Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress
Abstract
1. Introduction
2. Morphological and Dynamic Changes in Mitochondria
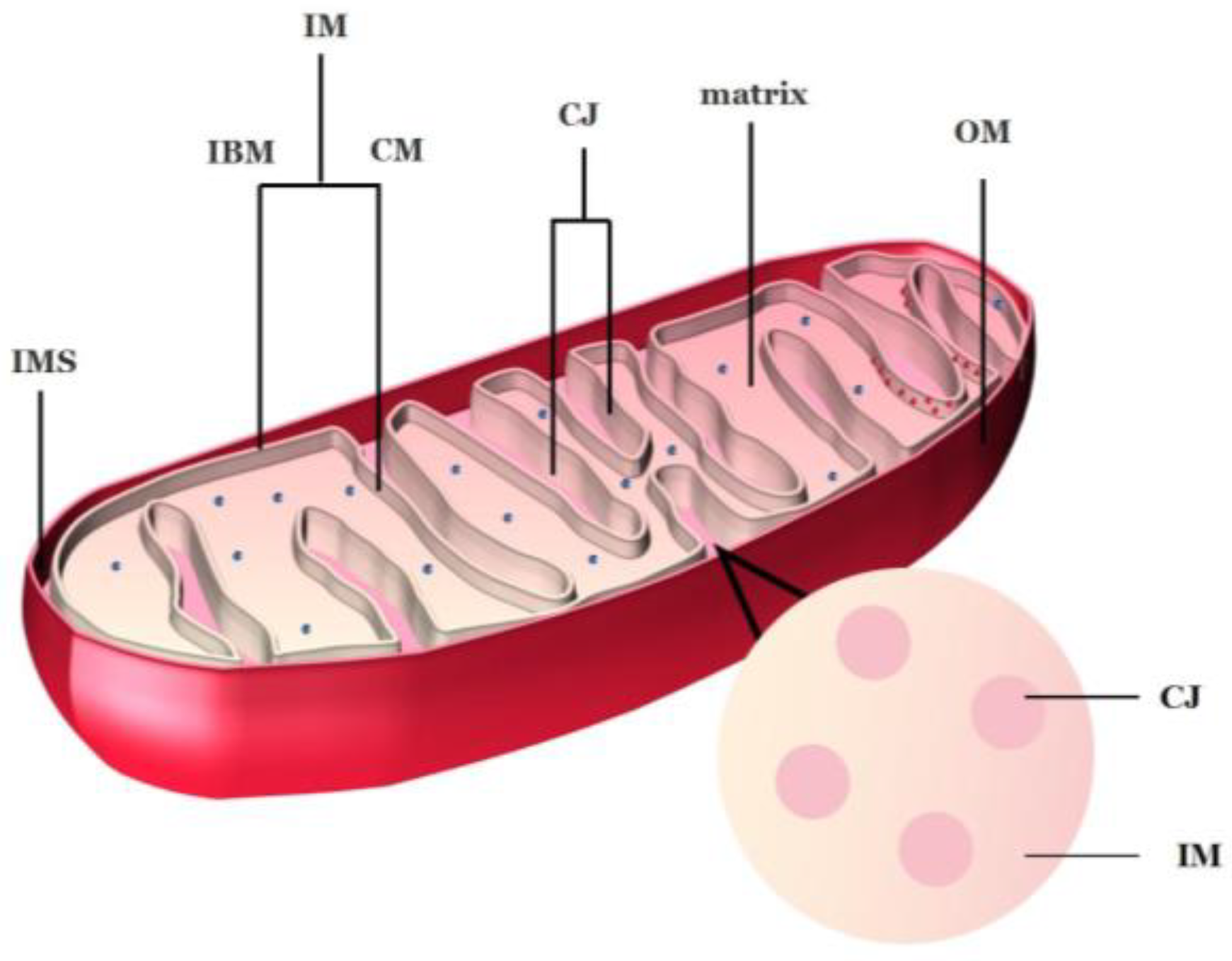
2.1. Ultrastructure of Mitochondria
2.2. Structure of Cristae
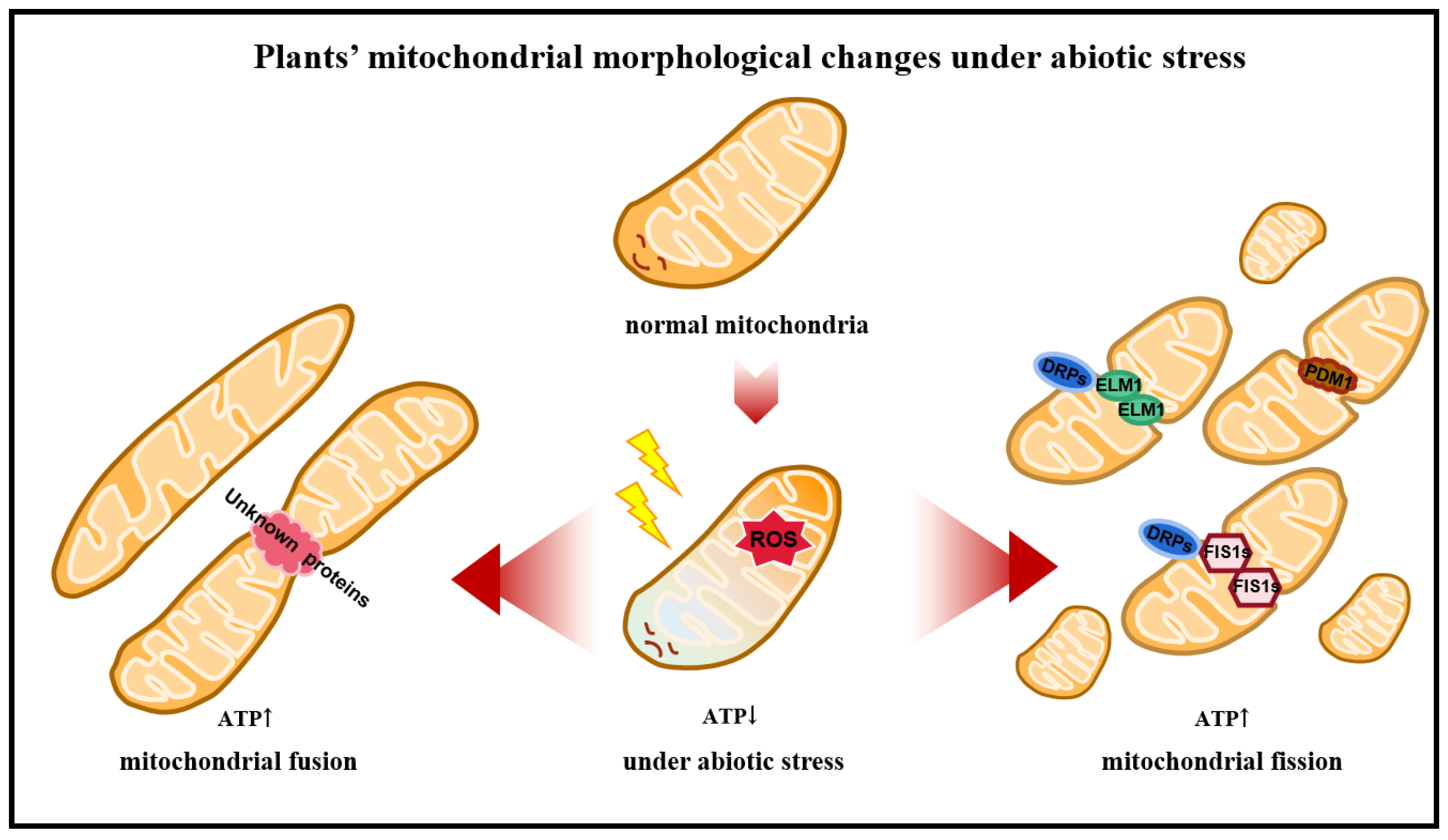
2.3. Dynamic Changes in Mitochondria in Plants
2.3.1. Proteins Associated with Mitochondrial Fusion
2.3.2. Proteins Associated with Mitochondrial Fission
2.3.3. Fusion and Fission of Cardiolipin with Mitochondria
3. Mitophagy
4. Interactions between Organelles Affect Mitochondrial Dynamics Processes
5. Morphological Changes in Mitochondria in Response to Abiotic Stress
5.1. Morphological Changes in Mitochondria under Temperature Stress
5.2. Morphological Changes in Mitochondria under Drought Stress
5.3. Morphological Changes in Mitochondria under Salt Stress
5.4. Morphological Changes in Mitochondria under Other Abiotic Stress
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Logan, D. Mitochondrial dynamics. New Phytol. 2003, 160, 463–478. [Google Scholar] [CrossRef] [PubMed]
- Stickens, D.; Verbelen, J.-P. Spatial structure of mitochondria and ER denotes changes in cell physiology of cultured tobacco protoplasts. Plant J. 1996, 9, 85–92. [Google Scholar] [CrossRef]
- Jaipargas, E.; Barton, K.; Mathur, N.; Mathur, J. Mitochondrial pleomorphy in plant cells is driven by contiguous ER dynamics. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.; Leaver, C. Mitochondria-targeted GFP highlights the heterogeneity of mitochondrial shape, size and movement within living plant cells. J. Exp. Bot. 2000, 51, 865–871. [Google Scholar] [CrossRef]
- Nekrasova, O.; Kulik, A.; Minin, A. Proteinkinase C regulates motility of mitochondria. Biol. Membr. 2007, 24, 126–131. [Google Scholar]
- Doniwa, Y.; Arimura, S.; Tsutsumi, N. Mitochondria use actin filaments as rails for fast translocation in Arabidopsis and tobacco cells. Plant Biotechnol. 2007, 24, 441–447. [Google Scholar] [CrossRef][Green Version]
- Palikaras, K.; Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef]
- Hoppins, S.; Nunnari, J. The molecular mechanism of mitochondrial fusion. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2009, 1793, 20–26. [Google Scholar] [CrossRef]
- Mareri, L.; Parrotta, L.; Cai, G. Environmental Stress and Plants. Int. J. Mol. Sci. 2022, 23, 5416. [Google Scholar] [CrossRef]
- Petrov, V.; Hille, J.; Mueller-Roeber, B.; Gechev, T.S. ROS-mediated abiotic stress-induced programmed cell death in plants. Front. Plant Sci. 2015, 6, 69. [Google Scholar] [CrossRef]
- Martínez-Fábregas, J.; Díaz-Moreno, I.; González-Arzola, K.; Janocha, S.; Navarro, J.A.; Hervás, M.; Bernhardt, R.; Antonio Díaz-Quintana, A.; De la Rosa, M.Á. New Arabidopsis thaliana cytochrome c partners: A look into the elusive role of cytochrome c in programmed cell death in plants. Mol. Cell. Proteomics 2013, 12, 3666–3676. [Google Scholar] [CrossRef] [PubMed]
- Breininger, S.P.; Malcomson, F.C.; Afshar, S.; Turnbull, D.M.; Greaves, L.; Mathers, J.C. Effects of obesity and weight loss on mitochondrial structure and function and implications for colorectal cancer risk. Proc. Nutr. Soc. 2019, 78, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, F.; Gonzalez, D.H.; Welchen, E. Plant mitochondria under pathogen attack: A sigh of relief or a last breath? Mitochondrion 2014, 19, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef]
- Logan, A.; Murphy, M.P. Using chemical biology to assess and modulate mitochondria: Progress and challenges. Interface Focus 2017, 7, 20160151. [Google Scholar] [CrossRef]
- Zhan, J.; Li, W.; He, H.; Li, C.Z.; He, L. Mitochondrial alterations during Al-induced PCD in peanut root tips. Plant Physiol. Biochem. 2014, 75, 105–113. [Google Scholar] [CrossRef]
- Yao, N.; Eisfelder, B.J.; Marvin, J.; Greenberg, J.T. The mitochondrion—An organelle commonly involved in programmed cell death in Arabidopsis thaliana. Plant J. 2004, 40, 596–610. [Google Scholar] [CrossRef]
- Hessenberger, M.; Zerbes, R.M.; Rampelt, H.; Kunz, S.; Xavier, A.H.; Purfürst, B.; Lilie, H.; Pfanner, N.; van der Laan, M.; Daumke, O. Regulated membrane remodeling by Mic60 controls formation of mitochondrial crista junctions. Nat. Commun. 2017, 8, 15258. [Google Scholar] [CrossRef]
- Harner, M.; Körner, C.; Walther, D.; Mokranjac, D.; Kaesmacher, J.; Welsch, U.; Griffith, J.; Mann, M.; Reggiori, F.; Neupert, W. The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 2011, 30, 4356–4370. [Google Scholar] [CrossRef]
- Frey, T.G.; Mannella, C.A. The internal structure of mitochondria. Trends Biochem. Sci. 2000, 25, 319–324. [Google Scholar] [CrossRef]
- Kühlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Frezza, C.; Soriano, M.E.; Varanita, T.; Quintana-Cabrera, R.; Corrado, M.; Sara Cipolat, S.; Costa, V.; Casarin, A.; Gomes, L.C.; et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 2013, 155, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Vogel, F.; Bornhovd, C.; Neupert, W.; Andreas, S. Reichert; Dynamic subcompartmentalization of the mitochondrial inner membrane. J. Cell Biol. 2006, 175, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A.; Pfeiffer, D.R.; Bradshaw, P.C.; Moraru, I.I.; Slepchenko, B.; Loew, L.M.; Hsieh, C.; Buttle, K.; Marko, M. Topology of the Mitochondrial Inner Membrane: Dynamics and Bioenergetic Implications. IUBMB Life (Int. Union Biochem. Mol. Biol. Life) 2011, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Glytsou, C.; Calvo, E.; Cogliati, S.; Mehrotra, A.; Anastasia, I.; Rigoni, G.; Raimondi, A.; Shintani, N.; Loureiro, M.; Vazquez, J.; et al. Optic atrophy 1 is epistatic to the core MICOS component MIC60 in mitochondrial cristae shape control. Cell Rep. 2016, 17, 3024–3034. [Google Scholar] [CrossRef] [PubMed]
- Pernas, L.; Scorrano, L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu. Rev. Physiol. 2016, 78, 505–531. [Google Scholar] [CrossRef]
- Perkins, G.; Bossy-Wetzel, E.; Ellisman, M.H. New insights into mitochondrial structure during cell death. Exp. Neurol. 2009, 218, 183–192. [Google Scholar] [CrossRef]
- Velours, J.; Dautant, A.; Salin, B.; Sagot, I.; Brèthes, D. Mitochondrial F1F0-ATP synthase and organellar internal architecture. Int. J. Biochem. Cell Biol. 2009, 41, 1783–1789. [Google Scholar] [CrossRef]
- Satoh, M.; Hamamoto, T.; Seo, N.; Kagawa, Y.; Endo, H. Differential sublocalization of the dynamin-related protein OPA1 isoforms in mitochondria. Biochem. Biophys. Res. Commun. 2003, 300, 482–493. [Google Scholar] [CrossRef]
- Olichon, A.; Emorine, L.J.; Descoins, E.; Pelloquin, L.; Brichese, L.; Gas, N.; Guillou, E.; Delettre, C.; Valette, A.; Hamel, C.P.; et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002, 523, 171–176. [Google Scholar] [CrossRef]
- Frezza, C.; Cipolat, S.; Martins de Brito, O.; Micaroni, M.; Beznoussenko, G.V.; Rudka, T.; Bartoli, D.; Polishuck, R.S.; Nika, N.; Danial, N.N.; et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 2006, 126, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Rampelt, H.; Zerbes, R.M.; van der Laan, M.; Pfanner, N. Role of the mitochondrial contact site and cristae organizing system in membrane architecture and dynamics. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2017, 1864, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Barbot, M.; Jans, D.C.; Schulz, C.; Denkert, N.; Kroppen, B.; Hoppert, M.; Jakobs, S.; Meinecke, M. Mic10 oligomerizes to bend mitochondrial inner membranes at cristae junctions. Cell Metab. 2015, 21, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Cogliati, S.; Enriquez, J.A.; Scorrano, L. Mitochondrial Cristae: Where Beauty Meets Functionality. Trends Biochem. Sci. 2016, 41, 261–273. [Google Scholar] [CrossRef]
- Hoppins, S.; Collins, S.R.; Cassidy-Stone, A.; Hummel, E.; DeVay, R.M.; Lackner, L.L.; Westermann, B.; Schuldiner, M.; Weissman, J.S.; Nunnari, J. A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 2011, 195, 323–340. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Marusich, M.F.; Souda, P.; Whitelegge, J.; Capaldi, R.A. The mitochondrial inner membrane protein mitofilin exists as a complex with SAM50, metaxins 1 and 2, coiled-coil-helix domain-containing protein 3 and 6 and DnaJC11. FEBS Lett. 2007, 581, 3545–3549. [Google Scholar] [CrossRef]
- John, G.B.; Shang, Y.; Li, L.; Renken, C.; Mannella, C.A.; Selker, J.M.L.; Rangell, L.; Bennett, M.J.; Zha, J. The mitochondrial inner membrane protein mitofilin controls cristae morphology. Mol. Biol. Cell 2005, 16, 1543–1554. [Google Scholar] [CrossRef]
- Rabl, R.; Soubannier, V.; Scholz, R.; Vogel, F.; Mendl, N.; Vasiljev-Neumeyer, A.; Korner, C.; Jagasia, R.; Keil, T.; Baumeister, W.; et al. Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J. Cell Biol. 2009, 185, 1047–1063. [Google Scholar] [CrossRef]
- Bohnert, M.; Zerbes, R.M.; Davies, K.M.; Mühleip, A.W.; Rampelt, H.; Horvath, S.E.; Boenke, T.; Kram, A.; Perschil, I.; Veenhuis, M.; et al. Central role of Mic10 in the mitochondrial contact site and cristae organizing system. Cell Metab. 2015, 21, 747–755. [Google Scholar] [CrossRef]
- Muñoz-Gómez, S.A.; Slamovits, C.H.; Dacks, J.B.; Baier, K.A.; Spencer, K.D.; Wideman, J.G. Ancient homology of the mitochondrial contact site and cristae organizing system points to an endosymbiotic origin of mitochondrial cristae. Curr. Biol. 2015, 25, 1489–1495. [Google Scholar] [CrossRef]
- Muñoz-Gómez, S.A.; Slamovits, C.H.; Dacks, J.B.; Wideman, J.G. The evolution of MICOS: Ancestral and derived functions and interactions. Commun. Integr. Biol. 2015, 8, e1094593. [Google Scholar] [CrossRef]
- Von der Malsburg, K.; Müller, J.M.; Bohnert, M.; Oeljeklaus, S.; Kwiatkowska, P.; Becker, T.; van der Laan, M. Dual Role of Mitofilin in Mitochondrial Membrane Organization and Protein Biogenesis. Dev. Cell 2011, 21, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Alkhaja, A.K.; Jans, D.C.; Nikolov, M.; Vukotic, M.; Lytovchenko, O.; Ludewig, F.; Schliebs, W.; Riedel, D.; Urlaub, H.; Jakobs, S.; et al. MINOS1 is a conserved component of mitofilin complexes and required for mitochondrial function and cristae organization. Mol. Biol. Cell 2012, 23, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Gros, V.; Tardif, M.; Brugière, S.; Ferro, M.; Prinz, W.A.; Toulmay, A.; Mathur, J.; Wozny, M.; Falconet, D.; et al. AtMic60 Is involved in plant mitochondria lipid trafficking and is part of a large complex. Curr. Biol. 2016, 26, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lavell, A.; Meng, X.; Berkowitz, O.; Selinski, J.; van de Meene, A.; Carrie, C.; Benning, C.; Whelan, J.; De Clercq, I.; et al. Arabidopsis DGD1 SUPPRESSOR 1 is a subunit of the mitochondrial contact site and cristae organizing system and affects mitochondrial biogenesis. Plant Cell 2019, 31, 1856–1878. [Google Scholar] [CrossRef] [PubMed]
- Dudkina, N.V.; Sunderhaus, S.; Braun, H.P.; Boekema, E.J. Characterization of dimeric ATP synthase and cristae membrane ultrastructure from Saccharomyces and Polytomella mitochondria. FEBS Lett. 2016, 580, 3427–3432. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Bron, P.; Weimann, T.; Dautant, A.; Giraud, M.F.; Paumard, P.; Salin, B.; Cavalier, A.; Velours, J.; Brèthes, D. Supramolecular organization of the yeast F1Fo-ATP synthase. Biol. Cell 2008, 100, 591–603. [Google Scholar] [CrossRef]
- Fuchs, P.; Rugen, N.; Carrie, C.; Elsässer, M.; Finkemeier, I.; Giese, J.; Hildebrandt, T.M.; Kuhn, K.; Maurino, V.G.; Ruberti, C.; et al. Single organelle function and organization as estimated from Arabidopsis mitochondrial proteomics. Plant J. 2019, 101, 420–441. [Google Scholar] [CrossRef]
- Paumard, P.; Vaillier, J.; Coulary, B.; Schaeffer, J.; Soubannier, V.; Mueller, D.M.; Brethes, D.; di Rago, J.P.; Velours, J. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002, 21, 221–230. [Google Scholar] [CrossRef]
- Strauss, M.; Hofhaus, G.; Schröder, R.R.; Kühlbrandt, W. Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 2008, 27, 1154–1160. [Google Scholar] [CrossRef]
- Gibala, M.; Kicia, M.; Sakamoto, W.; Gola, E.M.; Kubrakiewicz, J.; Smakowska, E.; Janska, H. The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. Plant J. 2009, 59, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in Apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef]
- Ong, S.B.; Hausenloy, D.J. Mitochondrial morphology and cardiovascular disease. Cardiovasc. Res. 2010, 88, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, L.; Wu, S.; Xing, D. Drp1, Mff, Fis1, and MiD51 are coordinated to mediate mitochondrial fission during UV irradiation–induced apoptosis. FASEB J. 2016, 30, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chan, D.C. The mitochondrial fission receptor Mff selectively recruits oligomerized Drp1. Mol. Biol. Cell 2015, 26, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, M.; Arimura, S.; Mano, S.; Kondo, M.; Saito, C.; Ueda, T.; Nakazono, M.; Nakano, A.; Nishimura, M.; Tsutsumi, N. Arabidopsis dynamin-related proteins DRP3A and DRP3B are functionally redundant in mitochondrial fission, but have distinct roles in peroxisomal fission. Plant J. 2009, 58, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.; Tobin, A.K.; Logan, D.C. BIGYIN, an orthologue of human and yeast FIS1 genes functions in the control of mitochondrial size and number in Arabidopsis thaliana. J. Exp. Bot. 2006, 57, 1275–1280. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, J. Two small protein families, DYNAMIN-RELATED PROTEIN3 and FISSION1, are required for peroxisome fission in Arabidopsis. Plant J. 2009, 57, 146–159. [Google Scholar] [CrossRef]
- Arimura, S.; Fujimoto, M.; Doniwa, Y.; Kadoya, N.; Nakazono, M.; Sakamoto, W.; Tsutsumi, N. Arabidopsis ELONGATED MITOCHONDRIA1 is required for localization of DYNAMIN-RELATED PROTEIN3A to mitochondrial fission sites. Plant Cell 2008, 20, 1555–1566. [Google Scholar] [CrossRef]
- Aung, K.; Hu, J. The Arabidopsis tail-anchored protein peroxisomal and mitochondrial division factor1 is involved in the morphogenesis and proliferation of peroxisomes and mitochondria. Plant Cell 2011, 23, 4446–4461. [Google Scholar] [CrossRef]
- Brandt, T.; Cavellini, L.; Kühlbrandt, W.; Cohen, M.M. A mitofusin-dependent docking ring complex triggers mitochondrial fusion in vitro. eLife 2016, 5, e14618. [Google Scholar] [CrossRef] [PubMed]
- Legros, F.; Lombès, A.; Frachon, P.; Rojo, M. Mitochondrial fusion in human cells is efficient, requires the inner membrane potential, and is mediated by mitofusins. Mol. Biol. Cell 2002, 13, 4343–4354. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Detmer, S.A.; Ewald, A.J.; Griffin, E.E.; Fraser, S.E.; Chan, D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003, 160, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chomyn, A.; Chan, D.C. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J. Biol. Chem. 2005, 280, 26185–26192. [Google Scholar] [CrossRef]
- Sinha, S.; Aradhyam, G.K. Identification and characterization of signal peptide of Mitofusin1 (Mfn1). Biochem. Biophys. Res. Commun. 2019, 509, 707–712. [Google Scholar] [CrossRef]
- Detmer, S.A.; Chan, D.C. Complementation between mouse Mfn1 and Mfn2 protects mitochondrial fusion defects caused by CMT2A disease mutations. J. Cell Biol. 2007, 176, 405–414. [Google Scholar] [CrossRef]
- Wong, E.D.; Wagner, J.A.; Scott, S.V.; Okreglak, V.; Holewinske, T.J.; Cassidy-Stone, A.; Nunnari, J. The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion. J. Cell Biol. 2003, 160, 303–311. [Google Scholar] [CrossRef]
- Ishihara, N.; Fujita, Y.; Oka, T.; Mihara, K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006, 25, 2966–2977. [Google Scholar] [CrossRef]
- Griparic, L.; Kanazawa, T.; van der Bliek, A.M. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J. Cell Biol. 2007, 178, 757–764. [Google Scholar] [CrossRef]
- Steiner, P.; Buchner, O.; Andosch, A.; Wanner, G.; Neuner, G.; Lütz-Meindl, U. Fusion of mitochondria to 3-D networks, autophagy and increased organelle contacts are important subcellular hallmarks during cold stress in plants. Int. J. Mol. Sci. 2020, 21, 8753. [Google Scholar] [CrossRef]
- Arimura, S.I.; Yamamoto, J.; Aida, G.P.; Nakazono, M.; Tsutsumi, N. Frequent fusion and fission of plant mitochondria with unequal nucleoid distribution. Proc. Natl. Acad. Sci. USA 2004, 101, 7805–7808. [Google Scholar] [CrossRef] [PubMed]
- Ohba, Y.; Sakuragi, T.; Kage-Nakadai, E.; Tomioka, N.H.; Kono, N.; Imae, R.; Inoue, A.; Aoki, J.; Ishihara, N.; Inoue, T.; et al. Mitochondria-type GPAT is required for mitochondrial fusion. EMBO J. 2013, 32, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Daum, G.; Vance, J.E. Import of lipids into mitochondria. Prog. Lipid Res. 1997, 36, 103–130. [Google Scholar] [CrossRef]
- Ardail, D.; Privat, J.P.; Egret-Charlier, M.; Levrat, C.; Lerme, F.; Louisot, P. Mitochondrial contact sites—Lipid composition and dynamics. J. Biol. Chem. 1990, 265, 18797–18802. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Tamura, Y.; Roy, M.; Adachi, Y.; Iijima, M.; Sesaki, H. Biosynthesis and roles of phospholipids in mitochondrial fusion, division and mitophagy. Cell. Mol. Life Sci. 2014, 71, 3767–3778. [Google Scholar] [CrossRef]
- Luévano-Martínez, L.A.; Pinto, I.F.D.; Yoshinaga, M.Y.; Miyamoto, S. In yeast, cardiolipin unsaturation level plays a key role in mitochondrial function and inner membrane integrity. Biochim. Biophys. Acta (BBA)-Bioenerg. 2022, 1863, 148587. [Google Scholar] [CrossRef]
- Smakowska, E.; Skibior-Blaszczyk, R.; Czarna, M.; Kolodziejczak, M.; Kwasniak-Owczarek, M.; Parys, K.; Funk, C.; Janska, H. Lack of FTSH4 protease affects protein carbonylation, mitochondrial morphology and phospholipid content in mitochondria of Arabidopsis: New insights into a complex interplay. Plant Physiol. 2016, 171, 2516–2535. [Google Scholar] [CrossRef]
- Kojima, R.; Kakimoto, Y.; Furuta, S.; Itoh, K.; Sesaki, H.; Endo, T.; Tamura, Y. Maintenance of Cardiolipin and Crista Structure Requires Cooperative Functions of Mitochondrial Dynamics and Phospholipid Transport. Cell Rep. 2019, 26, 518–528. [Google Scholar] [CrossRef]
- Luévano-Martínez, L.A.; Forni, M.F.; dos Santos, V.T.; Souza-Pinto, N.C.; Kowaltowski, A.J. Cardiolipin is a key determinant for mtDNA stability and segregation during mitochondrial stress. Biochim. Biophys. Acta (BBA)-Bioenerg. 2015, 1847, 587–598. [Google Scholar] [CrossRef]
- Rong, Z.; Tu, P.; Xu, P.; Sun, Y.; Yu, F.; Tu, N.; Guo, L.; Yang, Y. The mitochondrial response to DNA damage. Front. Cell Dev. Biol. 2021, 9, 669379. [Google Scholar] [CrossRef]
- Wei, H.; Liu, L.; Chen, Q. Selective removal of mitochondria via mitophagy: Distinct pathways for different mitochondrial stresses. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2015, 1853, 2784–2790. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Okamoto, K. Regulatory mechanisms of mitophagy in yeast. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129858. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, K.; Kondo-Okamoto, N.; Ohsumi, Y. Mitochondria-Anchored Receptor Atg32 Mediates Degradation of Mitochondria via Selective Autophagy. Dev. Cell 2009, 17, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Li, Z.; Liu, N.; Yan, Y.; Liu, B. Role of Mitophagy in Cardiovascular Disease. Aging Dis. 2020, 11, 419. [Google Scholar] [CrossRef]
- Fukuda, T.; Ebi, Y.; Saigusa, T.; Furukawa, K.; Yamashita, S.I.; Inoue, K.; Kobayashi, D.; Yoshida, Y.; Kanki, T. Atg43 tethers isolation membranes to mitochondria to promote starvation-induced mitophagy in fission yeast. eLife 2020, 9, e61245. [Google Scholar] [CrossRef]
- Nakamura, S.; Hagihara, S.; Izumi, M. Mitophagy in plants. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2021, 1865, 129916. [Google Scholar] [CrossRef]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Ohsumi, Y. Unveiling the molecular mechanisms of plant autophagy—From autophagosomes to vacuoles in plants. Plant Cell Physiol. 2018, 59, 1337–1344. [Google Scholar] [CrossRef]
- Nakamura, S.; Hagihara, S.; Otomo, K.; Ishida, H.; Hidema, J.; Nemoto, T.; Izumi, M. Autophagy contributes to the quality control of leaf mitochondria. Plant Cell Physiol. 2021, 62, 229–247. [Google Scholar] [CrossRef]
- Keech, O.; Pesquet, E.; Ahad, A.; Askne, A.; Nordvall, D.; Vodnala, S.M.; Tuominen, H.; Hurry, V.; Dizengremel, P.; Gardeström, P. The different fates of mitochondria and chloroplasts during dark-induced senescence in Arabidopsis leaves. Plant Cell Environ. 2007, 30, 1523–1534. [Google Scholar] [CrossRef]
- Chen, P.Y.; Wu, C.C.; Lin, C.C.; Jane, W.N.; Suen, D.F. 3D imaging of tapetal mitochondria suggests the importance of mitochondrial fission in pollen growth. Plant Physiol. 2019, 180, 813–826. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, D.; Müller, M.; Reichert, A.S.; Osiewacz, H.D. Simultaneous impairment of mitochondrial fission and fusion reduces mitophagy and shortens replicative lifespan. Sci. Rep. 2015, 5, 7885. [Google Scholar] [CrossRef] [PubMed]
- Michaud, M.; Prinz, W.A.; Jouhet, J. Glycerolipid synthesis and lipid trafficking in plant mitochondria. FEBS J. 2016, 284, 376–390. [Google Scholar] [CrossRef]
- White, R.R.; Lin, C.; Leaves, I.; Castro, I.G.; Metz, J.; Bateman, B.C.; Botchway, S.W.; Ward, A.D.; Ashwin, P.; Sparkes, I. Miro2 tethers the ER to mitochondria to promote mitochondrial fusion in tobacco leaf epidermal cells. Commun. Biol. 2020, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, K.; Köhler, R.H.; Verbelen, J. Plant mitochondria move on F-actin, but their positioning in the cortical cytoplasm depends on both F-actin and microtubules. J. Exp. Bot. 2002, 53, 659–667. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, R.; Yang, M.; Law, Y.; Sun, F.; Hon, N.; Ngai, S.; Lim, B. A balance between the activities of chloroplasts and mitochondria is crucial for optimal plant growth. Antioxidants 2021, 10, 935. [Google Scholar] [CrossRef]
- Aung, K.; Zhang, X.; Hu, J. Peroxisome division and proliferation in plants. Biochem. Soc. Trans. 2010, 38, 817–822. [Google Scholar] [CrossRef]
- Fransen, M.; Lismont, C.; Walton, P. The peroxisome-mitochondria connection: How and why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Oikawa, K.; Hayashi, M.; Hayashi, Y.; Nishimura, M. Re-evaluation of physical interaction between plant peroxisomes and other organelles using live-cell imaging techniques. J. Integr. Plant Biol. 2019, 61, 836–852. [Google Scholar] [CrossRef]
- Oikawa, K.; Matsunaga, S.; Mano, S.; Kondo, M.; Yamada, K.; Hayashi, M.; Kagawa, T.; Kadota, A.; Sakamoto, W.; Higashi, S.; et al. Physical interaction between peroxisomes and chloroplasts elucidated by in situ laser analysis. Nat. Plants 2015, 1, 15035. [Google Scholar] [CrossRef]
- United Nations Office of Disaster Risk Reduction. The Human Cost of Disasters: An Overview of the Last 20 Years (2000–2019); United Nations Office of Disaster Risk Reduction: Geneva, Switzerland, 2022. [Google Scholar]
- Rurek, M. Plant mitochondria under a variety of temperature stress conditions. Mitochondrion 2014, 19, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.P.; de Lima e Borges, E.E.; Brito, D.S.; Bernardes, R.C.; dos Santos Araújo, R. Heat stress-mediated effects on the morphophysiological, biochemical, and ultrastructural parameters of germinating Melanoxylon brauna Schott. seeds. Plant Cell Rep. 2021, 40, 1773–1787. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Tian, Q.; Yin, G.; Chen, X.; Zhang, J.; Ng, S.; Lu, X. Reduced mitochondrial and ascorbate–glutathione activity after artificial ageing in soybean seed. J. Plant Physiol. 2014, 171, 140–147. [Google Scholar] [CrossRef]
- Chen, R.; Liu, W.; Zhang, B.; Yang, L.; Li, Y. Effects of low-temperature stress on mitochondrial physiological and biochemical function in root of sugarcane seedlings. J. South. Agric. 2015, 46, 1385–1390. [Google Scholar]
- Fediuk, O.; Bilyavska, N.O.; Zolotareva, E.K. Effects of soil early-spring temperature on the morphometric parameters of mitochondria in Galanthus nivalis L. leaves. Plant Sci. Today 2018, 5, 149–154. [Google Scholar] [CrossRef]
- Arimura, S.; Kurisu, R.; Sugaya, H.; Kadoya, N.; Tsutsumi, N. Cold treatment induces transient mitochondrial fragmentation in Arabidopsis thaliana in a way that requires DRP3A but not ELM1 or an ELM1-Like homologue, ELM2. Int. J. Mol. Sci. 2017, 18, 2161. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.S.; Ali, M.; Ahmad, M.; Siddique, K.H. Polyamines: Natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 2011, 29, 300–311. [Google Scholar] [CrossRef]
- Yang, X.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine enhances the photosynthesis and ultrastructure of lettuce seedlings under high-temperature stress. Sci. Hortic. 2022, 291, 110570. [Google Scholar] [CrossRef]
- Li, X.; Gong, B.; Xu, K. Effect of exogenous spermidine on levels of endogenous hormones and chloroplast ultrastructure of ginger leaves under heat stress. Sci. Agric. Sin. 2015, 48, 120–129. [Google Scholar]
- Tian, J. Study on Physiological Regulation Mechanism and Proteomics of Exogenous Spermidine in Alleviating Injury of Cucumber Seedlings under High Temperature Stress. Ph.D. Thesis, Nanjing Agricultural University, Nanjing, China, 2012. [Google Scholar]
- Xia, F.; Wang, X.; Li, M.; Mao, P. Mitochondrial structural and antioxidant system responses to aging in oat (Avena sativa L.) seeds with different moisture contents. Plant Physiol. Biochem. 2015, 94, 122–129. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; Von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Laziti, K.; Zhang, M. Effect of soil drought stress on the ultramicrostructures of chloroplasts and mitochondria in three desert plants with different photosynthetic types. Acta Bot. Boreali-Occident. Sin. 2016, 36, 1155–1162. [Google Scholar]
- Zellnig, G.; Zechmann, B.; Perktold, A. Morphological and quantitative data of plastids and mitochondria within drought-stressed spinach leaves. Protoplasma 2014, 223, 221–227. [Google Scholar] [CrossRef]
- Grigorova, B.; Vassileva, V.; Klimchuk, D.; Vaseva, I.; Demirevska, K.; Feller, U. Drought, high temperature, and their combination affect ultrastructure of chloroplasts and mitochondria in wheat (Triticum aestivum L.) leaves. J. Plant Interact. 2012, 7, 204–213. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Meng, J.; Xi, Z. Effect of exogenous 24-epibrassinolide on chlorophyll fluorescence, leaf surface morphology and cellular ultrastructure of grape seedlings (Vitis vinifera L.) under water stress. Acta Physiol. Plant. 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Silva, E.N.; Ferreira-Silva, S.L.; Fontenele, A.d.V.; Ribeiro, R.V.; Viégas, R.A.; Silveira, J.A.G. Photosynthetic changes and protective mechanisms against oxidative damage subjected to isolated and combined drought and heat stresses in Jatropha curcas plants. J. Plant Physiol. 2010, 167, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Keck, R.W.; Boyer, J.S. Chloroplast response to low leaf water potentials: III. Differing inhibition of electron transport and photophosphorylation. Plant Physiol. 1974, 53, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef]
- Wan, L.; Shi, Y.; Li, X.; He, F.; Jia, Y. Alterations in leaf cellular ultra-structure of three varieties of Lolium perenne subjected to high temperature and soil drought stress. Acta Prataculturae Sin. 2009, 18, 25. [Google Scholar]
- Vassileva, V.; Simova-Stoilova, L.; Demirevska, K.; Feller, U. Variety-specific response of wheat (Triticum aestivum L.) leaf mitochondria to drought stress. J. Plant Res. 2009, 122, 445–454. [Google Scholar] [CrossRef]
- Liu, D.X.; Liu, H.L.; Du, H.Y.; Liu, H.P.; Kurtenbach, R. Relationship between polyamines conjugated to mitochondrion membrane and mitochondrion conformation from developing wheat embryos under drought stress. J. Biosci. 2021, 46, 1–11. [Google Scholar] [CrossRef]
- Aslami, H.; Pulskens, W.P.; Kuipers, M.T.; Bos, A.P.; Kuilenburg, A.B.P.; Wanders, R.J.A.; Roelofs, J.J.T.H.; Kerindongo, R.P.; Beurskens, C.J.P.; Schultz, M.J.; et al. Hydrogen sulfide donor NaHS reduces organ injury in a rat model of pneumococcal pneumosepsis, associated with improved bio-energetic status. PLoS ONE 2013, 8, e63497. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, W.H.; Wu, L.Y.; Yang, G.D.; Li, H.Z.; Wang, R. Hydrogen sulfide (H2S) metabolism in mitochondria and its regulatory role in energy production. Proc. Natl. Acad. Sci. USA 2012, 109, 2943–2948. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.D.; Zhao, K.X.; Ju, Y.J.; Mani, S.; Cao, Q.H.; Puukila, S.; Khaper, N.; Wu, L.Y.; Wang, R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013, 18, 1906–1919. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Sun, L.; Yang, G.; Pei, Y. Hydrogen sulfide regulates energy production to delay leaf senescence induced by drought stress in Arabidopsis. Front. Plant Sci. 2018, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.D.; Snyder, S.H.; Kashfi, K. Effects of hydrogen sulfide on mitochondrial function and cellular bioenergetics. Redox Biol. 2021, 38, 101772. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate Peroxidase and Catalase Activities and Their Genetic Regulation in Plants Subjected to Drought and Salinity Stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef]
- Pei, L.; Peng, L.; Wan, X.; Xiong, J.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. Expression Pattern and Function Analysis of AtPPRT1, a Novel Negative Regulator in ABA and Drought Stress Responses in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 394. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.; Ma, X.; Zhang, W. NRGA1, a Putative Mitochondrial Pyruvate Carrier, Mediates ABA Regulation of Guard Cell Ion Channels and Drought Stress Responses in Arabidopsis. Mol. Plant 2014, 7, 1508–1521. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant mitochondria synthesize melatonin and enhance the tolerance of plants to drought stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Zhigacheva, I.; Burlakova, E.; Generozova, I.; Shugaev, A.; Fattakhov, S.; Konovalov, A. Plant growth regulators melafen and pirafen prevent dysfunction of mitochondria caused by temporary water deficit. Dokl. Biochem. Biophys. 2011, 441, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wu, Y.; Chonglan, Z.; Xing, X.; Liu, L.; Jiang, H.; Xing, H. Triadimefon Induced C and N Metabolism and Root Ultra-Structural Changes for Drought Stress Protection in Soybean at Flowering Stage. J. Plant Growth Regul. 2015, 35, 222–231. [Google Scholar] [CrossRef]
- Sun, X.; Han, G.; Meng, Z.; Lin, L.; Sui, N. Roles of malic enzymes in plant development and stress responses. Plant Signal. Behav. 2019, 14, e1644596. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Vanlerberghe, G. A lack of mitochondrial alternative oxidase compromises capacity to recover from severe drought stress. Physiol. Plant. 2013, 149, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; Yang, M.; Wang, Y.; Chen, C.; Sui, N.; Meng, Q.; Zhuang, K.; Wei, L. SlWHY2 interacts with SlRECA2 to maintain mitochondrial function under drought stress in tomato. Plant Sci. 2020, 301, 110674. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Stoppelli, M.C.; Di Fonzo, N.; Passarella, S. The existence of the K+ channel in plant mitochondria. J. Biol. Chem. 1999, 274, 26683–26690. [Google Scholar] [CrossRef]
- Szewczyk, A.; Jarmuszkiewicz, W.; Kunz, W.S. Mitochondrial potassium channels. IUBMB Life 2009, 61, 134–143. [Google Scholar] [CrossRef]
- Ruy, F.; Vercesi, A.E.; Andrade, P.B.M.; Bianconi, M.L.; Chaimovich, H.; Kowaltowski, A.J. A highly active ATP-insensitive K+ import pathway in plant mitochondria. J. Bioenerg. Biomembr. 2004, 36, 195–202. [Google Scholar] [CrossRef]
- Laskowski, M.; Augustynek, B.; Kulawiak, B.; Koprowski, P.; Bednarczyk, P.; Jarmuszkiewicz, W.; Szewczyk, A. What do we not know about mitochondrial potassium channels? Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 1247–1257. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Taylor, N.L.; Millar, A.H. The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 2011, 16, 614–623. [Google Scholar] [CrossRef]
- Zhu, Q.; Dugardeyn, J.; Zhang, C.Y.; Muhlenbock, P.; Eastmond, P.J.; Valcke, R.; De Coninck, B.; Oden, S.; Karampelias, M.; Cammue, B.P.A. The Arabidopsis thaliana RNA editing factor SLO2, which affects the mitochondrial electron transport chain, participates in multiple stress and hormone responses. Mol. Plant 2014, 7, 290–310. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, D.M.; Umbach, A.L.; Subbaiah, C.C.; Siedow, J.N. Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 2006, 141, 357–366. [Google Scholar] [CrossRef]
- Virolainen, E.; Blokhina, O.; Fagerstedt, K. Ca2+-induced high amplitude swelling and cytochrome c release from wheat (Triticum aestivum L.) mitochondria under anoxic stress. Ann. Bot. 2002, 90, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Navrot, N.; Rouhier, N.; Gelhaye, E.; Jacquot, J.P. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 2007, 129, 185–195. [Google Scholar] [CrossRef]
- Greenbaum, N.L.; Wilson, D.F. Role of intramitochondrial pH in the energetics and regulation of mitochondrial oxidative phosphorylation. Biochim. Biophys. Acta (BBA)-Bioenerg. 1991, 1058, 113–120. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, W.; Cheng, H.; Wang, H.; Lv, D.; Wang, W.; Liang, M.; Miao, C. NADPH Oxidase-derived ROS promote mitochondrial alkalization under salt stress in Arabidopsis root cells. Plant Signal. Behav. 2020, 16, 1856546. [Google Scholar] [CrossRef]
- Steiner, P.; Luckner, M.; Kerschbaum, H.; Wanner, G.; Lütz-Meindl, U. Ionic stress induces fusion of mitochondria to 3-D networks: An electron tomography study. J. Struct. Biol. 2018, 204, 52–63. [Google Scholar] [CrossRef]
- Zhao, Y.; Pan, Z.; Zhang, Y.; Qu, X.; Zhang, Y.; Yang, Y.; Jiang, X.; Huang, S.; Yuan, M.; Schumaker, K.S.; et al. The actin-related Protein2/3 complex regulates mitochondrial-associated calcium signaling during salt stress in Arabidopsis. Plant Cell 2013, 25, 4544–4559. [Google Scholar] [CrossRef]
- Yu, C.; Wang, L.; Xu, S.; Zeng, Y.; He, C.; Chen, C.; Huang, w.; Zhu, Y.; Hu, J. Mitochondrial ORFH79 is essential for drought and salt tolerance in rice. Plant Cell Physiol. 2015, 56, 2248–2258. [Google Scholar] [CrossRef][Green Version]
- Linder, T.; Park, C.B.; Asin-Cayuela, J.; Pellegrini, M.; Larsson, N.G.; Falkenberg, M.; Samuelsson, T.; Gustafsson, C.M. A family of putative transcription termination factors shared amongst metazoans and plants. Curr. Genet. 2005, 48, 265–269. [Google Scholar] [CrossRef]
- Jiang, D.; Chen, J.; Zhang, Z.; Hou, X. Mitochondrial transcription termination factor 27 is required for salt tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2021, 22, 1466. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, K.; Verbelen, J.-P. Giant mitochondria are a response to low oxygen pressure in cells of tobacco (Nicotiana tabacum L.). J. Exp. Bot. 2002, 53, 1215–1218. [Google Scholar] [CrossRef] [PubMed]
- Ramonell, K.M.; Kuang, A.; Porterfield, D.M.; Crispi, M.L.; Xiao, Y.; Mcclure, G.; Musgrave, M.E. Influence of atmospheric oxygen on leaf structure and starch deposition in Arabidopsis thaliana. Plant Cell Environ. 2001, 24, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Vollenweider, P.; Günthardt-Goerg, M.S.; Menard, T.; Baumgarten, M.; Matyssek, R.; Schaub, M. Macro- and microscopic leaf injury triggered by ozone stress in beech foliage (Fagus sylvatica L.). Ann. For. Sci. 2019, 76, 71. [Google Scholar] [CrossRef]
- Kivimaenpaa, M. Cell structural changes in the needles of norway spruce exposed to long-term ozone and drought. Ann. Bot. 2003, 92, 779–793. [Google Scholar] [CrossRef]
- Holzinger, A.; Lütz, C.; Karsten, U.; Wiencke, C. The effect of ultraviolet radiation on ultrastructure and photosynthesis in the red macroalgae Palmaria palmata and Odonthalia dentata from arctic waters. Plant Biol. 2004, 6, 568–577. [Google Scholar] [CrossRef]
- Gao, C.; Xing, D.; Li, L.; Zhang, L. Implication of reactive oxygen species and mitochondrial dysfunction in the early stages of plant programmed cell death induced by ultraviolet-C overexposure. Planta 2007, 227, 755–767. [Google Scholar] [CrossRef]
- Gabara, B.; Skłodowska, M.; Wyrwicka, A.; Glińska, S.; Gapińska, M. Changes in the ultrastructure of chloroplasts and mitochondria and antioxidant enzyme activity in Lycopersicon esculentum Mill. leaves sprayed with acid rain. Plant Sci. 2003, 164, 507–516. [Google Scholar] [CrossRef]
- Fayez, K.A.; El-Deeb, B.A.; Mostafa, N.Y. Toxicity of biosynthetic silver nanoparticles on the growth, cell ultrastructure and physiological activities of barley plant. Acta Physiol. Plant. 2017, 39, 155. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, D. Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol. 2018, 49, 1092–1111. [Google Scholar] [CrossRef]
| Type of Stress | Species | Parts | Morphology of Mitochondria | Reference | |
|---|---|---|---|---|---|
| Size | Amount | ||||
| Low oxygen pressure | Nicotiana tabacum L. | Mesophyll cells | Giant mitochondria; eventually became an extensive mitochondrial reticulum, including large plates | The number of mitochondria decreased | [154] |
| Arabidopsis thaliana L. | Leaf cells | Large and elongated | -- | [155] | |
| Ozone stress | Fagus sylvatica L. | Beech foliage cells | Degeneration of cristae and matrix in mitochondria | -- | [156] |
| Picea abies L. | Mesophyll cells | The size of mitochondria decreased | Numerous | [157] | |
| UV stress | Palmaria palmata L. | Algal cells | Cristae were visible and even appeared swollen | -- | [158] |
| Arabidopsis thaliana L. | Leaf cells | The mitochondria clustered irregularly surrounding the chloroplasts or elsewhere within the cytoplasm | -- | [159] | |
| Arabidopsis thaliana L. | Leaf cells (agt mutant) | Small and fragmented | Numerous | [89] | |
| Arabidopsis thaliana L. | Leaf cells (wild type) | -- | The number of mitochondria decreases | [89] | |
| Acid rain | Lycopersicon esculentum M. | Leaf cells | Swollen, vacuolated, and cristae collapsed | -- | [160] |
| Silver nanoparticles (AgNPs) | Hordeum vulgare L. | Leaf cells | The mitochondrial cristae were partially or totally degenerated | -- | [161] |
| Methyl jasmonate, MeJa | Arabidopsis thaliana L. | Leaf cells | Swollen and spherical | -- | [162] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Zhu, H. Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress. Horticulturae 2023, 9, 11. https://doi.org/10.3390/horticulturae9010011
Tang H, Zhu H. Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress. Horticulturae. 2023; 9(1):11. https://doi.org/10.3390/horticulturae9010011
Chicago/Turabian StyleTang, Hui, and Hongliang Zhu. 2023. "Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress" Horticulturae 9, no. 1: 11. https://doi.org/10.3390/horticulturae9010011
APA StyleTang, H., & Zhu, H. (2023). Specific Changes in Morphology and Dynamics of Plant Mitochondria under Abiotic Stress. Horticulturae, 9(1), 11. https://doi.org/10.3390/horticulturae9010011






