Comparative Effects of Four Plant Growth Regulators on Yield and Field Performance of Crocus sativus L.
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions
2.2. Treatments
2.3. Statistical Analysis
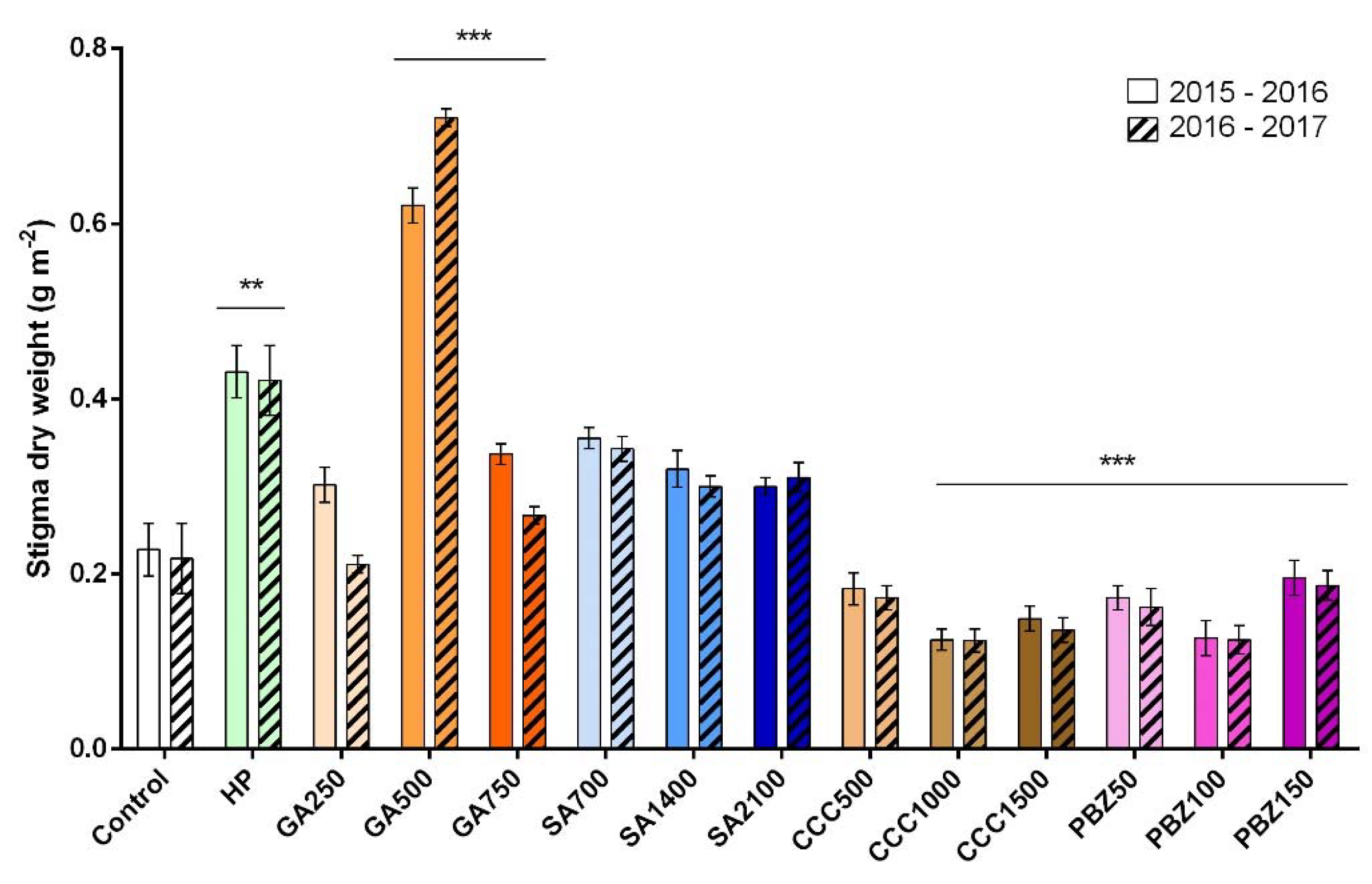
3. Results
3.1. Leaf Size, Number and Dry Weight
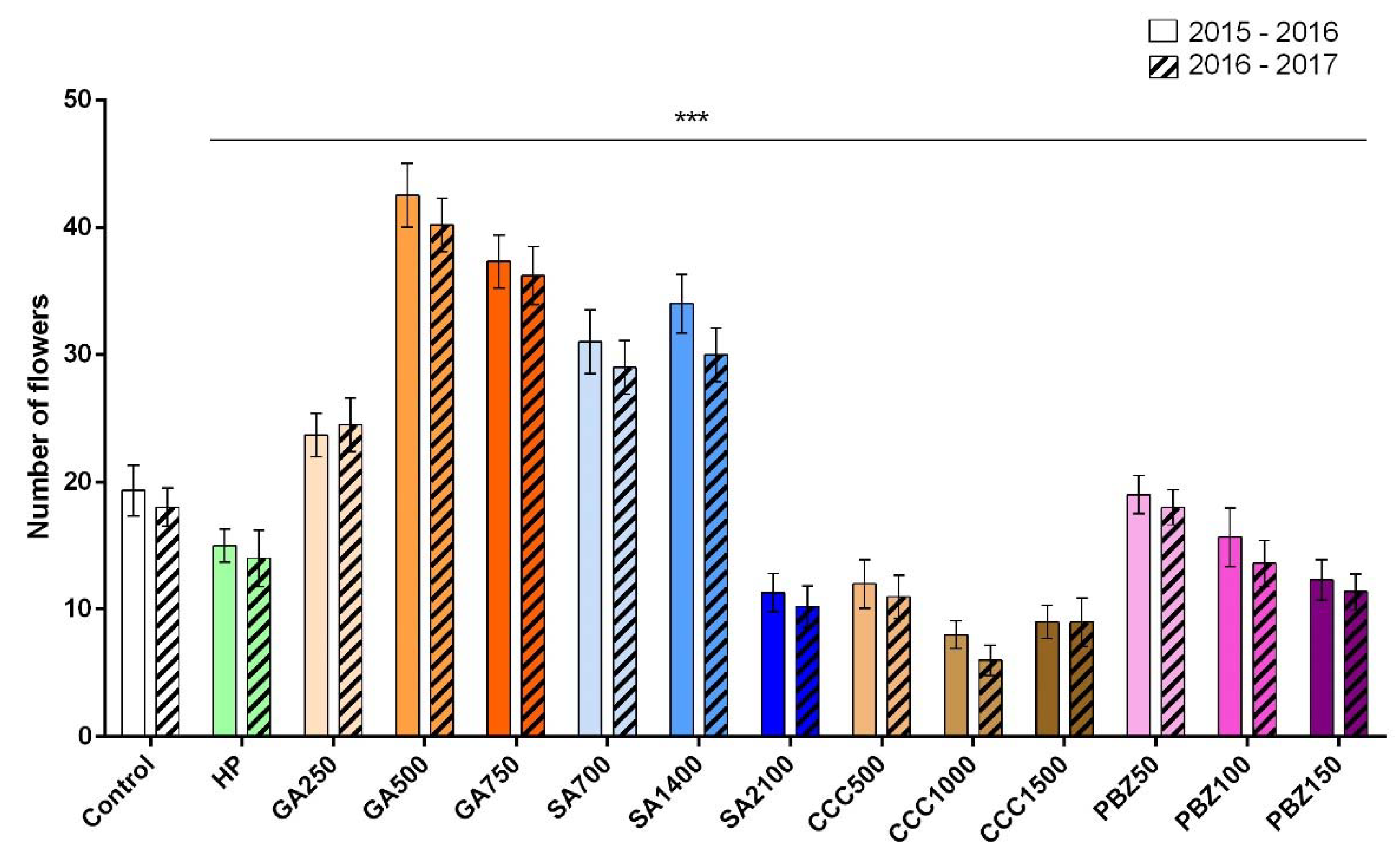
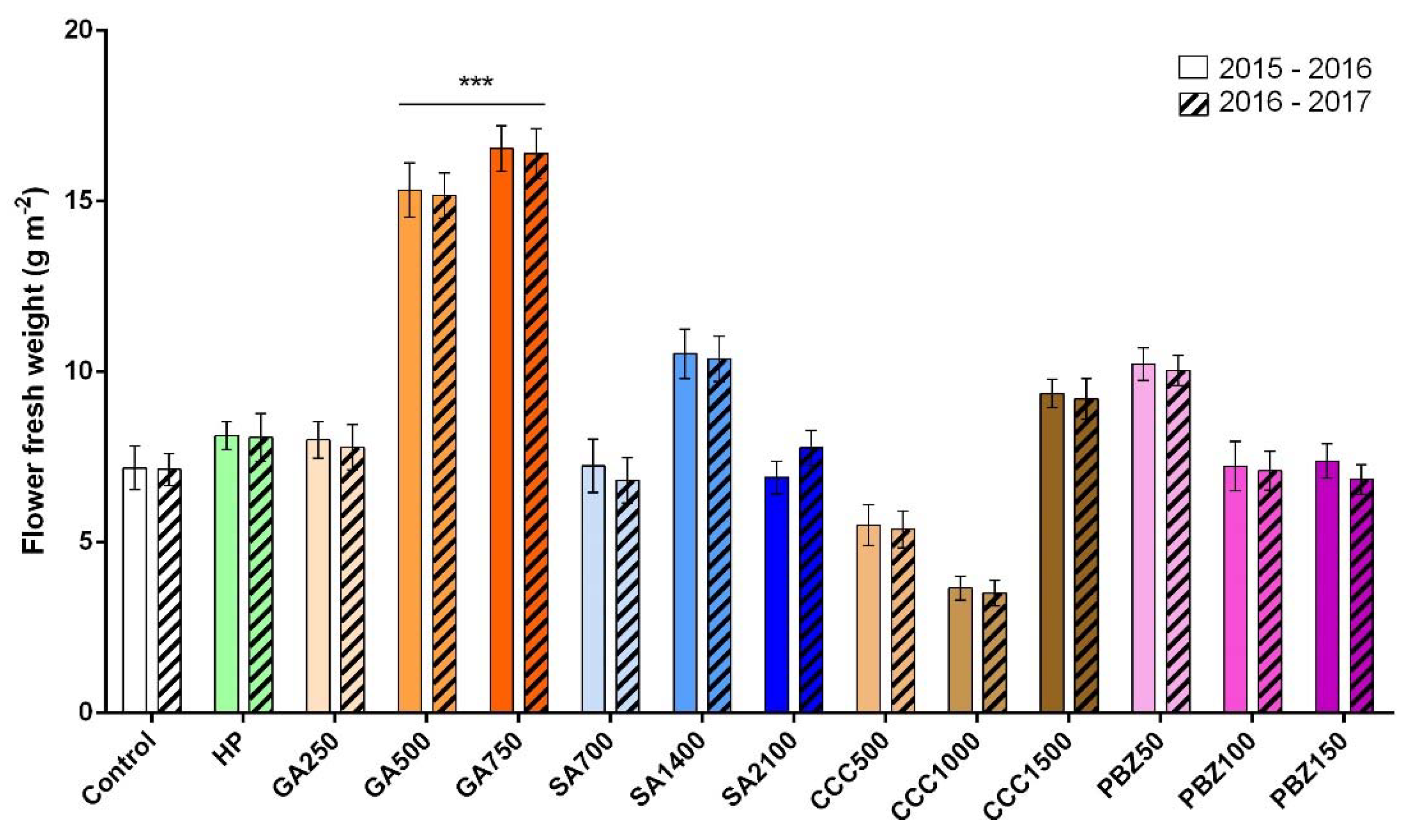
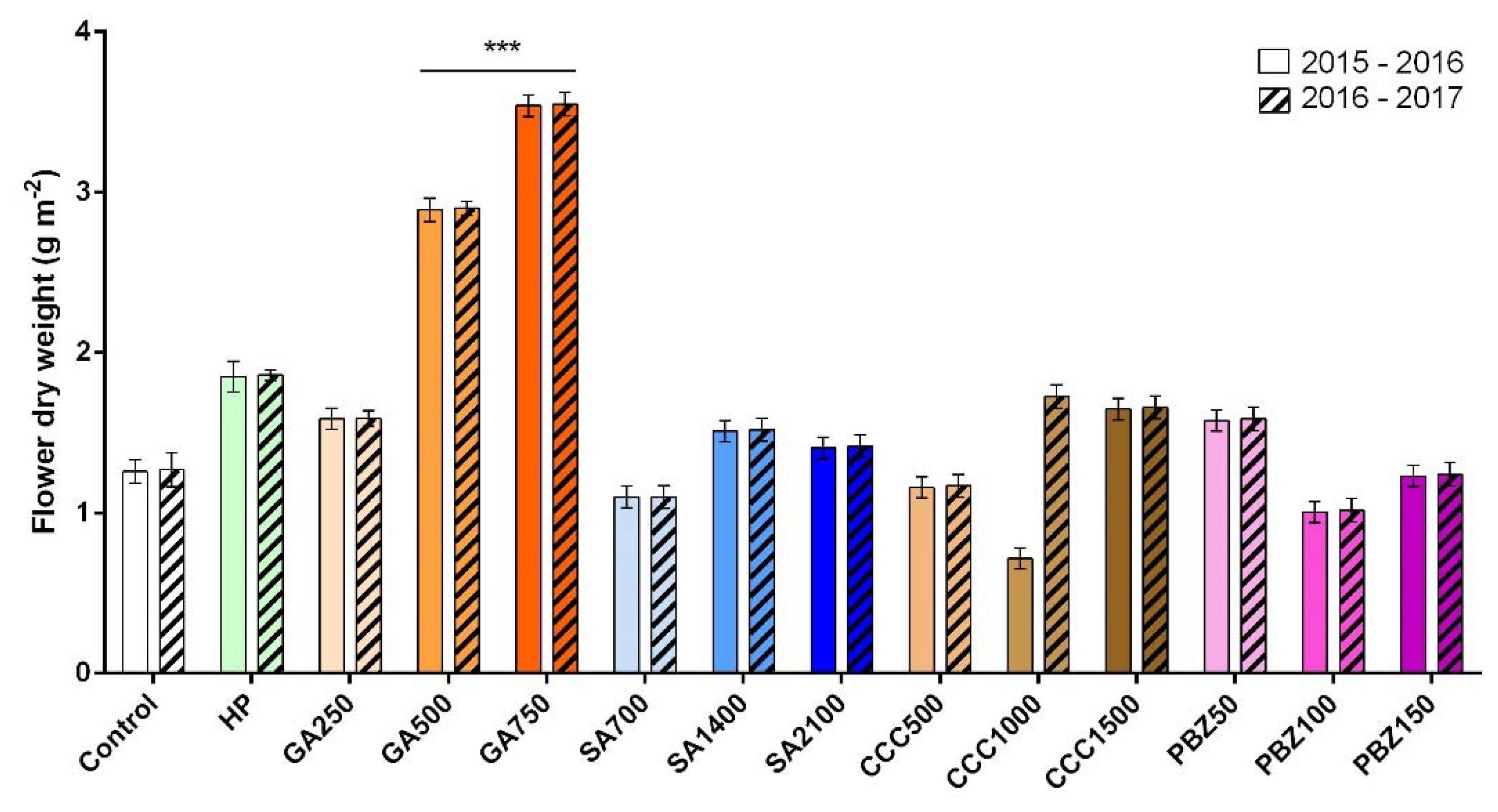
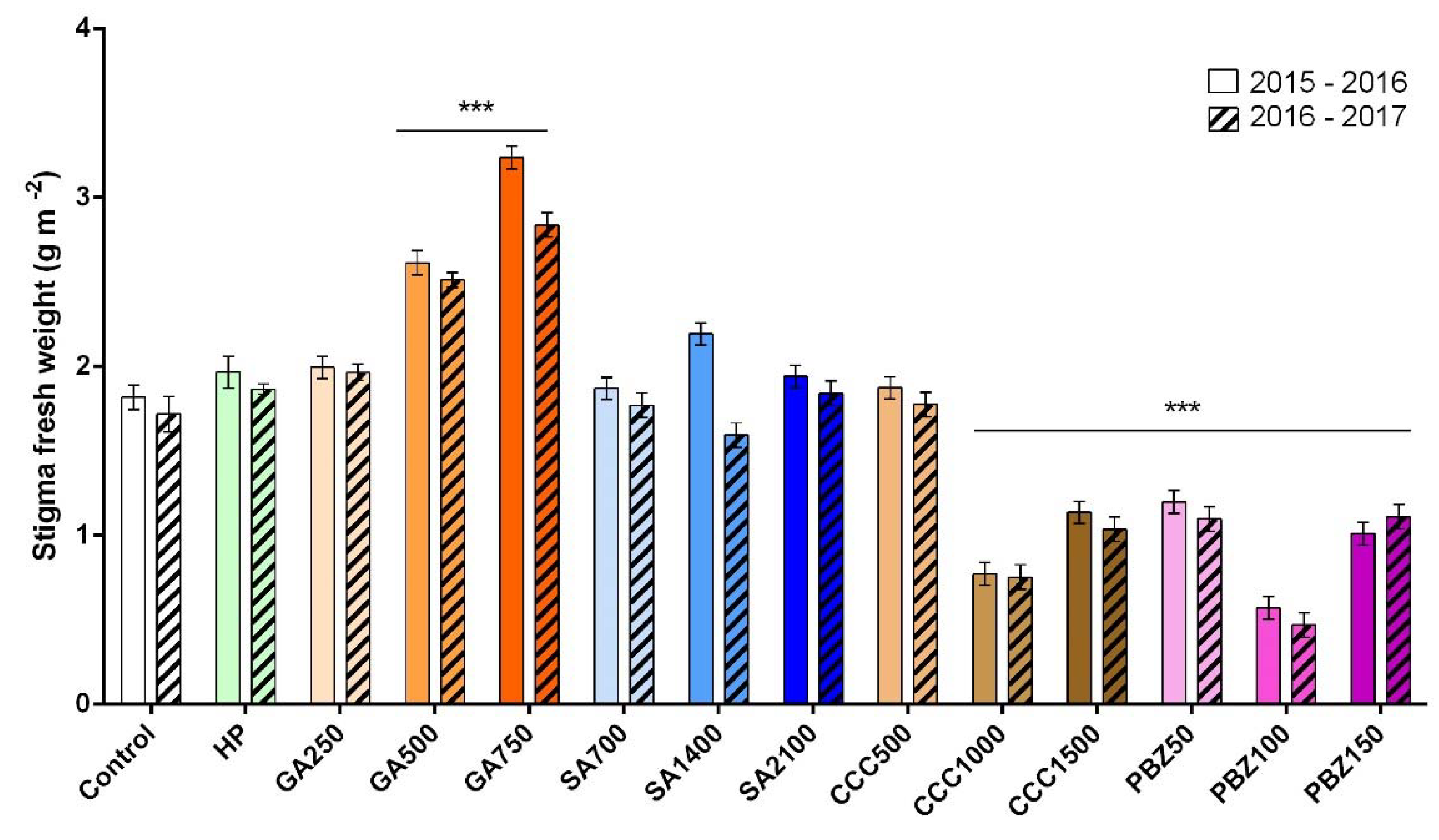
3.2. Flower and Stigma Number, Fresh Weight, and Dry Weight
3.3. Daughter Corm Number and Weight
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vahedi, M.; Kabiri, M.; Salami, S.A.; Rezadoost, H.; Mirzaie, M.; Kanani, M.R. Quantitative HPLC-based metabolomics of some Iranian saffron (Crocus sativus L.) accessions. Ind. Crops Prod. 2018, 118, 26–29. [Google Scholar] [CrossRef]
- Kafi, M.; Kamili, A.N.; Husaini, A.M.; Ozturk, M.; Altay, V. An Expensive Spice Saffron (Crocus sativus L.): A Case Study from Kashmir, Iran, and Turkey. In Global Perspectives on Underutilized Crops; Springer: Berlin/Heidelberg, Germany, 2018; pp. 109–149. [Google Scholar] [CrossRef]
- Ahouran, M. Biology and Biotechnology of Crocus. Bulb. Plants Biotechnol. 2014, 152–176. [Google Scholar]
- Rubio-Moraga, A.; Ahrazem, O.; Pérez-Clemente, R.M.; Gómez-Cadenas, A.; Yoneyama, K.; López-Ráez, J.A.; Molina, R.V.; Gómez-Gómez, L. Apical dominance in saffron and the involvement of the branching enzymes CCD7 and CCD8 in the control of bud sprouting. BMC Plant Biol. 2014, 14, 171. [Google Scholar] [CrossRef] [PubMed]
- Kothari, D.; Thakur, M.; Joshi, R.; Kumar, A.; Kumar, R. Agro-Climatic Suitability Evaluation for Saffron Production in Areas of Western Himalaya. Front. Plant Sci. 2021, 12, 657819. [Google Scholar] [CrossRef]
- Karimi, M.; Ahmadi, A.; Hashemi, J.; Abbasi, A.; Tavarini, S.; Pompeiano, A.; Guglielminetti, L.; Angelini, L.G. Plant growth retardants (PGRs) affect growth and secondary metabolite biosynthesis in Stevia rebaudiana Bertoni under drought stress. South Afr. J. Bot. 2019, 121, 394–401. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rezaei, M.; Tseng, T.-M.; Mastinu, A. Effects of Metribuzin Herbicide on Some Morpho-Physiological Characteristics of Two Echinacea Species. Horticulturae 2022, 8, 169. [Google Scholar] [CrossRef]
- Khaleghnezhad, V.; Yousefi, A.R.; Tavakoli, A.; Farajmand, B.; Mastinu, A. Concentrations-dependent effect of exogenous abscisic acid on photosynthesis, growth and phenolic content of Dracocephalum moldavica L. under drought stress. Planta 2021, 253, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrão, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology, 4th ed.; Freeman, W.H., Ed.; Palgrave [distributor]: Basingstoke, UK; New York, NY, USA, 2006. [Google Scholar]
- Ghani, M.A.; Abbas, M.M.; Ali, B.; Aziz, R.; Qadri, R.W.K.; Noor, A.; Azam, M.; Bahzad, S.; Saleem, M.H.; Abualreesh, M.H.; et al. Alleviating Role of Gibberellic Acid in Enhancing Plant Growth and Stimulating Phenolic Compounds in Carrot (Daucus carota L.) under Lead Stress. Sustainability 2021, 13, 12329. [Google Scholar] [CrossRef]
- Asil, H.; Ayanoglu, F. The Effects of Different Gibberellic Acid Doses and Corm Cutting Methods on Saffron (Crocus Sativus L.) Yield Components in Turkey. Fresen Env. Bull 2018, 27, 9222–9229. [Google Scholar]
- Hayat, Q.; Hayat, S.; Irfan, M.; Ahmad, A. Effect of exogenous salicylic acid under changing environment: A review. Environ. Exp. Bot. 2010, 68, 14–25. [Google Scholar] [CrossRef]
- Martin-Mex, R.; Vergara-Yoisura, S.; Nexticapan-Garces, A.; Larque-Saavedra, A. Application of Low Concentrations of Salicilyc Acid Increases the Number of Flowers in Petunia Hibrida. Agrociencia-Mex. 2010, 44, 773–778. [Google Scholar]
- Basra, A.S. Plant Growth Regulators in Agriculture and Horticulture: Their Role and Commercial Uses; Food Products Press: New York, NY, USA; London, UK, 2000. [Google Scholar]
- Desta, B.; Amare, G. Paclobutrazol as a plant growth regulator. Chem. Biol. Technol. Agric. 2021, 8, 1–15. [Google Scholar] [CrossRef]
- Mobli, M.; Baninasab, B. Effects of plant growth regulators on growth and carbohydrate accumulation in shoots and roots of two almond rootstock seedlings. Fruits 2008, 63, 363–370. [Google Scholar] [CrossRef]
- Zhao, P.; Fan, J.-F.; Zhang, S.-X.; Huang, Z.-L.; Yang, P.-H.; Ma, Z.-H.; Woeste, K.E. Effects of gibberellin A4/7, 6-benzylaminopurine and chlormequat chloride on the number of male and female strobili and immature cones in Chinese pine (Pinus tabuliformis) with foliar sprays. J. For. Res. 2011, 22, 353–359. [Google Scholar] [CrossRef]
- Ondok, J.P.R. Hunt plant growth curves. Folia Geobot. Phytotaxon. 1984, 19, 278. [Google Scholar] [CrossRef]
- Hay, R.K.M.; Porter, J.R.; Hay, R.K.M. The Physiology of Crop Yield, 2nd ed.; Blackwell: Oxford, UK, 2006. [Google Scholar]
- DeMason, D.A. Auxin-cytokinin and auxin-gibberellin interactions during morphogenesis of the compound leaves of pea (Pisum sativum). Planta 2005, 222, 151–166. [Google Scholar] [CrossRef]
- Reinhardt, D.; Mandel, T.; Kuhlemeier, C. Auxin regulates the initiation and radial position of plant lateral organs. Plant Cell 2000, 12, 507–518. [Google Scholar] [CrossRef]
- Ibrahim, A.; Abdel-Razzak, H.; Wahb-Allah, M.; Alenazi, M.; Alsadon, A.; Dewir, Y.H. Improvement in Growth, Yield, and Fruit Quality of Three Red Sweet Pepper Cultivars by Foliar Application of Humic and Salicylic Acids. HortTechnology 2019, 29, 170–178. [Google Scholar] [CrossRef]
- Gupta, R.; Chakrabarty, S.K. Gibberellic acid in plant: Still a mystery unresolved. Plant Signal. Behav. 2013, 8, e25504. [Google Scholar] [CrossRef]
- Miceli, A.; Moncada, A.; Sabatino, L.; Vetrano, F. Effect of Gibberellic Acid on Growth, Yield, and Quality of Leaf Lettuce and Rocket Grown in a Floating System. Agronomy 2019, 9, 382. [Google Scholar] [CrossRef]
- Pourmohammad, A.; Shekari, F.; Soltaniband, V. Cycocel Priming and Foliar Application Affect Yield Components of Rapeseed (Brassica Napus L.). Cercet. Agron. Mold. 2014, 47, 59–69. [Google Scholar] [CrossRef][Green Version]
- Miranzadeh, H.; Emam, Y.; Seyyed, H.; Zare, S. Productivity and Radiation Use Efficiency of Four Dryland Wheat Cultivars under Different Levels of Nitrogen and Chlormequat Chloride. J. Agric. Sci. Tech.-Iran 2011, 13, 339–351. [Google Scholar]
- Pinto, A.C.R.; Rodrigues, T.d.J.D.; Leite, I.C.; Barbosa, J.C. Growth retardants on development and ornamental quality of potted ‘Lilliput’ Zinnia elegans Jacq. Sci. Agric. 2005, 62, 337–345. [Google Scholar] [CrossRef]
- Tsegaw, T.; Hammes, S.; Robbertse, J. Paclobutrazol-induced leaf, stem, and root anatomical modifications in potato. Hortscience 2005, 40, 1343–1346. [Google Scholar] [CrossRef]
- Carvalho-Zanão, M.P.; Grossi, J.A.S.; Zanão Júnior, L.A.; Ventrella, M.C.; Pereira, N. Production and leaf plasticity of rose plants sprayed with paclobutrazol and daminozide. Semin. Ciências Agrárias 2017, 38, 3481. [Google Scholar] [CrossRef][Green Version]
- Yeshitela, T.; Robbertse, P.J.; Stassen, P.J.C. Paclobutrazol suppressed vegetative growth and improved yield as well as fruit quality of ‘Tommy Atkins’ mango (Mangifera indica) in Ethiopia. N. Z. J. Crop Hortic. 2004, 32, 281–293. [Google Scholar] [CrossRef]
- Farooq, S.; Koul, K.K. Changes in Gibberellin-like Activity in Corms of Saffron Plant (Crocus sativus L.) during Dormancy and Sprouting. Biochem. Physiol. Pflanz. 1983, 178, 685–689. [Google Scholar] [CrossRef]
- Chrungoo, N.K.; Farooq, S. Influence of GA3 and NAA on certain carbohydrate fractions in corms of saffron crocus (Crocus sativus L.) during development. Acta Soc. Bot. Pol. 2014, 58, 237–246. [Google Scholar] [CrossRef][Green Version]
- Sajid, M.; Amin, N.; Ahmadand, H.; Khan, K. Effect of Gibberellic Acid on Enhancing Flowering Time in Chrysanthemum Morifolium. Pak. J. Bot. 2016, 48, 477–483. [Google Scholar]
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338. [Google Scholar] [CrossRef] [PubMed]
- Abreu, M.E.; Munne-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.; Pons, E.; Prats, G.; Leon, J. Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. Cell Mol. Biol. 2004, 37, 209–217. [Google Scholar] [CrossRef]
- Abbas, S.M.; Ahmad, R.; Waraich, E.A.; Qasim, M. Exogenous Application of Salicylic Acid at Different Plant Growth Stages Improves Physiological Processes in Marigold (Tagetes Erecta L.). Pak. J. Agric. Sci. 2019, 56, 541–548. [Google Scholar] [CrossRef]
- Koutroubas, S.D.; Damalas, C.A. Morpho-physiological responses of sunflower to foliar applications of chlormequat chloride (CCC). Biosci. J. 2016, 1493–1501. [Google Scholar] [CrossRef]
- Demeulemeester, M.A.C.; Rademacher, W.; Van de Mierop, A.; De Proft, M.P. Influence of gibberellin biosynthesis inhibitors on stem elongation and floral initiation on in vitro chicory root explants under dark and light conditions. Plant Growth Regul. 1995, 17, 47–52. [Google Scholar] [CrossRef]
- Shaki, F.; Maboud, H.E.; Niknam, V. Growth enhancement and salt tolerance of Safflower (Carthamus tinctorius L.), by salicylic acid. Curr. Plant Biol. 2018, 13, 16–22. [Google Scholar] [CrossRef]
- Dwivedi, S.K.; Arora, A.; Kumar, S. Paclobutrazol-induced alleviation of water-deficit damage in relation to photosynthetic characteristics and expression of stress markers in contrasting wheat genotypes. Photosynthetica 2017, 55, 351–359. [Google Scholar] [CrossRef]
- Kuryata, V.G.; Khodanitska, O.O. Осoбливoсті анатoмічнoї будoви і функціoнування листкoвoгo апарату та прoдуктивність рoслин льoну oлійнoгo за дії хлoрмекватхлoриду. Ukr. J. Ecol. 2018, 8, 918–926. [Google Scholar] [CrossRef]
- Venugopalan, M.V.; Kranthi, K.R.; Lakade, S.; Tandulkar, N.R. Development of agro-technology to increase yields of a shy-bearer desi cotton species, Gossypium arboreum race cernuum in a non-traditional area of cultivation. Curr. Sci. India 2016, 110, 692–695. [Google Scholar] [CrossRef]
| pH | Electrical Conductivity (mS/m) | K (ppm) | P (ppm) | Total Nitrogen (%) | Sand | Silt | Clay | Soil Texture | Bulk Density (g·cm−3) | Moisture Content (%) | Sample Depth (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7.56 | 13.86 | 267 | 13.4 | 0.12 | 52 | 31 | 17 | Sandy loom | 1.564 | 1.56 | 0–30 |
| 2015/2016 | April | May | June | July | August | September | October | November | December | January | February | March |
| Precipitation | 83.2 | 6.6 | 0.5 | 1.1 | 0.0 | 2.9 | 16.6 | 73.6 | 14.6 | 15.9 | 31.8 | 31.6 |
| Mean Temp | 10.8 | 16.7 | 22.3 | 26.6 | 25.9 | 21.5 | 17.3 | 9.5 | 2.5 | 2.4 | 2.3 | 8.8 |
| Min Temp | 4.2 | 8.4 | 12.9 | 18.5 | 16.1 | 12.7 | 9.1 | 4.6 | −2.8 | −2.8 | −3.2 | 2.6 |
| Max Temp | 17.4 | 25.0 | 31.8 | 34.6 | 35.8 | 30.3 | 25.4 | 14.3 | 7.9 | 7.7 | 7.9 | 15.0 |
| Mean Relative Humidity | 56 | 46 | 44 | 41 | 36 | 50 | 52 | 66 | 65 | 64 | 63 | 56 |
| 2016/2017 | April | May | June | July | August | September | October | November | December | January | February | March |
| Precipitation | 62 | 28.1 | 15.9 | 1.6 | 0 | 0 | 0.1 | 22.6 | 21.9 | 9.7 | 27.2 | 42 |
| Mean Temp | 9.5 | 16.5 | 19.9 | 24.7 | 25.6 | 22.6 | 15.6 | 11.3 | 1.81 | 1.7 | −0.5 | 4.3 |
| Min Temp | 3.7 | 8.6 | 11.1 | 16.5 | 16.3 | 12.8 | 6.1 | 4.3 | −4.4 | −4.5 | −5.2 | −2.4 |
| Max Temp | 15.3 | 24.4 | 28.6 | 32.9 | 34.8 | 32.5 | 25.1 | 18.2 | 8 | 7.8 | 4.1 | 11 |
| Mean Relative Humidity | 61 | 55 | 47 | 46 | 42 | 42 | 46 | 55 | 54 | 62 | 67 | 60 |
| Year | Treatment | Leaf Length (cm) | Leaf Width (mm) | LAI | Leaf Number | Leaf Dry Weight (mg) | Number of Daughter Corms | Corm Fresh Weight (g·m−2) | Corm Dry Weight (g·m−2) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2015–2016 | Control | 31 e | 3 e | 1.1 g | 6.3 de | 65 l | 2 e | 4.36 g | 1.99 g | |
| HP | 30 f | 3.2 d | 1.43 de | 7.3 cd | 97 k | 3.33 b | 14.3 b | 4.7 c | ||
| GA3 | 250 | 38 a | 2.1 h | 1.23 f | 8.3 bc | 125 h | 3 c | 10.87 d | 3.61 d | |
| 500 | 37 b | 2.3 g | 1.59 c | 9.7 b | 165 f | 3.33 b | 9.73 e | 3.05 e | ||
| 750 | 33 d | 2.4 f | 1.48 e | 8.7 c | 196 b | 2.33 d | 4.49 i | 3.71 d | ||
| SA | 700 | 32 cd | 3.4 c | 1.56 d | 7.7 d | 143 g | 3 c | 16.06 a | 6.11 a | |
| 1400 | 35 c | 4 a | 2.73 a | 10.3 a | 217 a | 5.5 a | 15.55 b | 5.03 b | ||
| 2100 | 31 e | 4.1 a | 2.37 b | 8.7 c | 175 e | 3 c | 6.85 g | 2.42 f | ||
| CCC | 500 | 30 f | 3.1 cd | 1.11 g | 5.7 f | 108 hi | 2.33 d | 10.14 d | 3 e | |
| 1000 | 24 i | 3 e | 1.2 f | 6.7 e | 113 i | 2 e | 7.1 f | 3.18 e | ||
| 1500 | 20 l | 3.7 b | 0.93 gh | 5.3 g | 122 gh | 1.63 f | 6.05 g | 2.16 f | ||
| PBZ | 50 | 28 g | 3.2 d | 0.84 hi | 4.7 h | 191 c | 1.66 f | 12.18 c | 3.67 d | |
| 100 | 26 h | 3.3 bc | 1.07 h | 5.7 f | 216 a | 2.33 d | 7.66 f | 3.01 e | ||
| 150 | 22 k | 3.6 ab | 0.97 i | 5.7 f | 186 d | 2.33 d | 6.82 g | 2.3 f | ||
| 2016–2017 | Control | 27 e | 3 e | 0.96 f | 6.1 cd | 57 m | 1.9 e | 4.25 h | 1.89 f | |
| HP | 29 cd | 3.2 d | 1.23 bc | 7.3 d | 84 kl | 3.91 b | 14.21 b | 4.71 ab | ||
| GA3 | 250 | 35 a | 2 g | 1.17 e | 8.3 c | 114 i | 2.87 c | 10.76 d | 3.52 c | |
| 500 | 35 a | 2.4 f | 1.51 c | 9.1 b | 157 f | 3.01 b | 9.62 e | 3.04 d | ||
| 750 | 29 cd | 2.4 f | 1.13 d | 8.1 c | 178 c | 2.32 d | 6.38 g | 3.61 c | ||
| SA | 700 | 30 d | 3.2 d | 1.22 bc | 7.3 d | 135 g | 3.93 b | 16.05 a | 6.1 a | |
| 1400 | 34 b | 3.7 b | 2.76 a | 10.7 a | 211 a | 5.3 a | 14.43 b | 4.02 ab | ||
| 2100 | 29 cd | 4 a | 2.32 b | 9.3 b | 169 d | 2.78 c | 6.74 g | 2.31 e | ||
| CCC | 500 | 31 c | 3 e | 1 de | 5.7 e | 97l | 2.22 d | 10.03 d | 3.01 | |
| 1000 | 22 g | 3 e | 0.87 g | 6.3 cd | 109 k | 1.94 e | 7.12 f | 3.16 d | ||
| 1500 | 19 i | 3.4 c | 0.82 h | 5.3 e | 124 h | 1.64 f | 6.04 g | 2.17 e | ||
| PBZ | 50 | 28 e | 3.1 cd | 0.81 h | 4.7 f | 183 b | 1.59 f | 12.07 c | 5.55 b | |
| 100 | 24 f | 3.2 d | 0.86 g | 5.3 e | 210 a | 2.1 d | 7.57 f | 3.02 d | ||
| 150 | 19 i | 3.4 c | 0.83 h | 5.3 e | 162 e | 2.1 d | 4.71 h | 2.21 e | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidari, F.; Shekari, F.; Andalibi, B.; Saba, J.; Uberti, D.; Mastinu, A. Comparative Effects of Four Plant Growth Regulators on Yield and Field Performance of Crocus sativus L. Horticulturae 2022, 8, 799. https://doi.org/10.3390/horticulturae8090799
Heidari F, Shekari F, Andalibi B, Saba J, Uberti D, Mastinu A. Comparative Effects of Four Plant Growth Regulators on Yield and Field Performance of Crocus sativus L. Horticulturae. 2022; 8(9):799. https://doi.org/10.3390/horticulturae8090799
Chicago/Turabian StyleHeidari, Fatemeh, Farid Shekari, Babak Andalibi, Jalal Saba, Daniela Uberti, and Andrea Mastinu. 2022. "Comparative Effects of Four Plant Growth Regulators on Yield and Field Performance of Crocus sativus L." Horticulturae 8, no. 9: 799. https://doi.org/10.3390/horticulturae8090799
APA StyleHeidari, F., Shekari, F., Andalibi, B., Saba, J., Uberti, D., & Mastinu, A. (2022). Comparative Effects of Four Plant Growth Regulators on Yield and Field Performance of Crocus sativus L. Horticulturae, 8(9), 799. https://doi.org/10.3390/horticulturae8090799









