1. Introduction
Cut flower ornamental kale (
Brassica oleracea var.
acephala) is a 2-years herbaceous cultivar, whose leaves are colorful and changeable, with compact plant morphology and gorgeous inner leaves. Cut flower ornamental kale is mainly distributed in temperate regions, and is planted more in the United Kingdom, the Netherlands, Germany, and the United States. The ornamental period on land is over 4 months since its strong cold resistance and easy cultivation [
1]. Cut flower Ornamental Kale contains a large amount of anthocyanins, which as effective antioxidants have the potential to prevent some cancers, cardiovascular diseases and other chronic diseases [
2,
3,
4]. Cut flower ornamental kale can be self-pollinated or cross pollinated. It normally takes 5–7 years to obtain homozygous parent lineages through self-pollination from a hybrid. Isolated microspore culture (IMC) can quickly purify genotypes and accelerate breeding process [
5].
IMC was first reported by Nitsch [
6], and then scholars from worldwide made in-depth discussions and innovations on this technology. There are many factors that affect the IMC, involving internal factors, such as genotype, microspore viability, growth conditions, and physiological status of the material, while external factors such as donor environment, phytorregulators treatment, period of sampling and composition of the medium, etc. [
7]. In addition, there are several additive substances to the culture media which can be used to improve or induce microspore embryogenesis. Tuteja et al. [
8] found that surfactant treatment could promote plant tissue growth and increase cell viability. Nonionic surfactant treatment (Tween-20, Triton X-100, Pluronic F-68) can improve the microspore embryo induction rate and plant regeneration rate of purple flowering stalk (
Brassica campestris ssp.
chinensis var.
purpurea Bailey) [
9]. Chen et al. [
10] discovered that 10 nM methylene blue treatment of ornamental kale microspores could increase the microspore induction rate to 17.15 embryos per bud, which was 5 times that of the control group. Physical treatments applied to the microspores or bud flowers also promoted microspore sporophytic-pathway. Ari et al. [
11] reported that 4 °C cold shock treatment could promote the microspore embryogenesis rate of ornamental kale. We had tested the microspore culture of cut flower ornamental kale and detected that microspore embryogenesis was greatly difficult.
Histone deacetylation inhibitor (HDACI) is a class of compounds that change the gene expression pattern in cells by increasing the acetylation degree of histones in cells [
12]. Trichostatin A (TSA) is a kind of HDACI that induces cell apoptosis, cell differentiation, regulate transcription, reversal of changed cell morphology and cell cycle arrest [
13]. The function of TSA in plants was to reduce DNA methylation [
14] and increase histone acetylation, resulting in changes in gene expression [
15]. TSA treatment enabled recalcitrant
Arabidopsis microspores to develop into embryogenic clusters. TSA treatment prevented HDAC activity in IMC of
Brassica napus and induced male gametophyte to embryogenesis [
16]. TSA could induce embryogenesis in wheat [
12].
In this paper, the effects of different concentrations of TSA and genotypes on microspore embryo induction rate and plant regeneration in cut flower ornamental kale were investigated. The ploidy of regenerated plants was detected by flow cytometry and the horticultural characters of DH lines were investigated. This study can accelerate the breeding process and improve the breeding efficiency of cut flower ornamental kale.
2. Materials and Methods
2.1. Plant Materials
Cut flower ornamental kale F1 plant Crane Bicolor (CB), Crane Pink (CP) and Crane Feather Queen (CFQ) of Japan TAKII Company was used for IMC. CB showed bicolor round (white outside, pink inside). CP showed pink round leaves. CFQ showed red pinnate. In the autumn of 2020 (mid-July), the seeds were sown in the experimental base of the Shenyang Agricultural University. The seedlings were transplanted into 20 cm-diameter plastic pots in August (about 25 days), then plants vernalized in November and treated with 16 h long sunshine. The plants were moved to greenhouse in early December (about 140 days) in winter. From January to February (about 165–200 days) in spring, the plants bolted and bloomed, and in a sunny day, inflorescences with good growth conditions were selected for IMC.
2.2. IMC
IMC experiment was carried out after modification on the basis of Sato et al. [
17] and Hoseini et al. [
18]. Buds with a ratio of petal to anther length (P/A) of 0.5–0.8 were treated in a 4 °C refrigerator for 24 h. Buds were soaked in 75% (
v/v) ethanol for 30 s and 0.1% (
w/v) HgCl
2 for 6 min in turn for disinfection, afterwards rinsed with germ-free ultrapure water for 5 min, repeated 3 times. To isolate microspores, the buds were squeezed in 8 ml B5 liquid medium with sterile glass rods. The suspensions were first filtered through a 74 μm stainless steel cell sieve and then through a 40 μm cell sieve, later collected in a 50 mL centrifuge tube and for centrifugation (2000 rpm, 3 min). The precipitates were resuspended into NLN-15 medium [
19] at a cell density of 1 × 10
5 microspores/mL. The NLN medium containing microspores was sub-packed into sterile Petri dishes, 5 mL per dish, and 100 μL activated carbon (10 g/L) was added to each dish. The microspores were cultured at 33 °C for 1 d, finally transferred to dark culture (25 °C).
2.3. TSA Treatments
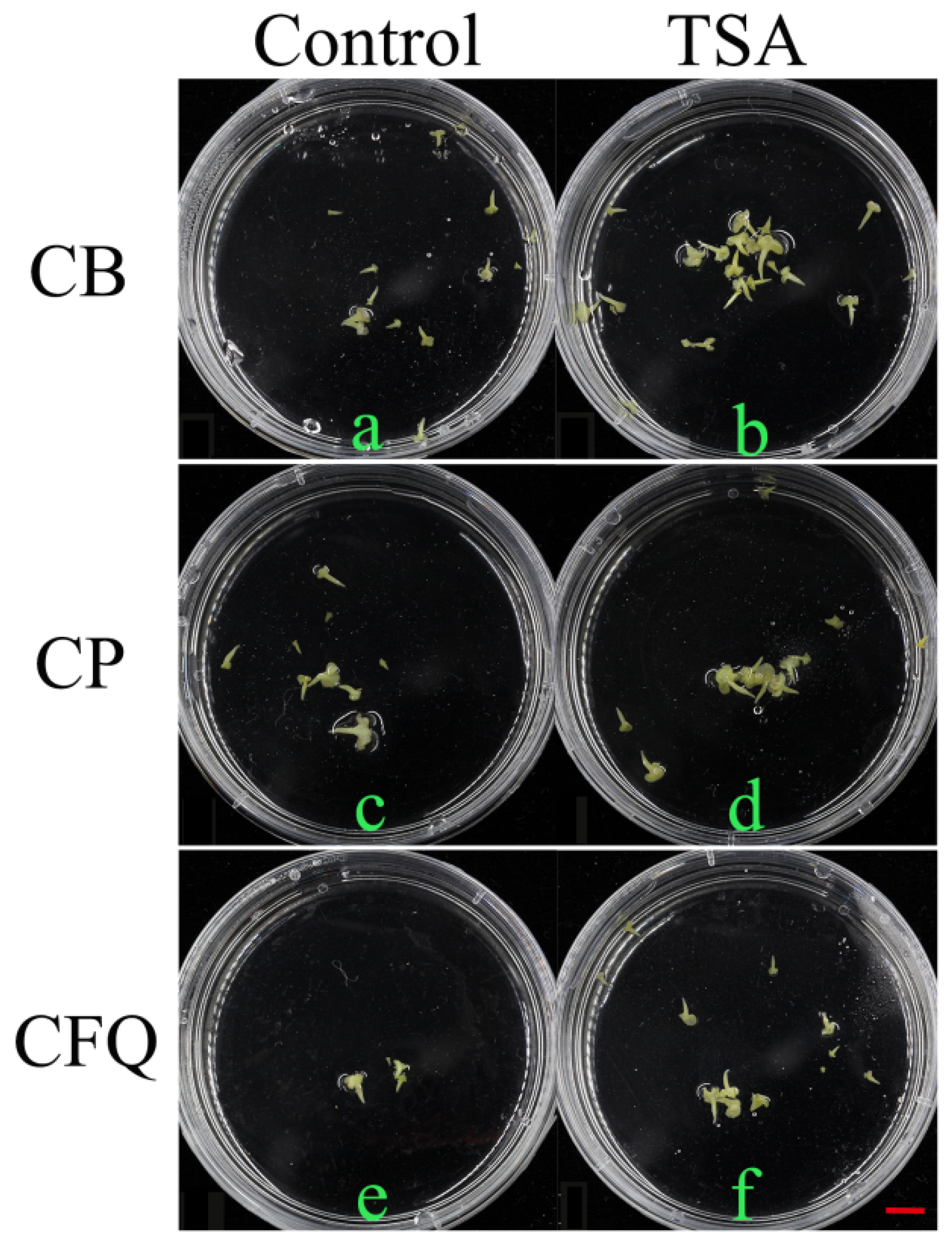
TSA was dissolved in dimethyl sulfoxide (pH 5.8). TSA concentrations were adjusted to 0, 5, 10, and 15 nM, respectively. In the process of NLN subpackaging to plastic petri dishes of IMC experiment, TSA with different concentrations was instilled.
2.4. Embryo Germination and Plantlet Regeneration
After 20 days of microspore culture, the embryos developed to approximately 0.5 cm in length, which were counted and shook (45 rpm, 25 °C) for 7 days. Whereafter, embryos were planted to solid Murashige and Skoog (MS) medium (pH 5.8, 30 g/L sucrose, 0.1 g/L AC, 7.5 g/L agar powder) [
19] in conical flask. After callus formation, they were cut and moved to solid differentiation MS medium (5.5 g/L agar powder).
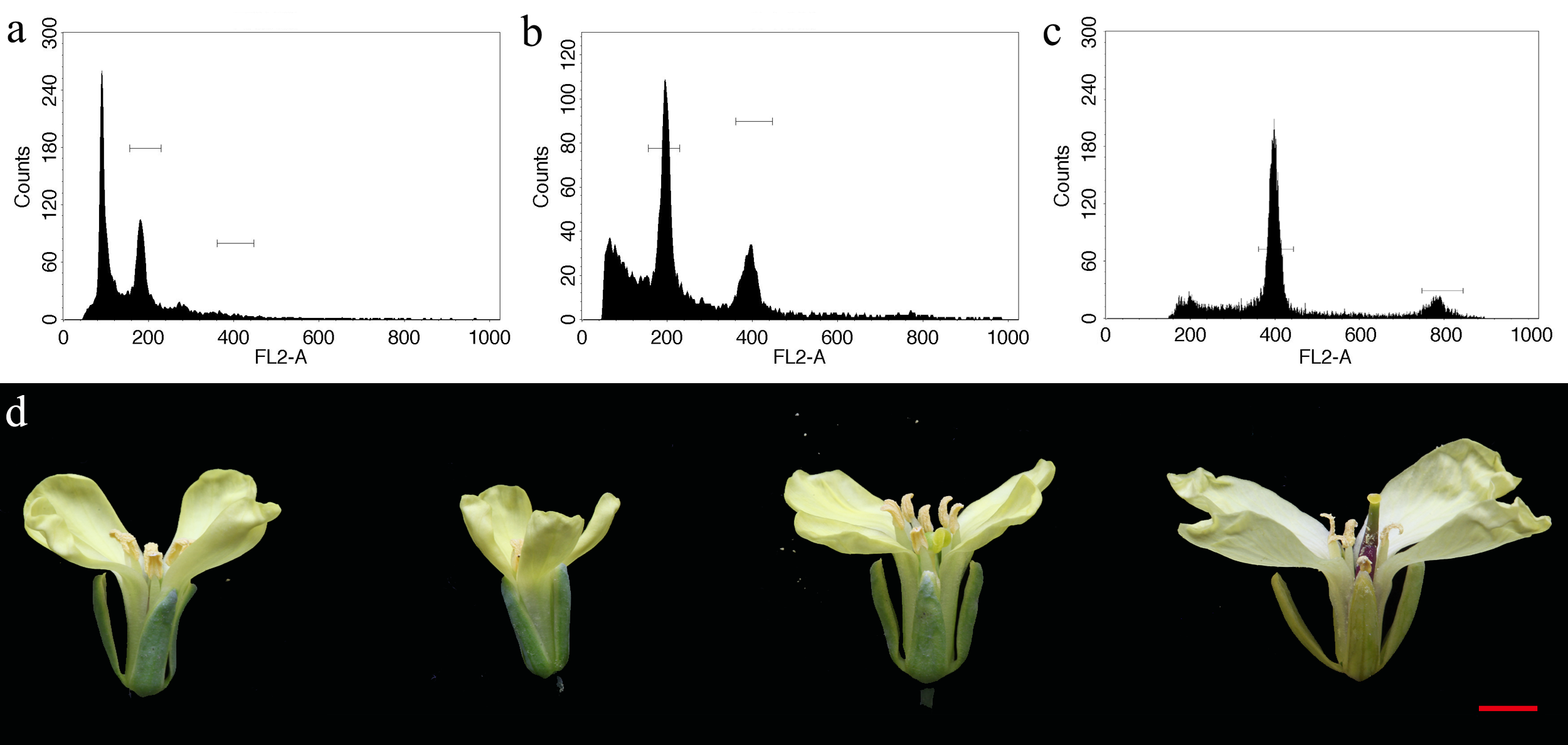
2.5. Ploidy Identification
The ploidy of regenerated seedlings was identified by FACSCalibur flow cytometer. The leaves were cut into 2 cm2 and put into Petri dishes containing chopping buffer solution (15 mM β-mercaptoethanol, 20 mM NaCl, 0.1% (v/v) TritonX-100, 0.5 mM spermine, 80 mM KCl, 15 mM Tris, and 2 mM EDTA-2Na at pH 7.2–7.5). Leaf samples were cut into pieces with scissors, later on, leached with a 300 mesh sieve to centrifuge tubes, centrifuged (10,000 rpm, 10 min). The supernatant was mixed with 1 mL PI dye and darkened for 15 min, after which the mixture was leached with a 500 mesh sieve. The DNA content of standard diploid was applied as control.
2.6. Experimental Design and Data Analysis
TSA treatment was repeated three times. When embryos grew to about 0.5 cm in length, the number of embryos was counted. The embryo yield measurement was to count the number of microspore embryos produced by per bud. Statistical evaluation was performed by SPSS software. To test whether the data difference was significant, Duncan’s least significant range test (p = 0.05) was used to separate the means.
4. Discussion
In this paper, the microspore embryogenesis rate and plant regeneration of CB, CP and CFQ were studied by adding various concentrations of TSA to NLN medium. The results manifested that the induction rate of embryos for diversified genotypes was varying, while CFQ had the most embryos, which was 13.27 embryos per bud. 5 nM TSA treatment of CFQ yielded the most embryos with 16.99 embryos per bud.
IMC can rapidly homozygous genotyping, promoting plant embryogenesis and improving plant regeneration rate. So far, IMC has been applied to many more species [
20], such as
Brassica [
21,
22,
23], barley (
Hordeum vulgare L.) [
24], triticale (
Secale cereale) [
25], eggplant (
Solanum melongena L.) [
26], pepper (
Capsicum frutescens L.) [
27], sweet pepper (
Capsicum frutescens L.) [
28] wheat (
Hordeum vulgare L.) [
29], rice (
Oryza sativa L.) [
30], potato (
Solanum tuberosum L) [
31], rye (
Secale cereale L.) [
32]. IMS changes the normal development direction of pollen to form haploid plants, which requires a variety of factors to regulate and control together. The genotype of the tested material affects both microspore embryogenesis and embryo yield [
33]. Microspore embryo induction of 15 genotype Chinese cabbage cultivars was analyzed, of which 13 cultivars obtained microspore embryos [
34]. Zeng et al. [
35] found that only 7 of the 11 genotypes could obtain microspore embryos in the study of stalks. Fang et al. [
36] applied 5 genotypes of flowering Chinese cabbage for IMC, and significant differences were sensed embryo induction rate among sundry genotypes. In this paper, three genotypes of cut flower ornamental kale cultivars were employed for IMC, and the embryo induction rates among the three genotypes were significantly different. Different genotypes of the same species have different ability to induce microspore embryos. This indicated that there was genotype dependence in the IMC of cut flower ornamental kale.
Microspores, as important morphologies throughout the plant life cycle, involve complex epigenetic modifications in both the normal gametophytic pathway and the stress-induced embryogenesis pathway. The impact of histone modification on embryonic development has long been concerned. Histone acetylation is regulated by histone acetylase and histone deacetylase (HDAC). Histone acetylation levels are altered when the activity of histone deacetylases is inhibited, thus affecting gene expression in microspore. It leads to the transformation for most cells from development to pollen pathway to embryogenesis, and eventually improves the embryogenesis rate of microspore [
37]. TSA, a metabolite of streptomycin, is one of many HDACI at present. Li et al. [
16] found that TSA treatment of
Brassica napus microspore could achieve the same effect of increasing embryo induction rate as heat shock stress. Castillo et al. [
38] found that 0.4 µM TSA and 0.7 m mannitol treated the anthers of bread wheat to obtain a four fold increase in the number of green DH plants. Wang et al. [
39] found that 0.01 µM TSA promotes wheat embryogenesis and green plant regeneration. Zhang et al. [
37] found that 0.05 µM TSA enabled pakchoi to produce the highest embryo yield and the highest plant regeneration frequency. Jiang et al. [
12] found that when wheat microspores were treated with TSA, low concentration of TSA had a promoting effect, and higher dosage of TSA had no effect or harm on embryonic development, 0.1 μM TSA treatment had the most significant effect. Low concentration of TSA can promote microspore embryogenesis In this study, we treated the microspores of CB, CP and CFQ with TSA, and the results depicted that 5 nM TSA had the highest embryogenesis rate. Very low concentrations of TSA could promote ‘CB’, ‘CP’ and ‘CFQ’ embryogenesis. Chen et al. [
10] also found that ornamental kale is low androgenic response: 10 nm Methylene blue can promote the embryogenesis of ornamental kale. However, high concentrations of TSA likewise inhibited microspore induction. TSA was the first application of IMC in cut flower ornamental kale.
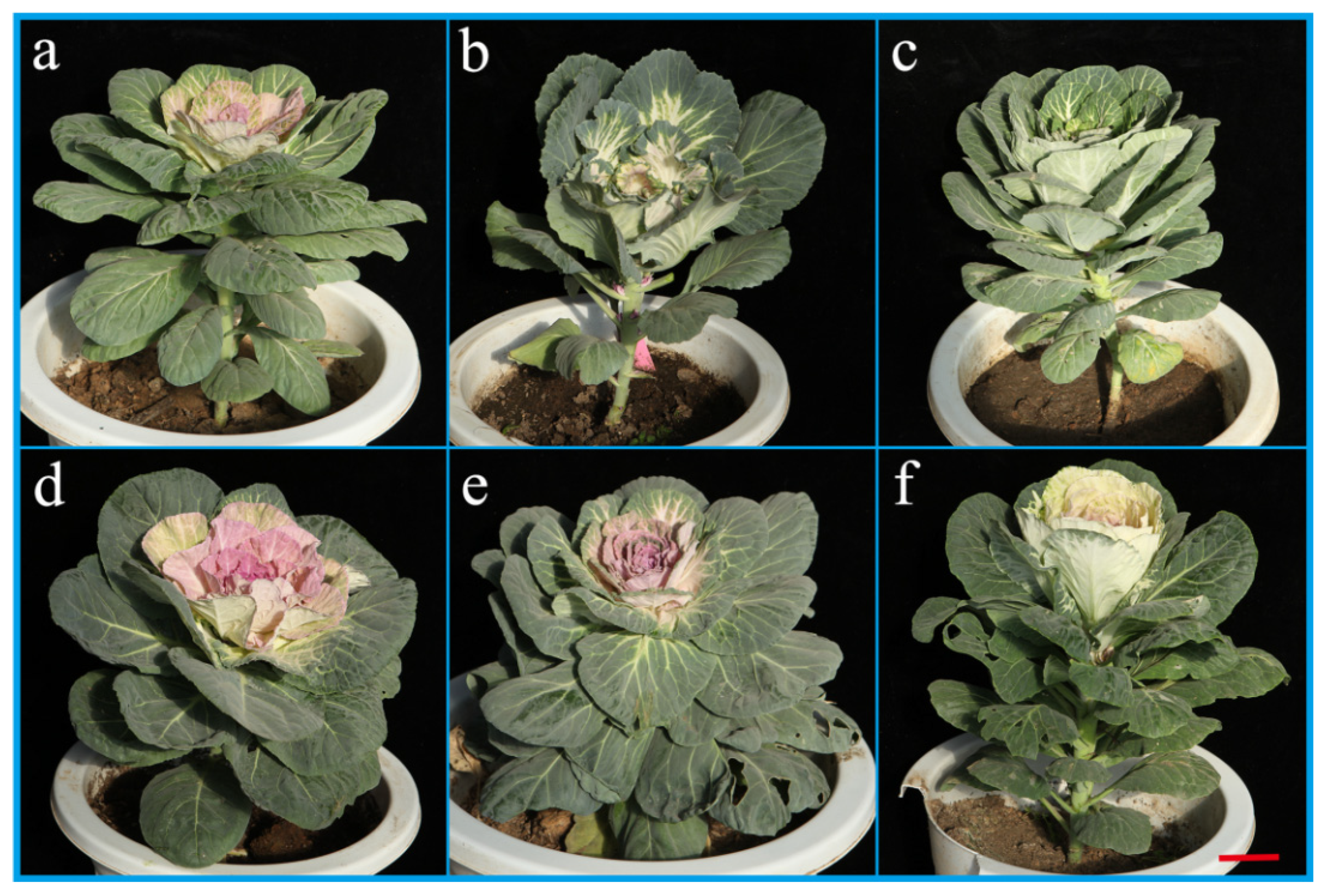
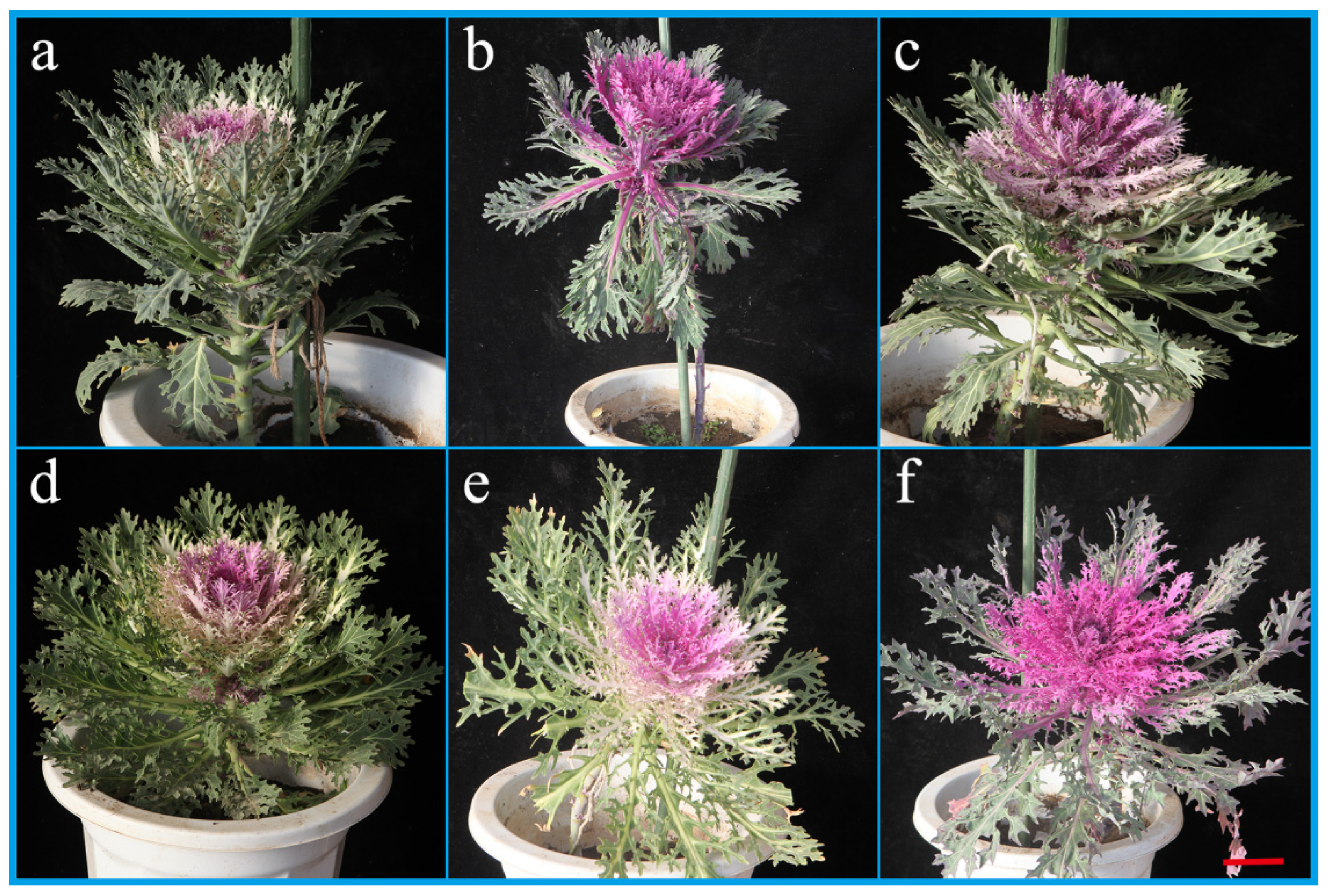
The use of hybrids as microspore culture materials can create abundant pure and mutant materials. CB with red leaves can yield materials with pink, white and green leaves. CP with pink leaves can generate materials with green, red and white leaves. CFQ with red leaves can manufacture materials with pink, red and white leaves. Plants with green leaves can produce grayish green and yellowish green materials. Compact morphology plants can be obtained from plants with loose morphology. Plants with round leaves can produce wavy round leaf materials. The leaf color turning time is likewise advanced or delayed. Homozygous lines with abundant variation are important for breeding cut flower ornamental kale. All gene loci of DH line of cut flower ornamental kale are homozygous and can be stably inherited. It is a valuable material for selecting combinations with high yield, high quality and strong superiority.
In this research, a suitable agent for promoting embryogenesis was discovered and the appropriate concentration for cut flower ornamental kale was tested, which was of great significance for the application of IMC in cut flower ornamental kale. This study contributed to improving the technology for IMC in cut flower ornamental kale. In the next step, DH line will be used as the parent to prepare hybrid combinations, determine their combining ability and screen excellent hybrids.