Salicylic Acid Applied via Irrigation Enhances Young Carica papaya L. Plant Performance under Water Deficit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Characteristics
2.2. Water Stress Conditions and Treatments
2.3. Soil Moisture Measurements
2.4. Growth Measurements and Sampling
2.5. Stomatal Conductance
2.6. Photosynthetic Pigment Determination
| Chl a (mg·g−1 frozen sample) = (12.25·Aλ = 663 nm − 2.79·Aλ = 647 nm)·V0/(m0·1000), |
| Chl b (mg·g−1 frozen sample) = (21.50·Aλ = 647 nm − 5.10·Aλ = 663 nm)·V0/(m0·1000), |
| Chl t (mg·g−1 frozen sample) = Chl a + Chl b, |
| Car (mg·g−1 dry sample) = (5.05·Aλ = 470 nm + 2.08·Aλ = 663 nm − 9.21·Aλ = 647 nm)·V0/(m0·1000). |
2.7. Proline Production
2.8. Statistical Analyses
3. Results
3.1. Soil Moisture
3.2. Plant Growth
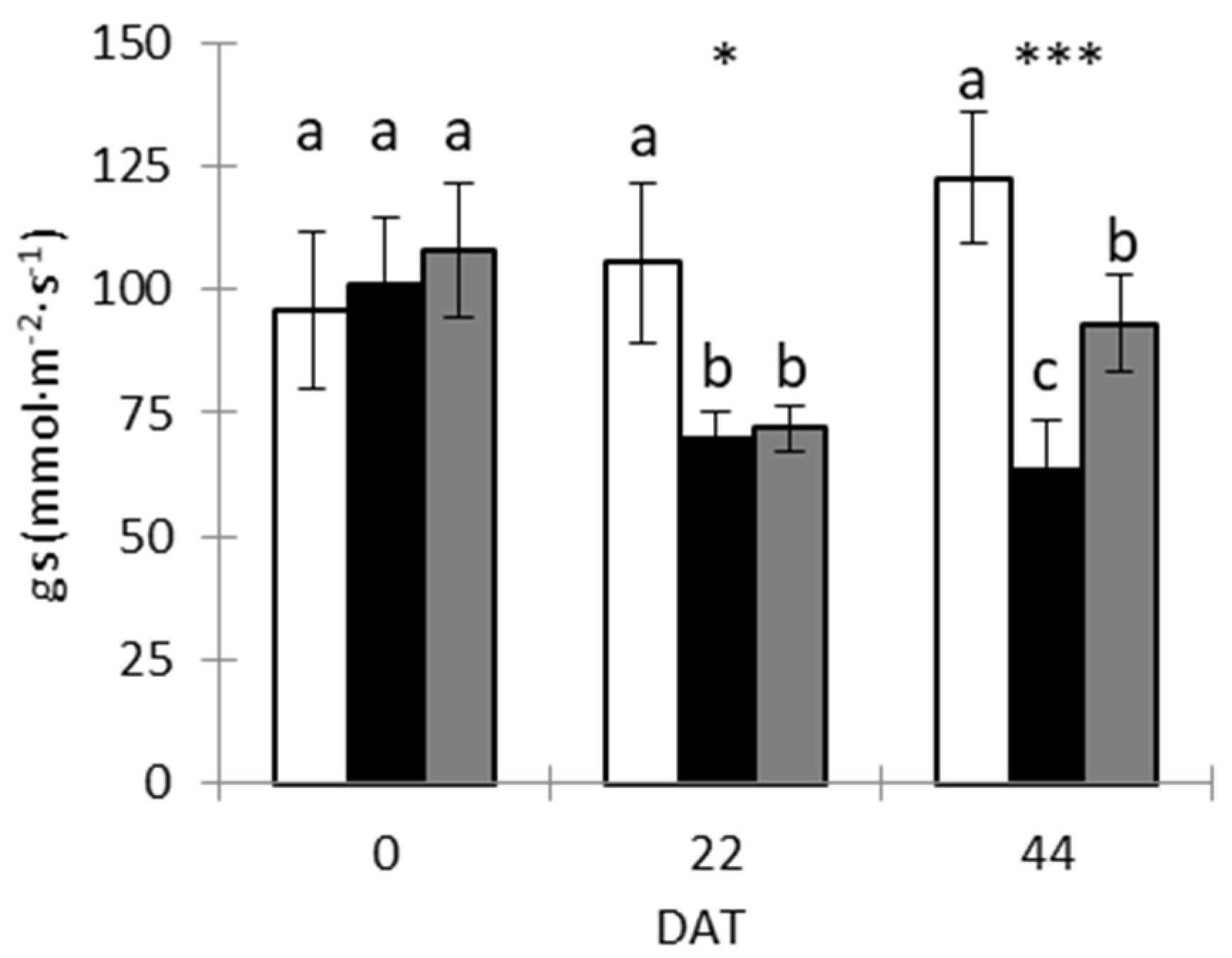
3.3. Stomatal Conductance
3.4. Photosynthetic Pigment Determination
3.5. Proline Production
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline treatment improves physiological responses in quinoa plants under drought stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, A.S.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Coutinho, J.; Moutinho-Pereira, J.; Correia, C.M. Salicylic acid increases drought adaptability of young olive trees by changes on redox status and ionome. Plant Physiol. Biochem. 2019, 141, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Farrell, C.; Szota, C.; Arndt, S.K. Does the turgor loss point characterize drought response in dry land plants? Plant Cell Envrion. 2017, 40, 1500–1511. [Google Scholar] [CrossRef]
- Martin-StPaul, N.; Delzon, S.; Cochard, H. Plant resistance to drought depends on timely stomatal closure. Ecol. Lett. 2017, 20, 1437–1447. [Google Scholar] [CrossRef]
- Lisar, S.Y.S.; Motafakkerazad, R.; Hossain, M.M.; Rahman, I.M.M. Water stress in plants: Causes, effects and responses. In Water Stress; Rahman, I.M.M., Ed.; InTech Publication: Reijka, Croatia, 2012; pp. 1–14. [Google Scholar]
- Hoshika, Y.; Omasa, K.; Paoletti, E. Both ozone exposure and soil water stress are able to induce stomatal sluggishness. Environ. Exp. Bot. 2013, 88, 19–23. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 3rd ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2002; p. 647. [Google Scholar]
- Li, P.; Zhu, Y.; Song, X.; Song, F. Negative effects of long-term moderate salinity and short-term drought stress on the photosynthetic performance of Hybrid Pennisetum. Plant Physiol. Biochem. 2020, 155, 93–104. [Google Scholar] [CrossRef]
- Juzoń, K.; Idziak-Helmcke, D.; Rojek-Jelonek, M.; Warzecha, T.; Warchoł, M.; Czyczyło-Mysza, I.; Dziurka, K.; Skrzypek, E. Functioning of the Photosynthetic Apparatus in Response to Drought Stress in Oat × Maize Addition Lines. Int. J. Mol. Sci. 2020, 21, 6958. [Google Scholar] [CrossRef]
- Gori, A.; Brunetti, C.; dos Santos Nascimento, L.B.; Marino, G.; Guidi, L.; Ferrini, F.; Centritto, M.; Fini, A.; Tattini, M. Photoprotective Role of Photosynthetic and Non-Photosynthetic Pigments in Phillyrea latifolia: Is Their “Antioxidant” Function Prominent in Leaves Exposed to Severe Summer Drought? Int. J. Mol. Sci. 2021, 22, 8303. [Google Scholar] [CrossRef] [PubMed]
- Zahra, N.; Hafeez, M.B.; Kausar, A.; Al Zeidi, M.; Asekova, S.; Siddique, K.H.; Farooq, M. Plant photosynthetic responses under drought stress: Effects and management. J. Agron. Crop Sci. 2023, 209, 651–672. [Google Scholar] [CrossRef]
- Ajithkumar, I.P.; Panneerselvam, R. ROS scavenging system, osmotic maintenance, pigment and growth status of Panicum sumatrense roth. under drought stress. Cell Biochem. Biophys. 2014, 68, 587–595. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Sheng, M.; Tang, M. Effects of Rhizophagus irregularis on photosynthesis and antioxidative enzymatic system in Robinia pseudoacacia L. under drought stress. Front. Plant Sci. 2017, 8, 183. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Zhang, M.; Liu, G.; Han, R.; Liang, Z. Proline accumulation in leaves of Periploca sepium via both biosynthesis up-regulation and transport during recovery from severe drought. PLoS ONE 2013, 8, e69942. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef]
- Moloi, M.J.; van der Merwe, R. Drought Tolerance Responses in Vegetable-Type Soybean Involve a Network of Biochemical Mechanisms at Flowering and Pod-Filling Stages. Plants 2021, 10, 1502. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant biotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Sankar, B.; Kishorekumar, A.; Somasundaram, R.; Lakshmanan, G.M.A.; Panneerselvam, R. Growth, biochemical modifications and proline metabolism in Helianthus annuus L. as induced by drought stress. Colloids Surf. B Biointerfaces 2007, 59, 141–149. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zheng, X.; Liu, Y.; Zhu, Y. Pre-storage application of oxalic acid alleviates chilling injury in mango fruit by modulating proline metabolism and energy status under chilling stress. Food Chem. 2014, 142, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Divyanshu, K.; Kumar, S.; Swapnil, P.; Zehra, A.; Shukla, V.; Yadav, M.; Upadhyay, R.S. Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 2019, 5, e02952. [Google Scholar] [CrossRef]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef]
- La, V.H.; Lee, B.R.; Islam, M.T.; Park, S.H.; Jung, H.I.; Bae, D.W.; Kim, T.H. Characterization of salicylic acid-mediated modulation of the drought stress responses: Reactive oxygen species, proline, and redox state in Brassica napus. Environ. Exp. Bot. 2019, 157, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X. Salicylic acid: Biosynthesis, perception, and contributions to plant immunity. Curr. Opin. Plant Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yousefvand, P.; Sohrabi, Y.; Heidari, G.; Weisany, W.; Mastinu, A. Salicylic Acid Stimulates Defense Systems in Allium hirtifolium Grown under Water Deficit Stress. Molecules 2022, 27, 3083. [Google Scholar] [CrossRef]
- Khodary, S.E.A. Effect of salicylic acid on the growth, photosynthesis and carbohydrate metabolism in salt-stressed maize plants. Inter. J. Agric. Biol. 2004, 6, 5–8. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef]
- Singh, P.K.; Gautam, S. Role of salicylic acid on physiological and biochemical mechanism of salinity stress tolerance in plants. Acta Physiol. Plant 2013, 35, 2345–2353. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef] [PubMed]
- Mohi-Ud-Din, M.; Talukder, D.; Rohman, M.; Ahmed, J.U.; Jagadish, S.; Islam, T.; Hasanuzzaman, M. Exogenous Application of Methyl Jasmonate and Salicylic Acid Mitigates Drought-Induced Oxidative Damages in French Bean (Phaseolus vulgaris L.). Plants 2021, 10, 2066. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Usha, K. Salicylic acid induced physiological and biochemical changes in wheat seedlings under water stress. Plant Grow. Regul. 2003, 39, 137–141. [Google Scholar] [CrossRef]
- Gunes, A.; Inal, A.; Alpaslan, M.; Cicek, N.; Guneri, E.; Eraslan, F.; Guzelordu, T. Effects of exogenously applied salicylic acid on the induction of multiple stress tolerance and mineral nutrition in maize (Zea mays L.). Arch. Agron. Soil. Sci. 2005, 51, 687–695. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. Integ Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Dong, C.J.; Wang, X.L.; Shang, Q.M. Salicylic acid regulates sugar metabolism that confers tolerance to salinity stress in cucumber seedlings. Sci. Hort. 2011, 129, 629–636. [Google Scholar] [CrossRef]
- Janda, T.; Gondor, O.K.; Yordanova, R.; Szalai, G.; Pál, M. Salicylic acid and photosynthesis: Signalling and effects. Acta Physiol. Plant 2014, 36, 2537–2546. [Google Scholar] [CrossRef]
- Torun, H. Time-course analysis of salicylic acid effects on ROS regulation and antioxidant defense in roots of hulled and hulless barley under combined stress of drought, heat and salinity. Physiol. Plant 2019, 165, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef]
- Kaya, C.; Ferhat Ugurlar, F.; Ashraf, M.; Ahmad, P. Salicylic acid interacts with other plant growth regulators and signal molecules in response to stressful environments in plants. Plant Physiol. Biochem. 2023, 196, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Teixeira Beltrão, J.G.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Kaur, H.; Hussain, S.J.; Kaur, G.; Poor, P.; Alamri, S.; Siddiqui, M.H.; Khan, M.I.R. Salicylic Acid Improves Nitrogen Fixation, Growth, Yield and Antioxidant Defense Mechanisms in Chickpea Genotypes Under Salt Stress. J. Plant Grow. Regul. 2022, 41, 2034–2047. [Google Scholar] [CrossRef]
- Estaji, A.; Niknamb, F. Foliar salicylic acid spraying effect’ on growth, seed oil content, and physiology of drought-stressed Silybum marianum L. plant. Agric. Water Manag. 2020, 234, 106116. [Google Scholar] [CrossRef]
- Gomes, M.M.A.; Netto, A.T.; Campostrini, E.; Bressan-Smith, R.; Zullo, M.A.T.; Ferraz, T.M.; Siqueira, L.D.; Leal, N.R.; Núñez-Vázquez, M. Brassinosteroid analogue affects the senescence in two papaya genotypes submitted to drought stress. Theor. Exp. Plant Physiol. 2013, 25, 186–195. [Google Scholar] [CrossRef]
- Marler, T.E.; Mickelbart, M.V. Drought, leaf gas exchange, and chlorophyll fluorescence of field-grown papaya. J. Am. Soc. Hort. Sci. 1998, 123, 714–718. [Google Scholar] [CrossRef]
- Campostrini, E.; Glenn, D.M. Ecophysiology of papaya: A review. Braz. J. Plant Physiol. 2007, 19, 413–424. [Google Scholar] [CrossRef]
- Espadas, J.L.; Castaño, E.; Marina, M.L.; Rodríguez, L.C.; Plaza, M. Phenolic compounds increase their concentration in Carica papaya leaves under drought stress. Acta Physiol. Plant 2019, 41, 180. [Google Scholar] [CrossRef]
- Ruas, K.F.; Baroni, D.F.; de Souza, G.A.R.; Bernado, W.D.; Paixão, J.S.; dos Santos, G.M.; Machado, J.A.; de Abreu, D.P.; de Sousa, E.F.; Rakocevic, M.; et al. A Carica papaya L. genotype with low leaf chlorophyll con-centration copes successfully with soil water stress in the field. Sci. Hortic. 2022, 293, 110722. [Google Scholar] [CrossRef]
- Girón-Ramírez, A.; Peña-Rodríguez, L.M.; Escalante-Erosa, F.; Fuentes, G.; Santamaría, J.M. Identification of the SHINE clade of AP2/ERF domain transcription factors genes in Carica papaya; Their gene expression and their possible role in wax accumulation and water deficit stress tolerance in a wild and a commercial papaya genotypes. Environ. Exp. Bot. 2021, 183, 104341. [Google Scholar] [CrossRef]
- Magdalita, P.M.; Noel, M.R.; Aguilar, E.A.; San Pascual, A.O. Morphological Characters of Papaya (Carica papaya L.) for Drought Tolerance. Sci. Diliman 2021, 33, 53–69. [Google Scholar]
- Mahouachi, J.; Socorro, A.R.; Talón, M. Responses of papaya seedlings (Carica papaya L.) to water stress and rehydration: Growth, photosynthesis and mineral nutrient imbalance. Plant Soil. 2006, 281, 137–146. [Google Scholar] [CrossRef]
- Mahouachi, J.; Arbona, V.; Gómez-Cadenas, A. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Mahouachi, J.; Argamasilla, R.; Gómez-Cadenas, A. Influence of exogenous glycine betaine and abscisic acid on papaya in responses to water-deficit stress. J. Plant Grow. Regul. 2012, 31, 1–10. [Google Scholar] [CrossRef]
- Silva, D.D.; Kane, M.E.; Beeson, R.C. Changes in root and shoot growth and biomass partition resulting from different irrigation intervals for Ligustrum japonicum Thunb. Hortic. Sci. 2012, 47, 1634–1640. [Google Scholar] [CrossRef]
- Lichtenthaler, H.M.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Gomes, M.M.A.; Siqueira, L.N.; Ferraz, T.M.; Rodrigues, W.P.; Figueiredo, F.A.M.M.A.; Reis, F.O.; Campostrini, E. Does abscisic acid and xylem sap pH regulate stomatal responses in papaya plants submitted to partial root-zone drying? Theor. Exp. Plant Physiol. 2023, 35, 185–197. [Google Scholar] [CrossRef]
- Koyro, H.W.; Ahmad, P.; Geissler, N. Abiotic stress responses in plants: An overview. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahouachi, J.; Marcelino-Castro, A.D.; Álvarez-Méndez, S.J.; Urbano-Gálvez, A. Salicylic Acid Applied via Irrigation Enhances Young Carica papaya L. Plant Performance under Water Deficit. Horticulturae 2023, 9, 1070. https://doi.org/10.3390/horticulturae9101070
Mahouachi J, Marcelino-Castro AD, Álvarez-Méndez SJ, Urbano-Gálvez A. Salicylic Acid Applied via Irrigation Enhances Young Carica papaya L. Plant Performance under Water Deficit. Horticulturae. 2023; 9(10):1070. https://doi.org/10.3390/horticulturae9101070
Chicago/Turabian StyleMahouachi, Jalel, Alexandre D. Marcelino-Castro, Sergio J. Álvarez-Méndez, and Antonio Urbano-Gálvez. 2023. "Salicylic Acid Applied via Irrigation Enhances Young Carica papaya L. Plant Performance under Water Deficit" Horticulturae 9, no. 10: 1070. https://doi.org/10.3390/horticulturae9101070
APA StyleMahouachi, J., Marcelino-Castro, A. D., Álvarez-Méndez, S. J., & Urbano-Gálvez, A. (2023). Salicylic Acid Applied via Irrigation Enhances Young Carica papaya L. Plant Performance under Water Deficit. Horticulturae, 9(10), 1070. https://doi.org/10.3390/horticulturae9101070




