Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Proximate Composition
2.3. Fatty Acid Composition
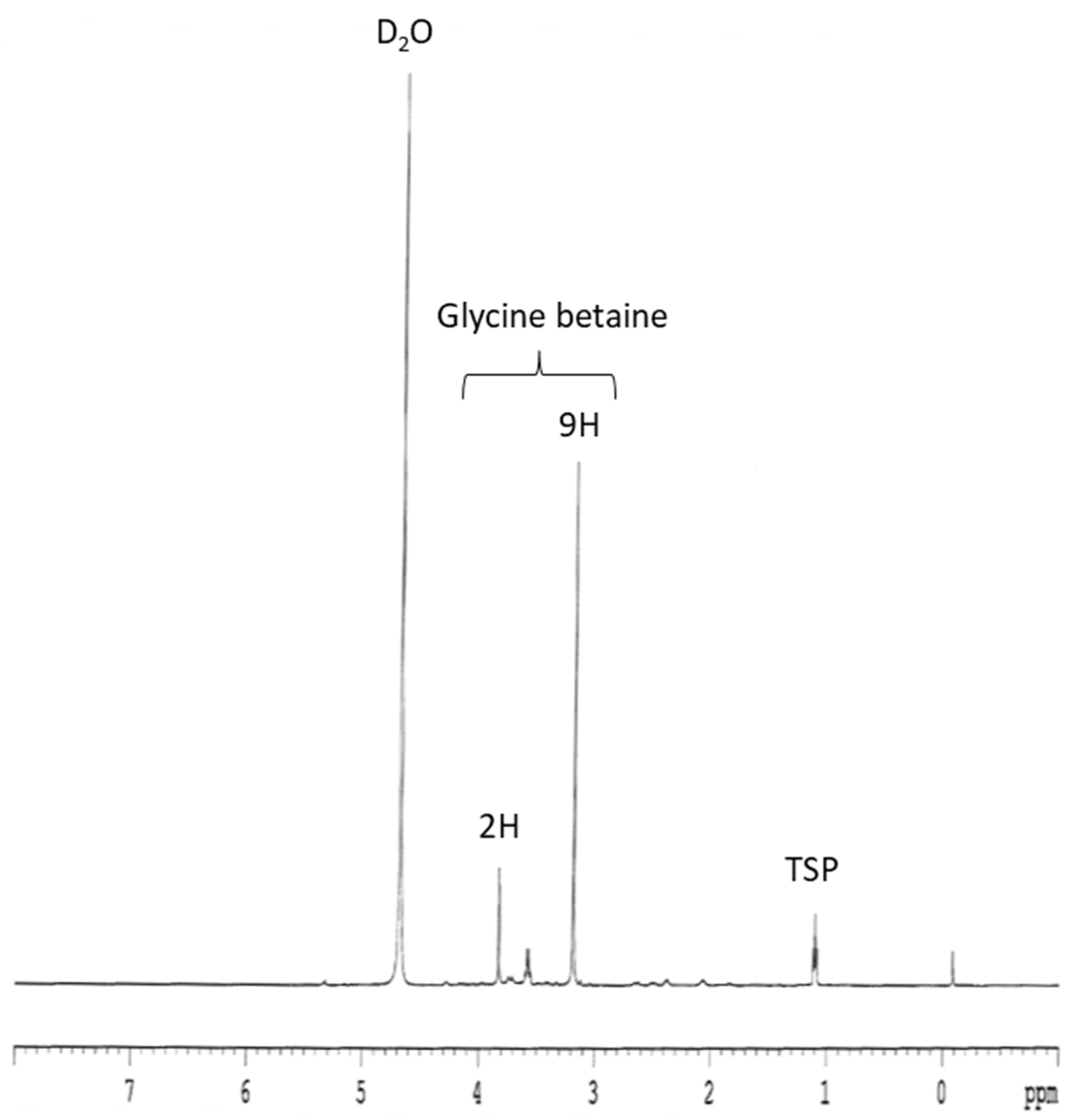
2.4. Glycine Betaine Extraction and Quantification
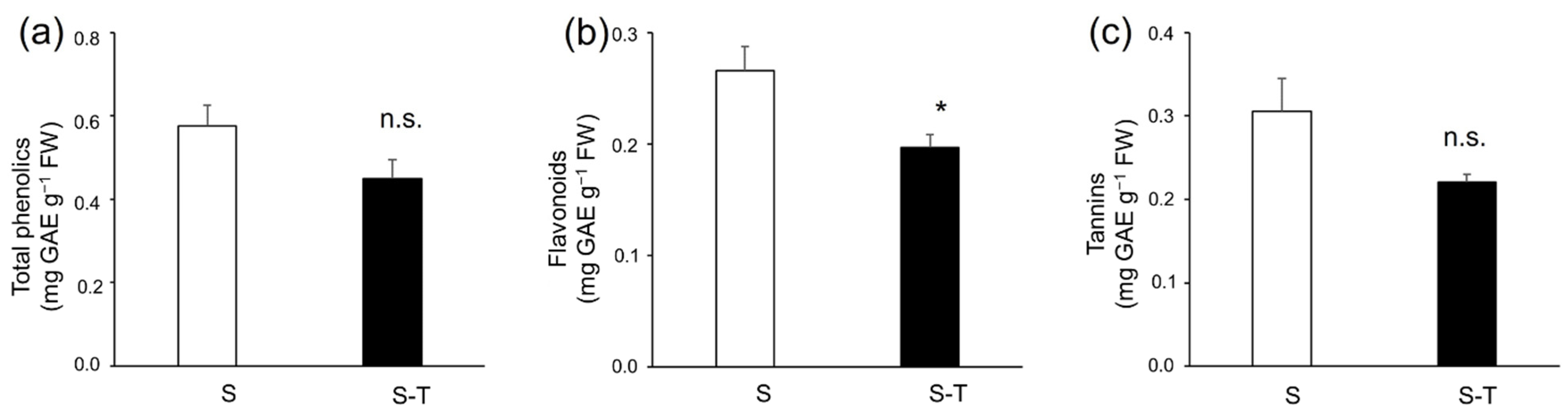
2.5. Extraction and Quantification of Total Phenolics, Flavonoids, and Tannins
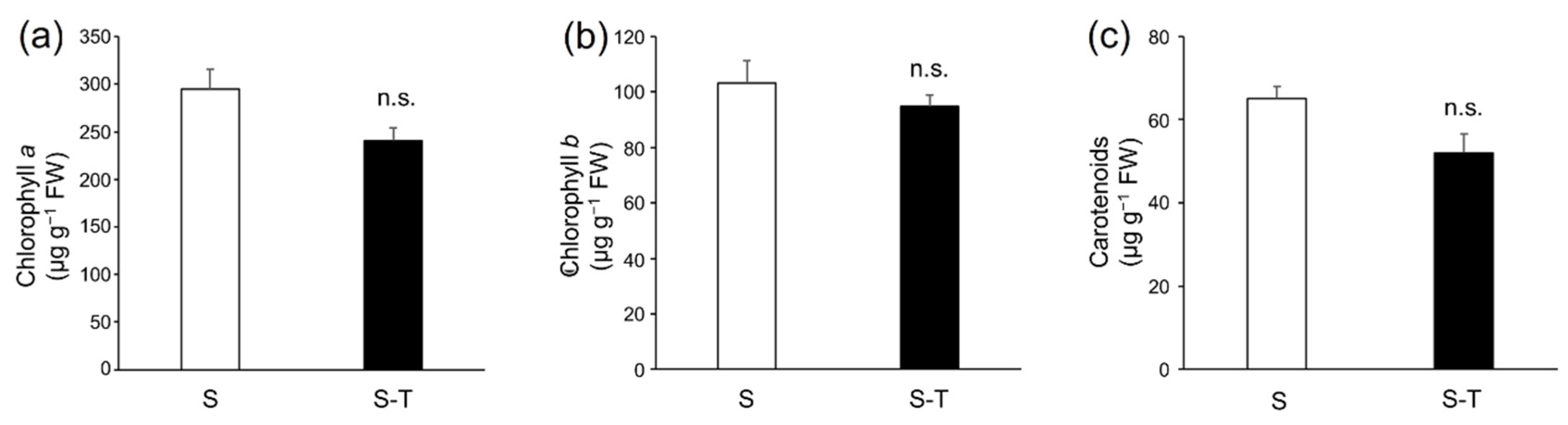
2.6. Extraction and Quantification of Chlorophylls and Carotenoids
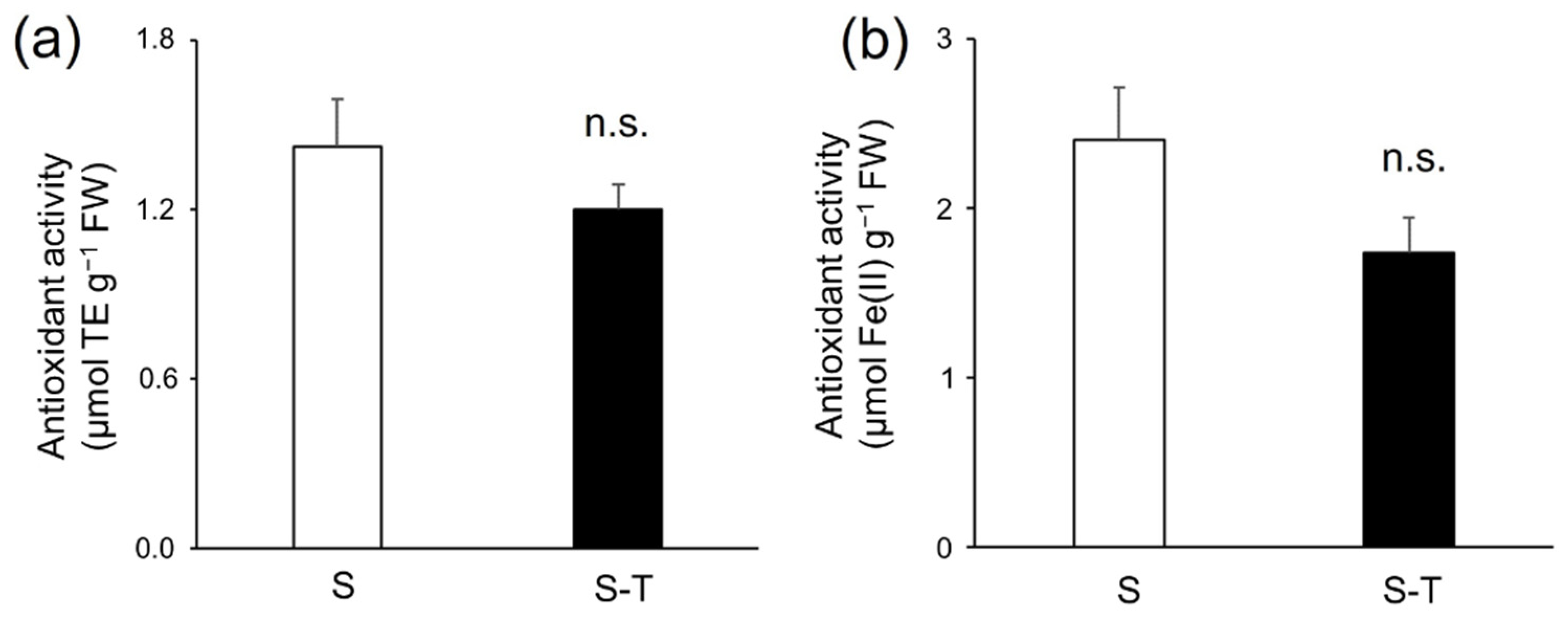
2.7. Evaluation of the In Vitro Antioxidant Activity
2.8. Evaluation of the In Vitro Antimicrobial Activity
2.9. Cytotoxic Effect and Evaluation of Inflammatory Mediators’ Expression in HT-29 Cells
2.10. Statistical Analysis
3. Results
3.1. Nutrient Composition
3.1.1. Proximate Percentage Composition
3.1.2. Fatty Acid Composition
3.2. Glycine Betaine
3.3. Chlorophylls and Carotenoids
3.4. Total Phenolics, Flavonoids, Tannins, and Antioxidant Activity
3.5. Antimicrobial Activity
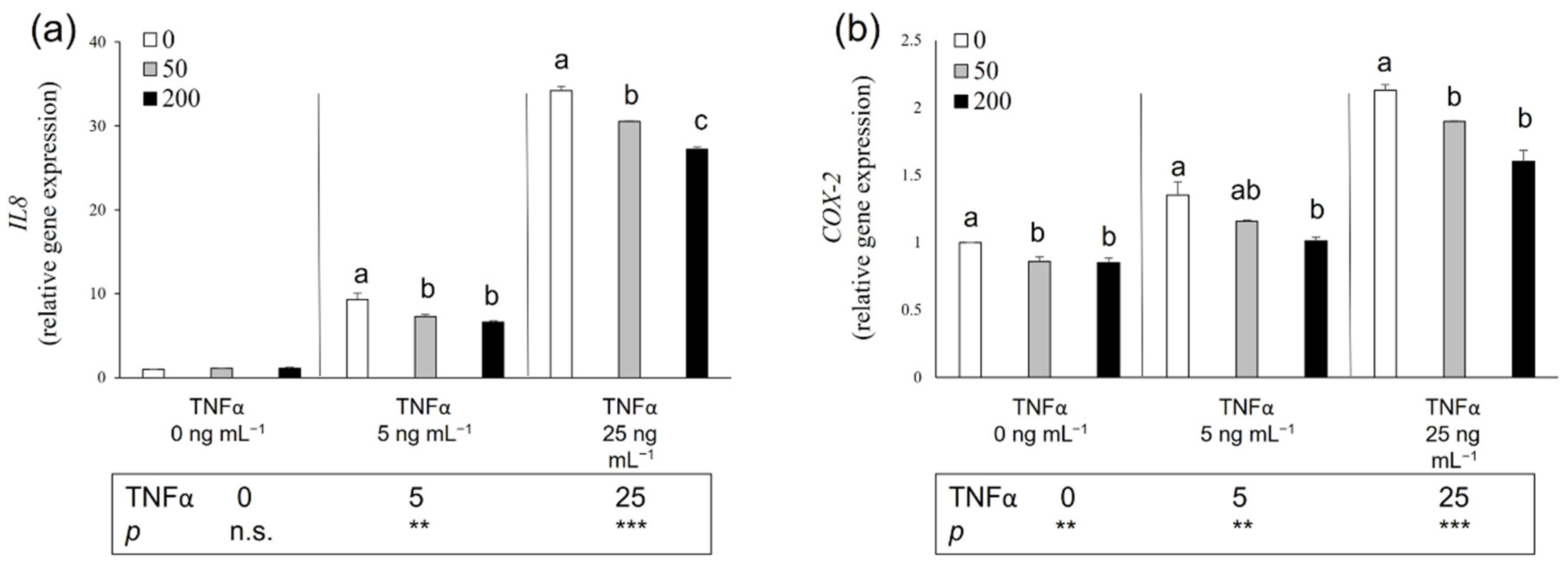
3.6. Potential Anti-Inflammatory Effect of Salicornia Extract on HT-29 Cells
4. Discussion
4.1. Nutrient Composition and Fatty Acid Profile
4.2. Glycine Betaine
4.3. Bioactive Compounds
4.4. Biological Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Volume I: Comprehensive Tables; United Nations, Department of Economic and Social Affairs, Population Division: New York, NY, USA, 2019. [Google Scholar]
- Abdelly, C.; Barhoumi, Z.; Ghnaya, T.; Debez, A.; Ben Hamed, K.; Ksouri, R.; Talbi, O.; Zribi, F.; Ouerghi, Z.; Smaoui, A.; et al. Potential utilisation of halophytes for the rehabilitation and valorisation of salt-affected areas in Tunisia. In Biosaline Agriculture and Salinity Tolerance in Plants; Oztürk, M., Waisel, Y., Khan, M.A., Görk, G.G., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2006; pp. 163–172. [Google Scholar]
- Daoud, S.; Elbrik, K.; Tachbibi, N.; Bouqbis, L.; Brakez, M.; Harrouni, M.C. The Potential Use of Halophytes for the Development of Marginal Dry Areas in Morocco. In Halophytes for Food Security in Dry Lands; Khan, M.A., Ozturk, M., Gul, B., Ahmed, M.Z., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 141–156. ISBN 978-0-12-801854-5. [Google Scholar]
- Duarte, B.; Feijão, E.; Pinto, M.V.; Matos, A.R.; Silva, A.; Figueiredo, A.; Fonseca, V.F.; Reis-Santos, P.; Caçador, I. Nutritional Valuation and Food Safety of Endemic Mediterranean Halophytes Species Cultivated in Abandoned Salt Pans under a Natural Irrigation Scheme. Estuar. Coast. Shelf Sci. 2022, 265, 107733. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; da Rosa Neng, N.; Nogueira, J.M.F.; Marques, C.; Santos, T.F.; Varela, J.; Custódio, L. Searching for New Sources of Innovative Products for the Food Industry within Halophyte Aromatic Plants: In Vitro Antioxidant Activity and Phenolic and Mineral Contents of Infusions and Decoctions of Crithmum Maritimum L. Food Chem. Toxicol. 2017, 107, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Souid, A.; Bellani, L.; Magné, C.; Zorrig, W.; Smaoui, A.; Abdelly, C.; Longo, V.; ben Hamed, K. Physiological and Antioxidant Responses of the Sabkha Biotope Halophyte Limonium Delicatulum to Seasonal Changes in Environmental Conditions. Plant Physiol. Biochem. 2018, 123, 180–191. [Google Scholar] [CrossRef]
- Souid, A.; Gabriele, M.; Longo, V.; Pucci, L.; Bellani, L.; Smaoui, A.; Abdelly, C.; ben Hamed, K. Salt Tolerance of the Halophyte Limonium Delicatulum Is More Associated with Antioxidant Enzyme Activities than Phenolic Compounds. Funct. Plant Biol. 2016, 43, 607–619. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible Halophytes of the Mediterranean Basin: Potential Candidates for Novel Food Products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Qasim, M.; Abideen, Z.; Adnan, M.Y.; Gulzar, S.; Gul, B.; Rasheed, M.; Khan, M.A. Antioxidant Properties, Phenolic Composition, Bioactive Compounds and Nutritive Value of Medicinal Halophytes Commonly Used as Herbal Teas. S. Afr. J. Bot. 2017, 110, 240–250. [Google Scholar] [CrossRef]
- Gyawali, R.; Hayek, S.A.; Ibrahim, S.A. Plant Extracts as Antimicrobials in Food Products: Mechanisms of Action, Extraction Methods, and Applications. In Handbook of Natural Antimicrobials for Food Safety and Quality; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 49–68. ISBN 9781782420422. [Google Scholar]
- ben Hamed, K.; Castagna, A.; Ranieri, A.; García-Caparrós, P.; Santin, M.; Hernandez, J.A.; Espin, G.B. Halophyte Based Mediterranean Agriculture in the Contexts of Food Insecurity and Global Climate Change. Environ. Exp. Bot. 2021, 191, 104601. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Rajabi Dehnavi, A.; Leszczyński, K.; Lubińska-Mielińska, S.; Ludwiczak, A.; Piernik, A. Salicornia europaea L. Functional Traits Indicate Its Optimum Growth. Plants 2022, 11, 1051. [Google Scholar] [CrossRef]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of Day Length on Flowering and Yield Production of Salicornia and Sarcocornia Species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Rebelo, J.; Silva, H.; Pinto, D.C.G.A. Gall Midge Baldratia Salicorniae Kieffer (Diptera: Cecidomyiidae) Infestation on Salicornia Europaea L. Induces the Production of Specialized Metabolites with Biotechnological Potential. Phytochemistry 2022, 200, 113207. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Helrich, K. Association of Official Analytical Chemist, Official Methods of Analysis of the AOAC, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fibre Analysis (Apparatus, Reagents, Procedures and Some Applications); US Agricultural Research Service: Washington, DC, USA, 1970. [Google Scholar]
- Vogel, K.P.; Pedersen, J.F.; Masterson, S.D.; Toy, J.J. Evaluation of a Filter Bag System for NDF, ADF, and IVDMD Forage Analysis. Crop Sci. 1999, 39, 276–279. [Google Scholar] [CrossRef]
- Christie, W.W. Preparation of ester derivatives of fatty acids for chromatographic analysis. In Advances in Lipid Methodology—Two; Christie, W.W., Ed.; Oily Press: Dundee, Scotland, 1993; pp. 69–111. [Google Scholar]
- Ulbricht, T.L.v.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Tavarini, S.; Castagna, A.; Conte, G.; Foschi, L.; Sanmartin, C.; Incrocci, L.; Ranieri, A.; Serra, A.; Angelini, L.G. Evaluation of Chemical Composition of Two Linseed Varieties as Sources of Health-Beneficial Substances. Molecules 2019, 24, 3729. [Google Scholar] [CrossRef] [PubMed]
- Alonso Borbalán, Á.M.; Zorro, L.; Guillén, D.A.; García Barroso, C. Study of the Polyphenol Content of Red and White Grape Varieties by Liquid Chromatography-Mass Spectrometry and Its Relationship to Antioxidant Power. J. Chromatogr. A 2003, 1012, 31–38. [Google Scholar] [CrossRef]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of Polyphenolics and Their Antioxidant Capacity in Fresh Plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Blummel, M.; Borowy, N.K.; Becker, K. Gravimetric Determination of Tannins and Their Correlations with Chemical and Protein Precipitation Methods. J. Sci. Food Agric. 1993, 61, 161–165. [Google Scholar] [CrossRef]
- Castagna, A.; di Baccio, D.; Tognetti, R.; Ranieri, A.; Sebastiani, L. Differential Ozone Sensitivity Interferes with Cadmium Stress in Poplar Clones. Biol. Plant. 2013, 57, 313–324. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Caddeo, C.; Gabriele, M.; Nácher, A.; Fernàndez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and Artemisinin Eudragit-Coated Liposomes: A Strategy to Tackle Intestinal Tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Pucci, L.; la Marca, M.; Lucchesi, D.; della Croce, C.M.; Longo, V.; Lubrano, V. A Fermented Bean Flour Extract Downregulates LOX-1, CHOP and ICAM-1 in HMEC-1 Stimulated by Ox-LDL. Cell. Mol. Biol. Lett. 2016, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, M.; Pucci, L.; Árvay, J.; Longo, V. Anti-Inflammatory and Antioxidant Effect of Fermented Whole Wheat on TNFα-Stimulated HT-29 and NF-ΚB Signaling Pathway Activation. J. Funct. Foods 2018, 45, 392–400. [Google Scholar] [CrossRef]
- Barreira, L.; Resek, E.; Rodrigues, M.J.; Rocha, M.I.; Pereira, H.; Bandarra, N.; da Silva, M.M.; Varela, J.; Custódio, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 59, 35–42. [Google Scholar] [CrossRef]
- Díaz, F.J.; Benes, S.E.; Grattan, S.R. Field Performance of Halophytic Species under Irrigation with Saline Drainage Water in the San Joaquin Valley of California. Agric. Water Manag. 2013, 118, 59–69. [Google Scholar] [CrossRef]
- Lopes, M.; Roque, M.J.; Cavaleiro, C.; Ramos, F. Nutrient Value of Salicornia Ramosissima—A Green Extraction Process for Mineral Analysis. J. Food Compos. Anal. 2021, 104, 104135. [Google Scholar] [CrossRef]
- Papandreou, D.; Noor, Z.T.; Rashed, M. The Role of Soluble, Insoluble Fibers and Their Bioactive Compounds in Cancer: A Mini Review. Food Nutr. Sci. 2015, 6, 53027. [Google Scholar] [CrossRef]
- Threapleton, D.E.; Greenwood, D.C.; Evans, C.E.L.; Cleghorn, C.L.; Nykjaer, C.; Woodhead, C.; Cade, J.E.; Gale, C.P.; Burley, V.J. Dietary Fibre Intake and Risk of Cardiovascular Disease: Systematic Review and Meta-Analysis. BMJ 2013, 347, f6879. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia Ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Garaffo, M.A.; Vassallo-Agius, R.; Nengas, Y.; Lembo, E.; Rando, R.; Maisano, R.; Dugo, G.; Giuffrida, D. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices, of Raw Roe of Blue Fin Tuna (Thunnus Thynnus L.) and Their Salted Product “Bottarga.”. Food Nutr. Sci. 2011, 2, 736–743. [Google Scholar] [CrossRef]
- Rodhes, D.; Hanson, A.D. Quaternary Ammonium and Tertiary Sulfonium Compounds in Higher Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2003, 44, 357–384. [Google Scholar]
- Moghaieb, R.E.A.; Saneoka, H.; Fujita, K. Effect of Salinity on Osmotic Adjustment, Glycinebetaine Accumulation and the Betaine Aldehyde Dehydrogenase Gene Expression in Two Halophytic Plants, Salicornia Europaea and Suaeda Maritima. Plant Sci. 2004, 166, 1345–1349. [Google Scholar] [CrossRef]
- Bohn, T.; Bonet, M.L.; Borel, P.; Keijer, J.; Landrier, J.F.; Milisav, I.; Ribot, J.; Riso, P.; Winklhofer-Roob, B.; Sharoni, Y.; et al. Mechanistic Aspects of Carotenoid Health Benefits—Where Are We Now? Nutr. Res. Rev. 2021, 34, 276–302. [Google Scholar] [CrossRef]
- Sgherri, C.; Pinzino, C.; Navari-Izzo, F.; Izzo, R. Contribution of Major Lipophilic Antioxidants to the Antioxidant Activity of Basil Extracts: An EPR Study. J. Sci. Food Agric. 2011, 91, 1128–1134. [Google Scholar] [CrossRef]
- Castañeda-Loaiza, V.; Oliveira, M.; Santos, T.; Schüler, L.; Lima, A.R.; Gama, F.; Salazar, M.; Neng, N.R.; Nogueira, J.M.F.; Varela, J.; et al. Wild vs Cultivated Halophytes: Nutritional and Functional Differences. Food Chem. 2020, 333, 127536. [Google Scholar] [CrossRef]
- Fan, X.; Fan, Z.; Yang, Z.; Huang, T.; Tong, Y.; Yang, D.; Mao, X.; Yang, M. Flavonoids—Natural Gifts to Promote Health and Longevity. Int. J. Mol. Sci. 2022, 23, 2176. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Barreca, D.; Trombetta, D.; Smeriglio, A.; Mandalari, G.; Romeo, O.; Felice, M.R.; Gattuso, G.; Nabavi, S.M. Food Flavonols: Nutraceuticals with Complex Health Benefits and Functionalities. Trends Food Sci. Technol. 2021, 117, 194–204. [Google Scholar] [CrossRef]
- Bonelli, F.; Turini, L.; Sarri, G.; Serra, A.; Buccioni, A.; Mele, M. Oral Administration of Chestnut Tannins to Reduce the Duration of Neonatal Calf Diarrhea. BMC Vet. Res. 2018, 14, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cappucci, A.; Mantino, A.; Buccioni, A.; Casarosa, L.; Conte, G.; Serra, A.; Mannelli, F.; Luciano, G.; Foggi, G.; Mele, M. Diets Supplemented with Condensed and Hydrolysable Tannins Affected Rumen Fatty Acid Profile and Plasmalogen Lipids, Ammonia and Methane Production in an in vitro Study. Ital. J. Anim. Sci. 2021, 20, 935–946. [Google Scholar] [CrossRef]
- Souid, A.; Della Croce, C.M.; Frassinetti, S.; Gabriele, M.; Pozzo, L.; Ciardi, M.; Abdelly, C.; Hamed, K.B.; Magné, C.; Longo, V. Nutraceutical Potential of Leaf Hydro-Ethanolic Extract of the Edible Halophyte Crithmum Maritimum L. Molecules 2021, 26, 5380. [Google Scholar] [CrossRef] [PubMed]
- Stanković, M.S.; Petrović, M.; Godjevac, D.; Stevanović, Z.D. Screening Inland Halophytes from the Central Balkan for Their Antioxidant Activity in Relation to Total Phenolic Compounds and Flavonoids: Are There Any Prospective Medicinal Plants? J. Arid. Environ. 2015, 120, 26–32. [Google Scholar] [CrossRef]
- Hulkko, L.S.S.; Chaturvedi, T.; Thomsen, M.H. Extraction and Quantification of Chlorophylls, Carotenoids, Phenolic Compounds, and Vitamins from Halophyte Biomasses. Appl. Sci. 2022, 12, 840. [Google Scholar] [CrossRef]
- Rodrigues, M.J.; Gangadhar, K.N.; Vizetto-Duarte, C.; Wubshet, S.G.; Nyberg, N.T.; Barreira, L.; Varela, J.; Custódio, L. Maritime Halophyte Species from Southern Portugal as Sources of Bioactive Molecules. Mar. Drugs 2014, 12, 2228–2244. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia Patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef]
- Santin, M.; Ranieri, A.; Castagna, A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants 2021, 10, 1485. [Google Scholar] [CrossRef]
- Kang, S.; Kim, M.R.; Chiang, M.; Hong, J. Evaluation and Comparison of Functional Properties of Freshwater-Cultivated Glasswort (Salicornia Herbacea L.) with Naturally-Grown Glasswort. Food Sci. Biotechnol. 2015, 24, 2245–2250. [Google Scholar] [CrossRef]
- Costa, C.S.B.; Chaves, F.C.; Rombaldi, C.v.; Souza, C.R. Bioactive Compounds and Antioxidant Activity of Three Biotypes of the Sea Asparagus Sarcocornia Ambigua (Michx.) M.A.Alonso & M.B.Crespo: A Halophytic Crop for Cultivation with Shrimp Farm Effluent. S. Afr. J. Bot. 2018, 117, 95–100. [Google Scholar] [CrossRef]
- Pinheiro, I.; Arantes, R.; do Espírito Santo, C.M.; do Nascimento Vieira, F.; Lapa, K.R.; Gonzaga, L.V.; Fett, R.; Barcelos-Oliveira, J.L.; Seiffert, W.Q. Production of the Halophyte Sarcocornia Ambigua and Pacific White Shrimp in an Aquaponic System with Biofloc Technology. Ecol. Eng. 2017, 100, 261–267. [Google Scholar] [CrossRef]
- Cosquer, A.; Ficamos, M.; Jebbar, M.; Corbel, J.C.; Choquet, G.; Fontenelle, C.; Uriac, P.; Bernard, T. Antibacterial Activity of Glycine Betaine Analogues: Involvement of Osmoporters. Bioorganic Med. Chem. Lett. 2004, 14, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, D.; Lee, B.H.; Kim, M.R.; Hong, J.; Chiang, M. Antioxidant Properties and Cytotoxic Effects of Fractions from Glasswort (Salicornia Herbacea) Seed Extracts on Human Intestinal Cells. Food Sci. Biotechnol. 2011, 20, 115–122. [Google Scholar] [CrossRef]
- Altay, A.; Celep, G.S.; Yaprak, A.E.; Baskose, I.; Bozoglu, F. Glassworts as Possible Anticancer Agents against Human Colorectal Adenocarcinoma Cells with Their Nutritive, Antioxidant and Phytochemical Profiles. Chem. Biodivers. 2017, 14, e1600290. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.C.; Zhang, H.B.; Gu, C.D.; Guo, S.D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.Q. Protective Effect of Acacetin on Sepsis-Induced Acute Lung Injury via Its Anti-Inflammatory and Antioxidative Activity. Arch. Pharmacal Res. 2018, 41, 1199–1210. [Google Scholar] [CrossRef]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The Ameliorative Effects of the Ethyl Acetate Extract of Salicornia Europaea L. and Its Bioactive Candidate, Irilin B, on LPS-Induced Microglial Inflammation and MPTP-Intoxicated PD-like Mouse Model. Oxidative Med. Cell. Longev. 2019, 2019, 6764756. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]

| Time (min) | Solvent A 1 (%) | Solvent B 1 (%) |
|---|---|---|
| 0 | 100 | 0 |
| 8 | 100 | 0 |
| 10 | 0 | 100 |
| 26 | 0 | 100 |
| 28 | 100 | 0 |
| 32 | 100 | 0 |
| S | S-T | SE | p | |
|---|---|---|---|---|
| Dry matter | 18.21 | 18.20 | 0.62 | n.s. |
| Crude protein | 13.82 | 12.35 | 0.63 | n.s. |
| Ether extract | 1.37 | 1.28 | 0.06 | n.s. |
| Ashes | 31.24 | 32.23 | 1.23 | n.s. |
| Carbohydrates | 14.39 | 16.41 | 0.88 | n.s. |
| NDF 1 | 39.18 | 37.63 | 1.43 | n.s. |
| ADF 2 | 23.29 | 23.08 | 1.00 | n.s. |
| ADL 3 | 5.23 | 4.19 | 0.34 | n.s. |
| S | S-T | SE | p | |
|---|---|---|---|---|
| C14:0 | 0.97 (0.20) | 0.90 (19) | 0.04 | n.s. |
| C16:0 | 103.48 (21.79) | 102.23 (21.50) | 2.03 | n.s. |
| C16:1c7 | 2.24 (0.47) | 2.56 (0.54) | 0.54 | n.s. |
| C16:1c9 | 0.69 (0.15) | 0.78 (0.16) | 0.03 | n.s. |
| C17:0 | 0.63 (0.13) | 0.54 (0.11) | 0.03 | n.s. |
| C18:0 | 7.54 (1.59) | 7.63 (1.60) | 1.03 | n.s. |
| C18:1c9 | 12.18 (2.57) | 12.27 (2.58) | 1.34 | n.s. |
| C18:1c11 | 1.77 (0.37) | 1.86(0.39) | 0.27 | n.s. |
| C18:2cc | 104.33 (21.97) | 104.52 (21.98) | 2.13 | n.s. |
| C20:0 | 14.00 (2.95) | 14.50 (3.05) | 1.56 | n.s. |
| C20:1c11 | 0.93 (0.20) | 0.88 (0.19) | 0.05 | n.s. |
| C18:3n3 | 209.41 (44.10) | 210.0 (44.17) | 4.32 | n.s. |
| C21:0 | 0.42 (0.09) | 0.51 (0.11) | 0.04 | n.s. |
| C22:0 | 3.83 (0.81) | 3.74 (0.79) | 0.41 | n.s. |
| C20:3n6 | 2.23 (0.47) | 2.32 (0.49) | 0.51 | n.s. |
| C22:1t13 | 0.46 (0.10) | 0.57 (0.12) | 0.04 | n.s. |
| C20:3n3 | 0.41 (0.09) | 0.32 (0.07) | 0.05 | n.s. |
| C22:1c13 | 0.26 (0.05) | 0.35 (0.07) | 0.05 | n.s. |
| C23:0 | 1.48 (0.31) | 1.39 (0.29) | 0.33 | n.s. |
| C20:5n3 | 0.39 (0.08) | 0.41 (0.09) | 0.05 | n.s. |
| C24:0 | 5.63 (1.19) | 5.54 (1.17) | 0.66 | n.s. |
| C22:5n6 | 0.43 (0.09) | 0.54 (0.11) | 0.07 | n.s. |
| C22:6n3 | 1.10 (0.23) | 1.09 (0.23) | 0.12 | n.s. |
| SFA 1 | 137.97 (29.06) | 136.98 (28.81) | 2.78 | n.s. |
| MUFA 2 | 18.52 (3.90) | 19.27 (4.05) | 1.67 | n.s. |
| PUFAn6 3 | 106.99 (22.53) | 107.38 (22.58) | 2.34 | n.s. |
| PUFAn3 4 | 211.30 (44.50) | 211.82 (44.55) | 4.56 | n.s. |
| n6/n3 ratio | 0.51 | 0.51 | 0.02 | n.s. |
| AI 5 | 0.32 | 0.31 | 0.04 | n.s. |
| TI 6 | 0.16 | 0.16 | 0.02 | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castagna, A.; Mariottini, G.; Gabriele, M.; Longo, V.; Souid, A.; Dauvergne, X.; Magné, C.; Foggi, G.; Conte, G.; Santin, M.; et al. Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae 2022, 8, 828. https://doi.org/10.3390/horticulturae8090828
Castagna A, Mariottini G, Gabriele M, Longo V, Souid A, Dauvergne X, Magné C, Foggi G, Conte G, Santin M, et al. Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae. 2022; 8(9):828. https://doi.org/10.3390/horticulturae8090828
Chicago/Turabian StyleCastagna, Antonella, Giada Mariottini, Morena Gabriele, Vincenzo Longo, Aymen Souid, Xavier Dauvergne, Christian Magné, Giulia Foggi, Giuseppe Conte, Marco Santin, and et al. 2022. "Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils" Horticulturae 8, no. 9: 828. https://doi.org/10.3390/horticulturae8090828
APA StyleCastagna, A., Mariottini, G., Gabriele, M., Longo, V., Souid, A., Dauvergne, X., Magné, C., Foggi, G., Conte, G., Santin, M., & Ranieri, A. (2022). Nutritional Composition and Bioactivity of Salicornia europaea L. Plants Grown in Monoculture or Intercropped with Tomato Plants in Salt-Affected Soils. Horticulturae, 8(9), 828. https://doi.org/10.3390/horticulturae8090828







