Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Location of Study and Plant Material
2.2. Onion Bulb Production and Selection of Fusarium Basal Rot (FBR)-Resistant Bulbs via Conidial Inoculation
2.3. Development of Different Populations of Each Cultivar
2.4. Evaluation of Mature Bulbs for FBR Susceptibility
2.5. Seed Production of Selected Bulbs to Form Next Generation
2.6. Statistical Analysis
3. Results
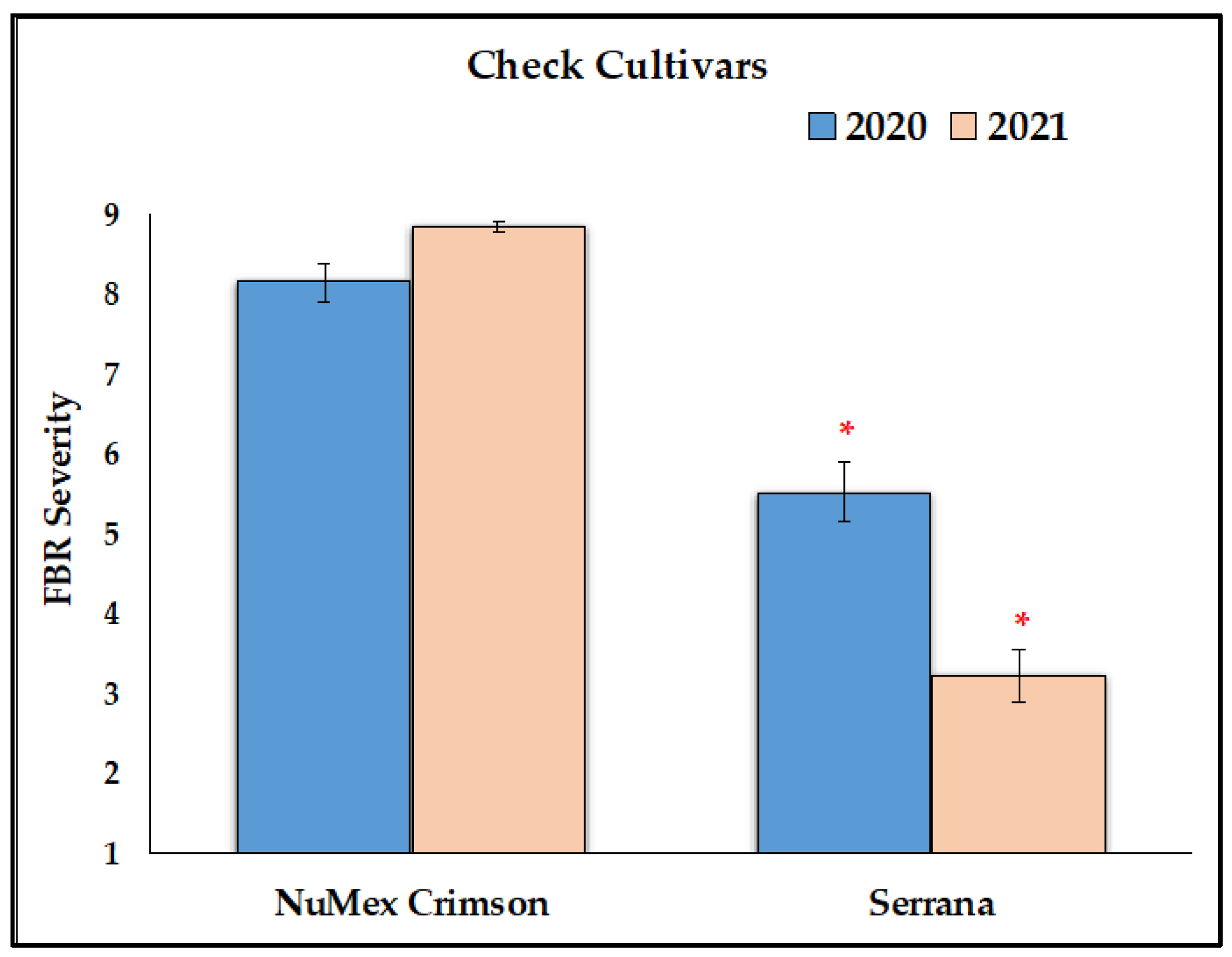
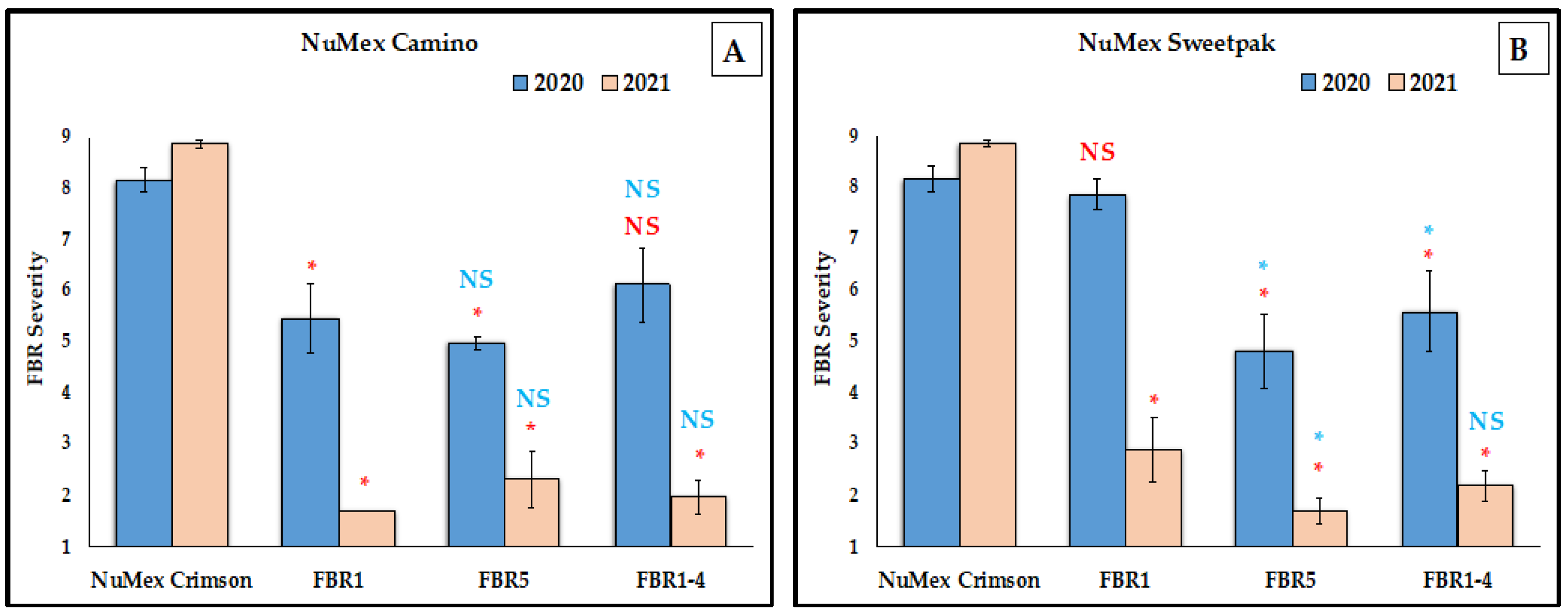
3.1. Reduction of FBR Severity of Advanced Selected Populations via Conidial Inoculation
3.2. Within Population Variability for FBR Severity
4. Discussion
4.1. No Difference between Two Advanced Selected Populations
4.2. Germplasm with Lesser FBR Severity Than the Susceptible Check
4.3. More FBR Resistance in the Most Advanced Selected Populations
4.4. Higher FBR Resistance Than the Partially Resistant Check
4.5. Reduction in within Population Variation
4.6. Yearly Differences of Same Cultivar Populations
4.7. Quantitative Nature of FBR Resistance
4.8. Successful Screening Method to Improve Resistance in Short-Day Onion Cultivars
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havey, M.J. Onion and Other Cultivated Alliums. Evol. Crop Plants 1995, 344–350. [Google Scholar]
- Mertz, W.C. Onion Utilized Production down 16 Percent from Previous Year in the Pacific Northwest Estimates also Published for Several Other Pacific Northwest Vegetable Crops; USDA, NASS-Northwest Regional Field Office: Olympia, Washington, DC, USA, 2021. [Google Scholar]
- USDA-NASS. New Mexico Onion Production. 2021. Available online: https://www.nass.usda.gov/ (accessed on 1 December 2022).
- Brewster, J.L. Onions and Other Vegetable Alliums; CABI: Wallingford, UK, 2008; Volume 15, ISBN 1845936221. [Google Scholar]
- Brayford, D. Fusarium oxysporum f. sp. cepae. Mycopathologia 1996, 133, 39–40. [Google Scholar]
- Cramer, C.S. Breeding and Genetics of Fusarium Basal Rot Resistance in Onion. Euphytica 2000, 115, 159–166. [Google Scholar] [CrossRef]
- Havey, M.J. Fusarium Basal Plate Rot. In Compendium of Onion and Garlic Diseases; Schwartz, H.F., Mohan, S.K., Eds.; Amer. Phytopathol. Soc.: St. Paul, MN, USA, 1995; pp. 10–11. [Google Scholar]
- Shalaby, G.I.; Struckmeyer, E. The Mode of Entrance of the Fusarium Rot Fungus into the Bulbs of Onions. J. Am. Soc. Hort. Sci. 1966, 89, 438–442. [Google Scholar]
- Sintayehu, A.; Sakhuja, P.K.; Fininsa, C.; Ahmed, S. Management of Fusarium Basal Rot (Fusarium oxysporum f. sp. cepae) on Shallot through Fungicidal Bulb Treatment. Crop Prot. 2011, 30, 560–565. [Google Scholar] [CrossRef]
- Cramer, C.S.; Corgan, J.N.; Mendoza, J.L.; Wall, M.M. 1998–1999 Onion Variety Trials at New Mexico State University; New Mexico State University, N.M. Agric. Expt. Stn. Res. Rpt. 739: Las Cruces, NM, USA, 2000. [Google Scholar]
- Katan, J.; Rotem, I.; Finkel, Y.; Daniel, J. Solar Heating of the Soil for the Control of Pink Root and Other Soilborne Diseases in Onions. Phytoparasitica 1980, 8, 39–50. [Google Scholar] [CrossRef]
- Higashida, S.; Ohsaki, I.; Narita, Y. Effects of Crop Rotation on Onion Yields and Its Microbial Factors [Control of Fusarium oxysporum]. Bull. Hokkaido Prefect. Agric. Exp. Station. 1982, 48, 1–9. [Google Scholar]
- Jaworski, C.A.; McCarter, S.M.; Johnson, A.W.; Williamson, R.E. Response of Onions Grown for Transplants to Soil Fumigation. J. Am. Soc. Hort. Sci. 1978, 103, 385–388. [Google Scholar] [CrossRef]
- Koriem, S.O.; Hussein, F.N.; Metwally, A.H. Chemical Control of Pink Root Rot, Basal Rot and Neck Rot Diseases of Onion Produced by Sets. Assiut J. Agric. Sci. (Egypt) 1991, 22, 81–96. [Google Scholar]
- Özer, N.; Köycü, N.D. Evaluation of Seed Treatments for Controlling Aspergillus niger and Fusarium oxysporum on Onion Seed. Phytopathol. Mediterr. 1998, 37, 33–40. [Google Scholar]
- Rajendran, K.; Ranganathan, K. Biological Control of Onion Basal Rot (Fusarium oxysporum f. sp. cepae) by Combined Application of Fungal and Bacterial Antagonists. J. Biol. Cont. 1996, 10, 97–102. [Google Scholar]
- Taylor, A.; Vagany, V.; Barbara, D.J.; Thomas, B.; Pink, D.A.C.; Jones, J.E.; Clarkson, J.P. Identification of Differential Resistance to Six Fusarium oxysporum f. sp. cepae Isolates in Commercial Onion Cultivars through the Development of a Rapid Seedling Assay. Plant Pathol. 2013, 62, 103–111. [Google Scholar] [CrossRef]
- Marzu, J.C. Genetic Analyses of Resistances to Fusarium Basal Rot and Pink Root in Onion. Ph.D. Thesis, University of Wisconsin-Madison, Madison, WI, USA, 2015. [Google Scholar]
- Mandal, S.; Saxena, A.; Cramer, C.S.; Steiner, R.L. Comparing Efficiencies of Two Selection Approaches for Improving Fusarium Basal Rot Resistance in Short-Day Onion after a Single Cycle of Selection. Horticulturae 2020, 6, 26. [Google Scholar] [CrossRef]
- Saxena, A. Screening of Onion Cultivars for Fusarium Basal Rot and Spatial Distribution of Fusarium oxysporum f. sp. cepae. Master’s Thesis, New Mexico State University, Las Cruces, NM, USA, 2007. [Google Scholar]
- Caligiore-Gei, P.F.; Ciotti, M.L.; Valdez, J.G.; Galmarini, C.R. Breeding Onion for Resistance to Fusarium Basal Rot: Comparison of Field Selection and Artificial Inoculation. Trop. Plant Pathol. 2020, 45, 493–498. [Google Scholar] [CrossRef]
- Saxena, A.; Cramer, C.S. Screening of Onion Seedlings for Resistance against New Mexico Isolates of Fusarium oxysporum f. sp. cepae. J. Plant Pathol. 2009, 91, 199–202. [Google Scholar]
- Mandal, S.; Cramer, C.S. An Artificial Inoculation Method to Select Mature Onion Bulbs Resistant to Fusarium Basal Rot. HortScience 2020, 55, 1840–1847. [Google Scholar] [CrossRef]
- Hallauer, A.R.; Darrah, L.L. Compendium of Recurrent Selection Methods and Their Application. CRC Crit. Rev. Plant Sci. 1985, 3, 1–33. [Google Scholar] [CrossRef]
- Cramer, C.S. Onion Trait Heritability and Response from Selection. J. Am. Soc. Hort. Sci. 2006, 131, 646–650. [Google Scholar] [CrossRef]
- Cramer, C.S.; Corgan, J.N. ‘NuMex Camino’ Onion. HortScience 2003, 38, 1251–1252. [Google Scholar] [CrossRef]
- Wall, M.; Corgan, J. ‘Numex Sweetpak’ Onion. HortScience 1999, 34, 1303–1304. [Google Scholar] [CrossRef]
- Cramer, C.S.; Corgan, J.N. ’NuMex Chaco’ Onion. HortScience 2001, 36, 1337–1338. [Google Scholar] [CrossRef]
- Corgan, J.N. ‘NuMex Crispy’ Onion. NM Agr. Expt. Sta. Var. Rel. Not. 1996, 1–4. [Google Scholar]
- Corgan, J.N. ‘NuMex Mesa’ Onion. NM Agr. Expt. Sta. Var. Rel. Not. 1996, 1–4. [Google Scholar]
- Corgan, J.N. ‘NuMex Vado’and ‘NuMex Luna’ Onion Varieties. NM Agr. Expt. Sta. Rel. Not. 1995, 1–6. [Google Scholar]
- Lopez, J.A.; Cramer, C.S. Screening Short-Day Onion Varieties for Resistance to Fusarium Basal Rot. In XXVI International Horticultural Congress: Advances in Vegetable Breeding 637; ISHS: Leuven, Belgium, 2002; pp. 169–173. [Google Scholar]
- Walker, S.; Ashigh, J.; Cramer, C.S.; Sammis, T.; Lewis, B. Bulb Onion Culture and Management for Southern New Mexico; Cooperative Extension Service Circular 563; New Mexico State University, Agricultural Experiment Station: Las Cruces, NM, USA, 2000. [Google Scholar]
- Black, L.; Chan, E.K.F.; Colcol, J.F.; Jones, R.; Kramer, C.; Xiang, W. QTLs Conferring Resistance to Fusarium Basal Rot, Pink Root and Complementary Pinks in Onions. U.S. Patent No. 11,457,582 B2, 10 April 2022. [Google Scholar]
- Cramer, C.S.; Corgan, J.N. ’NuMex Crimson’ Onion. HortScience 2003, 38, 306–307. [Google Scholar] [CrossRef]
- Mandal, S.; Cramer, C.S. Improving Fusarium Basal Rot Resistance of Onion Cultivars through Artificial Inoculation and Selection of Mature Bulbs. Horticulturae 2021, 7, 168. [Google Scholar] [CrossRef]
- Gutierrez, J.A.; Cramer, C.S. Screening Short-Day Onion Cultivars for Resistance to Fusarium Basal Rot. HortScience 2005, 40, 157–160. [Google Scholar] [CrossRef]
- Cramer, C.S.; Mandal, S.; Sharma, S.; Nourbakhsh, S.S.; Goldman, I.; Guzman, I. Recent Advances in Onion Genetic Improvement. Agronomy 2021, 11, 482. [Google Scholar] [CrossRef]
- Kablan, L.; Lagauche, A.; Delvaux, B.; Legrève, A. Silicon Reduces Black Sigatoka Development in Banana. Plant Dis. 2012, 96, 273–278. [Google Scholar] [CrossRef]
- Twizeyimana, M.; Ojiambo, P.S.; Tenkouano, A.; Ikotun, T.; Bandyopadhyay, R. Rapid Screening of Musa Species for Resistance to Black Leaf Streak Using in Vitro Plantlets in Tubes and Detached Leaves. Plant Dis. 2007, 91, 308–314. [Google Scholar] [CrossRef]
- Guo, Y.; Olsen, R.T.; Kramer, M.; Pooler, M. Use of Mycelium and Detached Leaves in Bioassays for Assessing Resistance to Boxwood Blight. Plant Dis. 2016, 100, 1622–1626. [Google Scholar] [CrossRef]
- Bernardo, R.N. Breeding for Quantitative Traits in Plants; Stemma Press: Woodbury, MN, USA, 2020; ISBN 9780972072434. [Google Scholar]
- Kountche, B.A.; Hash, C.T.; Dodo, H.; Laoualy, O.; Sanogo, M.D.; Timbeli, A.; Vigouroux, Y.; This, D.; Nijkamp, R.; Haussmann, B.I.G. Development of a Pearl Millet Striga-Resistant Genepool: Response to Five Cycles of Recurrent Selection under Striga-Infested Field Conditions in West Africa. Field Crops Res. 2013, 154, 82–90. [Google Scholar] [CrossRef]
- Hill, W.G. Understanding and Using Quantitative Genetic Variation. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 73–85. [Google Scholar] [CrossRef]
- Dweba, C.C.; Figlan, S.; Shimelis, H.A.; Motaung, T.E.; Sydenham, S.; Mwadzingeni, L.; Tsilo, T.J. Fusarium Head Blight of Wheat: Pathogenesis and Control Strategies. Crop Prot. 2017, 91, 114–122. [Google Scholar] [CrossRef]
- Comstock, R.E. Quantitative Genetics with Special Reference to Plant and Animal Breeding; Iowa State University Press: Ames, IA, USA, 1996. [Google Scholar]
- Taylor, A.; Teakle, G.R.; Walley, P.G.; Finch-Savage, W.E.; Jackson, A.C.; Jones, J.E.; Hand, P.; Thomas, B.; Havey, M.J.; Pink, D.A.C. Assembly and Characterisation of a Unique Onion Diversity Set Identifies Resistance to Fusarium Basal Rot and Improved Seedling Vigour. Theor. Appl. Genet. 2019, 132, 3245–3264. [Google Scholar] [CrossRef]
- Smith, C.S.; Slade, S.J.; Nordheim, E.V.; Cascino, J.J.; Harris, R.F.; Andrews’, J.H. Sources of Variability in the Measurement of Fungal Spore Yields. Appl. Environ. Microbiol. 1988, 54, 1430–1435. [Google Scholar] [CrossRef]
- Loveless, M.D.; Hamrick, J.L. Ecological Determinants of Genetic Structure in Plant Populations. Annu. Rev. Ecol. Syst. 1984, 15, 65–95. [Google Scholar] [CrossRef]
- Straley, E.; Colcol Marzu, J.; Havey, M.J. Genetic Analyses of Resistance to Fusarium Basal Rot in Onion. Horticulturae 2021, 7, 538. [Google Scholar] [CrossRef]
- Parlevliet, J.E.; Zadoks, J.C. The Integrated Concept of Disease Resistance: A New View Including Horizontal and Vertical Resistance in Plants. Euphytica 1977, 26, 5–21. [Google Scholar] [CrossRef]
- Orton, T.J. Breeding for Disease and Insect Resistance. In Horticultural Plant Breeding; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–382. [Google Scholar]
- Trdá, L.; Janda, M.; Macková, D.; Pospíchalová, R.; Dobrev, P.I.; Burketová, L.; Matušinsky, P. Dual Mode of the Saponin Aescin in Plant Protection: Antifungal Agent and Plant Defense Elicitor. Front. Plant Sci. 2019, 10, 1448. [Google Scholar] [CrossRef]
- Lanzotti, V.; Romano, A.; Lanzuise, S.; Bonanomi, G.; Scala, F. Antifungal Saponins from Bulbs of White Onion, Allium Cepa L. Phytochemistry 2012, 74, 133–139. [Google Scholar] [CrossRef]
- Teshima, Y.; Ikeda, T.; Imada, K.; Sasaki, K.; El-Sayed, M.A.; Shigyo, M.; Tanaka, S.; Ito, S. Identification and Biological Activity of Antifungal Saponins from Shallot (Allium Cepa L. Aggregatum Group). J. Agric. Food Chem. 2013, 61, 7440–7445. [Google Scholar] [CrossRef]
- Abdelrahman, M.; El-Sayed, M.; Sato, S.; Hirakawa, H.; Ito, S.; Tanaka, K.; Mine, Y.; Sugiyama, N.; Suzuki, M.; Yamauchi, N. RNA-Sequencing-Based Transcriptome and Biochemical Analyses of Steroidal Saponin Pathway in a Complete Set of Allium fistulosum—A. cepa Monosomic Addition Lines. PLoS ONE 2017, 12, e0181784. [Google Scholar] [CrossRef]
- Schroeder, B.K.; Waters, T.D.; Du Toit, L.J. Evaluation of Onion Cultivars for Resistance to Enterobacter Cloacae in Storage. Plant Dis. 2010, 94, 236–243. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Cramer, C.S. Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening. Horticulturae 2023, 9, 99. https://doi.org/10.3390/horticulturae9010099
Sharma S, Cramer CS. Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening. Horticulturae. 2023; 9(1):99. https://doi.org/10.3390/horticulturae9010099
Chicago/Turabian StyleSharma, Suman, and Christopher S. Cramer. 2023. "Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening" Horticulturae 9, no. 1: 99. https://doi.org/10.3390/horticulturae9010099
APA StyleSharma, S., & Cramer, C. S. (2023). Selection Progress for Resistance to Fusarium Basal Rot in Short-Day Onions Using Artificial Inoculation Mature Bulb Screening. Horticulturae, 9(1), 99. https://doi.org/10.3390/horticulturae9010099






