Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instruments and Equipment
2.3. Test Method
2.3.1. Sample Pretreatment
2.3.2. HS-SPME Conditions
2.3.3. GC-MS Conditions
2.4. Statistical Analysis
3. Results and Discussion
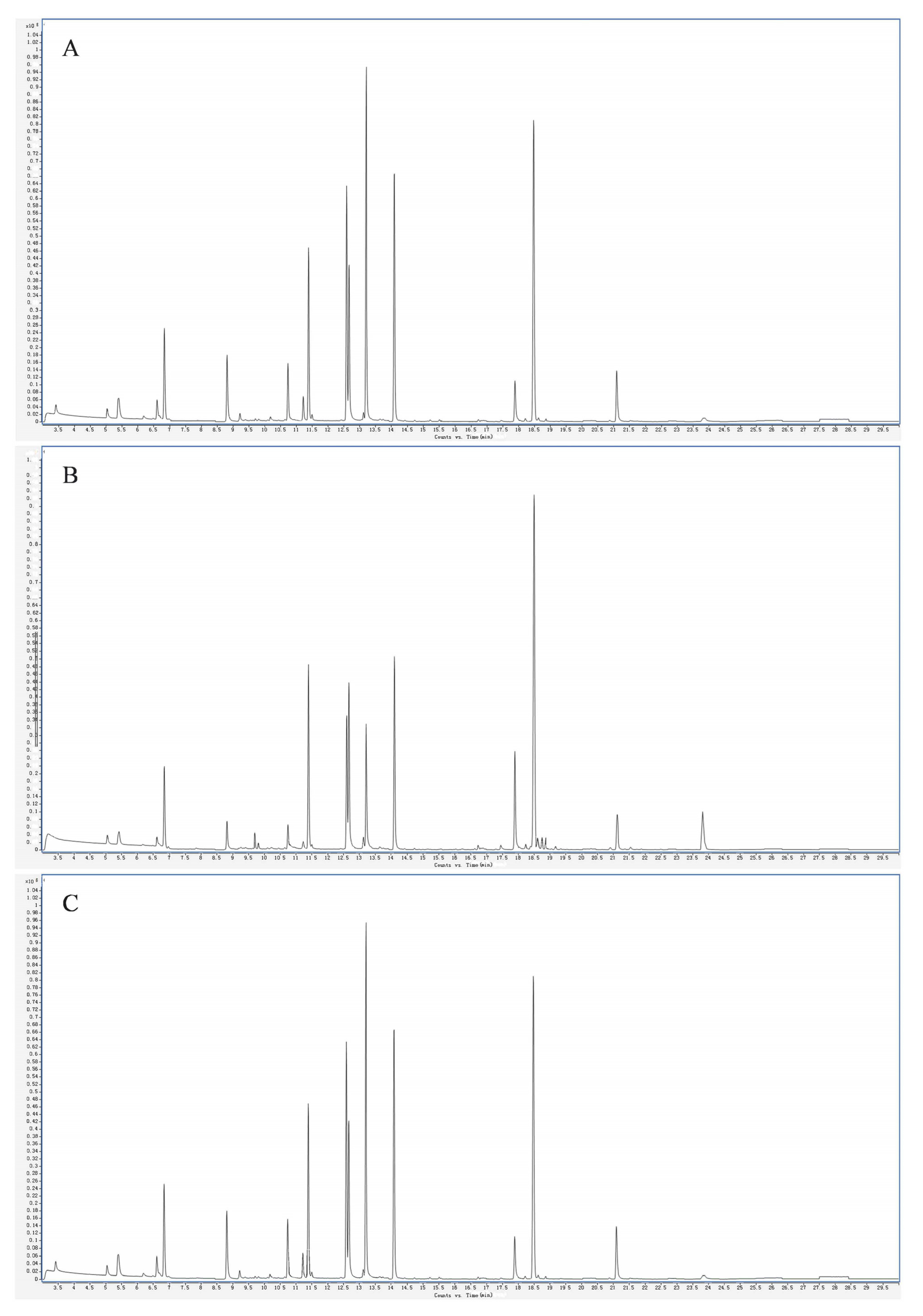
3.1. Analysis of Volatile Species of Plum Fruits of Different Varieties
3.2. Analysis of Volatile Content of Plum Fruits of Different Varieties
3.3. Principal Component Analysis of Volatile Substances of Plum Fruits of Different Varieties
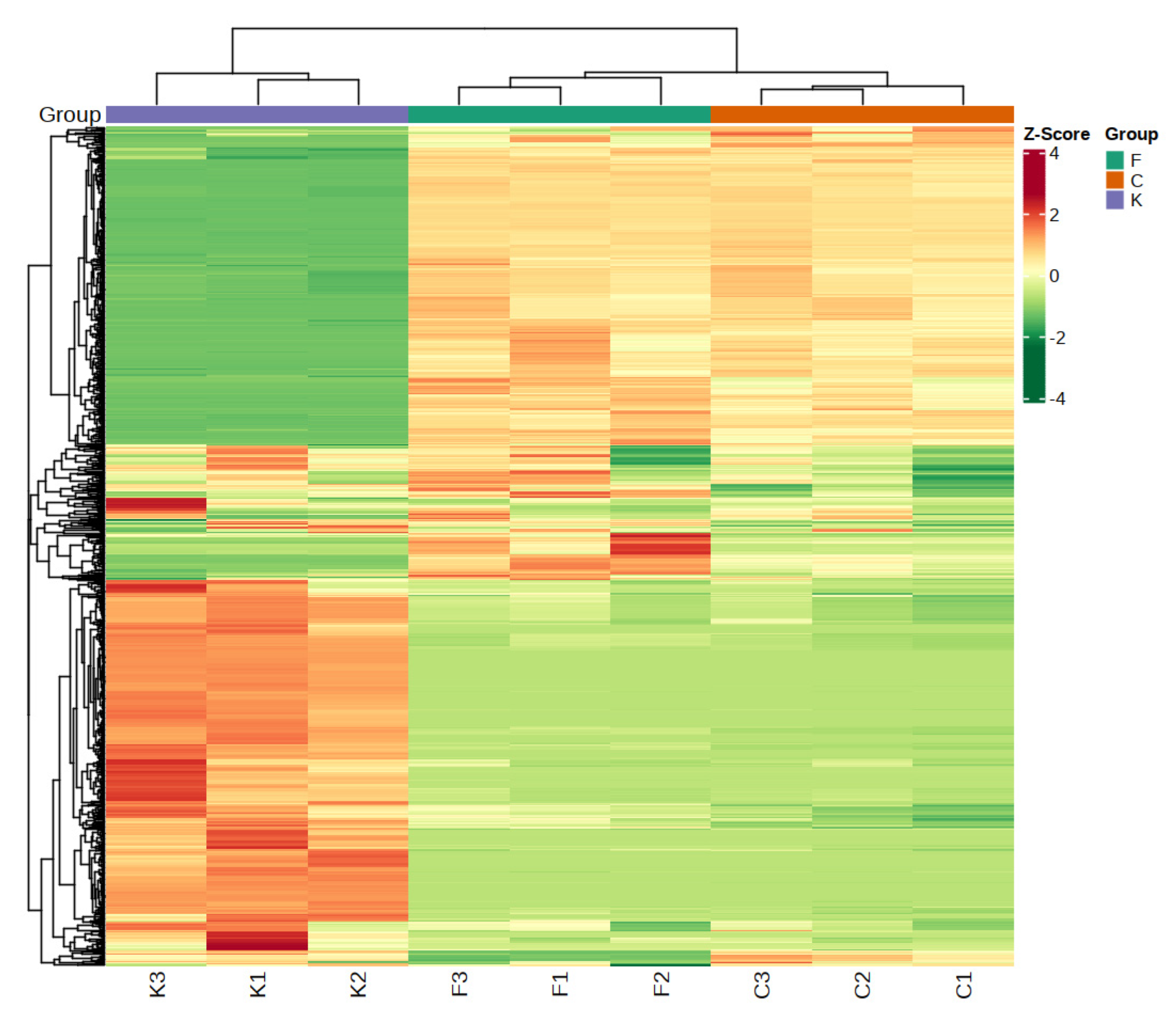
3.4. Cluster Analysis of Volatile Substances of Plum Fruits of Different Varieties
3.5. Orthogonal Partial Least Squares Discriminant Analysis of Volatile Substances of Plum Fruits of Different Varieties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, S.; Xu, M.; Zhang, Y.; Ma, X.; Zhang, Q.; Liu, N.; Liu, W. Retrospect, Problematical Issues and the Prospect of Plum Breeding in China. J. Fruit Sci. 2018, 35, 231–245. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Millson, M.; Huang, H.S.; Chuang, L.T.; Sanz, C.; Golding, J.B. Changes in Sugars, Acids and Fatty Acids in Naturally Parthenocarpic Date Plum Persimmon (Diospyros lotus L.) Fruit During Maturation and Ripening. Eur. Food Res. Technol. 2005, 221, 113–118. [Google Scholar] [CrossRef]
- Nowick, P.; Wojdlo, A.; Samoticha, J. Evaluation of Phytochemicals, Antioxidant Capacity, and Antidiabetic Activity of Novel Smoothies from Selected Punus Fruits. J. Funct. Food. 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Serradilla, M.J.; Martin, A.; Ruizmoyano, S. Physicochemical and Sensorial Characterisation of Four Sweet Cherry Cultivars Grown in Jerte Valley (Spain). Food Chem. 2012, 133, 1551–1559. [Google Scholar] [CrossRef]
- Hanmugam, S.; Donizete, S.R.; Rajan, M.; Denadi, M.; Narendraa, N.; Serafini, M. Volatile Profiling and UHPLC-QqQ-MS/MS Polyphenol Analysis of Passiflora Leschenaultii DC. Fruits and Its Anti-Radical and Anti-Diabetic Properties. Food Res. 2020, 133, 109202. [Google Scholar] [CrossRef]
- Yao, M.; Zhou, X.; Zhou, Q.; Shi, F.; Cheng, B.D.; Tan, S.C.; Zhou, J.; Shu, J. Low Temperature Conditioning Alleviates Loss of Aroma-Related Esters of ‘Nanguo’ Pears by Regulation of Ethylene Signal Transduction. Food Chem. 2018, 264, 263–269. [Google Scholar] [CrossRef]
- Zhang, W.L.; Chen, T.T.; Tang, J.M.; Zhou, Z. Tracing the Production Area of Citrus Fruits Using Aroma-Active Compounds and Their Quality Evaluation Models. J. Sci. Food Agric. 2020, 100, 517–526. [Google Scholar] [CrossRef]
- Cozzolino, R.; Giulio, B.; Petriccione, M.; Martignetti, A.; Pellicano, M. Comparative Analysis of Volatile Metabolites, Quality and Sensory Attributes of Actinidia Chinensis Fruit. Food Chem. 2020, 316, 126340. [Google Scholar] [CrossRef]
- Chai, Q.; Wu, B.; Liu, W.; Wang, L.; Yang, C.; Wang, Y.; Fang, J.; Liu, Y.; Li, S. Volatiles of Plums Evaluated by HS-SPME with GC-MS at The Germplasm Level. Food Chem. 2012, 130, 432–440. [Google Scholar] [CrossRef]
- Chai, Q.Q.; Wang, L.J.; Wu, B.H.; Fan, P.; Wei, D. Volatiles in Fruit of Three Plum Cultivars Belonging to Prunus Salicina,P. Cerasifera and Their Interspecific Hybrid. Acta Hortic. Sin. 2011, 38, 2357–2364. [Google Scholar] [CrossRef]
- Lozano, M.; Vidal, A.; Hernadez, M.T.; Ayuso, M.C.; Bernalte, M.J.; Velardo, J.G. Physicochemical and Nutritional Properties and Volatile Constituents of Six Japanese Plum (Prunus salicina Lindl.) Cultivars. Eur. Food Res. Technol. 2009, 228, 403–410. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, H.J.; Xu, N.W.; Cao, C.L.; Liu, J.Z.; Wu, C.C.; Zhang, L.B. Analysis of Volatile Components in Cerasus Humilis (Bge.) sok by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Sci. Agric. Sin. 2019, 52, 3448–3459. [Google Scholar] [CrossRef]
- Wang, H.R.; Ma, Y.H.; Wang, W.; Li, J.H.; Zhang, S.W. Changes in Volatile Composition during Fruit Development and Ripening of ‘Friar’ Plum. Food Sci. 2012, 33, 274–279. [Google Scholar]
- Yu, H.; Yang, L.; Zhao, F.; Yang, L. Study on Aroma Components of Six Kinds of Plum Fruits. J. Anhui Agri. Sci. 2012, 40, 13601–13604. [Google Scholar] [CrossRef]
- Shao, S.X.; Xu, M.G.; Lin, Y.Q.; Chen, X.M.; Fang, D.Y.; Cai, J.Y.; Wang, J.H.; Jin, S.; Ye, N.X. Differential Analysis of Aroma Components of Huangguanyin Oolong Ttea from Different Geographical Origins Using Electronic Nose and Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. Food Sci. 2023, 44, 232–239. [Google Scholar] [CrossRef]
- Fu, J.Y. The Sesquiterpene Metabolism and Response to Diverse Biotic Stresses in Tea Plant. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017; pp. 1–2. [Google Scholar]
- Shi, J.; Xie, D.C.; QI, D.D. Methyl Jasmonate-Induced Changes of Flavor Profiles During the Processing of Green, Oolong, and Black Tea. Front. Plant Sci. 2019, 10, 781. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Peng, Q.H.; Zhao, F.; Lu, H.W.; Bao, X.W.; Lin, Z. Flavor Chemistry Characteristics of New Jiuqu Hongmei Tea of Different Grade Levels. Food Sci. 2021, 42, 215–220. [Google Scholar] [CrossRef]
- Li, P.L.; Zhu, Y.; Lu, M.L. Variation Patterns in The Content of Glycosides During Green Tea Manufacturing by a Modification-Specific Metabolomics Approach: Enzymatic Reaction Promoting an Increase in the Glycosidically Bound Volatiles at the Pan Firing Stage. Food Chem. 2019, 279, 80–87. [Google Scholar] [CrossRef]
- Taiti, C.; Pandolfi, C.; Caparrotta, S.; Dei, M.; Giordani, E.; Mancuso, S.; Nencetti, V. Fruit Aroma and Sensorial Characteristics of Traditional and Innovative Japanese plum (Prunus salicina Lindl.) Cultivars Grown in Italy. Eur. Food Res. Technol. 2019, 245, 2655–2668. [Google Scholar] [CrossRef]
- Wang, S.S.; Zhao, C.H.; Li, H.L.; Zhang, B.B.; Liang, Y.H.; Song, H.W. Analysis of Fruit Aromatic Components of Ten Plum Germplasm Resources in Northeast China. Sci. Agric. Sin. 2021, 54, 2476–2486. [Google Scholar] [CrossRef]
- Niu, Y.W.; Yao, Z.M.; Xiao, Z.B.; Wu, M.L. The Study on Characteristic Aroma Compounds in Two Lavender Essential Oils by AEDA and OAVs. Food Ind. 2016, 37, 264–268. [Google Scholar]
- Pino, J.A.; Quijano, C.E. Study of The Volatile Compounds From Plum (Prunus domestica L. cv. Horvin) and Estimation of Their Contribution to the Fruit Aroma. Food Sci. Technol. 2012, 32, 76–83. [Google Scholar] [CrossRef]
- Ye, L.; Yang, C.; Li, W.; Hao, J.; Sun, M.; Zhang, J.; Zhong, Z. Evaluation of Volatile Compounds from Chinese Dwarf Cherry (Cerasus humilis (Bge.) Sok.) Germplasms by Headspace Solid-phase Microextraction and Gas Chromatography-Mass Spectrometry. Food Chem. 2017, 217, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhan, P.; Wang, P.; Tian, H.L. Analysis of Key Aroma Compounds in Kiwifruits. Food Sci. 2021, 42, 118–124. [Google Scholar] [CrossRef]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Wu, J. Identification of Aromaactive Compounds in Jiashi Muskmelon Juice by GC-O-MS and OAV Calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef]
- Wang, Y.; Hossain, D.; Perry, P.; Adams, B.; Lin, J. Characterization of Volatile and Aroma-impact Compounds in Persimmon (Diospyros kaki L., var. Triumph) Fruit by GC-MS and GC-O Analyses. Flavour Frag. J. 2012, 27, 141–148. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhang, M.Q.; Cai, S.J.; Jiang, K.L.; Ma, L.L.; Ma, Q.Y.; Wang, J.; Sun, J.F.; Wang, W.X. Analysis of The Differences in Major Bioactive Components and Flavor Characteristics of Toona Sinensis Buds from Six Production Areas. Food Sci. 2023, 44, 336–343. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.; Bi, J. Characterization of Volatile Profile from Ten Different Varieties of Chinese Jujubes by HS-SPME/GC–MS coupled with E-nose. Food Res. Int. 2018, 105, 605–615. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.H.; Yang, K. Comparative Analysis of Volatile Profiles in Kernel Oils of Ten Korean Pine (Pinus koraiensis) Varieties by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry and Electronic Nose. Food Sci. 2021, 42, 178–184. [Google Scholar] [CrossRef]
- Gao, H.; Xu, D.D.; Wang, W.P.; Zhao, Y.; Zhang, J.; Ding, J.; Tan, L.; Zhang, X. Identification of Characteristic Volatiles in Vinegar Prepared with Monascus-Fermented Rice During Acetic Acid Fermentation Using Multivariate Statistical Analysis. Food Sci. 2022, 43, 219–227. [Google Scholar] [CrossRef]
- Padilla-Jimenezs, M.; Angoa-Perez, M.V.; Mena-Violante, H.G. Identification of Organic Volatile Markers Associated with Aroma During Maturation of Strawberry Fruits. Molecules 2021, 26, 504. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Zhou, H.S.; Liu, Y.Q.; Wang, H.; Huang, J.Q.; Lei, P.D. Differences in Aroma Components of Black Tea Processed from Different Tea Cultivars in Huangshan by Using Headspace-Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry and Odor Activity Value. Food Sci. 2022, 43, 235–241. [Google Scholar] [CrossRef]
- Szeto, S.S.; Reinke, S.N.; Sykes, B.D.; Lemire, B.D. Mutations in the Saccharomyces Cerevisiae Succinate Dehydrogenase Result in Distinct Metabolic Phenotypes Revealed Tthrough 1H NMR-Based Metabolic Foot Printing. J. Proteome Res. 2010, 9, 6729–6739. [Google Scholar] [CrossRef]
- An, H.M.; Ou, X.C.; Xiong, Y.F.; Zhang, Y.B.; Li, J.; Li, Q.; Li, Q.; Li, S.; Huang, J.A. Study on the Characteristic Aroma Components of Jasmine Tea. J. Tea Sci. 2020, 40, 225–237. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Liu, W.; He, S.; Hu, X.Q.; Zhang, J.H.; Shan, Y. Analysis of Volatile Components in Three Varieties of Kumquat by Headspace Solid Phase Microextraction-Gas Chromatography-Mass Spectrometry. Food Sci. 2021, 42, 176–184. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, Y.; Zhang, Y.X.; Du, G.Q. Analysis and Evaluation of Fruit Quality and Aroma Components of ‘Yali’ Pear (Pyrus bretschneideri Rehd.) Pollinated with Eighteen Pollinizers. Food Sci. 2022, 43, 294–302. [Google Scholar] [CrossRef]


| Compound Type | Total Volatile Compounds | Total Volatile Common Substances | Types and Amounts of Volatile Substances in Plum Fruits of Different Varieties | ||
|---|---|---|---|---|---|
| ‘Fengtang’ Plum | ‘Kongxin’ Plum | ‘Cuihong’ Plum | |||
| Terpenoids | 200 | 109 | 154 | 155 | 154 |
| Esters | 171 | 77 | 120 | 124 | 120 |
| Hydrocarbons | 89 | 41 | 71 | 58 | 71 |
| Alcohols | 82 | 39 | 60 | 61 | 60 |
| Ketones | 82 | 35 | 60 | 55 | 60 |
| Aldehydes | 63 | 37 | 51 | 48 | 51 |
| Acids | 18 | 8 | 12 | 13 | 12 |
| Phenols | 17 | 5 | 9 | 13 | 9 |
| Amines | 21 | 8 | 13 | 16 | 13 |
| Aromatics | 54 | 24 | 37 | 41 | 37 |
| Heterocyclic | 116 | 78 | 101 | 92 | 101 |
| Nitrogen-containing compounds | 10 | 5 | 7 | 7 | 7 |
| Sulfur-containing compounds | 7 | 1 | 3 | 4 | 3 |
| Other categories | 8 | 3 | 6 | 4 | 6 |
| Total | 938 | 470 | 704 | 691 | 704 |
| Compound Type | Relative Content % | ||
|---|---|---|---|
| ‘Fengtang’ Plum | ‘Kongxin’ Plum | ‘Cuihong’ Plum | |
| Terpenoids | 34.889 ± 0.077 b | 38.312 ± 0.148 a | 34.985 ± 0.052 b |
| Ester | 12.573 ± 0.078 b | 12.816 ± 0.150 ab | 12.946 ± 0.023 a |
| Hydrocarbons | 5.189 ± 0.073 b | 5.540 ± 0.033 a | 4.828 ± 0.060 c |
| Alcohol | 7.654 ± 0.099 a | 7.582 ± 0.071 a | 7.639 ± 0.044 a |
| Ketone | 2.089 ± 0.022 b | 2.872 ± 0.001 a | 1.922 ± 0.060 c |
| Aldehyde | 6.952 ± 0.069 a | 5.488 ± 0.057 b | 6.890 ± 0.035 c |
| Acid | 0.543 ± 0.011 b | 0.623 ± 0.017 a | 0.555 ± 0.007 b |
| Phenol | 2.930 ± 0.044 ab | 2.980 ± 0.040 a | 2.812 ± 0.010 b |
| Amine | 0.498 ± 0.038 a | 0.249 ± 0.004 b | 0.673 ± 0.034 c |
| Aromatics | 3.801 ± 0.012 b | 7.233 ± 0.013 a | 3.672 ± 0.022 c |
| Heterocyclic | 20.647 ± 0.061 a | 14.676 ± 0.076 b | 20.787 ± 0.023 a |
| Nitrogen compounds | 1.566 ± 0.006 b | 0.935 ± 0.009 c | 1.607 ± 0.007 a |
| Sulfur compounds | 0.636 ± 0.013 a | 0.663 ± 0.017 a | 0.654 ± 0.003 a |
| Other categories | 0.014 ± 0.0002 a | 0.007 ± 0.0003 c | 0.011 ± 0.0004 b |
| NO. | Compound | CAS | RT/Min | Relative Contents % | ||
|---|---|---|---|---|---|---|
| ‘Fengtang’ Plum | ‘Kongxin’ Plum | ‘Cuihong’ Plum | ||||
| Terpenoids (13) | ||||||
| 1 | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | 495-60-3 | 18.43 | 1.260 ± 0.013 b | 1.678 ± 0.008 a | 1.260 ± 0.005 b |
| 2 | cis-.alpha.-Bisabolene | 29837-07-8 | 18.44 | 1.188 ± 0.010 b | 2.053 ± 0.023 a | 1.189 ± 0.005 b |
| 3 | [1α,4aα,8aα]-1,2,4a,5,6,8a-hexahydro-4-7-dimethyl-1-[1-methylethyl]naphthalene | 31983-22-9 | 17.46 | LOD | 1.556 ± 0.019 | LOD |
| 4 | Eremophilene | 10219-75-7 | 18.42 | 1.195 ± 0.009 b | 1.525 ± 0.015 a | 1.183 ± 0.003 c |
| 5 | Bicyclo[2.2.1]heptane, 2-methyl-3-methylene-2-(4-methyl-3-pentenyl)-, (1S-endo)- | 25532-78-9 | 17.87 | LOD | 1.122 ± 0.040 | LOD |
| 6 | (1S,2E,6E,10R)-3,7,11,11-Tetramethylbicyclo[8.1.0]undeca-2,6-diene | 24703-35-3 | 18.35 | 1.061 ± 0.010 b | 1.390 ± 0.016 a | 1.063 ± 0.002 b |
| 7 | .alpha.-Muurolene | 10208-80-7 | 18.47 | 1.189 ± 0.010 b | 1.556 ± 0.019 a | 1.260 ± 0.005 c |
| 8 | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, (1S-endo)- | 464-45-9 | 12.58 | 2.027 ± 0.016 a | 1.231 ± 0.015 b | 2.057 ± 0.002 a |
| 9 | Isoborneol | 124-76-5 | 12.57 | LOD | 1.33 ± 0.015 | LOD |
| 10 | endo-Borneol | 507-70-0 | 12.53 | 2.030 ± 0.013 a | 1.231 ± 0.015 b | 2.063 ± 0.004 a |
| 11 | Carvenone | 499-74-1 | 14.09 | 3.105 ± 0.014 b | 0.960 ± 0.014 c | 1.767 ± 0.020 a |
| 12 | 5,7-Octadien-4-one, 2,6-dimethyl-, (Z)- | 3588-18-9 | 12.59 | 2.031 ± 0.014 a | LOD | 2.144 ± 0.017 b |
| 13 | (-)-Aristolene | 6831-16-9 | 17.88 | 1.189 ± 0.010 a | 1.228 ± 0.013 b | 1.191 ± 0.005 a |
| Heterocyclic (6) | ||||||
| 14 | 2-((3,3-Dimethyloxiran-2-yl)methyl)-3-methylfuran | 92356-06-4 | 12.61 | 2.009 ± 0.019 a | LOD | 2.039 ± 0.001 a |
| 15 | 2-Hexanoylfuran | 14360-50-0 | 14.08 | 2.849 ± 0.012 b | 2.455 ± 0.015 c | 2.985 ± 0.016 a |
| 16 | Furo[3,4-c]pyridin-1(3H)-one, 7-hydroxy-6-methyl- | 4753-19-9 | 13.48 | 1.019 ± 0.014 b | 1.310 ± 0.016 a | 1.017 ± 0.006 b |
| 17 | 1H-Pyrazole-1-carboximidamide, 3,5-dimethyl- | 22906-75-8 | 11.38 | 1.353 ± 0.041 b | 1.821 ± 0.031 a | 0.913 ± 0.001 c |
| 18 | Furaneol | 3658-77-3 | 13.21 | 1.296 ± 0.027 b | LOD | 1.363 ± 0.018 a |
| 19 | Ethanone, 1-(1H-pyrazol-4-yl)- | 25016-16-4 | 11.58 | 2.017 ± 0.009 a | 1.233 ± 0.015 b | 2.150 ± 0.013 c |
| Aromatics (4) | ||||||
| 20 | (3-Bromo-1-methylpropoxymethyl)benzene | 51666-29-6 | 18.46 | 1.678 ± 0.013 b | 2.226 ± 0.023 a | 1.679 ± 0.009 b |
| 21 | (5-bromopentyl)-Benzene | 14469-83-1 | 18.45 | 1.678 ± 0.013 b | 2.221 ± 0.023 a | 1.679 ± 0.008 c |
| 22 | Benzene, 1,2-dimethoxy-4-(1-propenyl)- | 93-16-3 | 18.34 | LOD | 1.098 ± 0.006 | LOD |
| 23 | Benzoic acid, 4-hydroxy- | 99-96-7 | 18.29 | LOD | 1.388 ± 0.016 | LOD |
| Alcohol (2) | ||||||
| 24 | Cyclohexanemethanol, .alpha.,.alpha.-dimethyl-4-methylene- | 7299-42-5 | 12.66 | 1.113 ± 0.009 | 1.120 ± 0.006 | 1.125 ± 0.005 |
| 25 | Benzenemethanol, .alpha.-2-propenyl- | 936-58-3 | 14.10 | 1.282 ± 0.004 b | LOD | 1.322 ± 0.006 a |
| Aldehyde (3) | ||||||
| 26 | 2,6-Nonadienal, (E,Z)- | 557-48-2 | 13.02 | LOD | 1.621 ± 0.014 | LOD |
| 27 | 2-Hexenal, (E)- | 6728-26-3 | 12.66 | 1.640 ± 0.006 b | 1.033 ± 0.006 c | 2.047 ± 0.003 a |
| 28 | 5-Heptenal, 2,6-dimethyl- | 106-72-9 | 10.73 | 2.304 ± 0.048 b | LOD | 2.429 ± 0.035 a |
| Ester (3) | ||||||
| 29 | Ethyl acetate | 141-78-6 | 19.01 | 2.136 ± 0.006 c | 2.028 ± 0.005 a | 2.043 ± 0.004 b |
| 30 | Benzyl tiglate | 37526-88-8 | 18.51 | LOD | 2.228 ± 0.023 | LOD |
| 31 | Isobornyl formate | 1200-67-5 | 14.12 | 1.998 ± 0.007 b | LOD | 2.170 ± 0.010 a |
| Hydrocarbons (2) | ||||||
| 32 | 1,5-Cycloundecadiene, 8,8-dimethyl-9-methylene- | 62338-54-9 | 18.49 | 1.638 ± 0.014 b | 2.058 ± 0.022 a | 1.636 ± 0.009 b |
| 33 | Albene | 38451-64-8 | 11.60 | LOD | 1.233 ± 0.015 | LOD |
| Phenol (1) | ||||||
| 34 | 1,3-Benzenediol, 4,5-dimethyl- | 527-55-9 | 18.48 | 1.578 ± 0.023 b | 2.757 ± 0.039 a | 1.064 ± 0.016 c |
| Groups | R2X | R2Y | Q2 |
|---|---|---|---|
| F vs. C | 0.747 | 0.999 | 0.849 |
| C vs. K | 0.922 | 1 | 0.999 |
| F vs. K | 0.923 | 1 | 0.998 |
| Compound Type | Compound | F vs. C (23) | C vs. K (121) | F vs. K (108) | |||
|---|---|---|---|---|---|---|---|
| VIP Value | p Value | VIP Value | p Value | VIP Value | p Value | ||
| Ester | Benzoic acid, methyl ester | 1.694 | 0.008 | 1.067 | 0.003 | 1.070 | 0.001 |
| Butanoic acid, hexyl ester | 1.607 | 0.033 | - | - | - | - | |
| 2,6-Octadienoic acid, 3,7-dimethyl-, methyl ester | 1.649 | 0.034 | 1.070 | 0.006 | 1.073 | 0.019 | |
| Methyl acetate | 1.647 | 0.015 | 1.070 | 0.012 | 1.074 | 0.001 | |
| Ethyl acetate | 1.643 | 0.024 | 1.071 | 0.004 | 1.074 | 0.009 | |
| Bornyl acetate | - | - | 1.072 | 0.036 | 1.074 | 0.006 | |
| Citronellyl isobutyrate | - | - | 1.070 | 0.042 | 1.074 | 0.023 | |
| Linalyl acetate | - | - | 1.068 | 0.001 | 1.072 | 0.001 | |
| Diethyl Phthalate | - | - | 1.061 | 0.005 | - | - | |
| Benzeneacetic acid, 4-(1,1-dimethylethyl)-, methyl ester | - | - | - | - | 1.068 | 0.002 | |
| L-Aspartic acid, N-acetyl-, dimethyl ester | - | - | 1.058 | 0.015 | - | - | |
| 1,3-Cyclohexadiene-1-carboxylic acid, 2,6,6-trimethyl-, ethyl ester | - | - | 1.069 | 0.043 | - | - | |
| Carbamic acid, 2-chloroethyl ester | - | - | 1.050 | 0.016 | - | - | |
| Propanoic acid, 2-methyl-, 2-phenylethyl ester | - | - | 1.071 | 0.049 | 1.074 | 0.049 | |
| Benzyl tiglate | - | - | 1.071 | 0.011 | 1.074 | 0.010 | |
| cis-3-Hexenyl isovalerate | - | - | 1.071 | 0.039 | 1.074 | 0.039 | |
| Isobornyl formate | - | - | 1.070 | 0.036 | 1.074 | 0.009 | |
| Acetic acid, (propylthio)-, methyl ester | - | - | 1.066 | 0.001 | - | - | |
| 2-Butenoic acid, hexyl ester | - | - | 1.070 | 0.022 | 1.073 | 0.001 | |
| Acetic acid, phenyl ester | - | - | 1.070 | 0.009 | 1.073 | 0.026 | |
| 2-Oxepanone | - | - | 1.070 | 0.002 | 1.073 | 0.003 | |
| Ethyl 2-hexenoate, trans- | - | - | 1.071 | 0.037 | - | - | |
| cis-3-Hexenyl-.alpha.-methylbutyrate | - | - | 1.071 | 0.040 | 1.074 | 0.025 | |
| Methyl 6,6-dimethylbicyclo[3.1.1]hept-2-ene-2-carboxylate | - | - | - | - | 1.070 | 0.009 | |
| Benzoic acid, 1-methylethyl ester | - | - | - | - | 1.074 | 0.032 | |
| Benzoic acid, 2-propenyl ester | - | - | - | - | 1.074 | 0.010 | |
| Butanoic acid, 1,1-dimethyl-2-phenylethyl ester | - | - | - | - | 1.074 | 0.017 | |
| Terpenoids | 2-Buten-1-one, 1-(2,6,6-trimethyl-1,3-cyclohexadien-1-yl)-, (E)- | 1.686 | 0.010 | - | - | - | - |
| 2,6,10-Dodecatrien-1-ol, 3,7,11-trimethyl-, (Z,E)- | - | - | 1.059 | 0.023 | - | - | |
| Germacrene D | - | - | 1.071 | 0.020 | 1.074 | 0.020 | |
| cis-.alpha.-Bisabolene | - | - | 1.069 | 0.006 | 1.073 | 0.006 | |
| Naphthalene, decahydro-1,6-bis(methylene)-4-(1-methylethyl)-, (4.alpha.,4a.alpha.,8a.alpha.)- | - | - | 1.059 | 0.008 | - | - | |
| Humulene | - | - | 1.063 | 0.006 | 1.055 | 0.032 | |
| (S,1Z,6Z)-8-Isopropyl-1-methyl-5-methylenecyclodeca-1,6-diene | - | - | 1.061 | 0.026 | - | - | |
| (-)-trans-Isopiperitenol | - | - | 1.031 | 0.015 | 1.038 | 0.040 | |
| Bicyclo[2.2.1]heptane, 2-methyl-3-methylene-2-(4-methyl-3-pentenyl)-, (1S-endo)- | - | - | 1.063 | 0.007 | 1.054 | 0.030 | |
| (1S,4aR,7R)-1,4a-Dimethyl-7-(prop-1-en-2-yl)-1,2,3,4,4a,5,6,7-octahydronaphthalene | - | - | 1.064 | 0.004 | 1.056 | 0.030 | |
| Benzene, 1-(1,5-dimethyl-4-hexenyl)-4-methyl- | - | - | 1.071 | 0.007 | 1.074 | 0.007 | |
| Iridomyrmecin | - | - | 1.063 | 0.007 | 1.058 | 0.018 | |
| Isoborneol | - | - | 1.071 | 0.026 | 1.074 | 0.026 | |
| Citronellol | - | - | 1.063 | 0.003 | - | - | |
| 2,3-Dehydro-1,8-cineole | - | - | 1.071 | 0.005 | 1.074 | 0.005 | |
| .beta.-Phellandrene | - | - | 1.061 | 0.001 | 1.063 | 0.006 | |
| D-Carvone | - | - | 1.071 | 0.015 | 1.074 | 0.015 | |
| Carvone | - | - | 1.071 | 0.004 | 1.074 | 0.014 | |
| .gamma.-Terpinene | - | - | 1.071 | 0.003 | - | - | |
| Caryophyllene | - | - | 1.071 | 0.017 | - | - | |
| (2S,4R)-4-Methyl-2-(2-methylprop-1-en-1-yl)tetrahydro-2H-pyran | - | - | 1.071 | 0.032 | - | - | |
| Aristolochene | - | - | 1.071 | 0.047 | 1.074 | 0.022 | |
| Cyclohexene, 4-[(1E)-1,5-dimethyl-1,4-hexadien-1-yl]-1-methyl- | - | - | - | - | 1.067 | 0.047 | |
| 1H-Cyclopenta[1,3]cyclopropa[1,2]benzene, octahydro-7-methyl-3-methylene-4-(1-methylethyl)-, [3aS-(3a.alpha.,3b.beta.,4.beta.,7.alpha.,7aS*)]- | - | - | - | - | 1.066 | 0.012 | |
| Cyclohexanol, 5-methyl-2-(1-methylethyl)-, (1.alpha.,2.alpha.,5.alpha.)- | - | - | - | - | 1.048 | 0.008 | |
| 3,6-dihydro-4-methyl-2-(2-methyl-1-propenyl)- | - | - | - | - | 1.070 | 0.010 | |
| 1,3-Cyclohexadiene-1-carboxaldehyde, 2,6,6-trimethyl- | - | - | - | - | 1.073 | 0.045 | |
| 1,6-Octadiene, 3,7-dimethyl- | - | - | - | - | 1.075 | 0.011 | |
| Hydrocarbons | Tridecane | 1.581 | 0.008 | 1.071 | 0.001 | 1.073 | 0.024 |
| 1,4-Cyclohexadiene, 1-methyl- | 1.609 | 0.016 | 1.070 | 0.014 | 1.074 | 0.010 | |
| 1,5-Heptadiene, 2-methyl-, (Z)- | 1.534 | 0.017 | 1.070 | 0.023 | 1.074 | 0.009 | |
| Heptadecane, 2-methyl- | - | - | 1.071 | 0.042 | 1.074 | 0.042 | |
| Cyclohexane, 1-(1,5-dimethylhexyl)-4-methyl- | - | - | 1.063 | 0.002 | 1.058 | 0.032 | |
| 1-Pentadecene | - | - | 1.071 | 0.001 | 1.074 | 0.001 | |
| 1-Dodecene | - | - | 1.068 | 0.006 | - | - | |
| 4-Undecene, 3-methyl-, (Z)- | - | - | 1.069 | 0.017 | 1.073 | 0.002 | |
| Albene | - | - | 1.071 | 0.026 | 1.075 | 0.026 | |
| Decane, 4-methyl- | - | - | 1.069 | 0.026 | - | - | |
| Cyclohexane, (1,1-dimethylpropyl)- | - | - | 1.071 | 0.017 | 1.074 | 0.017 | |
| Bicyclo(3.3.1)non-2-ene | - | - | 1.068 | 0.004 | 1.072 | 0.002 | |
| Undecane, 2,9-dimethyl- | - | - | 1.071 | 0.005 | - | - | |
| Undecane, 2,4-dimethyl- | - | - | 1.070 | 0.023 | 1.073 | 0.043 | |
| Cyclohexene, 3,4-diethenyl-1,6-dimethyl- | - | - | 1.071 | 0.039 | 1.074 | 0.003 | |
| Octane, 2,3,3-trimethyl- | - | - | - | - | 1.073 | 0.038 | |
| (1S,5S)-2-Methyl-5-((R)-6-methylhept-5-en-2-yl)bicyclo[3.1.0]hex-2-ene | - | - | - | - | 1.074 | 0.035 | |
| 1-Undecene, 9-methyl- | - | - | - | - | 1.074 | 0.003 | |
| Alcohol | 1,2-Benzenedimethanol | 1.679 | 0.007 | - | - | 1.064 | 0.001 |
| 1,5,7-Octatrien-3-ol, 3,7-dimethyl- | 1.678 | 0.043 | - | - | - | - | |
| 5,9-Undecadien-2-ol, 6,10-dimethyl- | - | - | 1.071 | 0.016 | 1.074 | 0.016 | |
| 1-Cyclohexene-1-propanol, .alpha.,2,6,6-tetramethyl- | - | - | 1.063 | 0.003 | 1.066 | 0.005 | |
| 3-Buten-2-ol, 4-(2,6,6-trimethyl-2-cyclohexen-1-yl)-, (3E)- | - | - | 1.063 | 0.010 | - | - | |
| 1-Dodecanol | - | - | 1.051 | 0.042 | - | - | |
| Benzenemethanol, .alpha.-methyl- | - | - | 1.067 | 0.002 | - | - | |
| Cyclooctyl alcohol | - | - | 1.057 | 0.001 | - | - | |
| 3-Cyclopentyl-1-propanol | - | - | 1.070 | 0.041 | - | - | |
| 2-Pentyn-1-ol | - | - | 1.071 | 0.004 | - | - | |
| 1,2-Benzenedimethanol | - | - | - | - | 1.064 | 0.013 | |
| 1-Cyclopentene-1-methanol, 2-methyl-5-(1-methylethyl)- | - | - | - | - | 1.074 | 0.014 | |
| 2-Nonen-1-ol | - | - | - | - | 1.075 | 0.021 | |
| 7-Octene-2,6-diol, 2,6-dimethyl- | - | - | - | - | 1.075 | 0.011 | |
| Ketone | 1-Butanone, 2-hydroxy-1-phenyl- | 1.693 | 0.007 | - | - | - | - |
| 2-Propanone, 1-(4-methoxyphenyl)- | 1.688 | 0.010 | - | - | - | - | |
| 3-Nonen-5-one | 1.492 | 0.018 | 1.069 | 0.038 | 1.074 | 0.007 | |
| 3-Penten-2-one, 4-methyl- | 1.616 | 0.017 | 1.070 | 0.012 | 1.074 | 0.010 | |
| 6-Methyl-6-(5-methylfuran-2-yl)heptan-2-one | - | - | 1.067 | 0.030 | - | - | |
| 2-Butanone, 4-(2,6,6-trimethyl-1-cyclohexen-1-yl)- | - | - | 1.069 | 0.030 | 1.072 | 0.038 | |
| 2(1H)-Naphthalenone, 3,4,4a,5,6,7-hexahydro-1,1,4a-trimethyl- | - | - | 1.071 | 0.001 | 1.074 | 0.001 | |
| 3,4-Hexanedione, 2,2,5-trimethyl- | - | - | 1.063 | 0.029 | 1.068 | 0.002 | |
| Acetophenone | - | - | 1.068 | 0.002 | - | - | |
| 1(2H)-Naphthalenone, octahydro-4-hydroxy-, trans- | - | - | 1.064 | 0.003 | 1.055 | 0.040 | |
| 2-Cyclohexen-1-one, 4-hydroxy-3-methyl-6-(1-methylethyl)-, trans- | - | - | 1.071 | 0.041 | 1.074 | 0.041 | |
| 2H-Pyran-2-one, 6-pentyl- | - | - | 1.071 | 0.009 | 1.074 | 0.009 | |
| Cyclooctyl alcohol | - | - | 1.057 | 0.001 | - | - | |
| 3-Octen-2-one, (E)- | - | - | 1.051 | 0.045 | - | - | |
| Ethanone, 1-(2-hydroxy-4-methoxyphenyl)- | - | - | 1.071 | 0.047 | - | - | |
| spiro[3-oxatricyclo[3.3.1.02,4]nonane-8,1′-cyclopropane]-6-one | — | — | 1.069 | 0.018 | 1.074 | 0.043 | |
| 1,2-Cyclohexanedione | - | - | - | - | 1.073 | 0.031 | |
| Aldehyde | 10-Undecenal | 1.508 | 0.014 | 1.070 | 0.028 | 1.072 | 0.037 |
| 2-Hexenal, (Z)- | 1.630 | 0.016 | 1.070 | 0.011 | 1.074 | 0.010 | |
| 3-MethoxycinnamAldehyde | - | - | 1.059 | 0.008 | 1.058 | 0.019 | |
| 3-(4-Hydroxyphenyl)propanal | - | - | 1.070 | 0.005 | 1.074 | 0.005 | |
| 5-Heptenal, 2,6-dimethyl- | - | - | 1.070 | 0.026 | 1.074 | 0.022 | |
| BenzAldehyde, 3-methoxy- | - | - | 1.070 | 0.046 | - | - | |
| BenzAldehyde diethylacetal | - | - | 1.070 | 0.043 | - | - | |
| Tridecanal | - | - | - | - | 1.067 | 0.008 | |
| 2,6-Nonadienal, (E,Z)- | - | - | - | - | 1.074 | 0.019 | |
| Acid | Acetic acid, phenoxy- | 1.686 | 0.004 | - | - | 1.068 | 0.003 |
| 2-Hexenoic acid, (E)- | - | - | - | - | 1.074 | 0.001 | |
| Phenol | Methyleugenol | 1.649 | 0.038 | 1.070 | 0.016 | 1.074 | 0.028 |
| Phenol, 2-methyl-5-(1-methylethyl)- | - | - | 1.071 | 0.023 | 1.074 | 0.023 | |
| Phenol, 2-methyl- | - | - | 1.071 | 0.004 | 1.074 | 0.043 | |
| Phenol, 2,3,6-trimethyl- | - | - | - | - | 1.074 | 0.011 | |
| Amine | Butyramide, 2-cyano-2-ethyl- | - | - | 1.071 | 0.019 | - | - |
| Hordenine | - | - | 1.071 | 0.048 | 1.074 | 0.019 | |
| Aromatics | Benzene, 1-methyl-4-(1-methylethenyl)- | 1.673 | 0.023 | - | - | - | - |
| Benzene, 1,2-dimethoxy-4-(1-propenyl)- | - | - | 1.071 | 0.025 | 1.075 | 0.025 | |
| 2-Methoxy-4-vinylphenol | - | - | 1.071 | 0.034 | 1.075 | 0.034 | |
| Benzoic acid, 4-hydroxy- | - | - | 1.071 | 0.006 | 1.074 | 0.006 | |
| Indan, 1-methyl- | - | - | 1.071 | 0.027 | 1.074 | 0.027 | |
| Benzene, 1-methyl-2-(1-ethylpropyl)- | - | - | 1.071 | 0.046 | 1.074 | 0.002 | |
| Heterocyclic | Furan, 2-butyltetrahydro- | 1.601 | 0.004 | 1.069 | 0.015 | 1.074 | 0.004 |
| 2,2′-Ethylidenebis(5-methylfuran) | 1.688 | 0.010 | - | - | - | - | |
| Thiophene, 3-methyl- | 1.638 | 0.016 | 1.070 | 0.010 | 1.074 | 0.010 | |
| 8-Azabicyclo[3.2.1]octan-3-ol, 8-methyl-, endo- | - | - | 1.065 | 0.012 | - | - | |
| Meperidine | - | - | 1.061 | 0.002 | 1.069 | 0.005 | |
| 2-Methyl-1,3-dithiacyclopentane | - | - | 1.063 | 0.026 | - | - | |
| Ethanone, 1-(2-pyridinyl)- | - | - | 1.069 | 0.003 | - | - | |
| 1,2,4,5-Tetrazin-3-Amine | - | - | 1.068 | 0.001 | - | - | |
| 2H-Pyran-2-one, tetrahydro- | - | - | 1.068 | 0.023 | - | - | |
| 2-PyridinemethanAmine | - | - | 1.070 | 0.020 | 1.074 | 0.035 | |
| 2(5H)-Furanone, 5-ethyl-3-hydroxy-4-methyl- | - | - | 1.069 | 0.007 | 1.074 | 0.010 | |
| 4H-Pyran-4-one, 5-hydroxy-2-(hydroxymethyl)- | - | - | 1.063 | 0.002 | 1.056 | 0.044 | |
| 2-Ethoxy-3-methylpyrazine | - | - | 1.069 | 0.008 | 1.073 | 0.005 | |
| 5H-5-Methyl-6,7-dihydrocyclopentapyrazine | - | - | 1.071 | 0.028 | 1.074 | 0.028 | |
| 1H-Pyrazole, 1,3,5-trimethyl- | - | - | 1.068 | 0.001 | - | - | |
| 1H-1,2,4-Triazole, 3-chloro- | - | - | 1.071 | 0.023 | 1.075 | 0.018 | |
| Ethanone, 1-(1H-pyrrol-2-yl)- | - | - | 1.072 | 0.015 | 1.074 | 0.015 | |
| 3-Acetyl-2-oxo-1,3-oxazolidine | - | - | 1.070 | 0.029 | - | - | |
| Pyrazine, tetramethyl- | - | - | 1.071 | 0.016 | - | - | |
| 4-Piperidinone, 1,3-dimethyl- | - | - | 1.071 | 0.030 | 1.073 | 0.018 | |
| 4-Methylthiazole | - | - | 1.071 | 0.029 | - | - | |
| 2-((3,3-Dimethyloxiran-2-yl)methyl)-3-methylfuran | - | - | 1.071 | 0.037 | 1.074 | 0.004 | |
| 2-(3-Thienyl)pyridine | - | - | - | - | 1.074 | 0.000 | |
| Furaneol | - | - | - | - | 1.073 | 0.007 | |
| Nitrogen compounds | 2,6-Octadienenitrile, 3,7-dimethyl-, (Z)- | - | - | 1.071 | 0.049 | 1.074 | 0.049 |
| 2,6-Octadienenitrile, 3,7-dimethyl-, (Z)- | - | - | 1.070 | 0.035 | 1.074 | 0.007 | |
| Cyclopentanecarbonitrile, 3-(1-methylethylidene)- | - | - | 1.071 | 0.040 | 1.074 | 0.048 | |
| Sulfur compounds | Diallyl disulphide | - | - | 1.071 | 0.015 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Zhu, S.; Lin, X.; Peng, J.; Luo, D.; Wan, X.; Zhang, Y.; Dong, X.; Ma, Y. Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique. Horticulturae 2023, 9, 1069. https://doi.org/10.3390/horticulturae9101069
Zhang Q, Zhu S, Lin X, Peng J, Luo D, Wan X, Zhang Y, Dong X, Ma Y. Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique. Horticulturae. 2023; 9(10):1069. https://doi.org/10.3390/horticulturae9101069
Chicago/Turabian StyleZhang, Qin, Shouliang Zhu, Xin Lin, Junsen Peng, Dengcan Luo, Xuan Wan, Yun Zhang, Xiaoqing Dong, and Yuhua Ma. 2023. "Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique" Horticulturae 9, no. 10: 1069. https://doi.org/10.3390/horticulturae9101069
APA StyleZhang, Q., Zhu, S., Lin, X., Peng, J., Luo, D., Wan, X., Zhang, Y., Dong, X., & Ma, Y. (2023). Analysis of Volatile Compounds in Different Varieties of Plum Fruits Based on Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry Technique. Horticulturae, 9(10), 1069. https://doi.org/10.3390/horticulturae9101069







