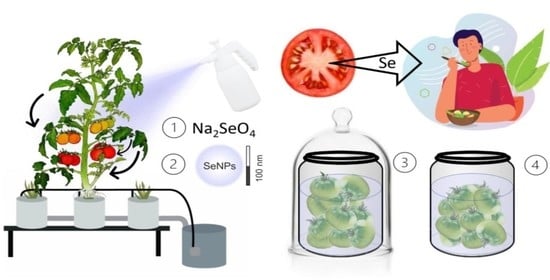
Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. On-Plant Fruit Enrichment
2.1.1. Cv. Kreos Experiment
2.1.2. Cv. Micro-Tom Experiment
2.2. Off-Plant Fruit Enrichment
2.2.1. Vacuum Infiltration
2.2.2. Passive Immersion
2.3. Sample Preparation and Se Determination
2.3.1. Oven Drying
2.3.2. Lyophilization (Freeze-Drying)
2.3.3. Selenium Determination
2.4. Statistical Analysis
3. Results and Discussion
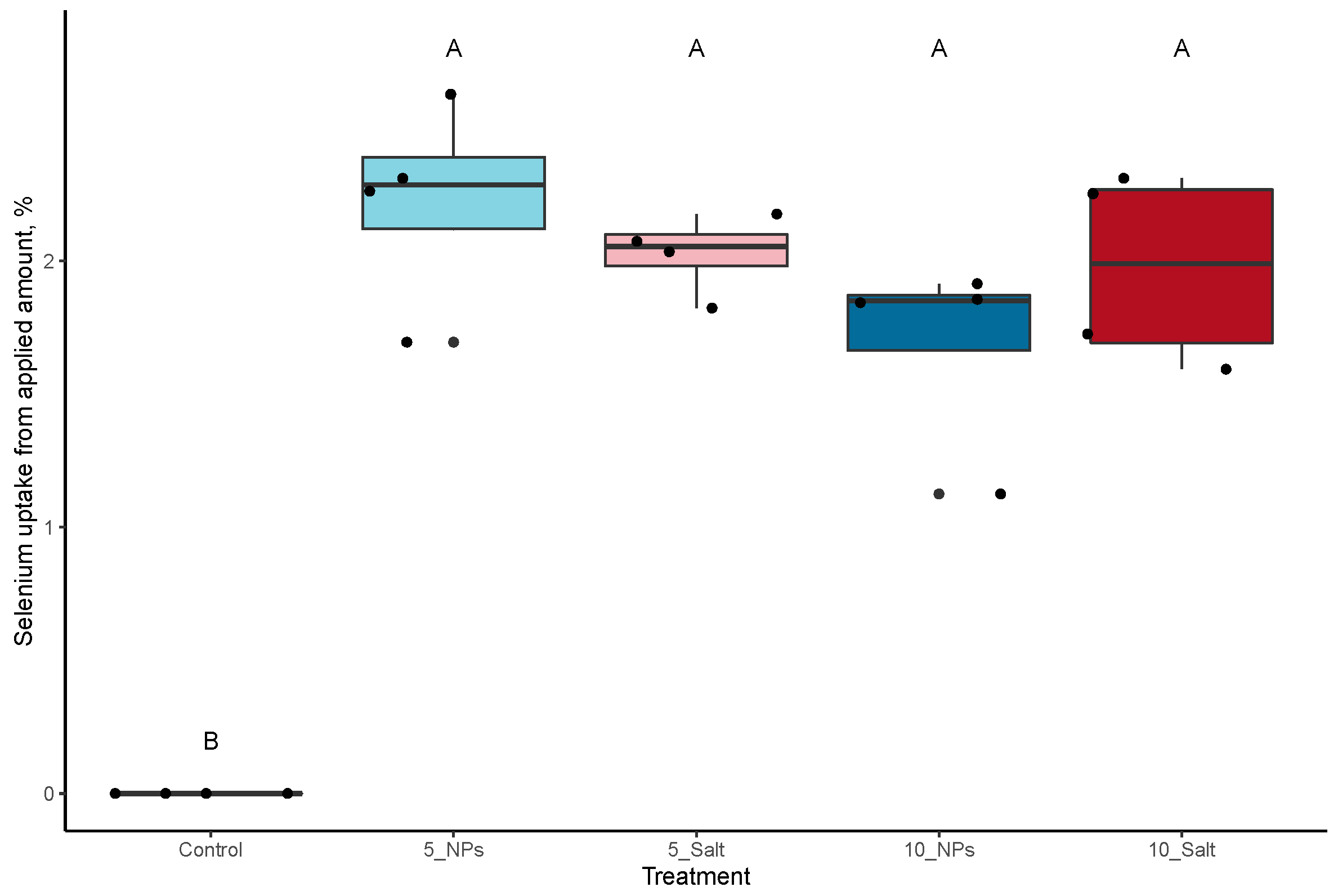
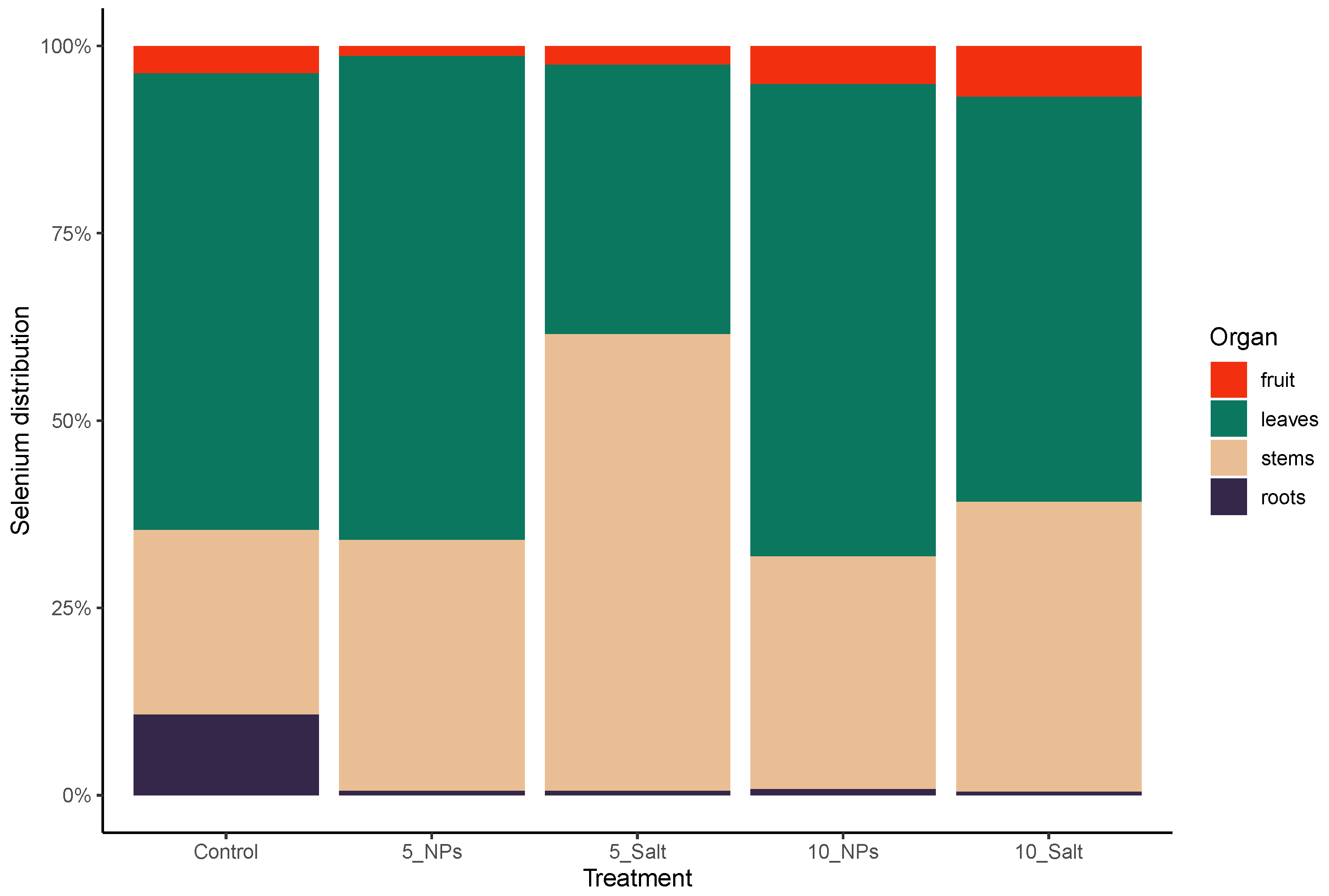
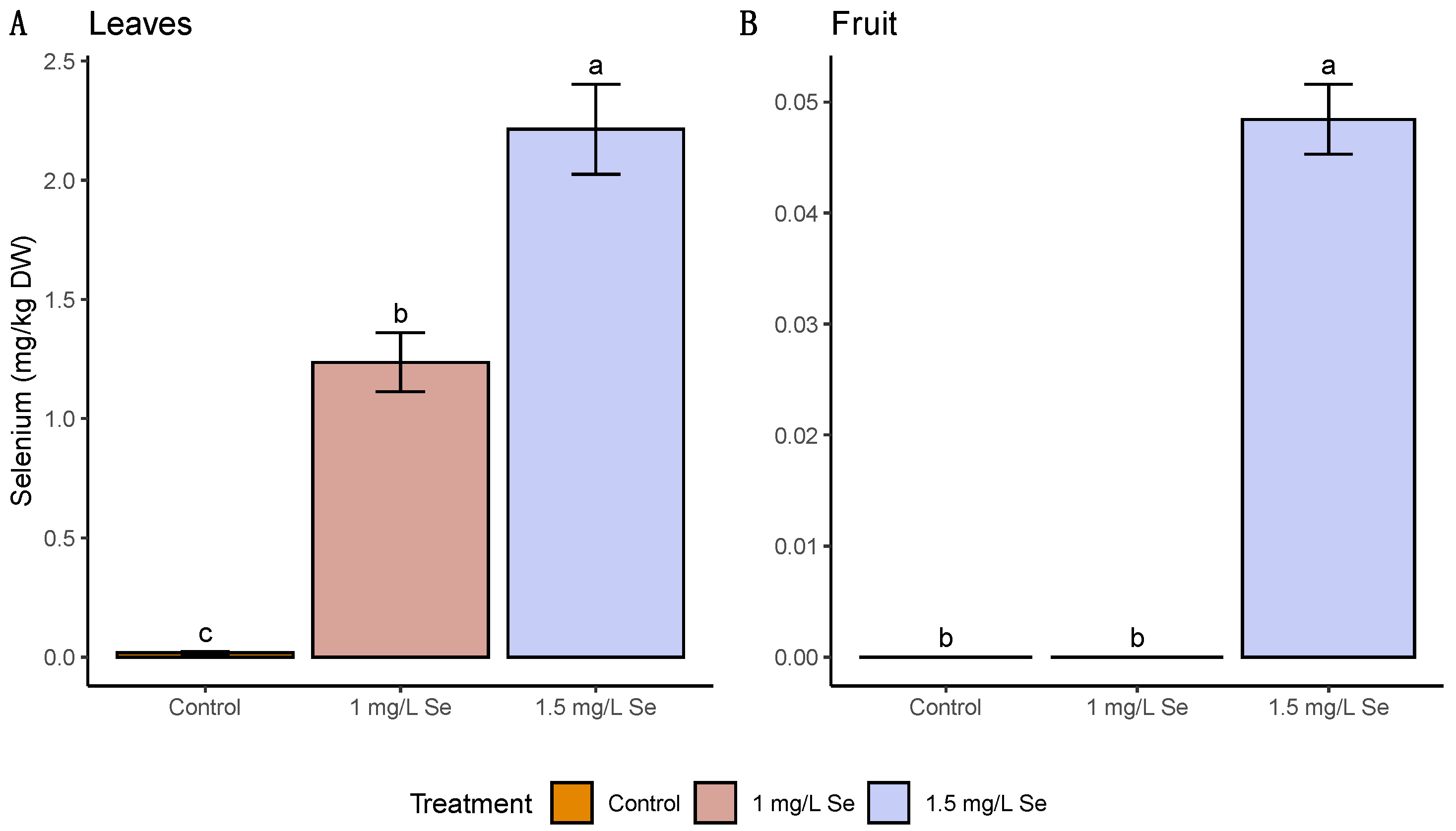
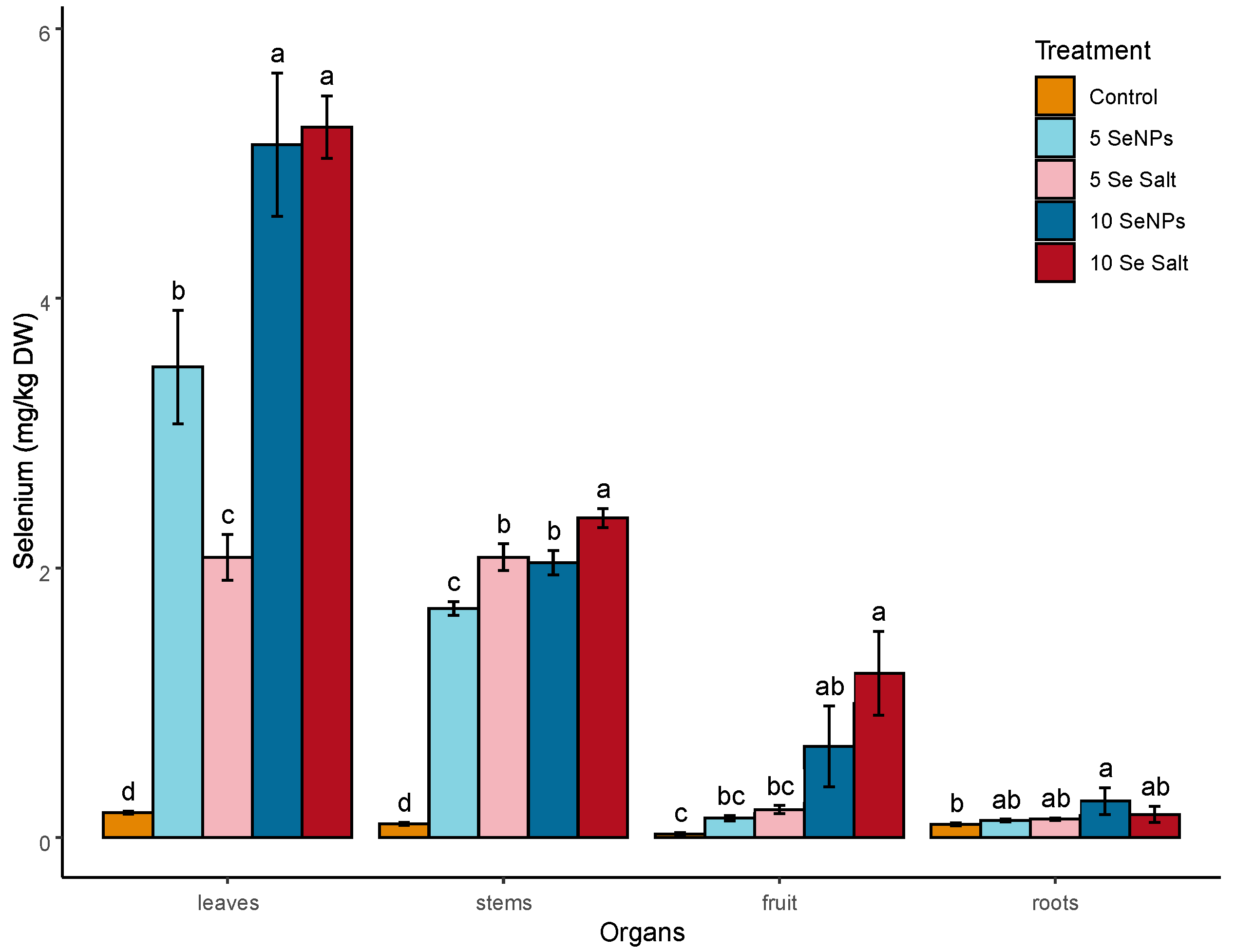
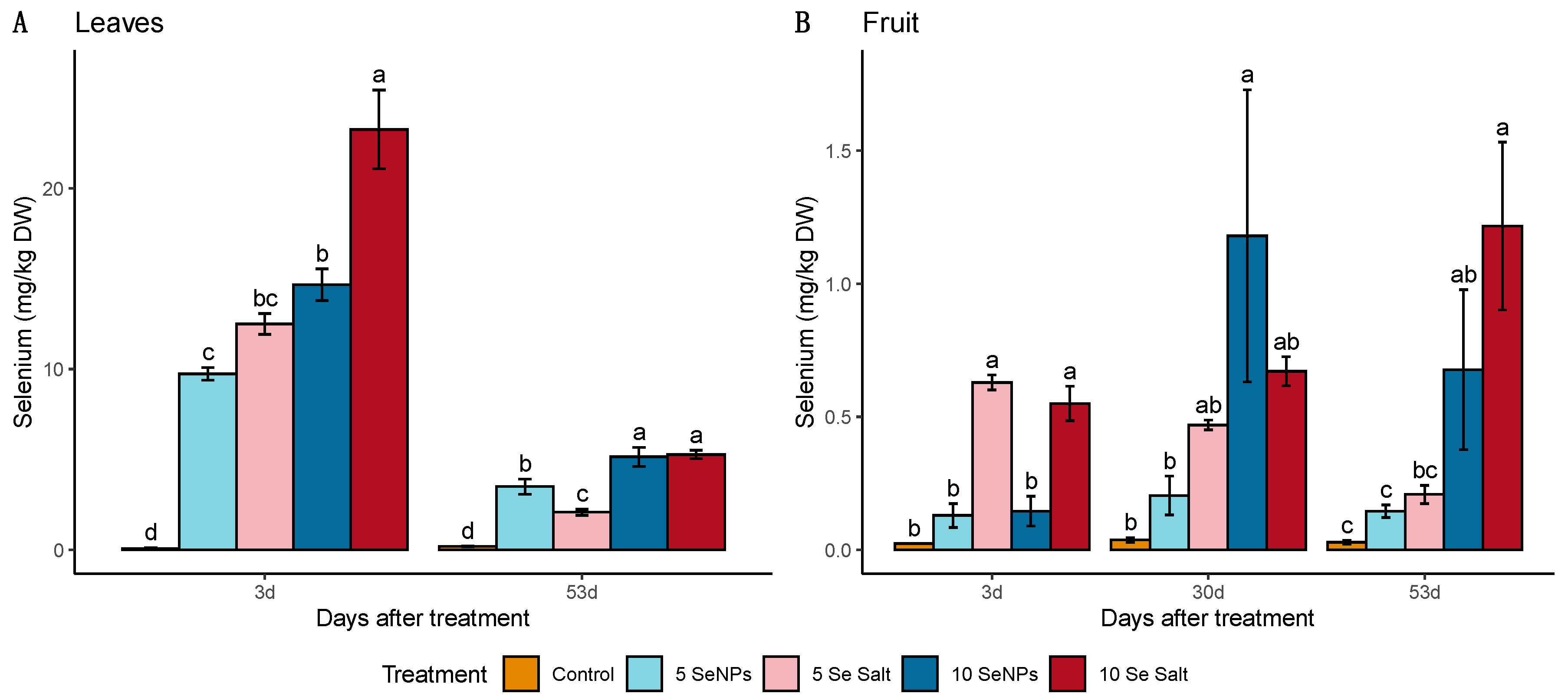
3.1. On-Plant Enrichment
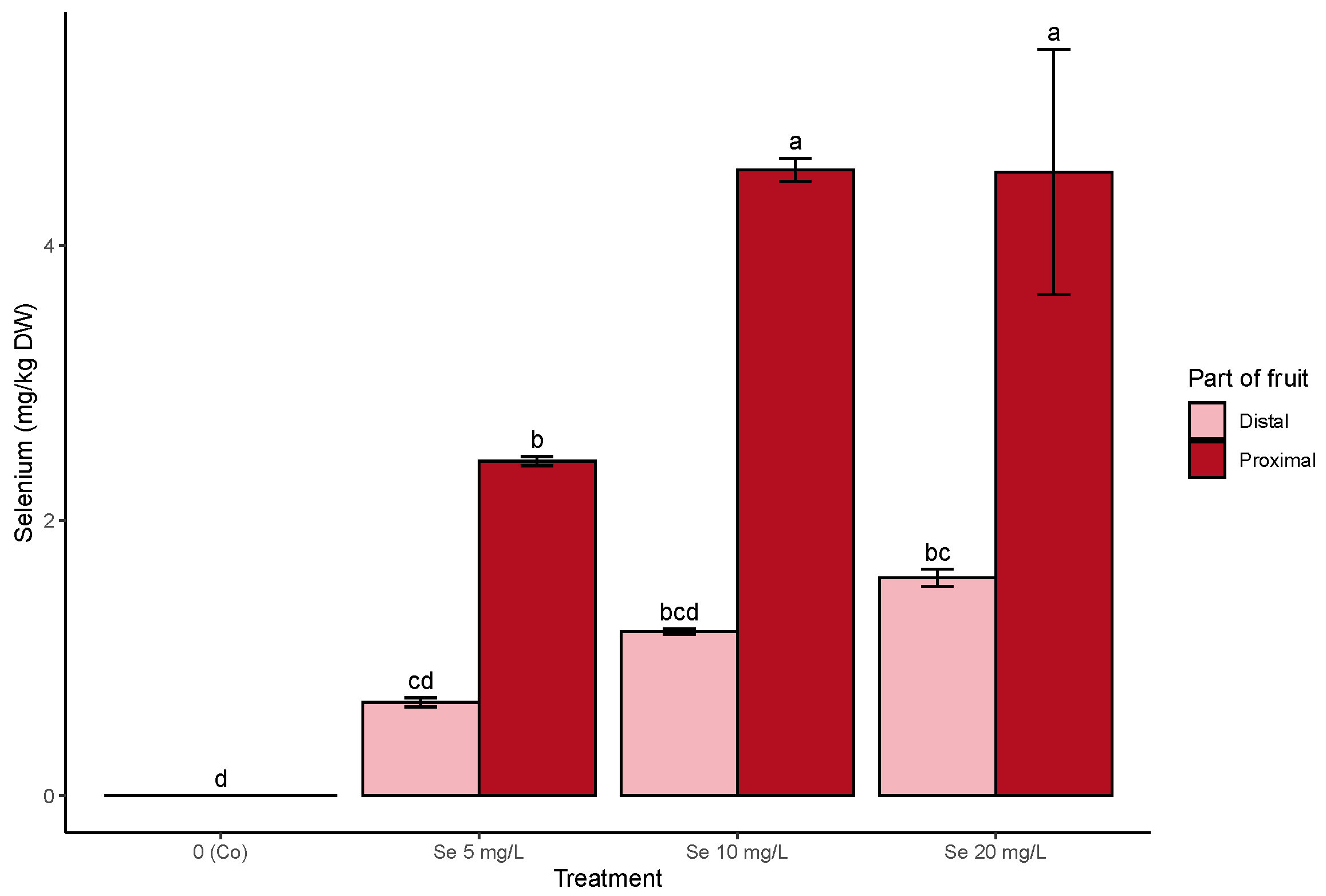
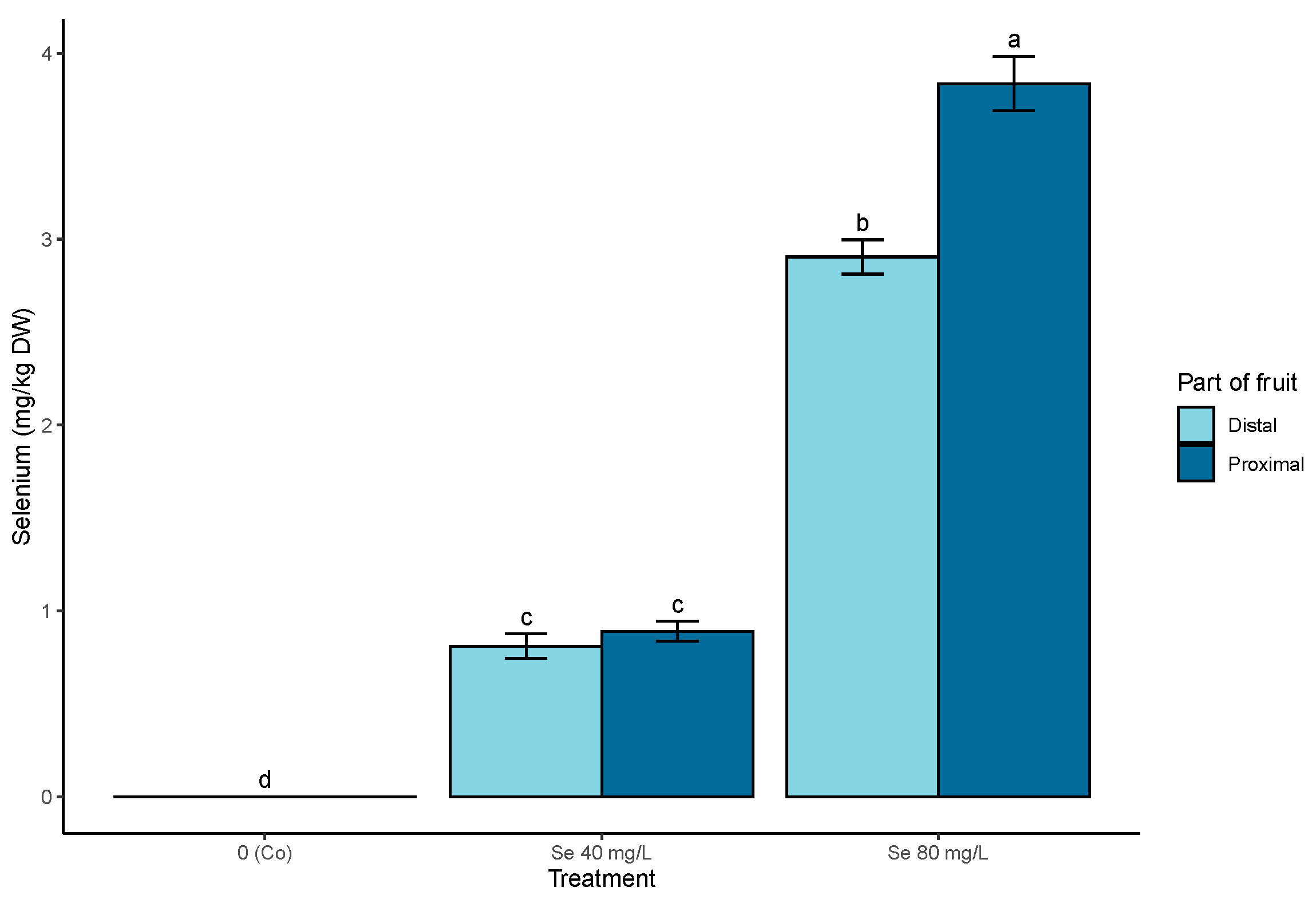
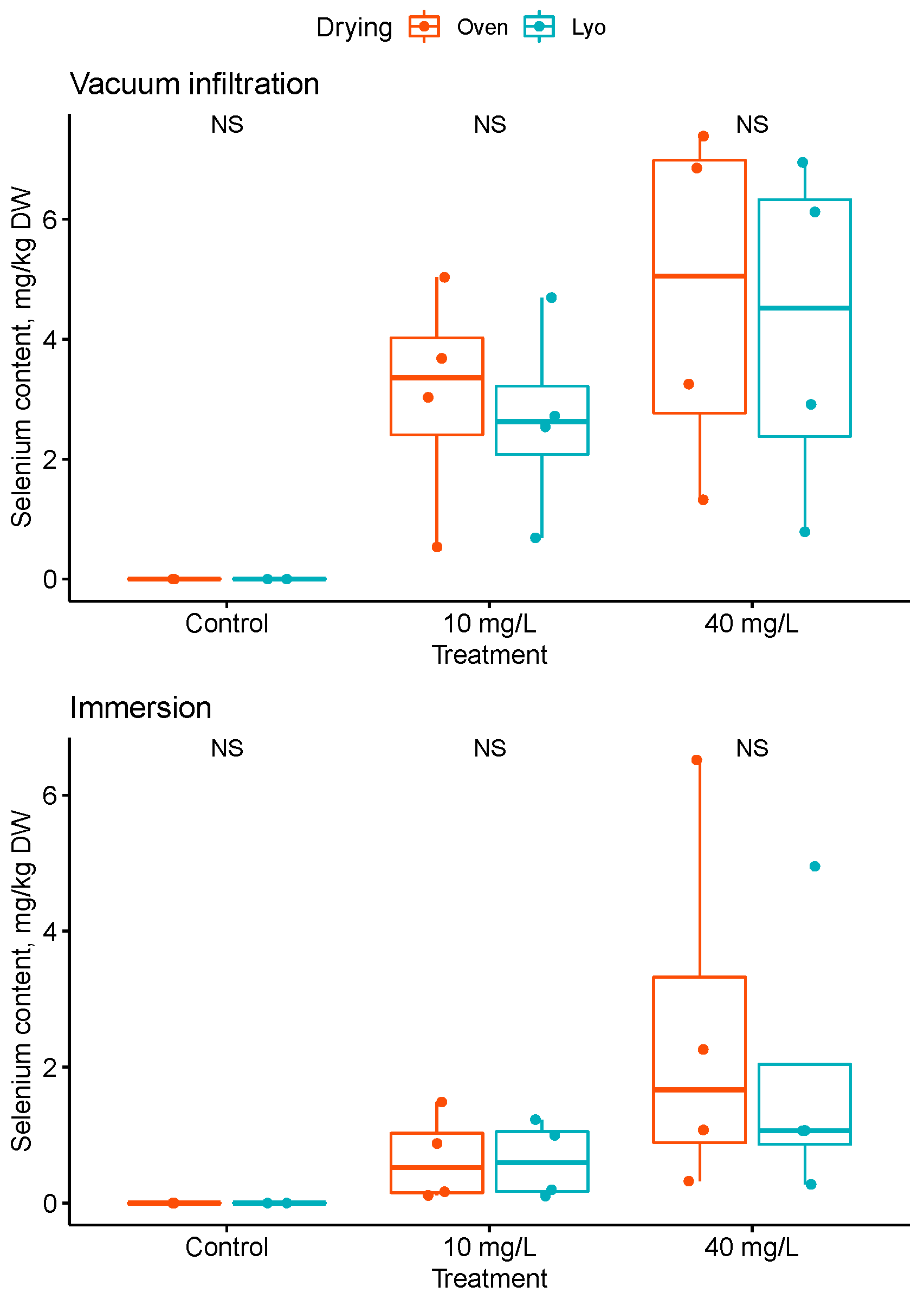
3.2. Off-Plant Enrichment
3.3. An Innovative Tool to Facilitate Sample Preparation for Se Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Golubkina, N.A. Selenium Accumulation by Cereals in Russia. Russ. Agric. Sci. 2007, 33, 288–291. [Google Scholar] [CrossRef]
- Sedykh, E.M.; Lyabusheva, O.A.; Tambiev, A.K.; Bannykh, L.N. Determination of the Elemental Composition of Cyanobacteria Cells and Cell Fractions by Atomic Emission and Atomic Absorption Spectrometry. J. Anal. Chem. 2005, 60, 29–33. [Google Scholar] [CrossRef]
- Tóth, R.J.; Csapó, J. The Role of Selenium in Nutrition—A Review. Acta Univ. Sapientiae Aliment. 2019, 11, 128–144. [Google Scholar] [CrossRef]
- Kieliszek, M.; Bano, I.; Zare, H. A Comprehensive Review on Selenium and Its Effects on Human Health and Distribution in Middle Eastern Countries. Biol. Trace Elem. Res. 2022, 200, 971–987. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Combs, G.F. Selenium in Global Food Systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular Pathways and Physiological Roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium Distribution in the Chinese Environment and Its Relationship with Human Health: A Review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and Human Health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Achibat, H.; AlOmari, N.A.; Messina, F.; Sancineto, L.; Khouili, M.; Santi, C. Organoselenium Compounds as Phytochemicals from the Natural Kingdom. Nat. Prod. Commun. 2015, 10, 1885–1892. [Google Scholar] [CrossRef] [Green Version]
- Li, H.F.; McGrath, S.P.; Zhao, F.J. Selenium Uptake, Translocation and Speciation in Wheat Supplied with Selenate or Selenite. New Phytol. 2008, 178, 92–102. [Google Scholar] [CrossRef]
- Buturi, C.V.; Mauro, R.P.; Fogliano, V.; Leonardi, C.; Giuffrida, F. Mineral Biofortification of Vegetables as a Tool to Improve Human Diet. Foods 2021, 10, 223. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; Rodrigo, S.; Santamaría, O.; Chen, Y.; McGrath, S.P. Agronomic Selenium Biofortification in Triticum Durum under Mediterranean Conditions: From Grain to Cooked Pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Wang, X.; Wong, Y.S. Generation of Selenium-Enriched Rice with Enhanced Grain Yield, Selenium Content and Bioavailability through Fertilisation with Selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium Accumulation by Plants. Ann. Bot. 2016, 117, 217–235. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Overv. Selenium Uptake Metab. Toxic. Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Qi, M.; Peng, Q.; Wang, M.; Bañuelos, G.S.; Miao, S.; Li, Z.; Dinh, Q.T.; Liang, D. Insights into Uptake, Accumulation, and Subcellular Distribution of Selenium among Eight Wheat (Triticum aestivum L.) Cultivars Supplied with Selenite and Selenate. Ecotoxicol. Environ. Saf. 2021, 207, 111544. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Su, X.; He, J.; Liang, Y.; Zhou, T.; Liu, C. Selenium (Se) Uptake and Dynamic Changes of Se Content in Soil–Plant Systems. Environ. Sci. Pollut. Res. 2018, 25, 34343–34350. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Zhumaev, A.A.; Dem’ianova-Roi, G.B. Pattern of Selenium Distribution in Tomatoes Lycopersicum Esculentum Mill. Izv. Akad. Nauk Ser. Biol. 2003, 30, 565–569. [Google Scholar]
- Schiavon, M.; Dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.H.; Sambo, P.; Masi, A.; Malagoli, M. Selenium Fertilization Alters the Chemical Composition and Antioxidant Constituents of Tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef]
- Nancy, D.; Arulselvi, P.I. Effect Od Selenium Fortification on Biochemical Activities of Tomato (Solanum lycopersicum) Plants. Indo Am. J. Pharm. Res. 2014, 4, 4. [Google Scholar]
- Meucci, A.; Shiriaev, A.; Rosellini, I.; Malorgio, F.; Pezzarossa, B. Se-Enrichment Pattern, Composition, and Aroma Profile of Ripe Tomatoes after Sodium Selenate Foliar Spraying Performed at Different Plant Developmental Stages. Plants 2021, 10, 1050. [Google Scholar] [CrossRef]
- Puccinelli, M.; Malorgio, F.; Terry, L.A.; Tosetti, R.; Rosellini, I.; Pezzarossa, B. Effect of Selenium Enrichment on Metabolism of Tomato (Solanum lycopersicum) Fruit during Postharvest Ripening. J. Sci. Food Agric. 2019, 99, 2463–2472. [Google Scholar] [CrossRef]
- Lee, G.J.; Kang, B.K.; Kim, T.I.; Kim, T.J.; Kim, J.H. Effects of Different Selenium Concentrations of the Nutrient Solution on the Growth and Quality of Tomato Fruit in Hydroponics. Acta Hortic. 2007, 761, 443–448. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Rosellini, I.; Borghesi, E.; Tonutti, P.; Malorgio, F. Effects of Se-Enrichment on Yield, Fruit Composition and Ripening of Tomato (Solanum lycopersicum) Plants Grown in Hydroponics. Sci. Hortic. 2014, 165, 106–110. [Google Scholar] [CrossRef]
- Keskinen, R.; Yli-Halla, M.; Hartikainen, H. Retention and Uptake by Plants of Added Selenium in Peat Soils. Commun. Soil Sci. Plant Anal. 2013, 44, 3465–3482. [Google Scholar] [CrossRef]
- Kaur, N.; Sharma, S.; Kaur, S.; Nayyar, H. Selenium in Agriculture: A Nutrient or Contaminant for Crops? Arch. Agron. Soil Sci. 2014, 60, 1593–1624. [Google Scholar] [CrossRef]
- Cremonini, E.; Zonaro, E.; Donini, M.; Lampis, S.; Boaretti, M.; Dusi, S.; Melotti, P.; Lleo, M.M.; Vallini, G. Biogenic Selenium Nanoparticles: Characterization, Antimicrobial Activity and Effects on Human Dendritic Cells and Fibroblasts. Microb. Biotechnol. 2016, 9, 758–771. [Google Scholar] [CrossRef]
- Cele, T. Preparation of Nanoparticles. In Engineered Nanomaterials Health and Safety; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Wang, K.; Wang, Y.; Li, K.; Wan, Y.; Wang, Q.; Zhuang, Z.; Guo, Y.; Li, H. Uptake, Translocation and Biotransformation of Selenium Nanoparticles in Rice Seedlings (Oryza sativa L.). J. Nanobiotechnol. 2020, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Marissa, P.; Delia, A.; Cabrera, M.; Fuente, D.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; et al. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Awasthi, S.; Srivastava, S.; Dwivedi, S.; Pilon-Smits, E.A.H.; Dhankher, O.P.; Tripathi, R.D. Understanding Selenium Metabolism in Plants and Its Role as a Beneficial Element. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1937–1958. [Google Scholar] [CrossRef]
- Neysanian, M.; Iranbakhsh, A.; Ahmadvand, R.; Ardebili, Z.O.; Ebadi, M. Comparative Efficacy of Selenate and Selenium Nanoparticles for Improving Growth, Productivity, Fruit Quality, and Postharvest Longevity through Modifying Nutrition, Metabolism, and Gene Expression in Tomato; Potential Benefits and Risk Assessment. PLoS ONE 2020, 15, e0244207. [Google Scholar] [CrossRef]
- Li, Q.; Chen, T.; Yang, F.; Liu, J.; Zheng, W. Facile and controllable one-step fabrication of selenium nanoparticles assisted by L-cysteine. Mater. Lett. 2010, 6, 614–617. [Google Scholar] [CrossRef]
- Zasoski, R.J.; Burau, R.G. A Rapid Nitric-perchloric Acid Digestion Method for Multi-element Tissue Analysis. Commun. Soil Sci. Plant Anal. 1977, 8, 425–436. [Google Scholar] [CrossRef]
- Karny, A.; Zinger, A.; Kajal, A.; Shainsky-Roitman, J.; Schroeder, A. Therapeutic Nanoparticles Penetrate Leaves and Deliver Nutrients to Agricultural Crops. Sci. Rep. 2018, 8, 7589. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of Foliar Treatment of Sodium Selenate on Postharvest Decay and Quality of Tomato Fruits. Sci. Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Narváez-Ortiz, W.; Becvort-Azcurra, A.; Fuentes-Lara, L.; Benavides-Mendoza, A.; Valenzuela-García, J.; González-Fuentes, J. Mineral Composition and Antioxidant Status of Tomato with Application of Selenium. Agronomy 2018, 8, 185. [Google Scholar] [CrossRef]
- Edelstein, M.; Berstein, D.; Shenker, M.; Azaizeh, H.; Ben-Hur, M. Effects of Selenium on Growth Parameters of Tomato and Basil under Fertigation Management. HortScience 2016, 51, 1050–1056. [Google Scholar] [CrossRef] [Green Version]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Senevirathna, P.; Daundasekera, W. Effect of Postharvest Calcium Chloride Vacuum Infiltration on the Shelf Life and Quality of Tomato (Cv. ’Thilina’). Ceylon J. Sci. Biological. Sci. 2010, 39, 35. [Google Scholar] [CrossRef]
- Pezzarossa, B.; Remorini, D.; Gentile, M.L.; Massai, R. Effects of Foliar and Fruit Addition of Sodium Selenate on Selenium Accumulation and Fruit Quality. J. Sci. Food Agric. 2012, 92, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, K.F.M.; Berton, R.S.; Coscione, A.R. Selenium Biofortification of Rice and Radish: Effect of Soil Texture and Efficiency of Two Extractants. Plant Soil Environ. 2014, 60, 105–110. [Google Scholar]
- Harvey, M.A.; Erskine, P.D.; Harris, H.H.; Brown, G.K.; Pilon-Smits, E.A.H.; Casey, L.W.; Echevarria, G.; Van Der Ent, A. Distribution and Chemical Form of Selenium in: Neptunia Amplexicaulis from Central Queensland, Australia. Metallomics 2020, 12, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Novak, T.; Maršić, N.K.; Šircelj, H.; Stibilj, V.; Jerše, A.; Kroflič, A.; Germ, M. Biofortification with Selenium and Iodine Changes Morphological Properties of Brassica oleracea L. Var. Gongylodes) and Increases Their Contents in Tubers. Plant Physiol. Biochem. 2020, 150, 234–243. [Google Scholar] [CrossRef]
- Wang, M.; Dinh, Q.T.; Qi, M.; Wang, M.; Yang, W.; Zhou, F.; Liang, D. Radicular and Foliar Uptake, and Xylem- and Phloem-Mediated Transport of Selenium in Maize (Zea mays L.): A Comparison of Five Se Exogenous Species. Plant Soil 2020, 446, 111–123. [Google Scholar] [CrossRef]
- Wang, M.; Yang, W.; Zhou, F.; Du, Z.; Xue, M.; Chen, T.; Liang, D. Effect of Phosphate and Silicate on Selenite Uptake and Phloem- Mediated Transport in Tomato (Solanum lycopersicum L.). Environ. Sci. Pollut. Res. 2019, 26, 20475–20484. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shiriaev, A.; Pezzarossa, B.; Rosellini, I.; Malorgio, F.; Lampis, S.; Ippolito, A.; Tonutti, P. Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes. Horticulturae 2022, 8, 800. https://doi.org/10.3390/horticulturae8090800
Shiriaev A, Pezzarossa B, Rosellini I, Malorgio F, Lampis S, Ippolito A, Tonutti P. Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes. Horticulturae. 2022; 8(9):800. https://doi.org/10.3390/horticulturae8090800
Chicago/Turabian StyleShiriaev, Anton, Beatrice Pezzarossa, Irene Rosellini, Fernando Malorgio, Silvia Lampis, Antonio Ippolito, and Pietro Tonutti. 2022. "Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes" Horticulturae 8, no. 9: 800. https://doi.org/10.3390/horticulturae8090800
APA StyleShiriaev, A., Pezzarossa, B., Rosellini, I., Malorgio, F., Lampis, S., Ippolito, A., & Tonutti, P. (2022). Efficacy and Comparison of Different Strategies for Selenium Biofortification of Tomatoes. Horticulturae, 8(9), 800. https://doi.org/10.3390/horticulturae8090800