The Effects of Growth Regulators and Apical Bud Removal on Growth, Flowering, and Corms Production of Two Gladiolus Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Experimental Design
2.2. Field Management
2.3. Plant Measurements and Sampling Methods
2.4. Statistical Analysis
3. Results
3.1. Variety
3.2. Growth Regulators
3.3. Apical Bud Removal
3.4. Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrich, L. Flowering in South African Iridaceae. In Bulbous Plants: Biotechnology; Ramawat, K.G., Merillon, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2013; pp. 248–269. [Google Scholar]
- Goldblatt, P.; Manning, J.C. Gladiolus in Southern Africa: Systematics, Biology, and Evolution; Fernwood Press: Cape Town, South Africa, 1998. [Google Scholar]
- Azimi, M.H. Evaluation yield and genetically factors in different cultivars of gladiolus. Ornam. Hortic. 2020, 26. [Google Scholar] [CrossRef]
- Cohat, J. Gladiolus. In Physiology of Flower Bulbs; Hertogh, A.D., Nard, M.L., Eds.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1992; pp. 297–320. [Google Scholar]
- Ahmed, M.J.; Akbar, Z.; Kosar, N.; Khan, Z.A. Introduction and Evaluation of Exotic Gladiolus (Gladiolus grandiflorus) Cultivars. Asian J. Plant Sci. 2002, 1, 560–562. [Google Scholar] [CrossRef]
- Memon, N.; Wahocho, N.; Miami, T.; Husain, M. Propagation of gladiolus corms and cormels: A review. African J. Biotechnol. 2016, 15, 1699–1710. [Google Scholar]
- Kumar, A.; Palni, L.M.; Sood, A.; Sharma, M.; Palni, U.T.; Gupta, A.K. Heat-shock induced somatic embryogenesis in callus cultures of gladiolus in the presence of high sucrose. J. Hortic. Sci. Biotechnol. 2002, 77, 73–78. [Google Scholar] [CrossRef]
- Singh, A.P.; Dohare, S.R. Maximization of corms and cormel production in Gladiolus. In Floriculture-Technology, Trades and Trends; Prakash, J., Bhandary, K.R., Eds.; Oxford and IBH Pub Co, Pvt Ltd.: New Delhi, India, 1994; pp. 205–208. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R.; Ahmad, I. Enhancement of corm and cormel production in gladiolus (Gladiolus spp.). N. Z. J. Crop Hortic. Sci. 2009, 37, 319–325. [Google Scholar] [CrossRef][Green Version]
- Kumar, P.N.; Misra, R.L. Effect of plant growth regulators on growth, flowering and corm production of gladiolus cv. Snow Princess. Indian J. Agric. Sci. 2012, 82, 632–637. [Google Scholar]
- Sajjad, Y.; Jaskani, M.; Qasim, M.; Mehmood, A.; Ahmed, N.; Akhtar, G. Pre-plant soaking of corms in growth regulators influences the multiple sprouting, floral and corm associated traits in Gladiolus grandiflorus L. J. Agric. Sci. 2015, 7, 173–181. [Google Scholar] [CrossRef]
- Shyla, R.M.R.; Rameshkumar, S. Effect of crop regulation practices on spike and corm yield of gladiolus (Gladiolus hybrids Hort.) cv Sarala. Ann. Plant Soil Res. 2021, 23, 93–98. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A.K. Effect of plant growth regulators and micronutrients on vegetative and flowering characters of gladiolus (Gladiolus grandiflorus L.) cv. Novalux. Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 1136–1143. [Google Scholar] [CrossRef]
- Nag, K.; Kuma, J. Influence of growth regulators and spacing’s on corms yield of gladiolus (Gladiolus grandifloras L.): A review. The Pharma Innov. J. 2021, 10, 595–597. [Google Scholar]
- Holkar, S.; Kumar, P.H.; Chandrashekar, S.Y. Effect of benzyl adenine and gibberellic acid on flowering and flower quality attributes of gladiolus. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 944–950. [Google Scholar]
- Wilfret, G.J. Gladiolus. In Introduction to Floriculture; Larson, R.A., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2012; pp. 143–157. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davis, F.T. Plant Propagation: Principles and Practices; Prentice/Hall International Inc.: Englewood Cliffs, NJ, USA, 1990. [Google Scholar]
- The Garden.org Plants Database. Available online: https://garden.org/plants/ (accessed on 10 August 2022).
- Khan, F.N.; Rahman, M.M.; Hossain, M.M. Effect of benzyladenine and gibberellic acid on dormancy breaking, growth and yield of gladiolus corms over different storage periods. J. Ornam. Horti. Plants 2013, 3, 59–71. [Google Scholar]
- Wilfret, G.J. Gladiolus. In Introduction to Floriculture; Larson, R.A., Ed.; Academic Press: San Diego, CA, USA, 1980; pp. 166–181. [Google Scholar]
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 23 January 2013).
- Ram, R.; Mukherjee, D.; Manuja, S. Plant growth regulators affect the development of both corms and cormels in gladiolus. HortScience 2002, 37, 343–344. [Google Scholar] [CrossRef]
- Baskaran, V.; Misra, R.L. Effect of plant growth regulators on growth and flowering of gladiolus. Indian J. Hort. 2007, 64, 479–482. [Google Scholar]
- Whipker, B.E.; Evans, M.R. Regulation of plant growth. In Greenhouse Operation and Management; Nelson, P.V., Ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012; pp. 373–390. [Google Scholar]
- Misra, R.L.; Tripathi, D.K.; Chaturvedi, O.P. Implication of gibberellic acid spraying on the standing crop of gladiolus var. Sylvia. Progress. Hort. 1993, 25, 147–150. [Google Scholar]
- Thomas, S.G.; Hedden, P. Gibberellin metabolism and signal transduction. In Annual Plant Reviews; Hedden, P., Thomas, S.G., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 147–184. [Google Scholar]
- Hanks, G.R.; Rees, A.R. Growth regulator treatments to improve the yield of twin-scaled narcissus. Sci. Hortic. 1977, 9, 399–411. [Google Scholar] [CrossRef]
- Foskett, D.E. Plant Growth and Development; Academic Press: San Diego, CA, USA, 1994. [Google Scholar]
- Wardlaw, I.F. The control and pattern of movement of carbohydrates in plants. Bot. Rev. 1968, 34, 79–105. [Google Scholar] [CrossRef]
- Ginzburg, C.; Ziv, M. Hormonal regulation of corn formation in gladiolus stolons grown in vitro. Ann. Bot. 1973, 37, 219–224. [Google Scholar] [CrossRef]
- Anju, P.; Kumar, S.; Srivastava, R. Effect of floral preservatives on postharvest management in gladiolus spikes. J. Ornam. Hortic. 2003, 6, 367–371. [Google Scholar]
- He, X.; Shi, L.; Yuan, Z.; Xu, Z.; Zhang, Z.; Yi, M. Effects of lipoxygenase on the corm formation and enlargement in Gladiolus hybridus. Sci. Hortic. 2008, 118, 60–69. [Google Scholar] [CrossRef]

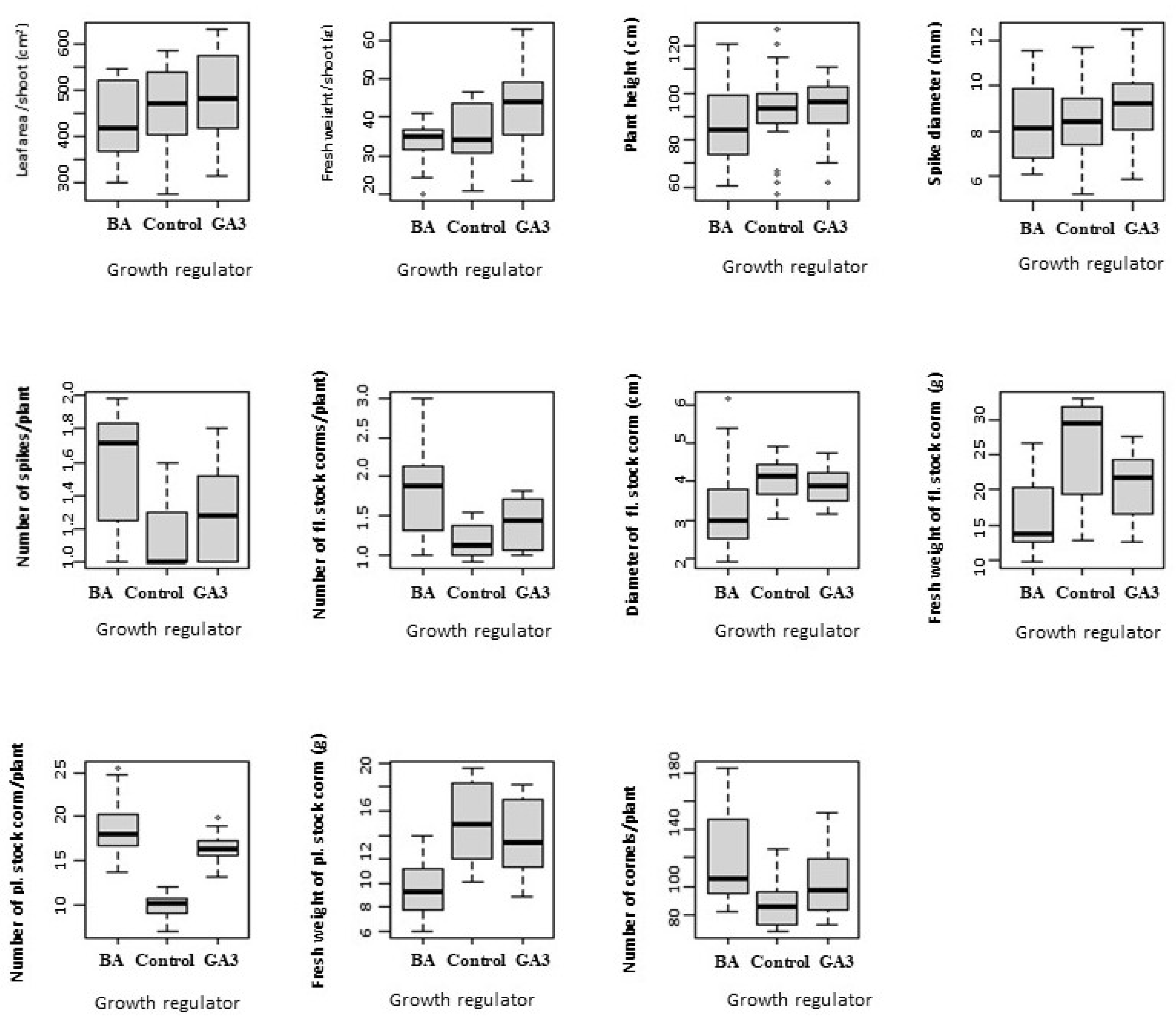
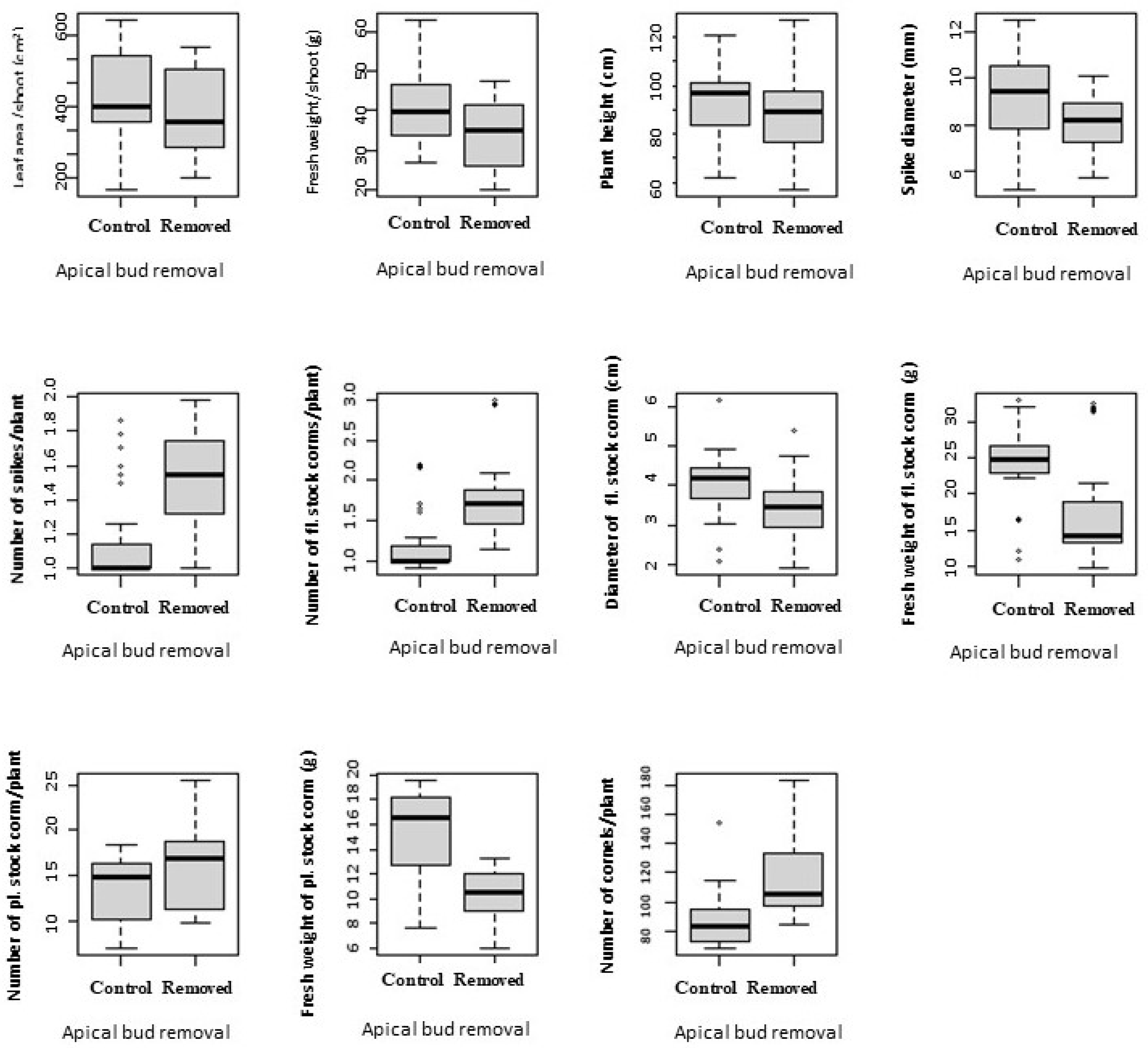
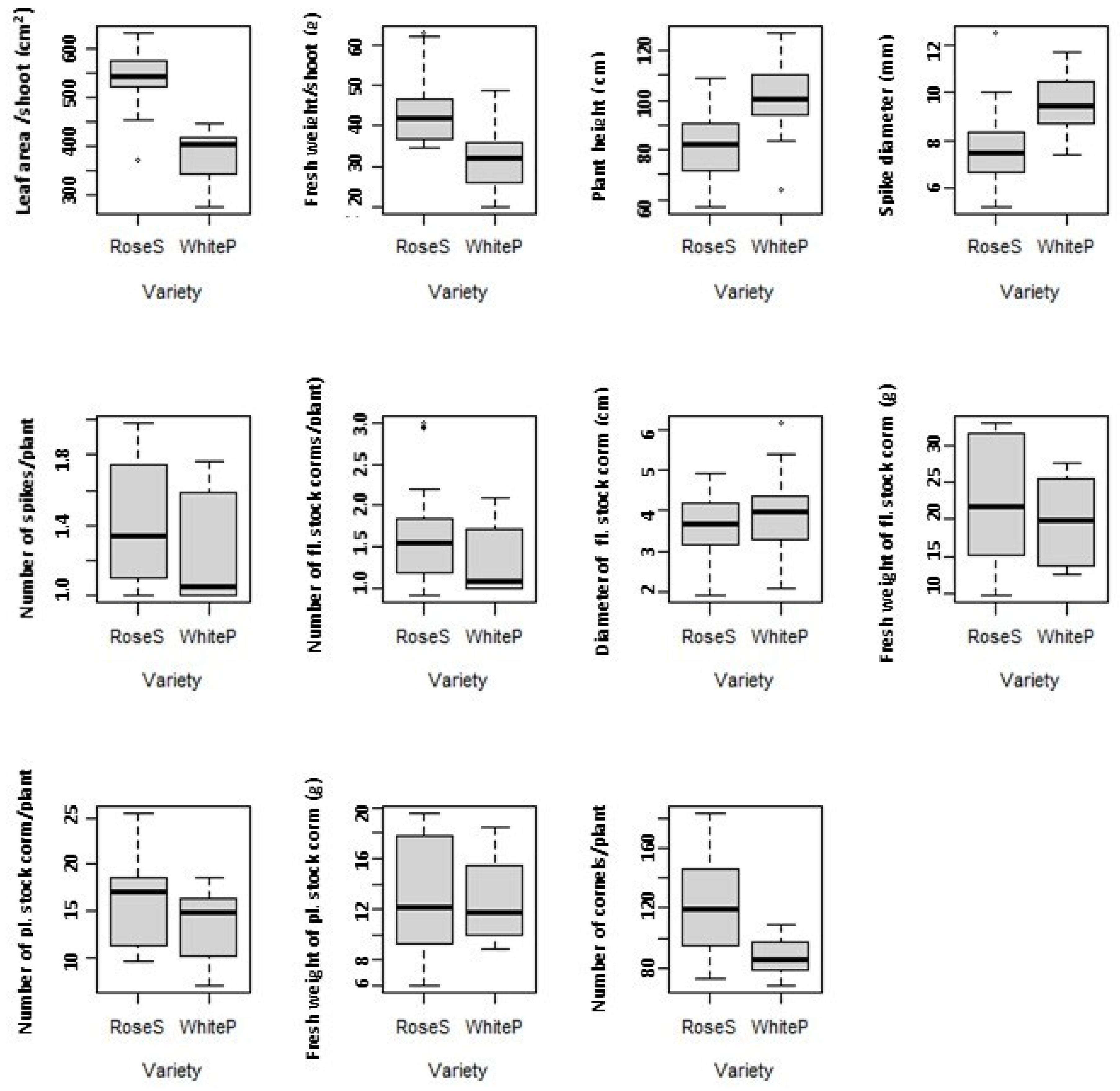
| Variables with Descriptions | Range |
|---|---|
| Flowering stock corms variables | |
| Number of flowering stock corms per plant | 1–3 |
| Diameter of flowering stock corm (cm) | 1.9–6.2 |
| Fresh weight of flowering stock corm (g) | 9.7–33.0 |
| Planting stock corm variables | |
| Number of planting stock corm per plant | 7.0–26 |
| Fresh weight of planting stock corm (g) | 6.0-19.6 |
| Cormels variables | |
| Number of cormels per plant | 69–183 |
| Vegetative and floral variables | |
| Total leaf area per shoot (cm) | 278–630 |
| Fresh weight of leaves per shoot (g) | 20.1–62.7 |
| Plant height (cm, at flowering) | 57.3–126.7 |
| Spike diameter (mm) | 5.2–12.5 |
| Number of spikes per plant | 1–2 |
| Combinations | Mean Fresh Weight (g) of Flowering Stock Corm Per Plant |
|---|---|
| BA + Removed apical bud + ‘Rose Supreme’ | 11.7 d |
| No growth regulator + Removed apical bud + ‘White Prosperity’ | 13.7 d |
| BA + Removed apical bud + ‘White Prosperity’ | 13.8 d |
| BA + No removal of apical bud + ‘Rose Supreme’ | 13.9 d |
| GA3 + Removed apical bud + ‘White Prosperity’ | 14.6 d |
| GA3 + Removed apical bud + ‘Rose Supreme’ | 19.1 c |
| GA3 + No removal of apical bud + ‘Rose Supreme’ | 23.6 b |
| BA + No removal of apical bud + ‘White Prosperity’ | 25.2 b |
| No growth regulator + No removal of apical bud + ‘White Prosperity’ | 25.3 b |
| GA3 + No removal of apical bud + ‘White Prosperity’ | 25.3 b |
| No growth regulator + Removed apical bud + ‘Rose Supreme’ | 31.9 a |
| No growth regulator + No removal of apical bud + ‘Rose Supreme’ | 32.0 a |
| Combinations | Mean Number of Planting Stock Corms Per Plant |
|---|---|
| No growth regulator + No removal of apical bud + ‘White Prosperity’ | 7.66 g |
| No growth regulator + Removed apical bud + ‘White Prosperity’ | 10.12 f |
| No growth regulator + No removal of apical bud + ‘Rose Supreme’ | 10.15 f |
| No growth regulator + Removed apical bud + ‘Rose Supreme’ | 11.37 f |
| GA3 + No removal of apical bud + ‘White Prosperity’ | 14.46 e |
| BA + No removal of apical bud + ‘White Prosperity’ | 15.03 de |
| GA3 + Removed apical bud + ‘White Prosperity’ | 16.31 cd |
| GA3 + No removal of apical bud + ‘Rose Supreme’ | 16.35 cd |
| BA + No removal of apical bud + ‘Rose Supreme’ | 17.62 bc |
| BA + Removed apical bud + ‘White Prosperity’ | 17.95 b |
| GA3 + Removed apical bud + ‘Rose Supreme’ | 18.46 b |
| BA + Removed apical bud + ‘Rose Supreme’ | 23.66 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalafalla, M.M.; Hegazi, M.A.; Eltarawy, A.M.; Magouz, M.R.; Elzaim, H.H.; Yndgaard, F.; Solberg, S.Ø. The Effects of Growth Regulators and Apical Bud Removal on Growth, Flowering, and Corms Production of Two Gladiolus Varieties. Horticulturae 2022, 8, 789. https://doi.org/10.3390/horticulturae8090789
Khalafalla MM, Hegazi MA, Eltarawy AM, Magouz MR, Elzaim HH, Yndgaard F, Solberg SØ. The Effects of Growth Regulators and Apical Bud Removal on Growth, Flowering, and Corms Production of Two Gladiolus Varieties. Horticulturae. 2022; 8(9):789. https://doi.org/10.3390/horticulturae8090789
Chicago/Turabian StyleKhalafalla, Magdy Mohamed, Mahmoud Abdelnabi Hegazi, Ahmed Mohamed Eltarawy, Mohamed Refaat Magouz, Hamdy Hassan Elzaim, Flemming Yndgaard, and Svein Øivind Solberg. 2022. "The Effects of Growth Regulators and Apical Bud Removal on Growth, Flowering, and Corms Production of Two Gladiolus Varieties" Horticulturae 8, no. 9: 789. https://doi.org/10.3390/horticulturae8090789
APA StyleKhalafalla, M. M., Hegazi, M. A., Eltarawy, A. M., Magouz, M. R., Elzaim, H. H., Yndgaard, F., & Solberg, S. Ø. (2022). The Effects of Growth Regulators and Apical Bud Removal on Growth, Flowering, and Corms Production of Two Gladiolus Varieties. Horticulturae, 8(9), 789. https://doi.org/10.3390/horticulturae8090789








