Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Extraction Process
2.3. DESs Preparation
2.4. Total Polyphenol Content
2.5. Determination of the Antioxidant Capacity
2.6. Statistical Analysis
2.7. Optimization
3. Results and Discussion
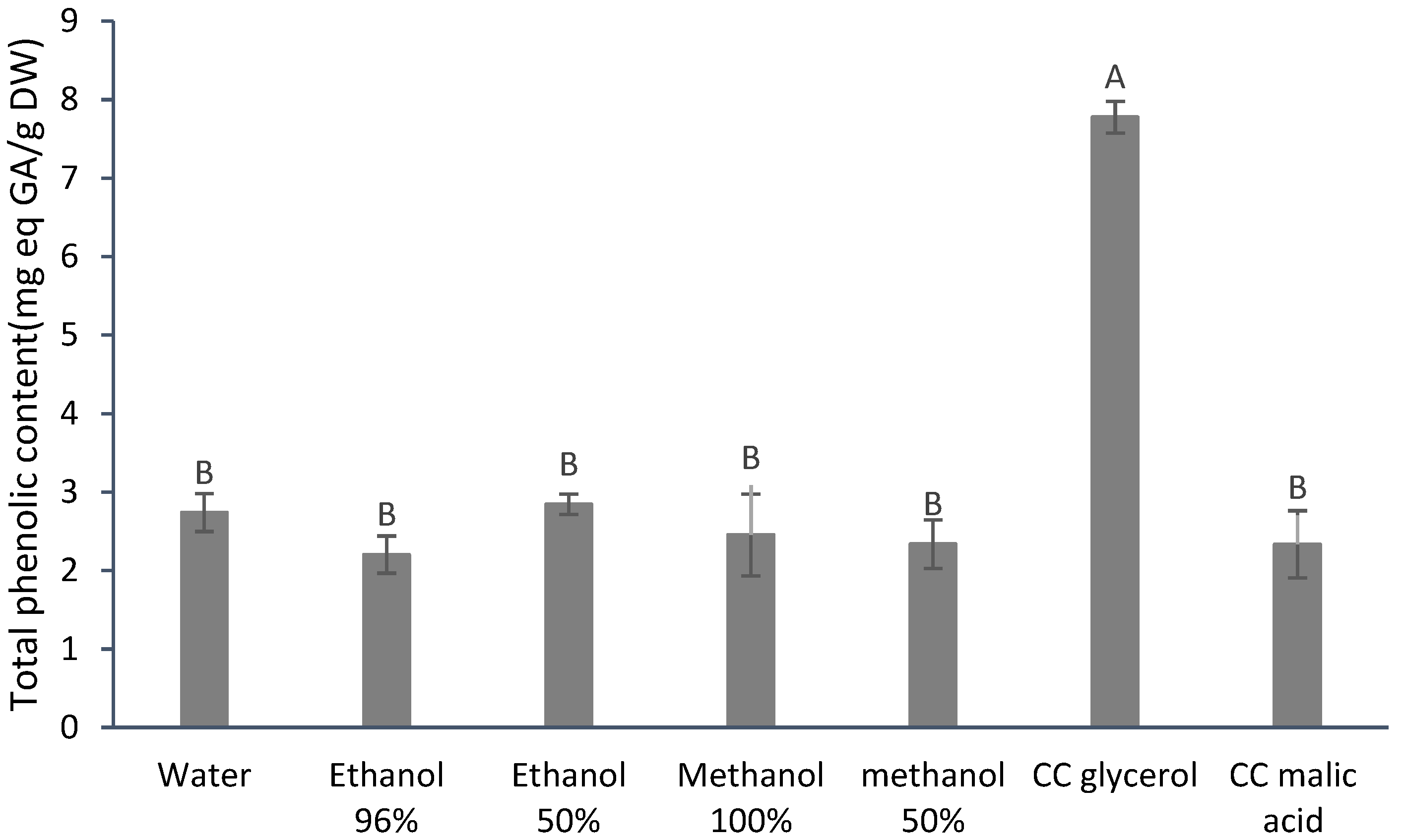
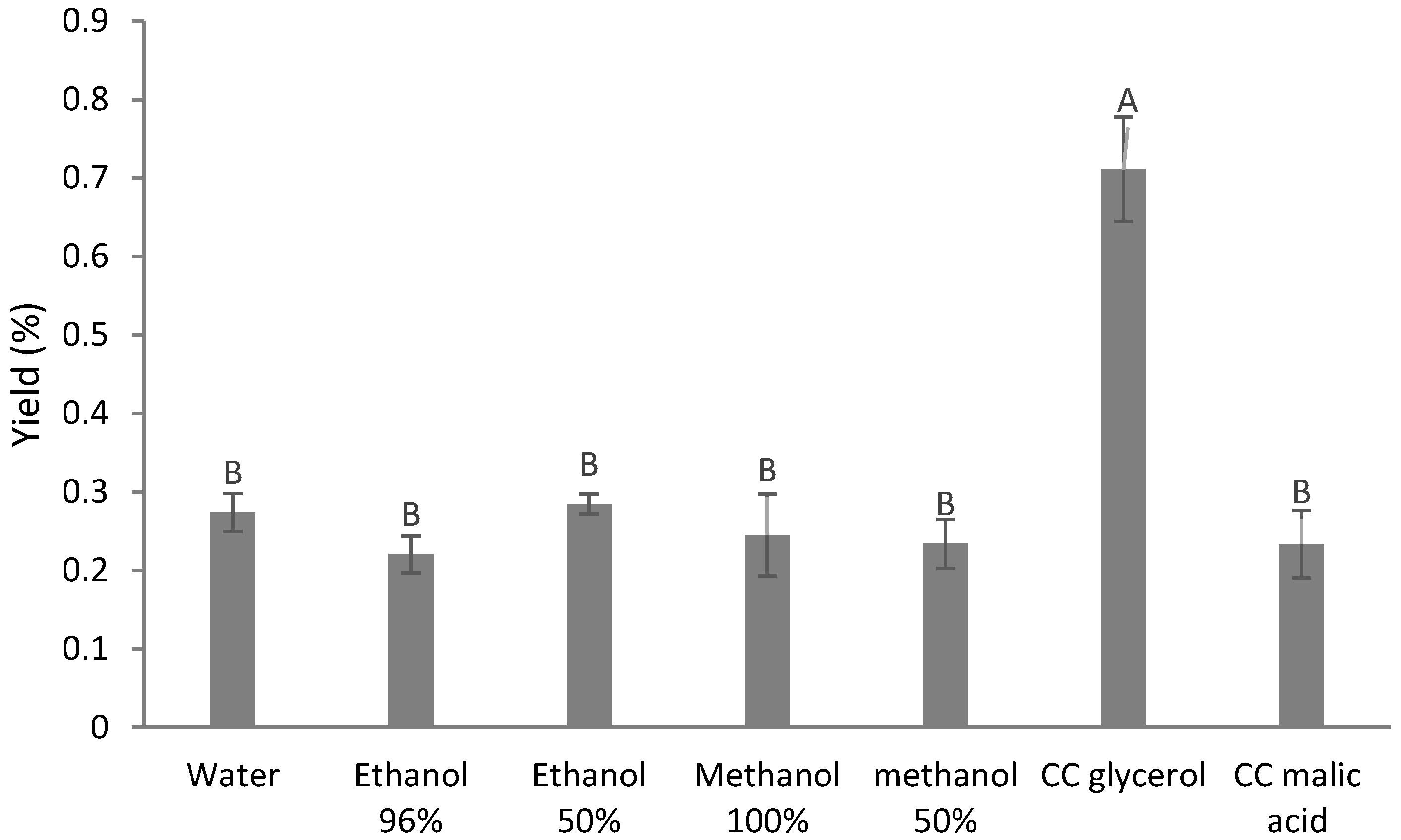
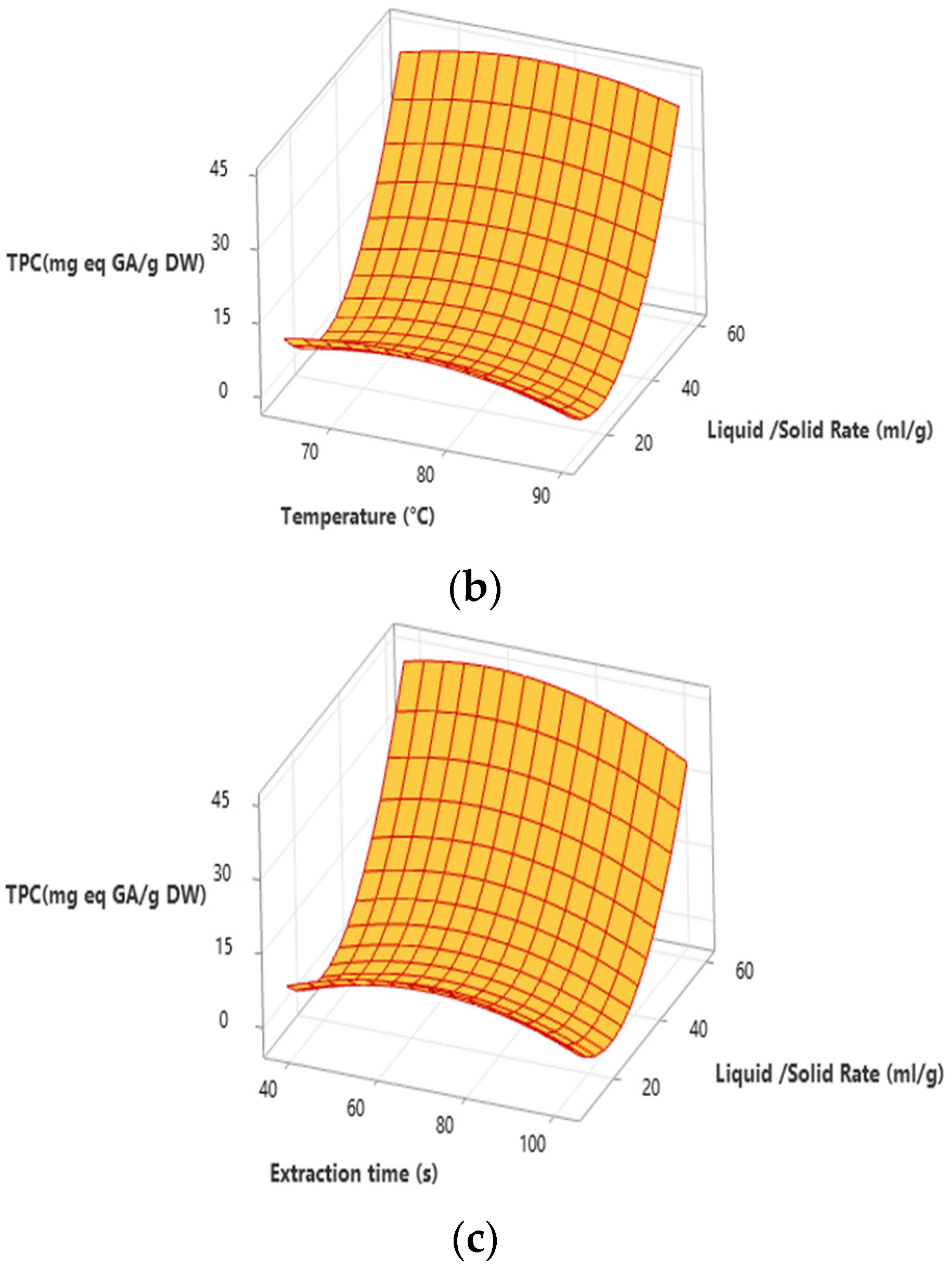
3.1. Total Phenolic Content
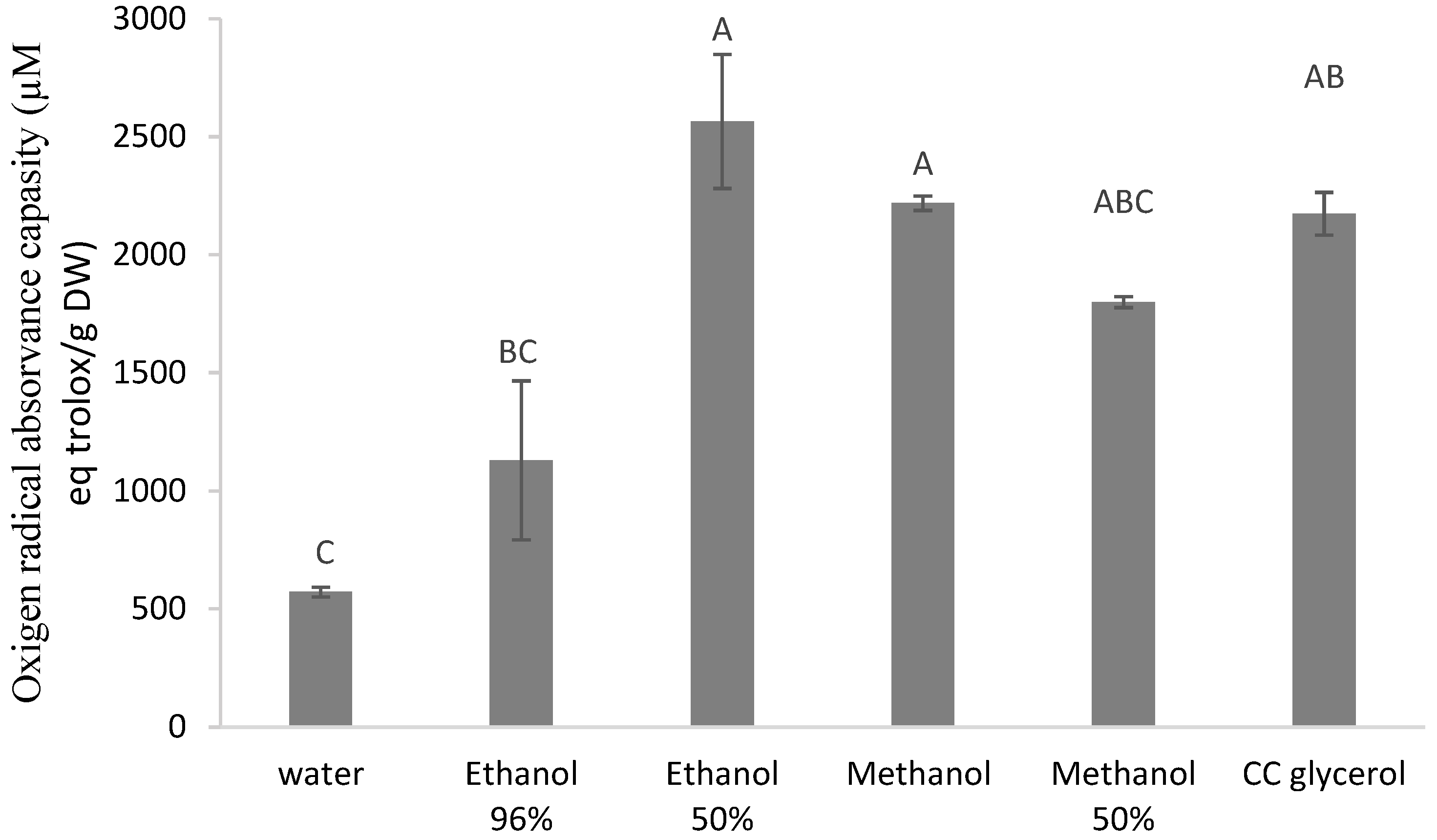
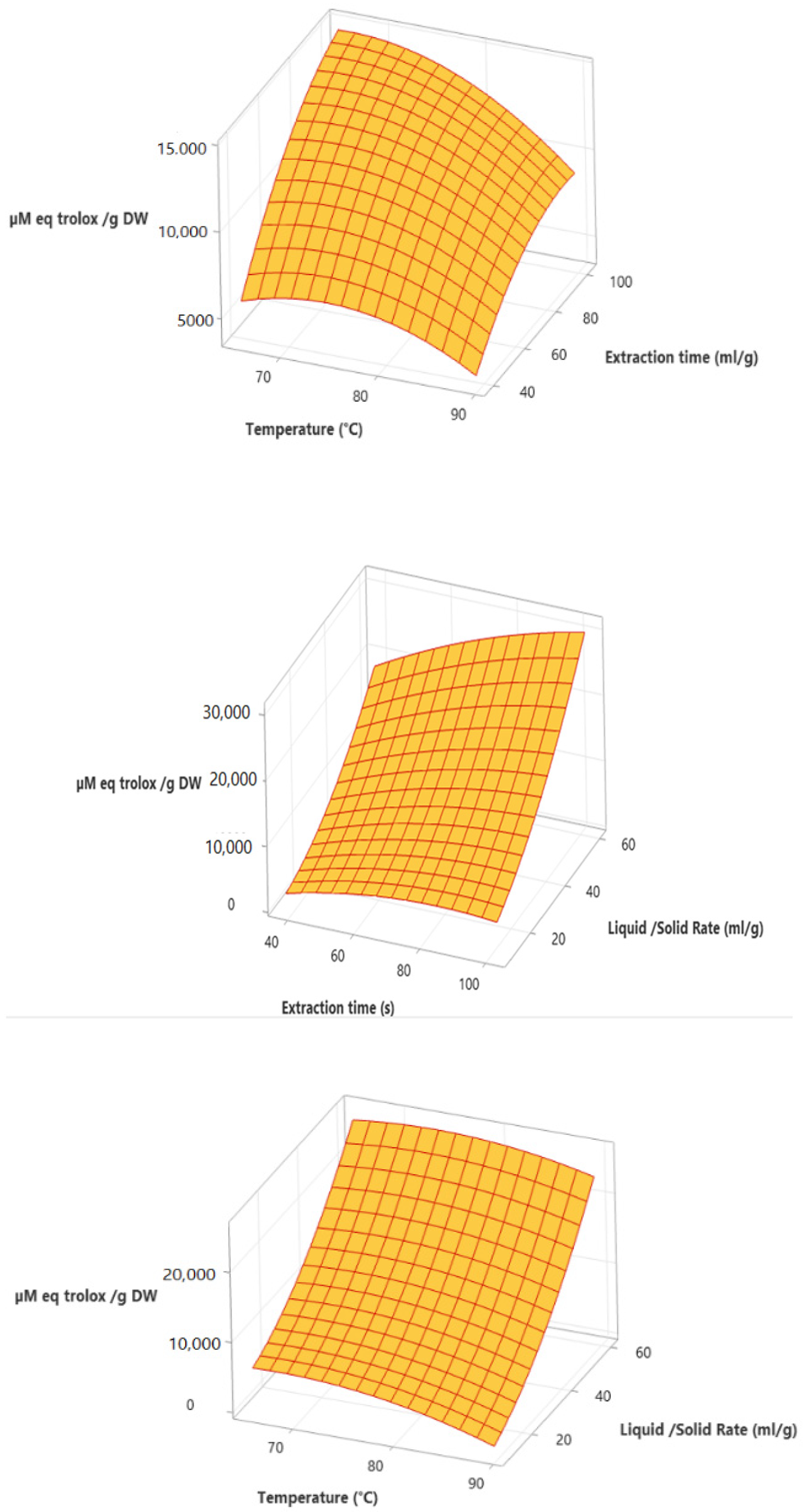
3.2. Antioxidant Activity
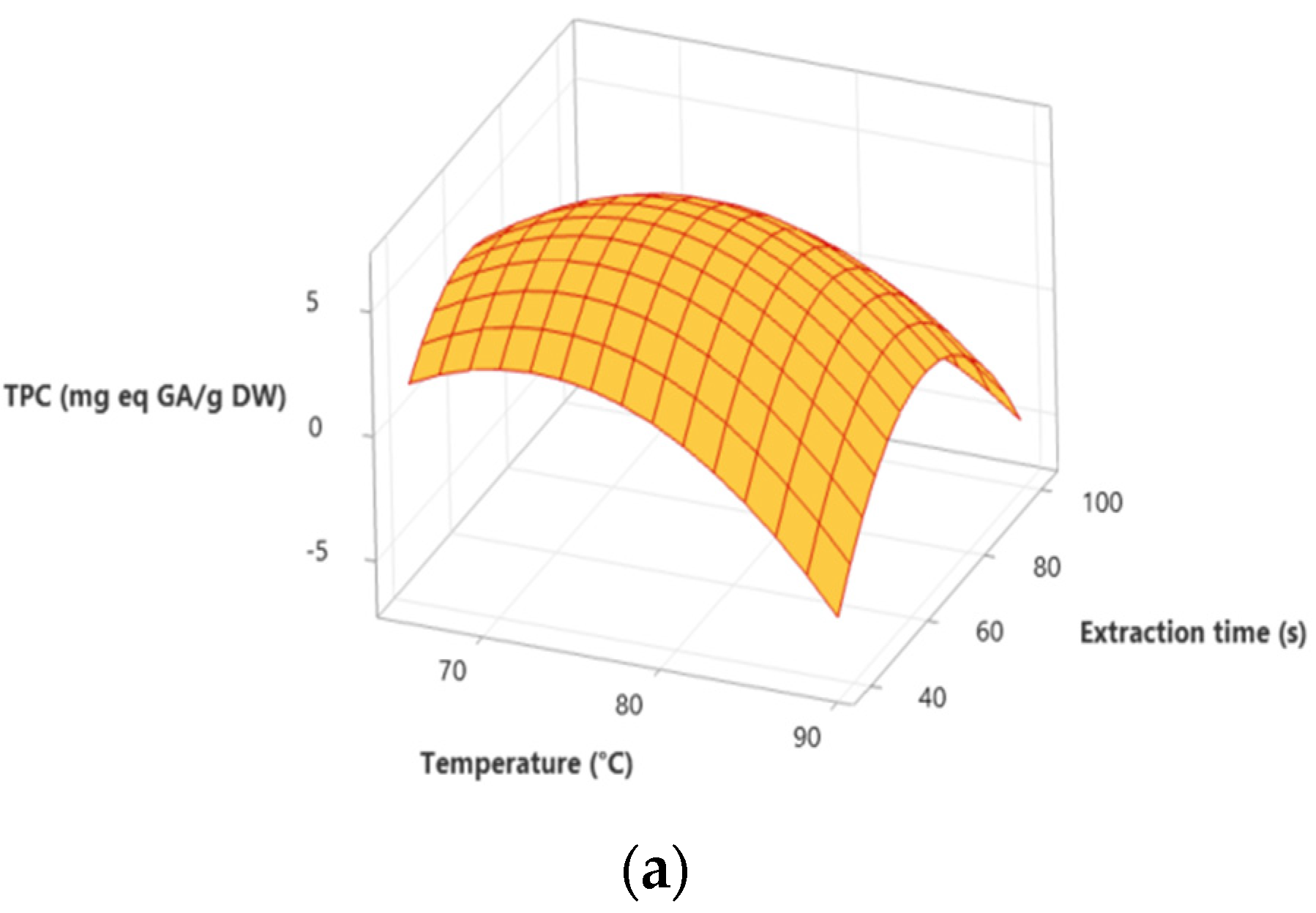
3.3. Optimization Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Organización de Las Naciones Unidas para la Limentación y la Agricultura FAOSTAT. Available online: http://www.fao.org/faostat/es/#data/QC (accessed on 6 April 2020).
- Sepúlveda, L.; Romaní, A.; Aguilar, C.N.; Teixeira, J. Valorization of Pineapple Waste for the Extraction of Bioactive Compounds and Glycosides Using Autohydrolysis. Innov. Food Sci. Emerg. Technol. 2018, 47, 38–45. [Google Scholar] [CrossRef]
- Zuhair, M.; Nor, M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Performance of a Two-Stage Membrane System for Bromelain Separation from Pineapple Waste Mixture as Impacted by Enzymatic Pretreatment and Diafiltration. Food Technol. Biotechnol. 2018, 56, 218–227. [Google Scholar] [CrossRef]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Integrated Ultrafiltration Process for the Recovery of Bromelain from Pineapple Waste Mixture. J. Food Process Eng. 2017, 40, e12492. [Google Scholar] [CrossRef]
- Campos, D.A.; Ribeiro, T.B.; Teixeira, J.A.; Pastrana, L.; Pintado, M.M. Integral Valorization of Pineapple (Ananas Comosus L.) By-Products through a Green Chemistry Approach towards Added Value Ingredients. Foods 2020, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Guzik, P.; Kulawik, P.; Zając, M.; Migdał, W. Microwave Applications in the Food Industry: An Overview of Recent Developments. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- He, J.; Wu, X.; Yu, Z. Microwave Pretreatment of Camellia (Camellia Oleifera Abel.) Seeds: Effect on Oil Flavor. Food Chem. 2021, 364, 130388. [Google Scholar] [CrossRef]
- Huang, J.; Chen, C.; Song, Z.; Chang, M.; Yao, L.; Jin, Q.; Wang, X. Effect of Microwave Pretreatment of Perilla Seeds on Minor Bioactive Components Content and Oxidative Stability of Oil. Food Chem. 2022, 388, 133010. [Google Scholar] [CrossRef]
- Leontopoulos, S.; Skenderidis, P.; Petrotos, K.; Mitsagga, C.; Giavasis, I. Preliminary Studies on Suppression of Important Plant Pathogens by Using Pomegranate and Avocado Residual Peel and Seed Extracts. Horticulturae 2022, 8, 283. [Google Scholar] [CrossRef]
- Barrios, S.; Lema, P.; Lareo, C. Modeling Respiration Rate of Strawberry (Cv. San Andreas) for Modified Atmosphere Packaging Design. Int. J. Food Prop. 2014, 17, 2039–2051. [Google Scholar] [CrossRef]
- Singh, K.; Srichairatanakool, S.; Chewonarin, T.; Brennan, C.S.; Brennan, M.A.; Klangpetch, W.; Utama-Ang, N. Manipulation of the Phenolic Quality of Assam Green Tea through Thermal Regulation and Utilization of Microwave and Ultrasonic Extraction Techniques. Horticulturae 2022, 8, 338. [Google Scholar] [CrossRef]
- Sridhar, A.; Vaishampayan, V.; Senthil Kumar, P.; Ponnuchamy, M.; Kapoor, A. Extraction Techniques in Food Industry: Insights into Process Parameters and Their Optimization. Food Chem. Toxicol. 2022, 166, 113207. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar] [CrossRef]
- Tatke, P.; Jaiswal, Y. An Overview of Microwave Assisted Extraction and Its Applications in Herbal Drug Research. Res. J. Med. Plant 2011, 5, 21–31. [Google Scholar] [CrossRef]
- Destandau, E.; Michel, T.; Elfakir, C. CHAPTER 4. Microwave-Assisted Extraction. In Natural Products Extraction: Principles and Aplication; The Royal Society of Chemistry: London, UK, 2013; pp. 113–156. ISBN 9781849737579. [Google Scholar]
- Dorta, E.; Lobo, M.G.; González, M. Using Drying Treatments to Stabilise Mango Peel and Seed: Effect on Antioxidant Activity. LWT-Food Sci. Technol. 2012, 45, 261–268. [Google Scholar] [CrossRef]
- Waghmare, R. Refractance Window Drying: A Cohort Review on Quality Characteristics. Trends Food Sci. Technol. 2021, 110, 652–662. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of Extraction Solvent/Technique on the Antioxidant Activity of Selected Medicinal Plant Extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Jablonský, M.; Škulcová, A.; Malvis, A.; Šima, J. Extraction of Value-Added Components from Food Industry Based and Agro-Forest Biowastes by Deep Eutectic Solvents. J. Biotechnol. 2018, 282, 46–66. [Google Scholar] [CrossRef]
- Peng, X.; Duan, M.-H.; Yao, X.-H.; Zhang, Y.-H.; Zhao, C.-J.; Zu, Y.-G.; Fu, Y.-J. Green Extraction of Five Target Phenolic Acids from Lonicerae Japonicae Flos with Deep Eutectic Solvent. Sep. Purif. Technol. 2016, 157, 249–257. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Alias, H.; Abbas, Z. Microwave-assisted extraction of phenolic compound from pineapple skins: The optimum operating condition and comparison with soxhlet extraction (Pengekstrakan Sebatian Fenolik Daripada Kulit Nenas Dengan Bantuan Ge. Malays. J. Anal. Sci. 2017, 21, 690–699. [Google Scholar] [CrossRef]
- Alias, N.H.; Abbas, Z. Preliminary Investigation on the Total Phenolic Content and Antioxidant Activity of Pineapple Wastes via Microwave-Assisted Extraction at Fixed Microwave Power. Chem. Eng Trans. 2017, 56, 1675–1680. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of Total Phenolic Content and Other Oxidation Substrates in Plant Tissues Using Folin–Ciocalteu Reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of Polysaccharides Extracted from Spent Coffee Grounds by Alkali Pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ganske, F. ORAC Assay to Determine Antioxidant Capacity. In Application Notes Binder; BMG Labtech: Ortenberg, Germany, 2014; pp. 105–106. [Google Scholar]
- Radosevic, K.; Curko, N.; Srcek, V.G.; Bubalo, M.C.; Tomasevic, M.; Kovacevic Ganic, K.; Radojcic Redovnikovi, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT-Food Sci. Technol. 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative Process of Polyphenol Recovery from Pomegranate Peels by Combining Green Deep Eutectic Solvents and a New Infrared Technology. LWT-Food Sci. Technol. 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Bentayeb, K.; Vera, P.; Rubio, C.; Nerín, C. The Additive Properties of Oxygen Radical Absorbance Capacity (ORAC) Assay: The Case of Essential Oils. Food Chem. 2014, 148, 204–208. [Google Scholar] [CrossRef]
- Su, D.; Zhang, R.; Hou, F.; Zhang, M.; Guo, J.; Huang, F.; Deng, Y.; Wei, Z. Comparison of the Free and Bound Phenolic Profiles and Cellular Antioxidant Activities of Litchi Pulp Extracts from Different Solvents. BMC Complement. Altern. Med. 2014, 14, 9. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Soxhlet Extraction of Phenolic Compounds from Vernonia Cinerea Leaves and Its Antioxidant Activity. J. Appl. Res. Med. Aromat. Plants 2018, 11, 12–17. [Google Scholar] [CrossRef]
- Dewage, E.; Sandun Abeyrathne, N.; Nam, K.; Ahn, D.U. Antioxidants Analytical Methods for Lipid Oxidation and Antioxidant Capacity in Food Systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef]


| Process Variables | Variable Levels | Unit | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| Drying temperature | 70 | 77.5 | 85 | °C |
| Extraction time | 50 | 70 | 90 | s |
| Liquid/solid ratio | 15 | 32.5 | 50 | mL/g |
| TPC | DPPH | ORAC | Yield | RSA | |
|---|---|---|---|---|---|
| Predicted value | 35.95 | 28630 | 1173.3 | 3.16 | 93.97 |
| Desirability | 0.57 | 0.94 | 0.99 | 0.59 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Serna, C.L.; Ochoa-Martínez, C.I.; Vélez-Pasos, C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae 2022, 8, 791. https://doi.org/10.3390/horticulturae8090791
Vargas-Serna CL, Ochoa-Martínez CI, Vélez-Pasos C. Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae. 2022; 8(9):791. https://doi.org/10.3390/horticulturae8090791
Chicago/Turabian StyleVargas-Serna, Claudia L., Claudia I. Ochoa-Martínez, and Carlos Vélez-Pasos. 2022. "Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents" Horticulturae 8, no. 9: 791. https://doi.org/10.3390/horticulturae8090791
APA StyleVargas-Serna, C. L., Ochoa-Martínez, C. I., & Vélez-Pasos, C. (2022). Microwave-Assisted Extraction of Phenolic Compounds from Pineapple Peel Using Deep Eutectic Solvents. Horticulturae, 8(9), 791. https://doi.org/10.3390/horticulturae8090791






