Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions
Abstract
1. Introduction
2. Microbial Safety Hazards in Conventional Farming
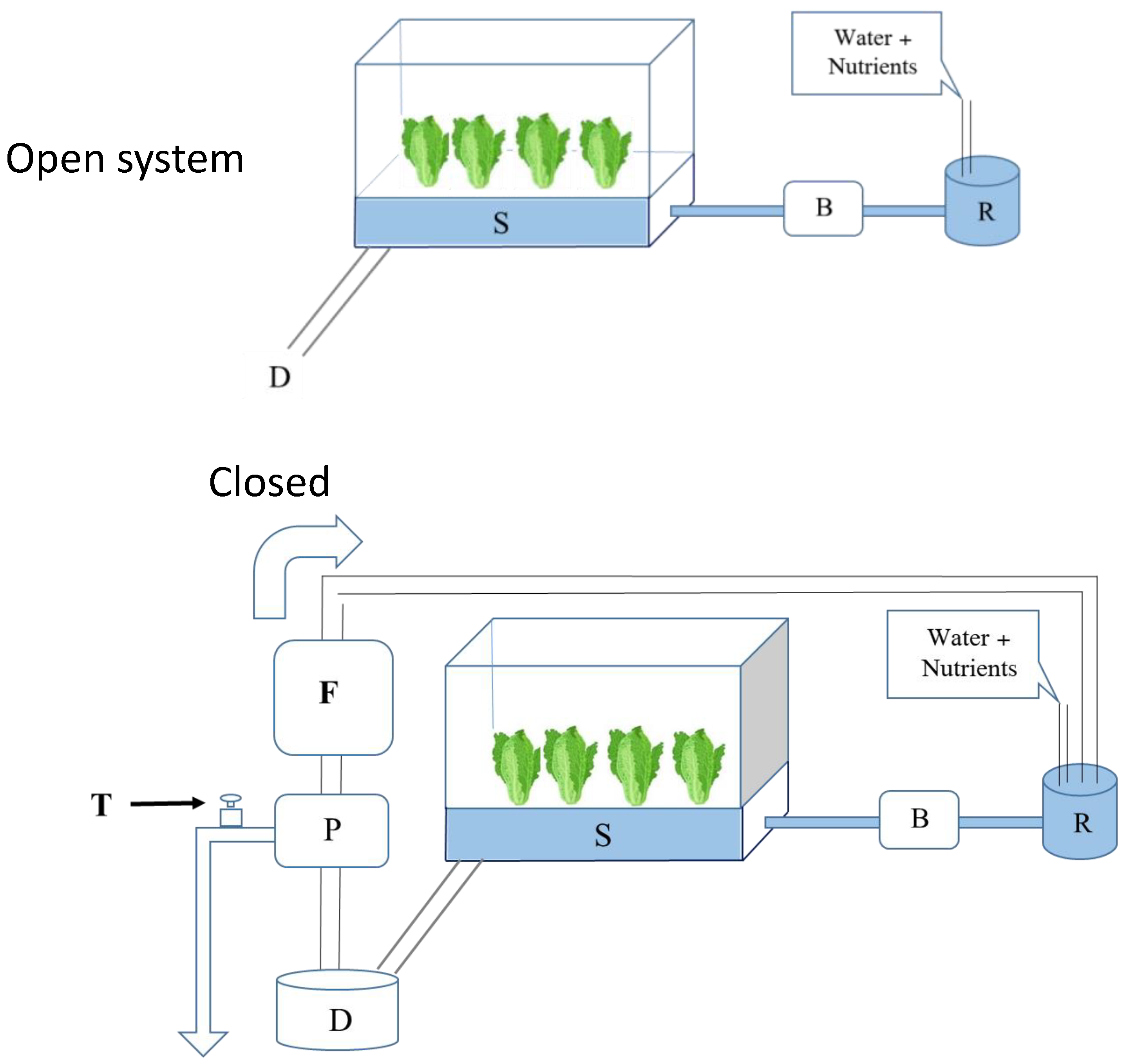
3. Hydroponic Cultivation Systems and Their Advantages
4. Role of Hydroponic Systems in Reducing the Microbial Hazards for Vegetable crops
4.1. Mechanism of Hydroponics for Reducing the Microbial Hazards
4.2. Effect of Culturing Technology on Reducing the Microbial Hazards
4.3. Effects of Nutrients Solutions Circulation on Microbial Hazards under Hydroponic Systems
5. Potential Sources and Routes of Contamination in Hydroponic Cultivation Systems
5.1. Potential Sources of Human Pathogens in Hydroponic Production Systems
| Hydroponic Systems | Crops | Microbial Hazards | Contamination Sources and Routes | References |
|---|---|---|---|---|
| Open system, pots; substrate: vermiculite | Tomato | E. coli, Salmonella | Pathogens were detected on tomatoes, water puddles, shoes, and local wild and farm animals; suspected sources: flood and wild animals. | [46,58] |
| Open system; substrates: coconut fiber and rock wool | Tomato | E. coli, Salmonella spp., Listeria spp. | E. coli was present in higher levels in reclaimed and surface water. Presumptive Salmonella spp. were detected in 7.7% of the water samples, mostly from reclaimed water. Listeria spp. numbers increased after adding the fertilizers. No pathogen detected on tomatoes. | [49] |
| Open system; substrate: coconut fiber | Bell pepper | E. coli | E. coli was present in higher levels in reclaimed and surface water. No link between E. coli prevalence and levels in water and pepper contamination. E. coli was present in fertilizer solutions and in water sprayed in humidifiers. | [60] |
| Open system, water from local wells; substrate: rockwool blocks, trickle irrigation | Cucumber | Fecal indicators: E. coli, total and fecal coliforms, Clostridium perfringens | E. coli and fecal coliforms were present on roots but only once detected on fruit. Suspected source: well water. | [62] |
| Deep culture. Not defined | Leafy greens | Salmonella Typhimurium | S. Typhimurium was isolated form 31 patients and linked to hydroponically grown leafy greens. The outbreak strain was detected in two nearby stormwater retention ponds. | [55] |
| Unknown; purchased from retail stores | Lettuce | E. coli O157:H7, Salmonella, L. monocytogenes | The three pathogens were detected in a number of lettuce samples. Source unknown. | [70] |
| Lettuce samples were obtained from retailers | Lettuce | Total count, coliforms, E. coli, yeast, mold | Aquaponically grown lettuce had significantly lower concentration of spoilage and fecal microorganisms compared to in-soil-grown lettuce. | [43] |
| Closed system, NFT; substrate: peat moss | lettuce | Indicator bacteria, Listeria spp. | Substrate, roots, and seedling water reservoir harbored high counts. No Listeria spp. was detected. Postharvest contamination of leaves occurred, potentially due to the transfer from substrate. | [54] |
| Closed system; combination of NFT and deep water culture compared to soil-based farm | Lettuce | Salmonella, E. coli, Stenotrophomonas maltophilia | E. coli and Salmonella detected in 7 and 4 (out of 50) water samples, respectively. All lettuce samples (25) had <10 CFU E. coli/g. One lettuce sample harbored Salmonella. | [61] |
| Closed system, pots; substrate: coconut fiber | Bell pepper | E. coli, Salmonella, L. monocytogenes | E. coli and Salmonella were detected on peppers. Salmonella was also present on conveyor belt; suspected source: nutrient solution; poor worker hygiene. | [59] |
5.2. Fate and Transmission Modes of Human Pathogens in Hydroponic Production Systems
| Model System | Crops | Microbial Hazards | Contamination Sources and Routes | References |
|---|---|---|---|---|
| Lab system; water in petri dish | Lettuce | E. coli O157:H7 strains | Water. Bacteria adhered preferentially to roots and seed coats; bacteria proliferated in seedlings. | [88] |
| Lab scale (tubes) | Lettuce | Salmonella Enteritidis | Salmonella was inoculated into the nutrient solution. The pathogen survived in the system and colonized the roots. Root internalization was higher in younger plants. pH and inoculum size affected the internalization and survival. | [83] |
| Lab scale, test tube; open system | Spinach | GFP-tagged E. coli O157:H7 | Following inoculation of the hydroponic medium, E. coli was found in the roots and shoots. Concentration in shoots increased from 14 to 21 days. Internalization was observed in hydroponically grown plants but not in soil-grown plants. | [80] |
| Lab-experiments | none | Generic E. coli | E. coli strains survive but do not proliferate in irrigation water and in several fertilizer solutions. Solution containing HNO3 inactivated E. coli. | [60] |
| Lab system (hydroponic tray) | Tomato | Salmonella | Artificially contaminated nutrient solution resulted in bacterial internalization. Salmonella found in the hypocotyls-cotyledons, stems, and leaves of 10-day-old plants. | [77] |
| Lab scale; hydroponic trays; open system | Spinach | E. coli O157:H7 | Inoculation of medium resulted in root internalization and transmission to the stem and leaves. Wounding the roots increased internalization. Internalization was higher in soil- versus hydroponically grown plants. | [78] |
| Lab-scale containers; open system | Spinach | E. coli, Salmonella, L. monocytogenes | Two contamination routes tested; hydroponic medium and leaves. Root and leaf contamination was rare with low inoculum (103 CFU/mL); leaf, but not root, contamination was rare with high concentrations (106 CFU/leaf). Root internalization is the principal route of leaf contamination. | [82] |
| Lab experiments. (Fertilizer solution with plant was taken from a deep flow technique system) | Basil | E. coli O157:H7; non-O157 STEC; Salmonella | Inoculation of the pathogens into a fertilizer solution resulted in proliferation over 24 h. E. coli O157:H7 grew better in fertilizer solution with plants, while non-O157:H7 E. coli and Salmonella grew better in solutions without plants. | [90] |
| Lab-scale deep culture open system | Lettuce | E. coli O157:H7 | Artificially contaminated water. Internalization was observed. Root injury increased internalization. | [81] |
| Lab-scale deep culture open system | Maize (young seedlings) | E. coli | Artificially contaminated nutrient solution resulted in decline of counts with time. E. coli internalized in the roots and was detected in the shoot. | [76] |
| Experimental system; open system | Lettuce | Human norovirus, murine norovirus, Tulane virus | Artificial water contamination resulted in viral internalization and dissemination to shoots and leaves | [86] |
| Pots with peat moss substrate; growing pads; open system | Radish seedlings (microgreens) | E. coli O157:H7 | Artificially contaminated seeds led to systemic contamination of the seedlings in both growing systems with a higher level in the hydroponic system; they survive and proliferate significantly. | [85] |
| Micro-Mats hydroponic growing pad and mini-seed tray with overhead irrigation; open system | Swiss chard (microgreen) | Salmonella | Artificial contamination of seeds and irrigation water. Salmonella growth was affected by serovar and inoculation level; irrigation water inoculation also resulted in proliferation that was affected by initial inoculation level and the growth medium. | [98] |
| Hydroponic mats; open system | Amaranth, Broccoli, Kale, Mustard, Coriander, Rocket, Parsley, Basil, Radish | E. coli O157:H7 | Inoculation of seeds or water. Bacteria proliferated and colonized eight different species of microgreens. | [91] |
| NFT system; closed system | Lettuce | E. coli, Salmonella, Entamoeba histolytica, Ancylostoma spp. | The effect of nutirent solutions on microbial quality was tested. No bacterial contamination was detected. Few samples contained Entamoeba histolytica, eggs, and larvae of Ancylostoma spp. No contamination was found when mineral nutrient solutions were used. | [99] |
| NFT system; closed system | Lettuce | S. Typhimurium L. monocytogenes | Artificial contamination of nutrient solution resulted in the persistence of the pathogens in the system throughout the growth period. The pathogens accumulated in rockwool medium and on lettuce roots and transferred to the leaves. L. monocytogenes, but not Salmonella, proliferated in the system following simulation of sporadic contamination (~104 CFU/mL) | [89] |
| NFT system; closed system | Spinach | Bioluminescence-labeled E. coli | Seed contamination resulted in surface and internal root colonization. The colonization was restricted to the roots in mature plants. In soil-grown plants colonization was restricted to the root surface | [100] |
| Experimental systems, water recirculation; closed system | Strawberry Basil, Lettuce | E. coli, Salmonella | Three out of seventy-nine water samples contained low levels of E.coli; no Salmonella was detected. | [44] |
| Experimental hydroponic closed systems | Lettuce, Basil, Tomato | Shiga-toxin-producing E. coli, Salmonella, L. monocytogenes | Only E. coli detected in water and on root surfaces. Source unknown. No contamination in edible parts. | [50] |
| Experimental; closed system | Lettuce | Salmonella | Seeds were artificially contaminated. Salmonella persisted in water and the farming environment for 6 weeks. | [101] |
| Hydroponic pads; closed system | Kale, Mustard (microgreens) | human norovirus surrogate (murine norovirus) | Inoculation of water resulted in root and leaf contamination The virus persisted in the system and caused cross-contamination. | [87] |
| Closed system, commercial | Three lettuce genotypes were studied | Coliforms, Lactic acid bacteria | Lactic acid bacteria and total coliform counts were lower in soilless-grown lettuce compared to soil-grown. | [52] |
| Green house. System not defined | Lettuce | E. coli, thermotolerant coliforms and total coliforms, Salmonella spp. and helminth eggs | Domestic wastewater effluents with different levels of treatment were used for irrigation. Leaves showed low levels of contamination with E. coli, thermotolerant coliforms, and total coliforms. Salmonella spp. and helminth eggs were not detected in the water. High bacterial loads were present on roots. | [51] |
6. Mitigation of Microbial Contamination in Hydroponically Grown Crops
7. Conclusions
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, S.D.; Sodha, S.V.; Ayers, T.L.; Lynch, M.F.; Gould, L.H.; Tauxe, R.V. Produce-associated foodborne disease outbreaks, USA, 1998–2013. Epidemiol. Infect. 2018, 146, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate outbreaks of foodborne illness in the United States associated with fresh produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- FAO. Microbial Hazards in Fresh Leafy Vegetables and Herbs; Meeting Report, Microbial Risk Assessment Series 14; World Health Organization, Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Gourama, H. Foodborne pathogens. In Food Safety Engineering; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer: Cham, Switzerland, 2020; pp. 25–49. [Google Scholar]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food. Sci. F 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Sapers, G.M.; Doyle, M.P. Chapter 1—Scope of the Produce Contamination Problem. In The Produce Contamination Problem, 2nd ed.; Matthews, K.R., Sapers, G.M., Gerba, C.P., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 3–20. [Google Scholar]
- Uyttendaele, M.; Jaykus, L.A.; Amoah, P.; Chiodini, A.; Cunliffe, D.; Jacxsens, L.; Holvoet, K.; Korsten, L.; Lau, M.; McClure, P. Microbial hazards in irrigation water: Standards, norms, and testing to manage use of water in fresh produce primary production. Compr. Rev. Food Sci. F 2015, 14, 336–356. [Google Scholar] [CrossRef]
- Beuchat, L.R. Vectors and conditions for preharvest contamination of fruits and vegetables with pathogens capable of causing enteric diseases. Br. Food J. 2006, 108, 38–53. [Google Scholar] [CrossRef]
- Lenzi, A.; Marvasi, M.; Baldi, A. Agronomic practices to limit pre- and post-harvest contamination and proliferation of human pathogenic Enterobacteriaceae in vegetable produce. Food Control 2021, 119, 107486. [Google Scholar] [CrossRef]
- Gil, M.I.; Selma, M.V.; Suslow, T.; Jacxsens, L.; Uyttendaele, M.; Allende, A. Pre-and postharvest preventive measures and intervention strategies to control microbial food safety hazards of fresh leafy vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 453–468. [Google Scholar] [CrossRef]
- Brandl, M.T. Fitness of human enteric pathogens on plants and implications for food safety. Annu. Rev. Phytopathol. 2006, 44, 367–392. [Google Scholar] [CrossRef]
- Teplitski, M.; Barak, J.D.; Schneider, K.R. Human enteric pathogens in produce: Un-answered ecological questions with direct implications for food safety. Curr. Opin. Biotech. 2009, 20, 166–171. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Chen, H.; Kinchla, A.J.; Richard, N.; Shaw, A.; Feng, Y. Produce growers’ on-farm food safety education: A review. J. Food Prot. 2020, 84, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Navratil, S.; Gregory, A.; Bauer, A.; Srinath, I.; Jun, M.; Szonyi, B.; Nightingale, K.; Anciso, J.; Ivanek, R. Generic Escherichia coli contamination of spinach at the preharvest Stage: Effects of farm management and environmental factors. Appl. Environ. Microbiol. 2013, 79, 4347–4358. [Google Scholar] [CrossRef] [PubMed]
- Pachepsky, Y.; Shelton, D.R.; McLain, J.E.T.; Patel, J.; Mandrell, R.E. Irrigation waters as a source of pathogenic microorganisms in produce. A review. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2011; Volume 113, pp. 73–138. [Google Scholar]
- Esteves, R.G.R.; Gerba, C.P.; Slack, D.C. Control of viral and bacterial contamination of lettuce by subsurface drip irrigation. J Irrig. Drain Eng. 2022, 148, 04022035. [Google Scholar] [CrossRef]
- Gorbatsevich, E.; Sela Saldinger, S.; Pinto, R.; Bernstein, N. Root internalization, transport and in-planta survival of Salmonella enterica serovar Newport in sweet basil. Environ. Microbiol. Rep. 2013, 5, 151–159. [Google Scholar] [CrossRef]
- Erickson, M.C.; Webb, C.C.; Davey, L.E.; Payton, A.S.; Flitcroft, I.D.; Doyle, M.P. Biotic and abiotic variables affecting internalization and fate of Escherichia coli O157:H7 isolates in leafy green roots. J. Food Prot. 2014, 77, 872–879. [Google Scholar] [CrossRef]
- Wang, Y.J.; Deering, A.J.; Kim, H.J. Effects of plant age and root damage on internalization of higa toxin-producing Escherichia coli in leafy vegetables and herbs. Horticulturae 2021, 7, 68. [Google Scholar] [CrossRef]
- FDA. USA Food and Drug Administration. Guidance for Industry: Guide to Minimize Microbial Food Safety Hazards for Fresh Fruits and Vegetables. 1998. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-guide-minimize-microbial-food-safety-hazards-fresh-fruits-and-vegetables (accessed on 20 September 2022).
- FAO. Development of a Framework for Good Agricultural Practices; Committee on Agriculture, 7th Session, 31 March–4 APRIL 2003; FAO: Rome, Italy, 2003. [Google Scholar]
- Raviv, M.; Lieth, J.H.; Bar-Tal, A. (Eds.) Significance of soilless culture in agriculture. In Soilless Culture: Theory and Practice; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 3–14. [Google Scholar]
- Mattson, N.; Lieth, J.H. Liquid culture hydroponic system operation. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 567–586. [Google Scholar]
- Gruda, N. Soilless culture systems and growing media in horticulture: An overview. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 1–22. [Google Scholar]
- Voogt, W.; Bar-Yosef, B. Water and nutrients management and crops response to nutrient solution recycling in soilless growing systems in greenhouses. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 425–507. [Google Scholar]
- Tzortzakis, N.; Nicola, S.; Savvas, D.; Voogt, W. Soilless cultivation through an intensive crop production scheme. Management strategies, challenges and future directions. Front. Plant Sci. 2020, 11, 363. [Google Scholar] [CrossRef]
- Eek Son, J.; In Ahn, T.; Moon, T. Advances in nutrient management modelling and nutrient concentration prediction for soilless culture systems. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 277–302. [Google Scholar]
- Okumura, T.; Saito, Y.; Takano, K.; Takahashi, K.; Takaki, K.; Satta, N.; Fujio, T. Inactivation of bacteria using discharge plasma under liquid fertilizer in a hydroponic culture system. Plasma Med. 2016, 6, 247–254. [Google Scholar] [CrossRef]
- Nikolaou, G.; Neocleous, D.; Kitta, E.; Katsoulas, N. Advances in irrigation/fertigation techniques in greenhouse soilless culture systems (SCS). In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 249–276. [Google Scholar]
- Silber, A.; Bar-Tal, A. Nutrition of substrates-grown plants. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 197–257. [Google Scholar]
- Van Os, E.A.; Gieling, T.H.; Lieth, J.H. Technical equipment in soilless production systems. In Soilless Culture: Theory and Practice; Raviv, M., Lieth, J.H., Bar-Tal, A., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2019; pp. 587–635. [Google Scholar]
- Blaustein, R.A.; Shelton, D.R.; Van Kessel, J.A.S.; Karns, J.S.; Stocker, M.D.; Pachepsky, Y.A. Irrigation waters and pipe-based biofilms as sources for antibiotic-resistant bacteria. Environ. Monit. Assess 2016, 188, 56. [Google Scholar] [CrossRef]
- Ragaveena, S.; Shirly Edward, A.; Surendran, U. Smart controlled environment agriculture methods: A holistic review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 887–913. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Tuzel, Y.; Balliu, A. Advances in liquid and solid-medium soilless culture systems. In Advances in Horticultural Soilless Culture; Gruda, N., Ed.; Burleigh Dodds Science Publishing: London, UK, 2020; pp. 213–248. [Google Scholar]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Xydis, G. Plant factories in the water-food-energy Nexus era: A systematic bibliographical review. Food Secur. 2020, 12, 253–268. [Google Scholar] [CrossRef]
- FDA; USA. FDA In Brief: FDA Warns Consumers not to Eat Romaine Lettuce Grown in Salinas, California. 2019. Available online: https://www.fda.gov/news-events/fda-brief/fda-brief-fda-warns-consumers-not-eat-romaine-lettuce-grown-salinas-california (accessed on 25 October 2022).
- Huang, L.C. Consumer attitude, concerns, and brand acceptance for the vegetables cultivated with sustainable plant factory production systems. Sustainability 2019, 11, 4862. [Google Scholar] [CrossRef]
- Alegbeleye, O.O.; Sant’Ana, A.S. Manure-borne pathogens as an important source of water contamination: An update on the dynamics of pathogen survival/transport as well as practical risk mitigation strategies. Int. J. Hyg. Environ. Health 2020, 227, 113524. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Holley, R.A. Factors influencing the microbial safety of fresh produce: A review. Food Microbiol. 2012, 32, 1–19. [Google Scholar] [CrossRef]
- Sirsat, S.A.; Neal, J.A. Microbial profile of soil-free versus in-soil grown lettuce and intervention methodologies to combat pathogen surrogates and spoilage microorganisms on lettuce. Foods 2013, 2, 488–498. [Google Scholar] [CrossRef]
- Weller, D.L.; Saylor, L.; Turkon, P. Total coliform and generic E. coli levels, and Salmonella presence in eight experimental aquaponics and hydroponics systems: A brief report highlighting exploratory data. Horticulturae 2020, 6, 42. [Google Scholar] [CrossRef]
- Sathyanarayana, S.R.; Warke, V.G.; Mahajan, G.B.; Annapure, U.S. Comparative studies of microbial and heavy metal safety assessment of the herbs cultivated in hydroponically and regular soil system. J. Food Saf. 2021, 41, e12936. [Google Scholar] [CrossRef]
- Orozco, R.L.; Iturriaga, M.H.; Tamplin, M.L.; Fratamico, P.M.; Call, J.E.; Luchansky, J.B.; Escartin, E.F. Animal and environmental impact on the presence and distribution of Salmonella and Escherichia coli in hydroponic tomato greenhouses. J. Food Prot. 2008, 71, 676–683. [Google Scholar] [CrossRef]
- Allende, A.; Monaghan, J. Irrigation water quality for leafy crops: A perspective of risks and potential solutions. Int. J. Environ. Res. Public Health 2015, 12, 7457–7477. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, J.B.; Gibson, K.E. Irrigation water and contamination of fresh produce with bacterial foodborne pathogens. Curr. Opin. Food Sci. 2022, 47, 100889. [Google Scholar] [CrossRef]
- Lopez-Galvez, F.; Allende, A.; Pedrero-Salcedo, F.; Alarcon, J.J.; Gil, M.I. Safety assessment of greenhouse hydroponic tomatoes irrigated with reclaimed and surface water. Int. J. Food Microbiol. 2014, 191, 97–102. [Google Scholar] [CrossRef]
- Wang, Y.J.; Deering, A.J.; Kim, H.J. The occurrence of shiga-Toxin-producing E. coli in aquaponic and hydroponic systems. Horticulturae 2020, 6, 1. [Google Scholar] [CrossRef]
- Keller, R.; Perim, K.; Semionato, S.; Zandonade, E.; Cassini, S.; Gonçalves, R.F. Hydroponic cultivation of lettuce (Lactuca sativa) using effluents from primary, secondary and tertiary +UV treatments. Water Supply 2005, 5, 95–100. [Google Scholar] [CrossRef]
- Selma, M.V.; Luna, M.C.; Martínez-Sánchez, A.; Tudela, J.A.; Beltrán, D.; Baixauli, C.; Gil, M.I. Sensory quality, bioactive constituents and microbiological quality of green and red fresh-cut lettuces (Lactuca sativa L.) are influenced by soil and soilless agricultural production systems. Postharvest Biol. Technol. 2012, 63, 16–24. [Google Scholar] [CrossRef]
- Kasozi, N.; Abraham, B.; Kaiser, H.; Wilhelmi, B. The complex microbiome in aquaponics: Significance of the bacterial ecosystem. Ann. Microbiol. 2021, 71, 1. [Google Scholar] [CrossRef]
- Dankwa, A.S.; Machado, R.M.; Perry, J.J. Sources of food contamination in a closed hydroponic system. Lett. Appl. Microbiol. 2020, 70, 55–62. [Google Scholar] [CrossRef]
- FDA. Investigation Report: Factors Potentially Contributing to the Contamination of Packaged Leafy Greens Implicated in the Outbreak of Salmonella typhimurium During the Summer of 2021. 2022. Available online: https://www.fda.gov/media/155402/download (accessed on 1 December 2022).
- Canadian Food Inspection Agency. 2018. Lufa Farms Inc. Brand Arugula Microgreens Recalled due to Salmonella. Recalls and Safety Alerts. Available online: http://healthycanadians.gc.ca/recall-alert-rappel-avis/inspection/2018/67156r-eng.php (accessed on 1 December 2022).
- Canadian Food Inspection Agency. 2018. Food Recall Warning—Goodleaf Brand Daikon Radish Microgreens Recalled due to Listeria Monocytogenes. Recalls and Safety Alerts. Available online: https://www.inspection.gc.ca/about-the-cfia/newsroom/food-recallwarnings/complete-listing/2018-06-28/eng/1530237479767/1530237483085 (accessed on 1 December 2022).
- Orozco, L.; Rico-Romero, L.; Escartin, E.F. Microbiological profile of greenhouses in a farm producing hydroponic tomatoes. J. Food Prot. 2008, 71, 60–65. [Google Scholar] [CrossRef]
- Avila-Vega, D.E.; Alvarez-Mayorga, B.; Arvizu-Medrano, S.M.; Pacheco-Aguilar, R.; Martinez-Peniche, R.; Hernandez-Iturriaga, M. Microbiological profile and incidence of Salmonella and Listeria monocytogenes on hydroponic bell peppers and greenhouse cultivation environment. J. Food Prot. 2014, 77, 1904–1910. [Google Scholar] [CrossRef]
- Lopez-Galvez, F.; Gil, M.I.; Pedrero-Salcedo, F.; Alarcon, J.J.; Allende, A. Monitoring generic Escherichia coli in reclaimed and surface water used in hydroponically cultivated greenhouse peppers and the influence of fertilizer solutions. Food Control 2016, 67, 90–95. [Google Scholar] [CrossRef]
- Tham, C.A.T.; Zwe, Y.H.; Li, D. Microbial study of lettuce and agriculture water used for lettuce production at Singapore urban farms. Food Control 2021, 126, 108065. [Google Scholar] [CrossRef]
- Xu, J.; Warriner, K. Coliphage as an indicator of fecal contamination in hydroponic cucumber (Cucumis sativus L) greenhouses. J. Sci. Food Agric. 2005, 85, 2397–2400. [Google Scholar] [CrossRef]
- Riggio, G.M.; Wang, Q.; Kniel, K.E.; Gibson, K.E. Microgreens—A review of food safety considerations along the farm to fork continuum. Int. J. Food Microbiol. 2019, 290, 76–85. [Google Scholar] [CrossRef]
- Misra, G.; Gibson, K.E. Characterization of microgreen growing operations and associated food safety practices. Food Prot. Trends 2021, 41, 56–69. [Google Scholar] [CrossRef]
- Park, S.; Szonyi, B.; Gautam, R.; Nightingale, K.; Anciso, J.; Ivanek, R. Risk factors for microbial contamination in fruits and vegetables at the preharvest level: A systematic review. J. Food Prot. 2012, 75, 2055–2081. [Google Scholar] [CrossRef]
- Moraru, P.I.; Rusu, T.; Mintas, O.S. Trial protocol for evaluating platforms for growing microgreens in hydroponic conditions. Foods 2022, 11, 1327. [Google Scholar] [CrossRef]
- Turner, E.R.; Luo, Y.; Buchanan, R.L. Microgreen nutrition, food safety, and shelf life: A review. J. Food Sci. 2020, 85, 870–882. [Google Scholar] [CrossRef]
- Yang, Y.S.; Meier, F.; Lo, J.A.; Yuan, W.Q.; Sze, V.L.P.; Chung, H.J.; Yuk, H.G. Overview of Recent Events in the Microbiological Safety of Sprouts and New Intervention Technologies. Compr. Rev. Food Sci. F 2013, 12, 265–280. [Google Scholar] [CrossRef]
- Chahar, M.; Gollop, R.; Kroupitski, Y.; Shemesh, M.; Sela Saldinger, S. Control of Salmonella in mung bean sprouts by antagonistic spore-forming Bacilli. Food Control 2023, 143, 109276. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Prado, I.d.; Sirsat, S.A. Comparative microbial analyses of hydroponic versus in-soil grown Romaine lettuce obtained at retail. Heliyon 2022, 8, e11050. [Google Scholar] [CrossRef]
- Heaton, J.C.; Jones, K. Microbial contamination of fruit and vegetables and the behaviour of enteropathogens in the phyllosphere: A review. J. Appl. Microbiol. 2008, 104, 613–626. [Google Scholar] [CrossRef]
- Martínez-Vaz, B.M.; Fink, R.C.; Diez-Gonzalez, F.; Sadowsky, M.J. Enteric pathogen-plant interactions: Molecular connections leading to colonization and growth and implications for food safety. Microbes Environ. 2014, 29, 123–135. [Google Scholar] [CrossRef]
- Murray, K.; Wu, F.; Shi, J.; Jun Xue, S.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual Saf-Oxf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- Warriner, K.; Huber, A.; Namvar, A.; Fan, W.; Dunfield, K. Recent advances in the microbial safety of fresh fruits and vegetables. Adv. Food Nutr. Res. 2009, 57, 155–208. [Google Scholar] [CrossRef]
- Riggio, G.M.; Jones, S.L.; Gibson, K.E. Risk of human pathogen internalization in leafy vegetables during lab-scale hydroponic cultivation. Horticulturae 2019, 5, 25. [Google Scholar] [CrossRef]
- Bernstein, N.; Sela, S.; Pinto, R.; Ioffe, M. Evidence for internalization of Escherichia coli into the aerial parts of maize via the root system. J. Food Prot. 2007, 70, 471–475. [Google Scholar] [CrossRef]
- Guo, X.A.; van Iersel, M.W.; Chen, J.R.; Brackett, R.E.; Beuchat, L.R. Evidence of association of salmonellae with tomato plants grown hydroponically in inoculated nutrient solution. Appl. Environ. Microbiol. 2002, 68, 3639–3643. [Google Scholar] [CrossRef]
- Macarisin, D.; Patel, J.; Sharma, V.K. Role of curli and plant cultivation conditions on Escherichia coli O157:H7 internalization into spinach grown on hydroponics and in soil. Int. J. Food Microbiol. 2014, 173, 48–53. [Google Scholar] [CrossRef]
- Warriner, K.; Ibrahim, F.; Dickinson, M.; Wright, C.; Waites, W.M. Interaction of Escherichia coli with growing salad spinach plants. J. Food Prot. 2003, 66, 1790–1797. [Google Scholar] [CrossRef]
- Sharma, M.; Ingram, D.T.; Patel, J.R.; Millner, P.D.; Wang, X.; Hull, A.E.; Donnenberg, M.S. A novel approach to investigate the uptake and internalization of Escherichia coli O157:H7 in spinach cultivated in soil and hydroponic medium. J. Food Prot. 2009, 72, 1513–1520. [Google Scholar] [CrossRef]
- Moriarty, M.J.; Semmens, K.; Bissonnette, G.K.; Jaczynski, J. Internalization assessment of E. coli O157: H7 in hydroponically grown lettuce. LWT 2019, 100, 183–188. [Google Scholar] [CrossRef]
- Koseki, S.; Mizuno, Y.; Yamamoto, K. Comparison of two possible routes of pathogen contamination of spinach leaves in a hydroponic cultivation system. J. Food Prot. 2011, 74, 1536–1542. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Skandamis, P.; Tzortzakis, N. Salmonella Enteritidis survival in different temperatures and nutrient solution pH levels in hydroponically grown lettuce. Food Microbiol. 2022, 102, 103898. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Sharma, M.; Kniel, K.E. Human enteric pathogen internalization by root uptake into food crops. Foodborne Pathog. Dis. 2012, 9, 396–405. [Google Scholar] [CrossRef]
- Xiao, Z.; Bauchan, G.; Nichols-Russell, L.; Luo, Y.; Wang, Q.; Nou, X. Proliferation of Escherichia coli O157:H7 in Soil-Substitute and Hydroponic Microgreen Production Systems. J. Food Prot. 2015, 78, 1785–1790. [Google Scholar] [CrossRef]
- DiCaprio, E.; Ma, Y.; Purgianto, A.; Hughes, J.; Li, J. Internalization and dissemination of human Norovirus and animal Caliciviruses in hydroponically grown romaine lettuce. Appl. Environ. Microbiol. 2012, 78, 6143–6152. [Google Scholar] [CrossRef]
- Wang, Q.; Kniel, K.E. Survival and transfer of murine norovirus within a hydroponic system during kale and mustard microgreen harvesting. Appl. Environ. Microbiol. 2016, 82, 705–713. [Google Scholar] [CrossRef]
- Wachtel, M.R.; Whitehand, L.C.; Mandrell, R.E. Association of Escherichia coli O157: H7 with preharvest leaf lettuce upon exposure to contaminated irrigation water. J. Food Prot. 2002, 65, 18–25. [Google Scholar] [CrossRef]
- Ilic, S.; Moodispaw, M.R.; Madden, L.V.; Lewis Ivey, M.L. Lettuce contamination and survival of Salmonella Typhimurium and Listeria monocytogenes in hydroponic nutrient film technique systems. Foods 2022, 11, 3508. [Google Scholar] [CrossRef]
- Shaw, A.; Helterbran, K.; Evans, M.R.; Curry, C. Growth of Escherichia coli O157:H7, non-O157 shiga toxin–producing Escherichia coli, and Salmonella in water and hydroponic fertilizer solutions. J. Food Prot. 2016, 79, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Holden, N.J. Quantification and colonisation dynamics of Escherichia coli O157:H7 inoculation of microgreens species and plant growth substrates. Int. J. Food Microbiol. 2018, 273, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.H.V.; Trientini, M.F.; Fisher, P.R. Biofilm management in irrigation lines and hydroponic lettuce solutions using sanitizing chemicals. Crops. Acta Hort 2022, 1335, 703–710. [Google Scholar] [CrossRef]
- Kyere, E.O.; Foong, G.; Palmer, J.; Wargent, J.J.; Fletcher, G.C.; Flint, S. Biofilm formation of Listeria monocytogenes in hydroponic and soil grown lettuce leaf extracts on stainless steel coupons. LWT 2020, 126, 109114. [Google Scholar] [CrossRef]
- Lim, J.A.; Lee, D.H.; Heu, S. The interaction of human enteric pathogens with plants. Plant Pathol. J. 2014, 30, 109–116. [Google Scholar] [CrossRef]
- Álvarez-Ordóñez, A.; Briandet, R. Biofilms from a food microbiology perspective: Structures, functions, and control strategies. Front. Microbiol. 2016, 7, 1938. [Google Scholar] [CrossRef]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.-C.; Naitali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Møretrø, T.; Langsrud, S. Residential bacteria on surfaces in the food industry and their implications for food safety and quality. Compr. Rev. Food Sci. F 2017, 16, 1022–1041. [Google Scholar] [CrossRef]
- Reed, E.; Ferreira, C.M.; Bell, R.; Brown, E.W.; Zheng, J. Plant-microbe and abiotic factors influencing Salmonella survival and growth on alfalfa sprouts and Swiss chard microgreens. Appl. Environ. Microbiol. 2018, 84, e02814-17. [Google Scholar] [CrossRef]
- Filho, A.F.M.; de Azevedo, C.A.V.; de Queiroz Almeida Azevedo, M.R.; Fernandes, J.D.; Correa, E.B.; dos Santos, S.A. Microbiological and parasitological contamination of hydroponic grown curly lettuce under different optimized nutrient solutions. Aust. J. Crop. Sci. 2018, 12, 400–406. [Google Scholar] [CrossRef]
- Warriner, K.; Spaniolas, S.; Dickinson, M.; Wright, C.; Waites, W. Internalization of bioluminescent Escherichia coli and Salmonella Montevideo in growing bean sprouts. J. Appl. Microbiol. 2003, 95, 719–727. [Google Scholar] [CrossRef]
- Li, Y.; Zwe, Y.H.; Tham, C.A.T.; Zou, Y.; Li, W.; Li, D. Fate and mitigation of Salmonella contaminated in lettuce (Lactuca sativa) seeds grown in a hydroponic system. J. Appl. Microbiol. 2022, 132, 1449–1456. [Google Scholar] [CrossRef] [PubMed]
- FDA. USA Food and Drug Administration. FSMA Final Rule on Produce Safety. 2015. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety (accessed on 25 October 2022).
- FDA. USA Food and Drug Administration. FSMA Final Rule on Produce Safety. Standards for the Growing, Harvesting, Packing, and Holding of Produce for Human Consumption. 2020. Available online: https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-finalrule-produce-safety (accessed on 20 September 2022).
- USDA. Aquaponics Good Agricultural Practices. Available online: https://www.ams.usda.gov/sites/default/files/media/AquaponicOperationGAP.pdf (accessed on 1 December 2022).
- Tavan, M.; Wee, B.; Brodie, G.; Fuentes, S.; Pang, A.; Gupta, D. Optimizing sensor-based irrigation management in a soilless vertical farm for growing microgreens. Front. Sustain. Food Syst. 2021, 4, 622720. [Google Scholar] [CrossRef]
- Phornvillay, S.; Yodsarn, S.; Oonsrithong, J.; Srilaong, V.; Pongprasert, N. A novel technique using advanced oxidation process (UV-C/H2O2) combined with micro-nano bubbles on decontamination, seed viability, and enhancing phytonutrients of roselle microgreens. Horticulturae 2022, 8, 53. [Google Scholar] [CrossRef]
- FDA. USA Food and Drug Administration. In Guidance for Industry: Reducing Microbial Food Safety Hazards for Sprouted Seeds; U.S. Food and Drug Administration: Silver Spring, MD, USA, 1999. [Google Scholar]
- Warriner, K.; Ibrahim, F.; Dickinson, M.; Wright, C.; Waites, W.M. Seed decontamination as an intervention step for eliminating Escherichia coli on salad vegetables and herbs. J. Sci. Food Agric. 2005, 85, 2307–2313. [Google Scholar] [CrossRef]
- Du, M.; Xiao, Z.; Luo, Y. Advances and emerging trends in cultivation substrates for growing sprouts and microgreens towards safe and sustainable agriculture. Curr. Opin. Food Sci. 2022, 46, 100863. [Google Scholar] [CrossRef]
- Palanivelu, S.D.; Armir, N.A.Z.; Zulkifli, A.; Hair, A.H.A.; Salleh, K.M.; Lindsey, K.; Che-Othman, M.H.; Zakaria, S. Hydrogel application in urban farming: Potentials and limitations—A review. Polymers 2022, 14, 2590. [Google Scholar] [CrossRef]
- Teng, Z.; Luo, Y.G.; Pearlstein, D.J.; Zhou, B.; Johnson, C.M.; Mowery, J.; Wang, Q.; Fonseca, J.M. Agarose hydrogel composite supports microgreen cultivation with enhanced porosity and continuous water supply under terrestrial and microgravitational conditions. Int. J. Biol. Macromol. 2022, 220, 135–146. [Google Scholar] [CrossRef]
- Zohuriaan-Mehr, M.J.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Diez, A.M.D.; Gonzalez, J.A.; Perez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial activity of starch hydrogel incorporated with copper nanoparticles. ACS Appl. Mater. Inter. 2016, 8, 16280–16288. [Google Scholar] [CrossRef]
- Wang, T.; Dai, Z.P.; Kang, J.M.; Fu, F.Y.; Zhang, T.; Wang, S. A TiO2 nanocomposite hydrogel for Hydroponic plants in efficient water improvement. Mater. Chem. Phys. 2018, 215, 242–250. [Google Scholar] [CrossRef]
- Ehret, D.L.; Alsanius, B.; Wohanka, W.; Menzies, J.G.; Utkhede, R. Disinfestation of recirculating nutrient solutions in greenhouse horticulture. Agronomie 2001, 21, 323–339. [Google Scholar] [CrossRef]
- Takahashi, K.; Saito, Y.; Oikawa, R.; Okumura, T.; Takaki, K.; Fujio, T. Development of automatically controlled corona plasma system for inactivation of pathogen in hydroponic cultivation medium of tomato. J. Electrostat. 2018, 91, 61–69. [Google Scholar] [CrossRef]
- Song, W.T.; Zhou, L.G.; Yang, C.Z.; Cao, X.D.; Zhang, L.Q.; Liu, X.L. Tomato Fusarium wilt and its chemical control strategies in a hydroponic system. Crop. Prot. 2004, 23, 243–247. [Google Scholar] [CrossRef]
- Chatzidimopoulos, M.; Pappas, A.C. Control of bottom rot in hydroponic lettuce, caused by strains of Botrytis cinerea with multiple fungicide resistance. Phytopathol. Mediterr. 2019, 58, 507–517. [Google Scholar] [CrossRef]
- Lee, S.; Ge, C.T.; Bohrerova, Z.; Grewal, P.S.; Lee, J. Enhancing plant productivity while suppressing biofilm growth in a windowfarm system using beneficial bacteria and ultraviolet irradiation. Can. J. Microbiol. 2015, 61, 457–466. [Google Scholar] [CrossRef]
- Hultberg, M.; Holmkvist, A.; Alsanius, B. Strategies for administration of biosurfactant-producing pseudomonads for biocontrol in closed hydroponic systems. Crop. Prot. 2011, 30, 995–999. [Google Scholar] [CrossRef]
- Ye, J.; Kostrzynska, M.; Dunfield, K.; Warriner, K. Control of Salmonella on sprouting mung bean and alfalfa seeds by using a biocontrol preparation based on antagonistic bacteria and lytic bacteriophages. J. Food Prot. 2010, 73, 9–17. [Google Scholar] [CrossRef]
- Barak, J.D.; Liang, A.; Narm, K.-E. Differential attachment to and subsequent contamination of agricultural crops by Salmonella enterica. Appl. Environ. Microbiol. 2008, 74, 5568–5570. [Google Scholar] [CrossRef]
- Jacob, C.; Melotto, M. Human pathogen colonization of lettuce dependent upon plant genotype and defense response activation. Front. Plant Sci. 2020, 10, 1769. [Google Scholar] [CrossRef]
- Sela, S.; Manulis-Sasson, S. What else can we do to mitigate contamination of fresh produce by foodborne pathogens? Microb. Biotechnol. 2015, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Robert Antony, A.; Janani, R.; Rajesh Kannan, V. Biofilm instigation of plant pathogenic bacteria and its control measures. Biofilms Plant Soil Health 2017, 409–438. [Google Scholar]
- Yamaguchi, N.; Roberts, M.; Castro, S.; Oubre, C.; Makimura, K.; Leys, N.; Grohmann, E.; Sugita, T.; Ichijo, T.; Nasu, M. Microbial monitoring of crewed habitats in space-current status and future perspectives. Microbes Environ. 2014, 29, 250–260. [Google Scholar] [CrossRef]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An updated overview on metal nanoparticles toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef]
- Bliedung, A.; Dockhorn, T.; Germer, J.; Mayerl, C.; Mohr, M. Experiences of running a hydroponic system in a pilot scale for resource-efficient water reuse. J. Water Reuse Desal. 2020, 10, 347–362. [Google Scholar] [CrossRef]
- Eregno, F.E.; Moges, M.E.; Heistad, A. Treated greywater Reuse for hydroponic lettuce production in a green wall system: Quantitative health risk assessment. Water 2017, 9, 454. [Google Scholar] [CrossRef]
- Dannehl, D.; Schuch, I.; Gao, Y.; Cordiner, S.; Schmidt, U. Effects of hypochlorite as a disinfectant for hydroponic systems on accumulations of chlorate and phytochemical compounds in tomatoes. Eur. Food Res. Technol. 2016, 242, 345–353. [Google Scholar] [CrossRef]
- Webber, M.A.; Whitehead, R.N.; Mount, M.; Loman, N.J.; Pallen, M.J.; Piddock, L.J.V. Parallel evolutionary pathways to antibiotic resistance selected by biocide exposure. J. Antimicrob. Chemoth. 2015, 70, 2241–2248. [Google Scholar] [CrossRef]
- Goncalves, D.R.; de Jesus, A.L.; Pires-Zottarelli, C.L.A. Pythium and Phytopythium species associated with hydroponically grown crops around the City of So Paulo, Brazil. Trop. Plant Pathol. 2016, 41, 397–405. [Google Scholar] [CrossRef]
- Teplitski, M.; Warriner, K.; Bartz, J.; Schneider, K.R. Untangling metabolic and communication networks: Interactions of enterics with phytobacteria and their implications in produce safety. Trends Microbiol. 2011, 19, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Feng, H. Microbial community analysis and food safety practice survey-based hazard identification and risk assessment for controlled environment hydroponic/aquaponic farming systems. Front. Microbiol. 2022, 13, 879260. [Google Scholar] [CrossRef] [PubMed]


| Approach | Specific Measures | References |
|---|---|---|
| Reducing contamination of planting material | Seed decontamination: sanitizers, ozone gas, ethanol, advanced oxidation, microbubbles | [105,106,107,108] |
| Hydrogel use as seedling growing substrate | [109,110,111] | |
| Antimicrobial hydrogel substrates, e.g., chitosan | [109] | |
| Natural antimicrobials added to substrates | [109] | |
| Inorganic nanoparticles added to substrates | [112,113,114] | |
| Sanitation of recirculating water | Filtration | [47,75,115] |
| Sanitizer additives: chlorine, iodine, H2O2, etc. | [47,75,115] | |
| Physical water treatment: UV, ultrasound | [47,75,115] | |
| Physical water treatment: plasma | [29,116] | |
| Controlling plant colonization by human pathogens | Controlling plant diseases as route for human pathogen penetration: agrochemicals | [117,118] |
| Biocontrol agents: bacteria, bacteriophages | [69,119,120,121] | |
| Selection of resistant plant genotypes | [122,123,124] | |
| Controlling biofilms that harbor human pathogens | Sanitizers | [92] |
| Surface modification | [125] | |
| Physical measures (e.g., ultrasound, UV) | [119,125] | |
| Micobiome manipulation, biocontrol | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sela Saldinger, S.; Rodov, V.; Kenigsbuch, D.; Bar-Tal, A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae 2023, 9, 51. https://doi.org/10.3390/horticulturae9010051
Sela Saldinger S, Rodov V, Kenigsbuch D, Bar-Tal A. Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae. 2023; 9(1):51. https://doi.org/10.3390/horticulturae9010051
Chicago/Turabian StyleSela Saldinger, Shlomo, Victor Rodov, David Kenigsbuch, and Asher Bar-Tal. 2023. "Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions" Horticulturae 9, no. 1: 51. https://doi.org/10.3390/horticulturae9010051
APA StyleSela Saldinger, S., Rodov, V., Kenigsbuch, D., & Bar-Tal, A. (2023). Hydroponic Agriculture and Microbial Safety of Vegetables: Promises, Challenges, and Solutions. Horticulturae, 9(1), 51. https://doi.org/10.3390/horticulturae9010051







