Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Environmental Conditions
2.2. Treatments
2.3. Environmental Conditions
2.4. Vegetative Growth
2.5. Physiological Measurements
2.6. Physicochemical Characteristics of the Fruit
2.6.1. Fruit Quality
2.6.2. Pigment Content
2.6.3. Phenolic Extraction
2.6.4. Determination of the Total Phenolic Content
2.6.5. Determination of Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Environmental Conditions
3.2. Vegetative Growth
3.3. Plant Physiological Variables
3.4. Leaf Pigments
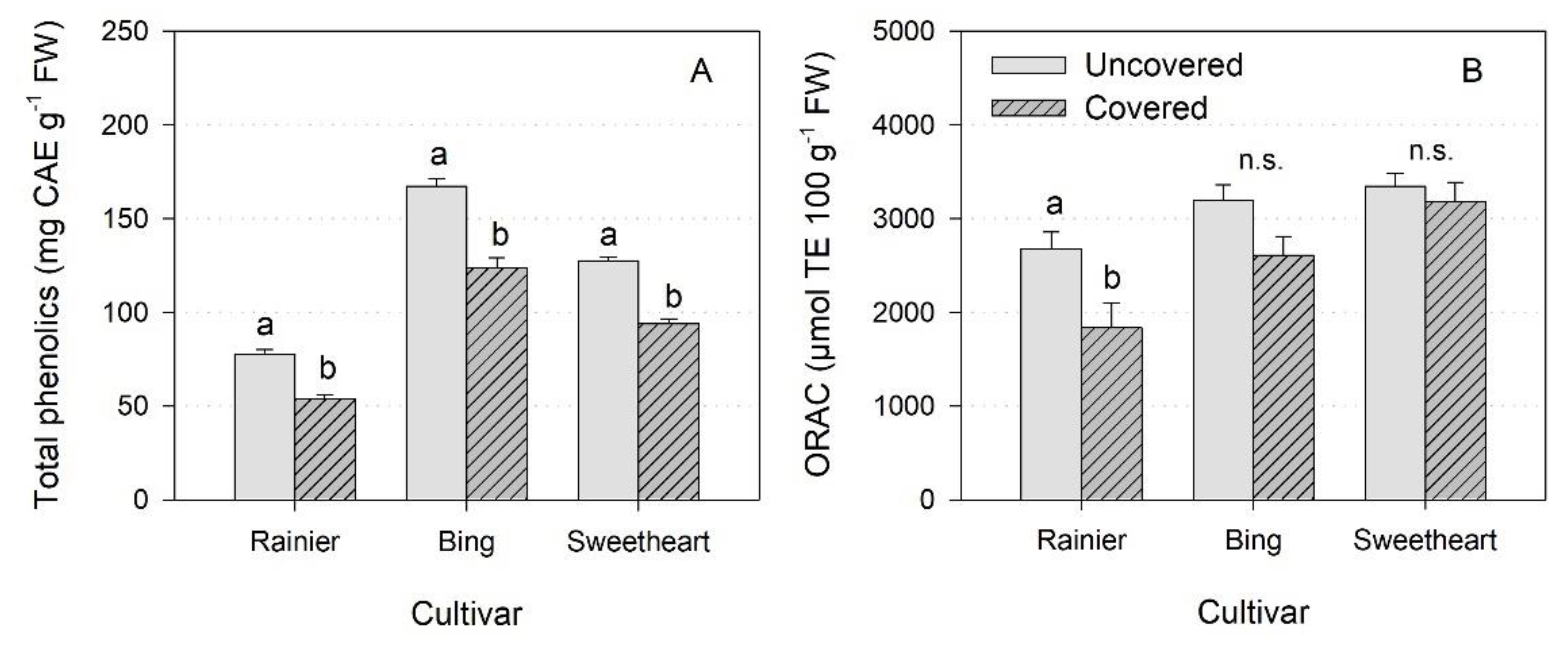
3.5. Physicochemical Characteristics of the Fruit
4. Discussion
4.1. Environmental Conditions
4.2. Vegetative Growth
4.3. Plant Physiological Variables
4.4. Leaf Pigments
4.5. Physicochemical Characteristics of the Fruit
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ODEPA Catastros Frutícolas. Available online: https://reportes.odepa.gob.cl/#/catastro-superficie-fruticola-regional (accessed on 19 December 2022).
- Lang, G.A.; Sage, L.; Wilkinson, T. Ten Years of Studies on Systems to Modify Sweet Cherry Production Environments: Retractable Roofs, High Tunnels, and Rain-Shelters. Acta Hortic. 2016, 1130, 83–89. [Google Scholar] [CrossRef]
- Balbontín, C.; Ayala, H.; Bastías, R.M.; Tapia, G.; Ellena, M.; Torres, C.; Yuri, J.A.; Quero-garcía, J.; Ríos, J.C.; Silva, H. Cracking in Sweet Cherries: A Comprehensive Review from a Physiological, Molecular, and Genomic Perspective. Chil. J. Agric. Res. 2013, 73, 66–72. [Google Scholar] [CrossRef]
- Ellena, M. Partidura y Proteccion de La Fruta en Cerezo Dulce. In Formación y Sistemas de Conducción del Cerezo Dulce; Ellena, M., Ed.; INIA: Santiago, Chile, 2012; pp. 185–194. [Google Scholar]
- Blanke, M.M.; Lang, G.A.; Meland, M. Orchard Microclimate Modification. In Cherries: Botany, Production and Uses; Quero-García, J., Iezzoni, A., Putawska, J., Lang, G., Eds.; CABI Publishing: Boston, MA, USA, 2017; pp. 244–268. [Google Scholar]
- Rom, C.R. Light Thresholds for Apple Tree Canopy Growth and Development. HortScience 1991, 26, 989–992. [Google Scholar] [CrossRef]
- Wallberg, B.N.; Sagredo, K.X. Vegetative and Reproductive Development of “Lapins” Sweet Cherry Trees under Rain Protective Covering. Acta Hortic. 2014, 1058, 411–418. [Google Scholar] [CrossRef]
- Usenik, V.; Zadravec, P.; Štampar, F. Influence of Rain Protective Tree Covering on Sweet Cherry Fruit Quality. Eur. J. Hortic. Sci. 2009, 74, 49–53. [Google Scholar]
- Mika, A.; Buler, Z.; Wójcik, K.; Konopacka, D. Influence of the Plastic Cover on the Protection of Sweet Cherry Fruit against Cracking, on the Microclimate under Cover and Fruit Quality. J. Hortic. Res. 2020, 27, 31–38. [Google Scholar] [CrossRef]
- Vosnjak, M.; Mrzlic, D.; Usenik, V. Summer Pruning of Sweet Cherry: A Way to Control Sugar Content in Different Organs. J. Sci. Food Agric. 2022, 102, 1216–1224. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. High Tunnel Cultivation of Sweet Cherry (Prunus avium L.): Physiological and Production Variables. Sci. Hortic. 2019, 251, 108–117. [Google Scholar] [CrossRef]
- Blanco, V.; Zoffoli, J.P.; Ayala, M. Eco-Physiological Response, Water Productivity and Fruit Quality of Sweet Cherry Trees under High Tunnels. Sci. Hortic. 2021, 286, 110180. [Google Scholar] [CrossRef]
- Serrano, M.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Valero, D. Chemical Constituents and Antioxidant Activity of Sweet Cherry at Different Ripening Stages. J. Agric. Food Chem. 2005, 53, 2741–2745. [Google Scholar] [CrossRef]
- Pacifico, S.; Di Maro, A.; Petriccione, M.; Galasso, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Scortichini, M.; Monaco, P. Chemical Composition, Nutritional Value and Antioxidant Properties of Autochthonous Prunus avium Cultivars from Campania Region. Food Res. Int. 2014, 64, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Acero, N.; Gradillas, A.; Beltran, M.; García, A.; Muñoz Mingarro, D. Comparison of Phenolic Compounds Profile and Antioxidant Properties of Different Sweet Cherry (Prunus avium L.) Varieties. Food Chem. 2019, 279, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Stanley, C.J.; Tustin, D.S.; Lupton, G.B.; Mcartney, S.; Cashmore, W.M.; De Silva, H.N. Towards Understanding the Role of Temperature in Apple Fruit Growth Responses in Three Geographical Regions within New Zealand. J. Hortic. Sci. Biotechnol. 2000, 75, 413–422. [Google Scholar] [CrossRef]
- Anderson, J.; Richardson, E.; Kesner, C. Validation of Chill Unit and Flower Bud Phenology Models for “Montmorency” Sour Cherry. Acta Hortic. 1986, 184, 71–78. [Google Scholar] [CrossRef]
- Torres, C.A.; Sepúlveda, A.; Leon, L.; Yuri, J.A. Early Detection of Sun Injury on Apples (Malus domestica Borkh.) through the Use of Crop Water Stress Index and Chlorophyll Fluorescence. Sci. Hortic. 2016, 211, 336–342. [Google Scholar] [CrossRef]
- Fuleki, T.; Francis, F.J. Quantitative Methods for Anthocyanins. 3. Purification of Cranberry Anthocyanins. J. Food Sci. 1968, 33, 266–274. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Coseteng, M.Y.; Lee, C.Y. Changes in Apple Polyphenoloxidase and Oolyphenol Concentrations in Relation to Degree of Browning. J. Food Sci. 1987, 52, 985–989. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and Validation of Oxygen Radical Absorbance Capacity Assay for Lipophilic Antioxidants Using Randomly Methylated β-Cyclodextrin as the Solubility Enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for Hydrophilic and Lipophilic Antioxidant Capacity (Oxygen Radical Absorbance Capacity (ORACFL)) of Plasma and Other Biological and Food Samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Børve, J.; Skaar, E.; Sekse, L.; Meland, M.; Vangdal, E. Rain Protective Covering of Sweet Cherry Trees-Efeects of Different Covering Methods on Fruit Quality and Microclimate. HortTechnnology 2003, 13, 143–148. [Google Scholar] [CrossRef]
- Blanke, M.M.; Balmer, M. Cultivation of Sweet Cherry under Rain Covers. Acta Hortic. 2008, 795, 479–484. [Google Scholar] [CrossRef]
- Lang, G.; Valentino, T.; Demirsoy, H.; Demirsoy, L. High Tunnel Sweet Cherry Studies: Innovative Integration of Precision Canopies, Precocious Rootstocks, and Environmental Physiology. Acta Hortic. 2011, 903, 717–723. [Google Scholar] [CrossRef]
- Overbeck, V.; Schmitz, M.; Tartachnyk, I.; Blanke, M. Identification of Light Availability in Different Sweet Cherry Orchards under Cover by Using Non-Destructive Measurements with a DualexTM. Eur. J. Agron. 2018, 93, 50–56. [Google Scholar] [CrossRef]
- Schäfer, S. Überdachungssysteme im Obstbau–Auswirkungen auf Mikro-klima, Baumwachstum, Fruchtqualität Sowie Den Krankheits- und Schädlingsbefall von Süßkirschen. Ph.D. Thesis, Horticultural and Agricultural Faculty, Humboldt University, Berlin, Germany, 2005. [Google Scholar]
- Mark, U.; Tevini, M. Combination Effects of UV-B Radiation and Temperature on Sunflower (Helianthus annuus L., cv. Polstar) and Maize (Zea mays L., cv. Zenit 2000) Seedlings. J. Plant Physiol. 1996, 148, 49–56. [Google Scholar] [CrossRef]
- Sotiropoulos, T.; Petridis, A.; Koundouras, S.; Therios, I.; Koutinas, N.; Kazantzis, K.; Pappa, M. Efficacy of Using Rain Protective Plastic Films against Cracking of Four Sweet Cherry (Prunus avium L.) Cultivars in Greece. Int. J. Agric. Innov. Res. 2014, 2, 1035–1040. [Google Scholar]
- Zhang, H.; Hou, Q.; Tu, K.; Qiao, G.; Li, Q.; Wen, X. The Effects of Rain-Shelter Cultivation on the Photosynthetic Characteristics and Chlorophyll Fluorescence of Sweet Cherry (Prunus avium L.). Erwerbs-Obstbau 2021, 63, 359–368. [Google Scholar] [CrossRef]
- Tartachnyk, I.I.; Blanke, M.M. Effect of Delayed Fruit Harvest on Photosynthesis, Transpiration and Nutrient Remobilization of Apple Leaves. New Phytol. 2004, 164, 441–450. [Google Scholar] [CrossRef]
- Gonçalves, B.; Santos, A.; Silva, A.P.; Moutinho-Pereira, J.; Torres-Pereira, J.M.G. Effect of Pruning and Plant Spacing on the Growth of Cherry Rootstocks and Their Influence on Stem Water Potential of Sweet Cherry Trees. J. Hortic. Sci. Biotechnol. 2003, 78, 667–672. [Google Scholar] [CrossRef]
- Carrasco-Benavides, M.; Antunez-Quilobrán, J.; Baffico-Hernández, A.; Ávila-Sánchez, C.; Ortega-Farías, S.; Espinoza, S.; Gajardo, J.; Mora, M.; Fuentes, S. Performance Assessment of Thermal Infrared Cameras of Different Resolutions to Estimate Tree Water Status from Two Cherry Cultivars: An Alternative to Midday Stem Water Potential and Stomatal Conductance. Sensors 2020, 20, 3596. [Google Scholar] [CrossRef]
- Shackel, K.A.; Ahmadi, H.; Biasi, W.; Buchner, R.; Goldhamer, D.; Gurusinghe, S.; Hasey, J.; Kester, D.; Krueger, B.; Lampinen, B.; et al. Plant Water Status as an Index of Irrigation Need in Deciduous Fruit Trees. HortTechnology 1997, 7, 23–29. [Google Scholar] [CrossRef]
- Gucci, R.; Flore, J.A.; Petracek, P.D. The Effect of Fruit Harvest on Photosyntetic Rate, Starch Content, and Chloroplast Ultrastructure in Leaves of Prunus avium L. Adv. Hortic. Sci. 1991, 5, 19–22. [Google Scholar]
- Cartelat, A.; Cerovic, Z.G.; Goulas, Y.; Meyer, S.; Lelarge, C.; Prioul, J.L.; Barbottin, A.; Jeuffroy, M.H.; Gate, P.; Agati, G.; et al. Optically Assessed Contents of Leaf Polyphenolics and Chlorophyll as Indicators of Nitrogen Deficiency in Wheat (Triticum aestivum L.). Field Crops Res. 2005, 91, 35–49. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive Diagnostic Test for Nitrogen Nutrition of Grapevine (Vitis vinifera L.) Based on Dualex Leaf-Clip Measurements in the Field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef] [PubMed]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A New Optical Leaf-Clip Meter for Simultaneous Non-Destructive Assessment of Leaf Chlorophyll and Epidermal Flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Barthod, S.; Cerovic, Z.; Epron, D. Can Dual Chlorophyll Fluorescence Excitation Be Used to Assess the Variation in the Content of UV-Absorbing Phenolic Compounds in Leaves of Temperate Tree Species along a Light Gradient? J. Exp. Bot. 2007, 58, 1753–1760. [Google Scholar] [CrossRef]
- Ruisa, S.; Feldmane, D.; Skrivele, M.; Rubauskis, E.; Kaufmane, E. The Effect of Rain Protective Covering on Sweet Cherry Fruit Quality. Acta Hortic. 2017, 1161, 143–147. [Google Scholar] [CrossRef]
- Børve, J.; Meland, M. Rain Cover Protection against Cracking of Sweet Cherries. I. The Effects on Marketable Yield. Acta Hortic. 1998, 468, 449–453. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Christopoulos, M.V.; Ktistaki, M.E.; Sotiropoulos, T.; Tsantili, E. Influence of Rain Cover on Respiration, Quality Attributes and Storage of Cherries (Prunus avium L.). J. Appl. Bot. Food Qual. 2015, 88, 87–96. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.A.; Blanke, M.M. Bioactive Components in Forced Sweet Cherry Fruit (Prunus avium L.), Antioxidative Capacity and Allergenic Potential as Dependent on Cultivation under Cover. LWT-Food Sci. Technol. 2012, 46, 388–392. [Google Scholar] [CrossRef]
- Lang, G.A. High Tunnel Tree Fruit Production: The Final Frontier? HortTechnology 2009, 19, 50–55. [Google Scholar] [CrossRef]
- Kataoka, I.; Sugiyama, A.; Beppu, K. Involvement of UV Rays in Sweet Cherry Fruit Coloration during Maturation. Acta Hortic. 2005, 667, 461–466. [Google Scholar] [CrossRef]
- Correia, S.; Schouten, R.; Silva, A.P.; Gonçalves, B. Factors Affecting Quality and Health Promoting Compounds during Growth and Postharvest Life of Sweet Cherry (Prunus avium L.). Front. Plant Sci. 2017, 8, 2166. [Google Scholar] [CrossRef]
- Yuri, J.A.; Neira, A.; Quilodran, A.; Razmilic, I.; Motomura, Y.; Torres, C.; Palomo, I. Sunburn on Apples Is Associated with Increases in Phenolic Compounds and Antioxidant Activity as a Function of the Cultivar and Areas of the Fruit. J. Food Agric. Environ. 2010, 8, 920–925. [Google Scholar]

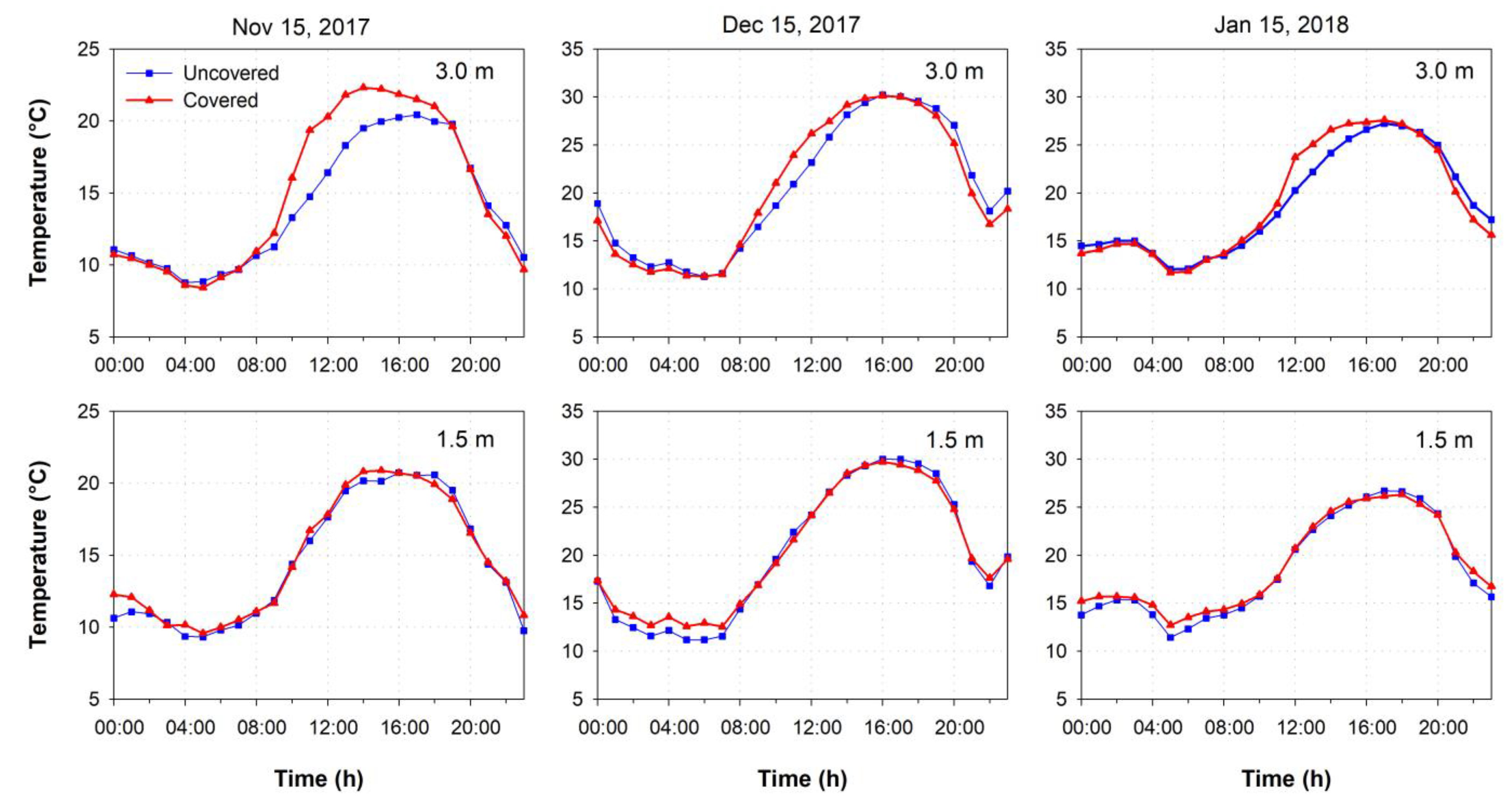
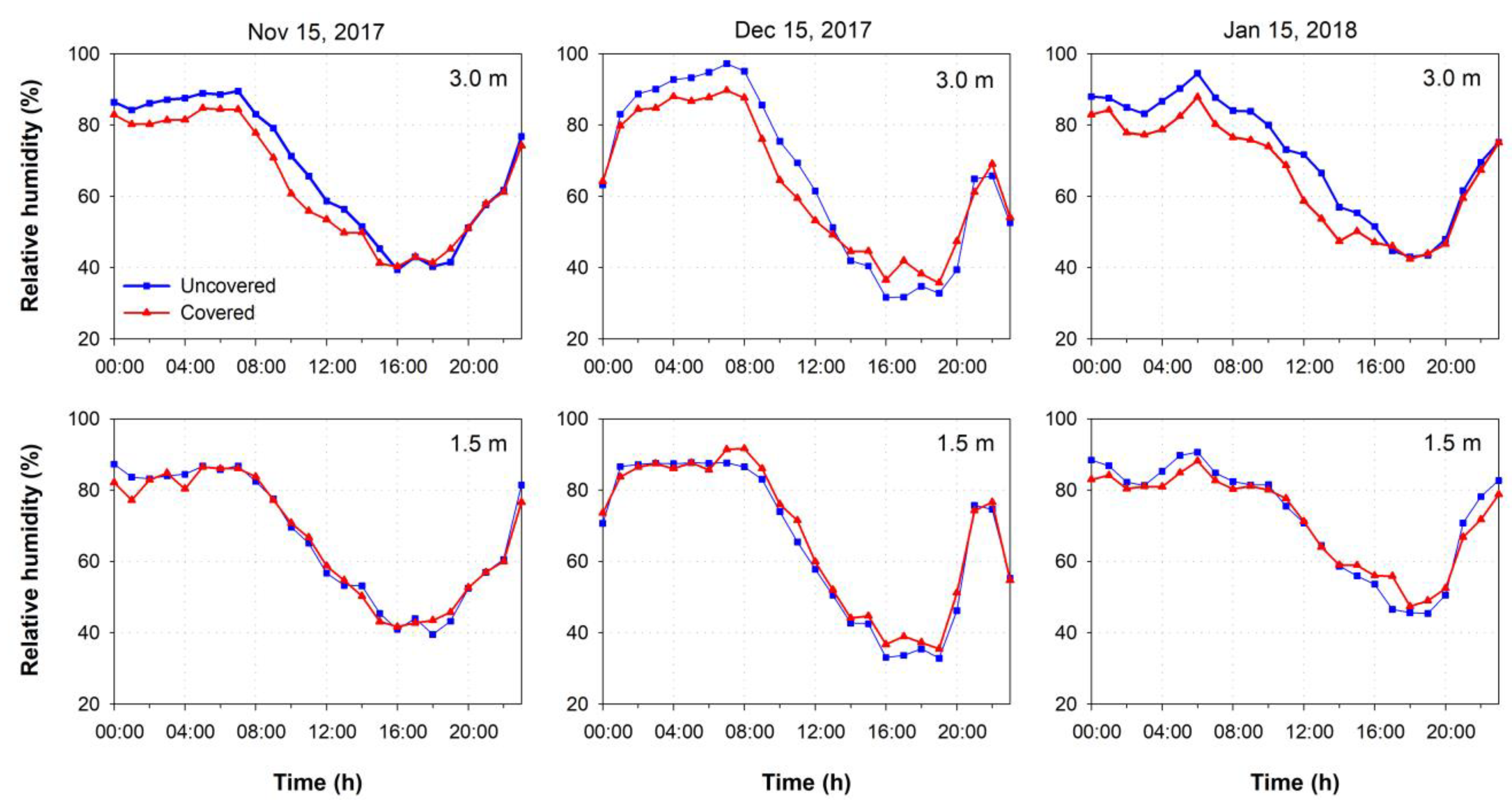

| Height (m) | Treatment | Air Temperature (°C) | Relative Humidity (%) | Thermal Accumulation | Stress Unit | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Mean | Min | Max | Mean | Min | GDH | GD | |||
| 3.0 | Uncovered | 24.8 | 16.3 | 9.1 | 97.4 | 75.9 | 44.8 | 12,385 | 296 | 23,591 |
| Covered | 25.7 | 16.9 | 9.2 | 89.8 | 71.2 | 46.4 | 12,562 | 323 | 26,717 | |
| 1.5 | Uncovered | 24.5 | 16.2 | 9.1 | 88.9 | 74.3 | 46.6 | 12,436 | 293 | 21,804 |
| Covered | 24.2 | 16.7 | 10.2 | 93.2 | 75.4 | 49.4 | 13,238 | 307 | 18,741 | |
| Height (m) | Treatment | Air Temperature (°C) | Relative Humidity (%) | Thermal Accumulation | Stress Units | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Max | Mean | Min | Max | Mean | Min | GDH | GD | |||
| 3.0 | Uncovered | 27.4 | 18.3 | 10.4 | 96.1 | 72.3 | 40.7 | 35,850 | 1011 | 86,914 |
| Covered | 27.6 | 18.5 | 10.2 | 88.3 | 67.0 | 41.3 | 35,243 | 1041 | 102,845 | |
| 1.5 | Uncovered | 26.9 | 18.1 | 10.1 | 89.4 | 71.8 | 42.2 | 35,701 | 985 | 81,393 |
| Covered | 26.4 | 18.4 | 11.2 | 92.6 | 72.9 | 45.4 | 38,157 | 1016 | 69,248 | |
| Cultivar | Treatment | Canopy Volume (m3) | TCSA (cm2) | Shoot | Leaf | Dry Matter (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Internode Length (cm) | Leaf Area (cm2) | Fresh Weight (g) | N° Leaves | Area (cm2) | Fresh Weight (g) | |||||
| ‘Rainier’ | Uncovered | 16 a | 289 b | 47 b | 2.2 b | 1496 b | 42 a | 21 a | 71 b | 2.0 a | 40 a |
| Covered | 13 a | 354 a | 60 a | 3.0 a | 1854 a | 45 a | 20 a | 91 a | 2.2 a | 37 b | |
| p-value | 0.14 | 0.04 | 0.04 | 0.01 | 0.01 | 0.46 | 0.57 | 0.00 | 0.16 | 0.00 | |
| ‘Bing’ | Uncovered | 17 a | 343 a | 33 b | 1.7 b | 1094 b | 31 a | 19 a | 55 b | 1.6 a | 44 a |
| Covered | 18 a | 399 a | 49 a | 2.3 a | 1510 a | 37 a | 22 a | 67 a | 1.7 a | 38 b | |
| p-value | 0.40 | 0.12 | 0.04 | 0.01 | 0.03 | 0.23 | 0.08 | 0.03 | 0.47 | 0.00 | |
| ‘Sweetheart’ | Uncovered | 13 a | 194 a | 33 b | 2.2 b | 1034 b | 28 b | 15 b | 69 b | 1.9 a | 43 a |
| Covered | 15 a | 219 a | 52 a | 2.6 a | 1558 a | 37 a | 20 a | 80 a | 1.9 a | 39 b | |
| p-value | 0.18 | 0.28 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.98 | 0.02 | |
| Cultivar | Treatment | Assimilation (µmol CO2 m−2 s−1) | Transpiration (mmol H2O m−2 s−1) | Water Use Efficiency (µmol CO2 mmol H2O−1) | Stomatal Conductance (mol H2O m−2 s−1) | Stem Water Potential (MPa) | Photon Maximal Efficiency Fv/Fm | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11-Dec | 11-Jan | 11-Dec | 11-Jan | 11-Dec | 11-Jan | 11-Dec | 11-Jan | 11-Dec | 11-Jan | 11-Dec | 11-Jan | ||
| ‘Rainier’ | Uncovered | 9.4 aA | 8.4 aA | 2.9 bA | 1.6 aB | 3.2 aB | 5.4 aA | 0.27 aA | 0.11 aB | −0.9 bA | −1.9 aB | 0.78 aA | 0.81 aA |
| Covered | 9.9 aA | 7.1 aB | 3.7 aA | 1.3 aB | 2.7 aB | 5.5 aA | 0.25 aA | 0.09 aB | −0.6 aA | −1.4 aB | 0.82 aA | 0.82 aA | |
| ‘Bing’ | Uncovered | 9.1 aA | 6.8 aB | 2.5 bA | 2.0 aB | 3.7 aA | 3.5 aA | 0.20 bA | 0.13 aB | −1.0 aA | −1.4 bB | 0.81 aA | 0.83 aA |
| Covered | 8.5 aA | 7.3 aA | 3.1 aA | 2.1 aB | 2.7 bA | 3.4 aA | 0.26 aB | 1.15 aA | −0.9 aA | −0.9 aA | 0.81 aA | 0.84 aA | |
| ‘Sweetheart’ | Uncovered | 9.4 aA | 5.7 aB | 2.7 bA | 1.2 aB | 3.4 aB | 4.8 aA | 0.18 bA | 0.07 bB | −0.9 aA | −1.3 aB | 0.80 aA | 0.81 aA |
| Covered | 8.7 aA | 7.5 aA | 3.6 aA | 2.0 bB | 2.5 bB | 3.7 bA | 0.27 aA | 0.14 aB | −0.6 aA | −1.2 aB | 0.78 aA | 0.77 bA | |
| Cultivar | Treatment | Dualex index | SPAD Index | |||
|---|---|---|---|---|---|---|
| Chlorophyll (Chl) | Flavonoid (Flav) | Anthocyanin (Anth) | Nitrogen Balance (NBI) | |||
| ‘Rainier’ | Uncovered | 34 a | 1.3 b | 0.18 a | 10 a | 37 b |
| Covered | 37 a | 2.0 a | 0.19 a | 10 a | 42 a | |
| p-value | 0.06 | 0.01 | 0.32 | 0.68 | 0.04 | |
| ‘Bing’ | Uncovered | 34 a | 1.6 a | 0.18 a | 10 a | 41 a |
| Covered | 29 b | 1.7 a | 0.15 b | 8 b | 39 a | |
| p-value | 0.01 | 0.23 | 0.01 | 0.02 | 0.42 | |
| ‘Sweetheart’ | Uncovered | 27 a | 7.0 a | 0.68 a | 14 a | 33 a |
| Covered | 27 a | 6.5 a | 0.68 a | 15 a | 33 a | |
| p-value | 0.83 | 0.24 | 0.85 | 0.49 | 0.69 | |
| Cultivar | Treatment | Diameter (mm) | Weight (g) | SSC (°Brix) | Firmness (g mm−1) | Color (%*; 1–5) |
|---|---|---|---|---|---|---|
| ‘Rainier’ | Uncovered | 27 a | 13 b | 20 a | 260 a | 72 * a |
| Covered | 28 a | 14 a | 18 a | 239 b | 42 * b | |
| p-value | 0.06 | 0.01 | 0.08 | 0.01 | 0.00 (β) | |
| ‘Bing’ | Uncovered | 28 a | 11 b | 23 a | 275 a | 4.1 a |
| Covered | 29 a | 13 a | 20 b | 236 b | 4.3 a | |
| p-value | 0.53 | 0.00 | 0.00 | 0.00 | 0.05 (β) | |
| ‘Sweetheart’ | Uncovered | 26 b | 12 b | 21 a | 271 a | 2.0 a |
| Covered | 27 a | 13 a | 20 a | 249 b | 2.4 a | |
| p-value | 0.00 | 0.00 | 0.13 | 0.00 | 0.05 (β) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino, S.; Palma, M.; Sepúlveda, Á.; Sánchez-Contreras, J.; Moya, M.; Yuri, J.A. Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees. Horticulturae 2023, 9, 109. https://doi.org/10.3390/horticulturae9010109
Pino S, Palma M, Sepúlveda Á, Sánchez-Contreras J, Moya M, Yuri JA. Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees. Horticulturae. 2023; 9(1):109. https://doi.org/10.3390/horticulturae9010109
Chicago/Turabian StylePino, Simón, Miguel Palma, Álvaro Sepúlveda, Javier Sánchez-Contreras, Mariana Moya, and José Antonio Yuri. 2023. "Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees" Horticulturae 9, no. 1: 109. https://doi.org/10.3390/horticulturae9010109
APA StylePino, S., Palma, M., Sepúlveda, Á., Sánchez-Contreras, J., Moya, M., & Yuri, J. A. (2023). Effect of Rain Cover on Tree Physiology and Fruit Condition and Quality of ‘Rainier’, ‘Bing’ and ‘Sweetheart’ Sweet Cherry Trees. Horticulturae, 9(1), 109. https://doi.org/10.3390/horticulturae9010109





