Assessment of Fruit Quality and Genes Related to Proanthocyanidins Biosynthesis and Stress Resistance in Persimmon (Diospyros kaki Thunb.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of the Exterior Quality of Fruit
2.3. Measurement of the Interior Quality of Fruit
2.4. Statistical Analysis of Data
2.5. RNA Isolation and cDNA Synthesis
2.6. qRT—PCR
3. Results and Discussion
3.1. Analysis of Variation and the Quality of Different Persimmon Fruit
3.2. Correlation Analysis of Indicators
3.3. Principal Component Analysis of Fruit Quality
3.4. Comprehensive Evaluation of Different Persimmon Fruits
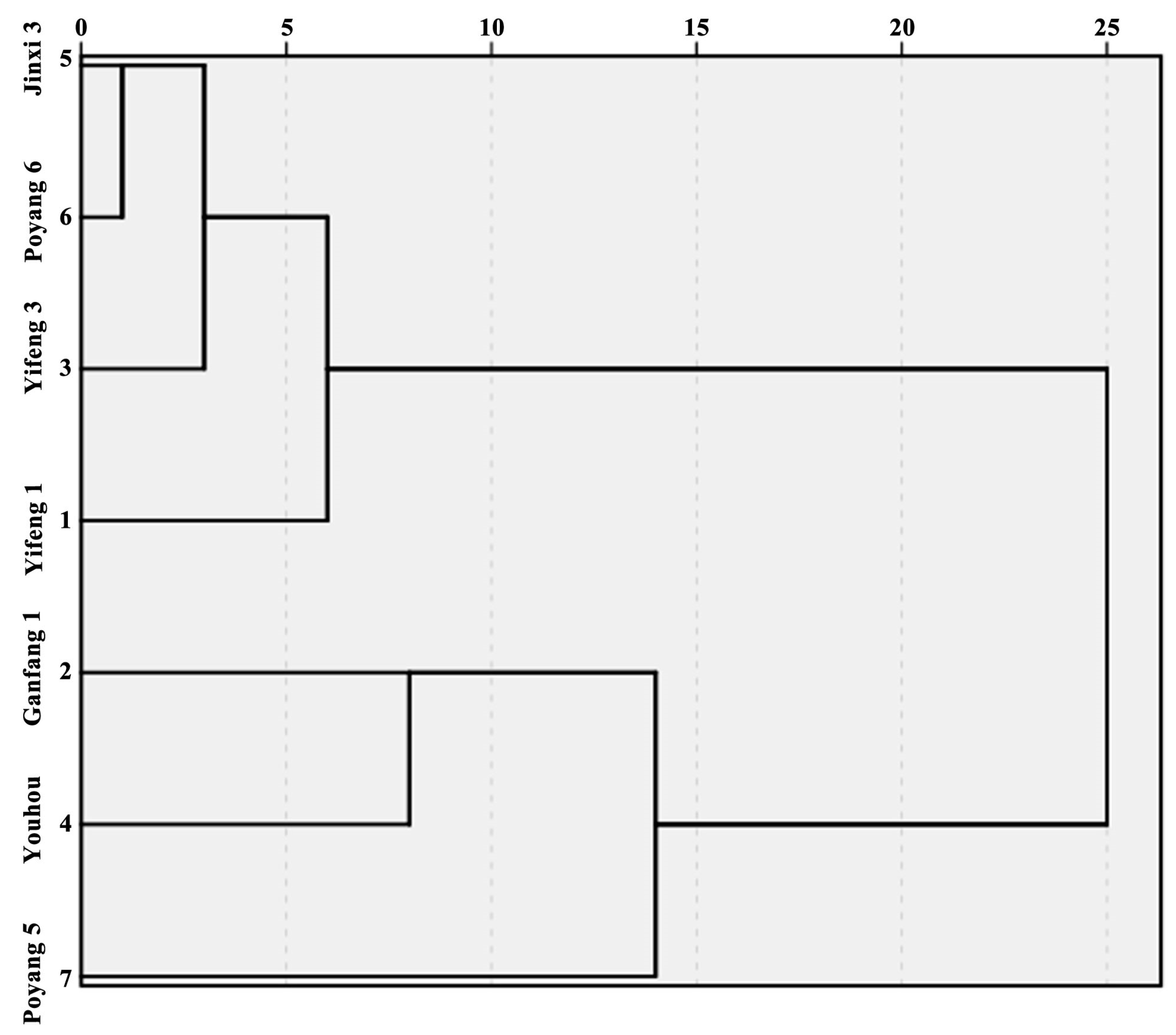
3.5. Cluster Analysis of Different Persimmon Fruits
3.6. Expression of Adversity and Stress Genes in Different Persimmon Varieties
3.7. Expression of PA Pathway Genes in Different Persimmon Varieties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Z.R.; Zhang, Q.L.; Xu, L.Q.; Guo, D.Y.; Chen, W.X. Fruit scientific research in new China in the past 70 years: Persimmon. J. Fruit Sci. 2019, 36, 1382–1388. [Google Scholar] [CrossRef]
- Guan, C.F.; Zhang, Y.F.; Zhang, P.X.; Chachar, S.; Wang, R.Z.; Du, X.Y.; Yang, Y. Germplasm conservation, molecular identity and morphological characterization of persimmon (Diospyros kaki Thunb.) in the NFGP of China. Hortic. Sci. 2020, 272, 109490. [Google Scholar] [CrossRef]
- Guan, C.F.; Che, Q.H.; Zhang, P.X.; Huang, J.M.; Chachar, S.; Ruan, X.F.; Wang, R.Z.; Yang, Y. Codification and description of growth stages in persimmon (Diospyros kaki Thunb.) using the extended BBCH scale. Sci. Hortic. 2021, 280, 109895. [Google Scholar] [CrossRef]
- Qiu, Z.N.; Zhang, R.Y.; Peng, M.L.; Zhang, W.; Li, T.Z.; Zhu, Y.D. Comprehensive evaluation system of the fruit quality of wild Actinidia argute in Beijing. J. China Agric. Univ. 2017, 22, 45–53. [Google Scholar] [CrossRef]
- Lv, Y.Z.; Li, Z.; Zhang, Y.B.; Liang, Z.H. Analysis and comprehensive evaluation on fruit quality of different persimmon varieties. Food Ferment. Ind. 2020, 46, 180–186. [Google Scholar] [CrossRef]
- Wang, Y.; Li, K.K.; Li, C.M. Effects of interaction between pectin and tannin on the deastringency of different varieties of persimmons during maturing. Mod. Food Sci. Technol. 2019, 35, 87–94. [Google Scholar] [CrossRef]
- Chen, L.; Liao, G.L.; Yang, C.; Huang, C.H.; Zhong, M.; Tao, J.J.; Qu, X.Y.; Xu, X.B. Comprehensive evaluation of main fruit characters of yellow flesh kiwifruit cultivars (strains) based on principal component analysis and cluster analysis. Acta Agric. Univ. Jiangxiensis 2018, 40, 1231–1240. [Google Scholar] [CrossRef]
- Akagi., T.; Katayama-Ikegami, A.; Yonemori, K. Proanthocyanidin biosynthesis of persimmon (Diospyros kaki Thunb.) fruit. Sci. Hortic. 2011, 130, 373–380. [Google Scholar] [CrossRef]
- Du, X.Y.; Wang, Y.B.; Zeng, M.; Xiong, Y.T.; Huang, J.M.; Le, M.W.; Luo, Z.R. Preliminary investigation on persimmon variety resources in Jiangxi Province. Acta Agric. Jiangxi 2013, 25, 69–71. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wu, M.H.; Zeng, M.; Wang, S.C.; Li, G.Q.; Xiong, Y.T.; Xie, F.; Xiao, H.Y.; Du, X.Y.; Wang, F.; et al. Further investigation on persimmon germplasm resources in Jiangxi Province. Acta Agric. Jiangxi 2016, 28, 32–35. [Google Scholar] [CrossRef]
- Zeng, M.; Du, X.Y.; Wu, M.H.; Wang, Y.B. Genetic diversity analysis of Diospyros spp. germplasm resources in Jiangxi by using IRAP molecular marker. Acta Agric. Jiangxi 2017, 29, 29–34. [Google Scholar] [CrossRef]
- Chien, P.J.; Sheu, F.; Yang, F.H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Li, S.L.; Huo, J.W.; Li, F.X.; Qin, D. Comparing of methods for determination of ascorbic acid content of black currant. J. Jilin Agric. Sci. 2014, 39, 74–77. [Google Scholar] [CrossRef]
- Suntornsuk, L.; Gritsanapun, W.; Nilkamhank, S.; Paochom, A. Quantitation of vitamin C content in herbal juice using direct titration. J. Pharmaceut. Biomed. 2002, 28, 849–855. [Google Scholar] [CrossRef]
- Li, X.X.; Li, J.Z. Determination of the content of soluble sugar in sweet corn with optimized anthrone colorimetric method. Storage Process 2013, 13, 24–27. [Google Scholar] [CrossRef]
- Zhu, L.C.; Tang, S.J.; Zhou, L. Teaching practice and methodological investigation of protein content determination using coomassie brilliant blue G250. Educ. Teach. Forum 2020, 266–269. [Google Scholar]
- He, H. Screening of Professional PCNA Pollinizer and F1 Progenie Sexuality Identification in Persimmon. MD Dissertation, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Oshida, M.; Yonemori, K.; Sugiura, A. On the nature of coagulated tannins in astringent-type persimmon fruit after an artificial treatment of astringency removal. Postharvest Biol. Technol. 1996, 8, 317–327. [Google Scholar] [CrossRef]
- Li, Z.F.; Xu, J.G. Determination of pectinase activity by spectrophotometry. Heilongngjiang Med. 2002, 15, 428–430. [Google Scholar] [CrossRef]
- Ikegami, A.; Eguchi, S.; Yonemori, K.; Yamada, M.; Sato, A.; Mitani, N.; Kitajima, A. Segregation of astringent progenies in the F1 populations derived from crosses between a Chinese pollination-constant nonastringent (PCNA) ‘Luo Tian Tian Shi’, and pollination-constant astringent (PCA) cultivars of Japanese origin. HortScience 2006, 41, 561–563. [Google Scholar] [CrossRef]
- Tessmer, M.A.; Appezzato-Da-Glória, B.; Kluge, R.A. Astringency in ‘Giombo’ persimmon and its relationship with the harvest time. Rev. Ceres 2016, 63, 646–652. [Google Scholar] [CrossRef]
- Guillén-Casla, V.; Rosales-Conrado, N.; León-González, M.E.; Pérez-Arribas, L.V.; Polo-Díez, L.M. Principal component analysis (PCA) and multiple linear regression (MLR) statistical tools to evaluate the effect of E-beam irradiation on ready-to-eat food. J. Food Compos. Anal. 2010, 24, 456–464. [Google Scholar] [CrossRef]
- An, J.P.; Li, R.; Qu, F.J.; You, C.X.; Wang, X.F.; Hao, Y.J. An apple NAC transcription factor negatively regulates cold tolerance via CBF-dependent pathway. J. Plant Physiol. 2018, 221, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, C.P.; Yang, H.; Kuang, R.B.; Huang, B.X.; Wei, Y.R. Identification of WRKY transcription factor genes in papaya and response of their expression to Colletotrichum brevisporum infection. J. Northwest A&F Univ. 2022, 50, 127–154. [Google Scholar] [CrossRef]
- Chen, W.X.; Xiong, Y.L.; Xu, L.Q.; Zhang, Q.L.; Luo, Z.R. An integrated analysis based on transcriptome and proteome reveals deastringency-related genes in CPCNA persimmon. Sci. Rep. 2017, 7, 44671. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Yamane, H.; Ukai, Y. Genetic analysis of Japanese persimmon fruit weight. J. Am. Soc. Hortic. Sci. 1994, 119, 1298–1302. [Google Scholar] [CrossRef]
- Yonemori, K.; Sugiura, A.; Yamada, M. Persimmon genetics and breeding. Plant Breed Rev. 2000, 19, 191–225. [Google Scholar] [CrossRef]
- Ikeda, I.; Yamada, M.; Kurihara, A.; Nishida, T. Inheritance of astringency in Japanese persimmon. J. Japan. Soc. Hortic. Sci. 1985, 54, 39–45. [Google Scholar] [CrossRef]
- Yamada, M.; Sato, A. Segregation for fruit astringency type in progenies derived from crosses of ‘Nishimurawase’ × pollination constant non-astringent genotypes in oriental persimmon (Diospyros kaki Thunb.). Sci. Hortic. 2002, 92, 107–111. [Google Scholar] [CrossRef]
- Ikegami, A.; Yonemori, K.; Sugiura, A.; Sato, A.; Yamada, M. Segregation of astringency in F1 progenies derived from crosses between pollination-constant, nonastringent persimmon cultivars. HortScience 2004, 39, 371–374. [Google Scholar] [CrossRef]
- Du, X.Y.; Zhang, Q.L.; Luo, Z.R. Development of retrotransposon primers and their utilization for germplasm identification in Diospyros spp. (Ebenaceae). Tree Genet. Genomes 2009, 5, 235–245. [Google Scholar] [CrossRef]
- Luo, Z.R.; Li, F.F.; Cai, L.H. Molecular systematics of China native nonastringent persimmon based on random amplified polymorphic DNA. Acta Hortic. Sin. 1999, 26, 297–301. [Google Scholar]
- Zhang, N.; Xu, J.C.; Mo, R.L.; Zhang, Q.L.; Luo, Z.R. Androecious genotype ‘Male 8’ carries the CPCNA gene locus controlling natural deastringency of Chinese PCNA persimmons. Hortic. Plant J. 2016, 2, 309–314. [Google Scholar] [CrossRef]
- Jung, W.J.; Seo, Y.W. Identification of novel C-repeat binding factor (CBF) genes in rye (Secale cereale L.) and expression studies. Gene 2019, 684, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.H.; Huang, J.Y.; Sun, T.S.; Wang, X.F.; Zhu, C.Q.; Ai, Y.X.; Gu, H.Y. The precise regulation of different COR genes by individual CBF transcription factors in Arabidopsis thaliana. J. Integr. Plant Biol. 2017, 59, 118–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Genotypes | Exterior Quality | Interior Quality | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fruit Weight (g) | Horizontal Length (mm) | Vertical Length (mm) | Firmness (kg/cm2) | Titratable Acidity (FW%) | Vitamin C (mg/100 g FW) | Soluble Sugar (FW%) | Pectinase Activity (U/g FW) | Anthocyanin Content (mg/g FW) | Flavonoid Content (mg/g FW) | Soluble Protein (mg/g FW) | Tannin Cell Size (μm2) | Tannin Content (FW%) | |
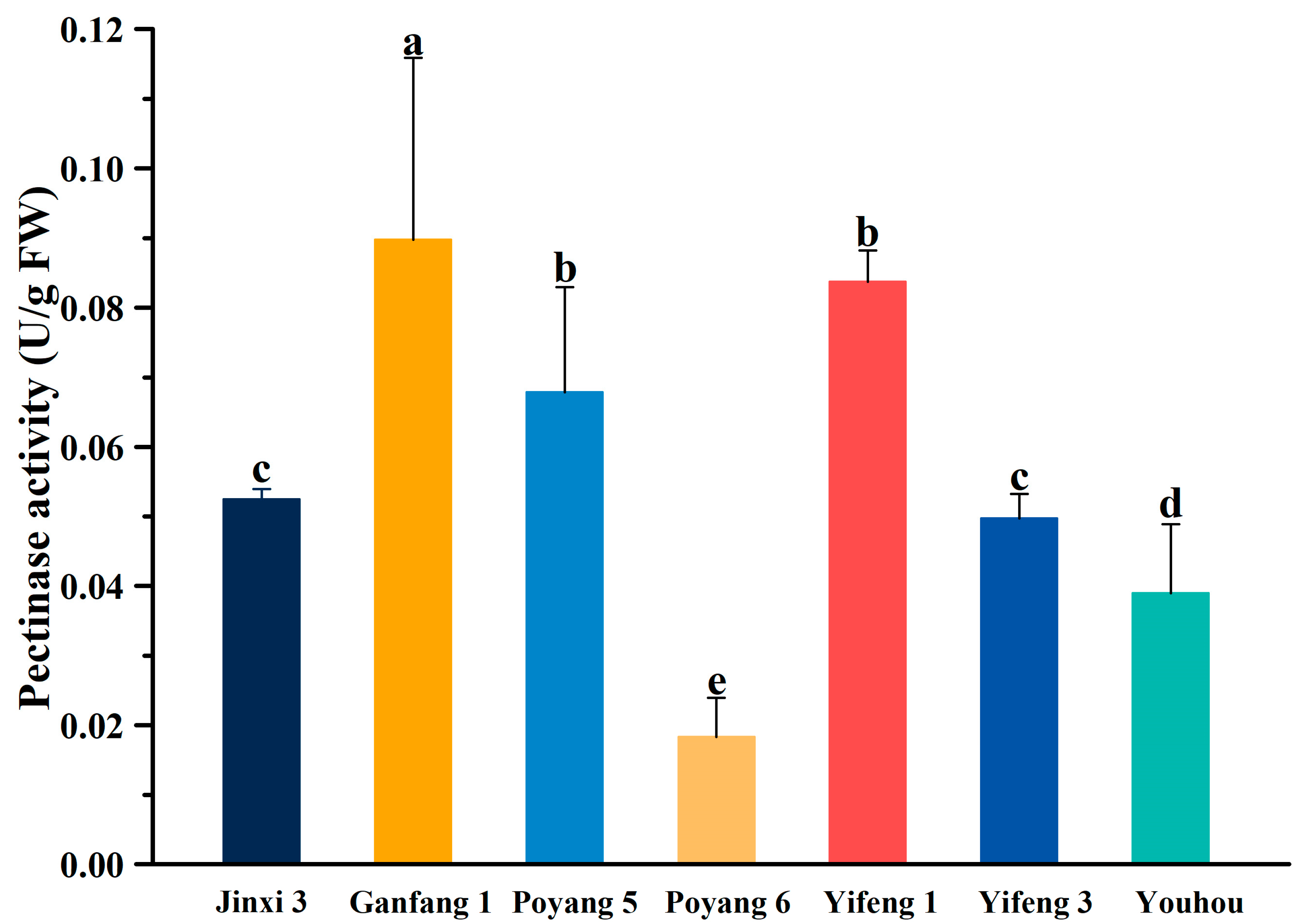
| Jinxi 3 | 136.308 | 68.893 | 53.191 | 1.833 | 1.831 | 268.094 | 0.005 | 0.053 | 0.004 | 2.375 | 0.497 | 15,243.906 | 6.882 |
| Ganfang 1 | 177.350 | 76.732 | 57.919 | 2.433 | 1.973 | 209.524 | 0.038 | 0.090 | 0.007 | 4.543 | 0.496 | 9914.305 | 11.768 |
| Poyang 5 | 165.439 | 71.554 | 60.700 | 2.567 | 2.052 | 238.893 | 0.051 | 0.068 | 0.008 | 4.979 | 0.980 | 7391.723 | 5.217 |
| Poyang 6 | 52.446 | 44.676 | 50.932 | 2.250 | 2.047 | 100.477 | 0.036 | 0.018 | 0.007 | 4.038 | 0.423 | 15,047.179 | 14.550 |
| Yifeng 1 | 186.712 | 75.431 | 60.770 | 3.056 | 1.845 | 192.857 | 0.122 | 0.084 | 0.005 | 2.102 | 0.523 | 16,809.189 | 7.353 |
| Yifeng 3 | 141.181 | 69.650 | 55.230 | 2.144 | 1.867 | 166.667 | 0.131 | 0.050 | 0.003 | 1.611 | 0.535 | 14,216.744 | 14.685 |
| Youhou | 159.523 | 76.941 | 52.788 | 3.500 | 1.771 | 260.476 | 0.116 | 0.039 | 0.002 | 1.387 | 0.986 | 3995.812 | 1.235 |
| Average value | 145.570 | 69.130 | 55.930 | 2.540 | 1.910 | 205.280 | 0.071 | 0.057 | 0.005 | 3.005 | 0.630 | 11,802.690 | 8.810 |
| Standard deviation | 44.850 | 11.270 | 3.940 | 0.569 | 0.110 | 58.840 | 0.051 | 0.025 | 0.002 | 1.478 | 0.240 | 4782.460 | 5.040 |
| Coefficient of variation (%) | 30.810 | 16.310 | 7.040 | 22.380 | 5.830 | 28.660 | 71.190 | 43.860 | 40.000 | 49.185 | 37.990 | 40.520 | 57.180 |
| Correlation Matrix | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trait | Tx1 | Tx2 | Tx3 | Tx4 | Tx5 | Tx6 | Tx7 | Tx8 | Tx9 | Tx10 | Tx11 | Tx12 | Tx13 | |
| Fruit weight | Tx1 | 1 | ||||||||||||
| (g FW) | ||||||||||||||
| Horizontal length | Tx2 | 0.967 ** | 1 | |||||||||||
| (mm) | ||||||||||||||
| Vertical length | Tx3 | 0.762 * | 0.583 | 1 | ||||||||||
| (mm) | ||||||||||||||
| Firmness | Tx4 | −0.500 | −0.443 | −0.568 | 1 | |||||||||
| (kg/cm2) | ||||||||||||||
| Titratable acid | Tx5 | −0.388 | −0.534 | 0.185 | 0.069 | 1 | ||||||||
| content (FW%) | ||||||||||||||
| Vitamin C content | Tx6 | 0.657 | 0.752 | 0.226 | 0.114 | −0.495 | 1 | |||||||
| (mg/100 g FW) | ||||||||||||||
| Flavonoid content | Tx7 | −0.144 | −0.285 | 0.428 | 0.18 | 0.928 ** | −0.206 | 1 | ||||||
| (mg/g FW) | ||||||||||||||
| Anthocyanin content | Tx8 | −0.444 | −0.532 | 0.033 | 0.439 | 0.786 * | −0.476 | 0.818 * | 1 | |||||
| (mg/g FW) | ||||||||||||||
| Soluble sugar content | Tx9 | 0.361 | 0.375 | 0.217 | −0.835 * | −0.494 | −0.990 | −0.571 | −0.052 | 1 | ||||
| (FW%) | ||||||||||||||
| Pectinase activity | Tx10 | 0.855 * | 0.734 | 0.863 * | −0.348 | −0.020 | 0.368 | 0.219 | 0.269 | 0.061 | 1 | |||
| (U/g FW) | ||||||||||||||
| Soluble protein | Tx11 | 0.372 | 0.427 | 0.216 | −0.326 | −0.093 | 0.578 | −0.575 | 0.248 | 0.248 | −0.085 | 1 | ||
| content (mg/g FW) | ||||||||||||||
| Tannin content | Tx12 | −0.506 | −0.562 | −0.185 | −0.031 | 0.448 | −0.820 * | 0.639 | −0.105 | −0.105 | −0.035 | −0.780 * | 1 | |
| (FW%) | ||||||||||||||
| Tannin cell size | Tx13 | −0.307 | −0.412 | −0.030 | 0.205 | 0.026 | −0.513 | 0.459 | −0.097 | −0.097 | −0.042 | −0.863 * | 0.611 | 1 |
| (μm2) | ||||||||||||||
| Index | Eigenvectors | |||
|---|---|---|---|---|
| Principal Component 1 | Principal Component 2 | Principal Component 3 | Principal Component 4 | |
| Fruit weight | 0.889 | 0.351 | 0.204 | 0.202 |
| Horizontal length | 0.939 | 0.173 | 0.095 | 0.205 |
| Vertical length | 0.491 | 0.743 | 0.382 | 0.017 |
| Firmness | −0.502 | −0.149 | −0.598 | 0.596 |
| Titratable acid content | −0.623 | 0.709 | −0.089 | −0.309 |
| Vitamin C content | 0.759 | 0.046 | −0.505 | 0.367 |
| Soluble sugar | 0.533 | −0.362 | 0.567 | −0.497 |
| Pectinase activity | 0.523 | 0.653 | 0.370 | 0.385 |
| Anthocyanin content | −0.468 | 0.869 | −0.075 | −0.060 |
| Flavonoid content | −0.424 | 0.859 | −0.256 | −0.093 |
| Soluble protein content | 0.629 | 0.115 | −0.538 | −0.524 |
| Tannin cell size | −0.513 | −0.140 | 0.609 | 0.477 |
| Tannin content | −0.728 | 0.038 | 0.575 | −0.025 |
| Eigenvalues | 5.276 | 3.316 | 2.337 | 1.578 |
| Contribution rates (%) | 40.582 | 25.506 | 17.980 | 12.141 |
| Cumulative contribution rates (%) | 40.582 | 66.088 | 84.068 | 96.209 |
| Cultivar | Principal Component Comprehensive Score | Total Score | Ranking | |||
|---|---|---|---|---|---|---|
| Z1 | Z2 | Z3 | Z4 | |||
| Jinxi 3 | 7413.229 | −2019.359 | 9203.670 | 7413.180 | −968.647 | 5 |
| Ganfang 1 | −4681.491 | −1254.332 | 6001.277 | 4858.517 | −550.876 | 3 |
| Poyang 5 | −3374.207 | −902.564 | 4444.376 | 3662.245 | −355.802 | 2 |
| Poyang 6 | −7544.180 | −2032.932 | 9152.512 | 7235.214 | −1056.053 | 6 |
| Yifeng 1 | −8218.115 | −2217.529 | 10,209.935 | 8143.000 | −1076.296 | 7 |
| Yifeng 3 | −6961.686 | −1876.996 | 8636.920 | 6885.962 | −915.000 | 4 |
| Youhou | −1615.278 | −436.677 | 2360.218 | 2050.675 | −93.557 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Zhang, M.; Zeng, M.; Wu, M.; Zhang, Q.; Luo, Z.; Hu, X. Assessment of Fruit Quality and Genes Related to Proanthocyanidins Biosynthesis and Stress Resistance in Persimmon (Diospyros kaki Thunb.). Horticulturae 2022, 8, 844. https://doi.org/10.3390/horticulturae8090844
Yang S, Zhang M, Zeng M, Wu M, Zhang Q, Luo Z, Hu X. Assessment of Fruit Quality and Genes Related to Proanthocyanidins Biosynthesis and Stress Resistance in Persimmon (Diospyros kaki Thunb.). Horticulturae. 2022; 8(9):844. https://doi.org/10.3390/horticulturae8090844
Chicago/Turabian StyleYang, Sichao, Meng Zhang, Ming Zeng, Meihua Wu, Qinglin Zhang, Zhengrong Luo, and Xinlong Hu. 2022. "Assessment of Fruit Quality and Genes Related to Proanthocyanidins Biosynthesis and Stress Resistance in Persimmon (Diospyros kaki Thunb.)" Horticulturae 8, no. 9: 844. https://doi.org/10.3390/horticulturae8090844
APA StyleYang, S., Zhang, M., Zeng, M., Wu, M., Zhang, Q., Luo, Z., & Hu, X. (2022). Assessment of Fruit Quality and Genes Related to Proanthocyanidins Biosynthesis and Stress Resistance in Persimmon (Diospyros kaki Thunb.). Horticulturae, 8(9), 844. https://doi.org/10.3390/horticulturae8090844






