accumulation, respectively, in all plants tested, and the V increase in lettuce cultivars entail the important spaceflight effects on plant seeds.
Author Contributions
Conceptualization: N.G., V.K. and G.C.; formal analysis: L.S., O.C.M. and L.V.; investigation: N.G. and V.K.; methodology: L.S. and O.C.M.; validation, V.K. and G.C.; draft manuscript writing, N.G. and G.C.; manuscript revision and final editing, N.G., L.S. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge to the Institute of Medical and Biological Problems for providing the opportunity to subject seeds to the ISS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buchheim, J.I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts after Long-Duration Space Flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Visscher, A.M.; Seal, C.E.; Newton, R.J.; Frances, A.L.; Pritchard, H.W. Dry seeds and environmental extremes: Consequences for seed lifespan and germination. Funct. Plant Biol. 2016, 43, 656–668. [Google Scholar] [CrossRef]
- Tepfer, D.; Leach, S. Survival and DNA damage in plant seeds exposed for 558 and 682 days outside the International Space Station. Astrobiology 2017, 17, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Musgrave, M.E. Seeds in space. Seed Sci. Res. 2002, 12, 1–16. [Google Scholar] [CrossRef]
- Zeng, D.; Cui, J.; Yin, Y.; Zhang, M.; Shan, S.; Liu, M.Y. Proteomic analysis in different development stages on SPO generation of rice seeds after space flight. Life Sci. Space Res. 2020, 26, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Djoss, H.; Golubkina, N.; Kondratyeva, I.; Koshevarov, A.; Shkaplerov, A.; Zavarikina, T.; Nechitailo, G.; Caruso, G. Effect of spaceflight on tomato seed quality and biochemical characteristics of mature plants. Horticulturae 2021, 7, 89. [Google Scholar] [CrossRef]
- Shi, J.; Yang, B.; Feng, P.; Li, D.; Zhu, J. Induction of apoptosis by tomato using space mutation breeding in human colon cancer SW480 and HN-29 cells. J. Sci. Food Agr. 2010, 90, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Chandler, J.O.; Haas, F.B.; Khan, S.; Bowden, L.; Ignatz, M.; Enfissi, E.M.A.; Gawthrop, F.; Griffiths, A.; Fraser, P.D.; Rensing, S.A.; et al. Rocket science: The effect of spaceflight on germination physiology, ageing, and transcriptome of Eruca sativa seeds. Life 2020, 10. [Google Scholar] [CrossRef]
- Zhang, J.-Z.; Gao, W.-Y.; Gao, Y.; Liu, D.-L.; Huang, L.-Q. Analysis of influences of spaceflight on chemical constituents in licorice by HPLC– ESI-MS/MS. Acta Physiol. Plant. 2011, 33, 2511–2520. [Google Scholar] [CrossRef]
- Dong, Y.-Y.; Gao, W.-Y.; Zhang, J.-Z.; Zuo, B.-M.; Huang, L.-Q. Quantification of four active ingredients and fingerprint analysis of licorice (Glycyrrhiza uralensis fisch.) after spaceflight by HPLC–DAD. Res. Chem. Intermed. 2012, 38, 1719–1731. [Google Scholar] [CrossRef]
- Yuan, C.Q.; Li, Y.F.; Sun, P.; Sun, Y.H.; Zhang, G.J.; Yang, M.S.; Zhang, Y.Y.; Li, Y.; Wang, L. Asswssment of Genetic diversity and variation of Robina pseudoacacia seeds induced by short-term spaceflight based on two molecular marker systems and morphological traits. Gen. Mol. Res. 2012, 11, 4268–4277. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, Y.; Yuan, C.; Yang, Q.; Long, C.; Li, Y.; Yang, M. Assessment of genetic diversity and variation of Acer Mono Max seedlings after spaceflight. Pakistan J. Bot. 2015, 47, 197–202. [Google Scholar]
- Xianfang, W.; Long, Z.; Weixu, D.; Chunhua, L. Study of space mutation breeding in China. Appl. Life Sci. 2004, 18, 241–246. [Google Scholar]
- He, X.; Liu, M.; Lu, J.; Xue, H.; Pan, Y. Space mutation breeding: A brief Introduction of screening New floricultural, vegetable and medicinal varieties from earth-grown plants returned from China’s satellites and spaceships. In Floriculture, Ornamental and Plant Biotechnology: Advances and Topical Issues, 1st ed.; da Silva, J.A.T., Ed.; Global Science Books: Isleworth, UK, 2006; pp. 266–271. [Google Scholar]
- Liu, L.X.; Guo, H.J.; Zhao, L.; Gu, J.; Zhao, S. Advances in crop improvement by space mutagenesis in China. ICSC 2008, 4, 274. [Google Scholar]
- Prasad, B.; Richter, P.; Vadakedath, N.; Haag, F.W.M.; Strauch, S.M.; Mancinelli, R.; Schwarzwälder, A.; Etcheparre, E.; Gaume, N.; Lebert, M. How the space environment influences organisms: An astrobiological perspective and review. Int. J. Astrobiol. 2021, 20, 159–177. [Google Scholar] [CrossRef]
- Dutcher, F.; Hess, E.L.; Halstead, T.W. Progress in plant research in space. Adv. Space Res. 1994, 14, 159–171. [Google Scholar] [CrossRef]
- Ren, W.B.; Zhang, Y.; Deng, B.; Guo, H.; Cheng, L.; Liu, Y. Effect of space flight factors on alfalfa seeds. Afr. J. Biotechbol. 2010, 9, 7273–7279. [Google Scholar] [CrossRef]
- De Micco, V.; Arena, C.; Pignalosa, D.; Durante, M. Effects of Sparsely and Densely Ionizing Radiation on Plants. Radiat. Environ. Biophys. 2011, 50, 1–19. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; De Maio, A. Growth alteration and leaf biochemical responses in Phaseolus Vulgaris exposed to different doses of ionizing radiation. Plant Biol. 2014, 16 (Suppl. S1), 194–202. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Aronne, G.; Pugliese, M.G.; Virzo, A.; DeMaio, A. Response of Phaseolus vulgaris L. plants to low-LET ionizing radiation: Growth and oxidative stress. Acta Astronaut. 2014, 91, 107–114. [Google Scholar] [CrossRef]
- Deyong, Z.; Jie, C.; Yishu, Y.; Meng, Z.; Shan, S.; Xin, G. Effects of space flight on expression of key proteins in rice leaves. Rice Sci. 2020, 27, 423–433. [Google Scholar] [CrossRef]
- Ferl, R.J.; Koh, J.; Denison, F.; Paul, A.L. Spaceflight induces specific alterations in the proteomes of Arabidopsis. Astrobiology 2015, 15, 32–56. [Google Scholar] [CrossRef]
- Kruse, C.P.S.; Meyers, A.D.; Basu, P.; Hutchinson, S.; Luesse, D.R.; Wyatt, S.E. Spaceflight induces novel regulatory responses in Arabidopsis seedling as recealed by combined proteomic and transcriptomic analysis. BMC Plant Biol. 2020, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Ciu, J.; Yin, Y.; Xiong, Y.; Liu, M.; Guan, S. Metabolomic analysis in different development stages on SP0 generation of rice seeds after space flight. Front. Plant Sci. 2021, 12, 1235. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Li, O.; Liang, Z.; Zhang, X.; Yan, K. Spaceflight breeding could improve the volatile constituents of Andrographis paniculate. Ind. Crops Prod. 2021, 171, 113967. [Google Scholar] [CrossRef]
- Liu, L.; Zheng, Q. Report No: CNIC01139/CSNAS-0111. Space-Induced Mutation for Crop Improvement; CNIC-01139; CSNAS-0111; China Nuclear Information Centre: Beijing, China, 1997; p. 10. ISBN 7-5022-1646-4. [Google Scholar]
- Zeng, D.; Cui, J.; Yin, Y.; Dai, C.; Zhao, H.; Song, C.; Guan, S.; Cheng, D.; Sun, Y.; Lu, W. Combining proteomics and metabolomics to analyze the effects of spaceflight on rice progeny. Front. Plant Sci. 2022, 13, 900143. [Google Scholar] [CrossRef]
- AOAC Crude Protein in Cereal Grains and Oil Seeds. Official Methods of Analysis of Association of Official Analytical Chemists, 17th ed.; Method: Gaithersburg, MD, USA, 2000; pp. 23, 992. [Google Scholar]
- Golubkina, N.A.; Kekina, H.G.; Molchanova, A.V.; Antoshkina, M.S.; Nadezhkin, S.; Soldatenko, A.V. Plants Antioxidants and Methods of Their Determination; Infra-M: Moscow, Russia, 2020; (In Russian). [Google Scholar] [CrossRef]
- Ouertani, R.N.; Abid, G.; Karmous, C.; Chikha, M.B.; Boudaya, O.; Mahmoudi, H.; Mejri, S.; Jansen, K.; Ghorbel, A. Evaluating the contribution of osmotic and oxidative stress components on barley growth under salt stress. AoB Plants 2021, 13, plab034. [Google Scholar] [CrossRef]
- Heath, R.L.; Parker, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic bio-membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Jia, C.-Z.; Wang, J.-J.; Chen, D.-L.; Hu, X.-W. Seed Germination and Seed Bank Dynamics of Eruca sativa (Brassicaceae): A Weed on the Northeastern Edge of Tibetan Plateau. Front. Plant Sci. 2022, 13, 820925. [Google Scholar] [CrossRef]
- Baskin, C.; Baskin, J. Seed dormancy in Asteraveae: A global vegetation zone and taxonomic/phylogenetic assessment. Seeds Sci. Res. 2023, 1–35. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Liu, H.; Tao, J.; Zhang, K. Seed Dormancy and Germination Requirements of Torilis scabra (Apiaceae). Agronomy 2023, 13, 1250. [Google Scholar] [CrossRef]
- Arena, C.; De Micco, V.; Macaevac, E.; Quintens, R. Space radiation effects on plant and mammalian cells. Acta Austranaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Fleming, M.B.; Patterson, E.L.; Reeves, P.A.; Richards, C.M.; Gaines, T.A.; Walters, C. Exploring the fate of mRNA in aging seeds: Protection, destruction, or slow decay? J. Exp. Bot. 2018, 69, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.B.; Hill, L.M.; Walters, C. The kinetics of ageing in dry-stored seeds: A comparison of viability loss and RNA degradation in unique legacy seed collections. Ann. Bot. 2019, 123, 1133–1146. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J.Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Jia, K.; Baz, L.; Al-Babili, S. From carotenoids to strigolactones. J. Exp. Bot. 2017, 69, 2189–2204. [Google Scholar] [CrossRef]
- Hashioto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. In Carotenoids in Nature; Springer: Cham, Switzerland, 2016; pp. 111–139. [Google Scholar]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Mishra, A.K.; Mohanta, Y.K.; Al-Harrasi, A. Space breeding: The next generation crops. Front. Plant Sci. 2021, 12, 771985. [Google Scholar] [CrossRef]
- Małecka, A.; Konkolewska, A.; Hanć, A.; Barałkiewicz, D.; Ciszewska, L.; Ratajczak, E.; Staszak, A.M.; Kmita, H.; Jarmuszkiewicz, W. Insight into the phytoremediation capability of Brassica juncea (v. Malopolska): Metal accumulation and antioxidant enzyme activity. Int. J. Mol. Sci. 2019, 20, 4355. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Zayachkovsky, V.; Sheshnitsan, S.; Skrypnik, L.; Smirnova, T.; Antoshkina, M.; Fedotov, M.; Caruso, C. Prospects of garlic extracts, selenium and silicon application for plants protection against herbivory. Review Agriculture 2022, 12, 64. [Google Scholar] [CrossRef]
- Selvaraj, K.; Ramasabramanian, V.; Makesj Kumar, B. Phytoremediation potential of Brassica juncea in nickel contaminated soil. Paripex. Indian J. Res. 2021, 10, 150–159. [Google Scholar]
- Lim, J.-M.; Salido, A.L.; Butcher, D.J. Phytoremediation of lead using Indian mustard (Brassica juncea) with EDTA and electrodics. Microchem. J. 2004, 76, 3–9. [Google Scholar] [CrossRef]
- Rathore, S.S.; Shekhawat, K.; Dass, A.; Kandpal, B.K.; Singh, V.K. Phytoremediation mechanism in Indian mustard (Brassica juncea) and its enhancement through agronomic interventions. Proc. Natl. Acad. Sci. USA—India Sect. B Biol. Sci. 2017, 89, 419–427. [Google Scholar] [CrossRef]
- López-Bucio, J.S.; Ravelo-Ortega, G.; López-Bucio, J. Chromium in plant growth and development: Toxicity, tolerance and hormesis. Environ. Pollut. 2022, 312, 120084. [Google Scholar] [CrossRef]
- Sharma, A.; Kapoor, D.; Wang, J.; Shahzad, B.; Kumar, V.; Bali, A.S.; Jasrotia, S.; Zheng, B.; Yuan, H.; Yan, D. Chromium bioaccumulation and its impacts on plants: An overview. Plants 2020, 9, 100. [Google Scholar] [CrossRef]
- Ofoe, R.; Thomas, R.H.; Asiedu, S.K.; Wang-Pruski, G.; Fofana, B.; Abbey, L. Aluminum in plant: Benefits, toxicity and tolerance mechanisms. Front. Plant Sci. 2023, 13, 1085998. [Google Scholar] [CrossRef]
- Kastori, R.R.; Putnik-Delić, M.I.; Maksimović, I.V. Functions of nickel in higher plants—A review. Acta Agr. Serbica 2022, 27, 89–101. [Google Scholar] [CrossRef]
- García-Jiménez, A.; Trejo-Téllez, L.I.; Guillén-Sánchez, D.; Gómez-Merino, F.C. Vanadium stimulates pepper plant growth and flowering, increases concentrations of amino acids, sugars and chlorophylls, and modifies nutrient concentrations. PLoS ONE 2018, 13, e0201908. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wiszniewska, A.; Kaminska, I. A dual role of vanadium in environmental systems—Beneficial and detrimental effects on terrestrial plants and humans. Plants 2021, 10, 1110. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.A.; Kharchenko, V.A.; Caruso, G. Selenium: Prospects of functional food production with high antioxidant activity Reference series in phyto-chemistry. In Plant Antioxidants and Health; Ekiert, H.M., Ramawat, K.G., Arora, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Liu, H.; Xiao, C.; Qiu, T.; Deng, J.; Cheng, H.; Cong, X.; Cheng, S.; Rao, S.; Zhang, Y. Selenium regulates antioxidant, photosynthesis, and cell permeability in plants under various abiotic stresses: A review. Plants 2023, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium supplementation and crop plant tolerance to metal/metalloid toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef] [PubMed]
- Cordones, M.; Al Shiblawi, F.R.; Sentenac, H. Roles and Transport of Sodium and Potassium in Plants. Met Ions Life Sci. 2016, 16, 291–324. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).