Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods
Abstract
:1. Introduction
2. Conceptual Framework
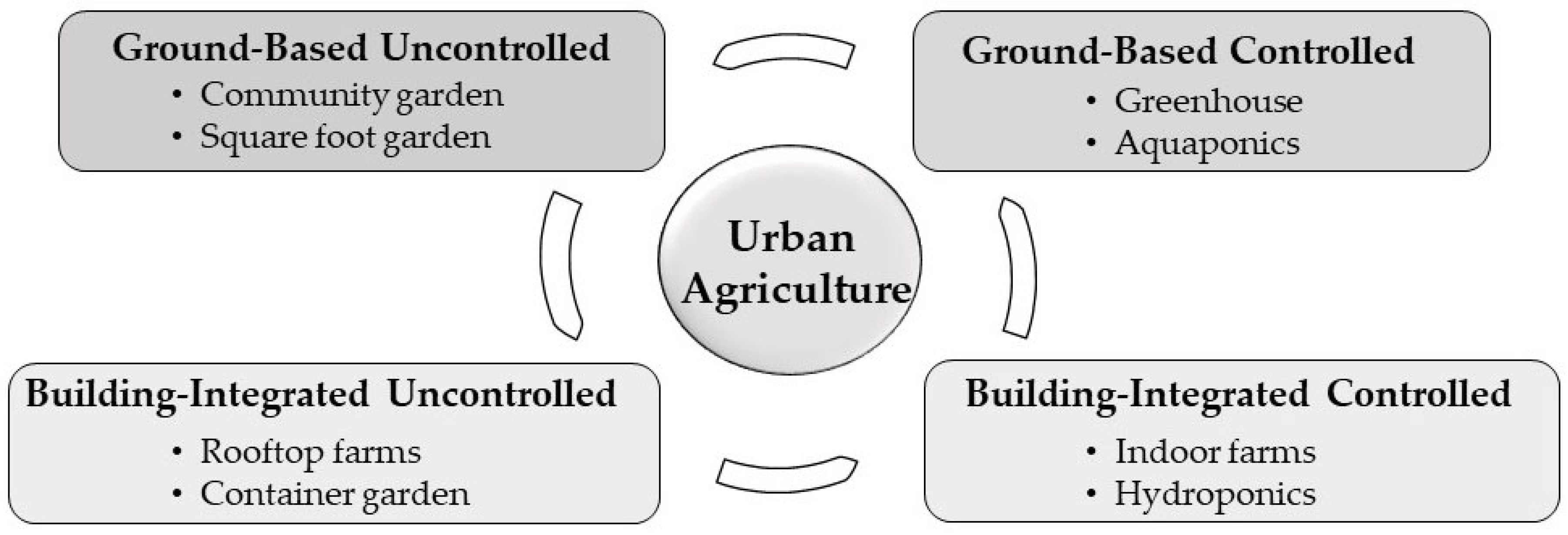
2.1. Recent Classification of Urban Agriculture
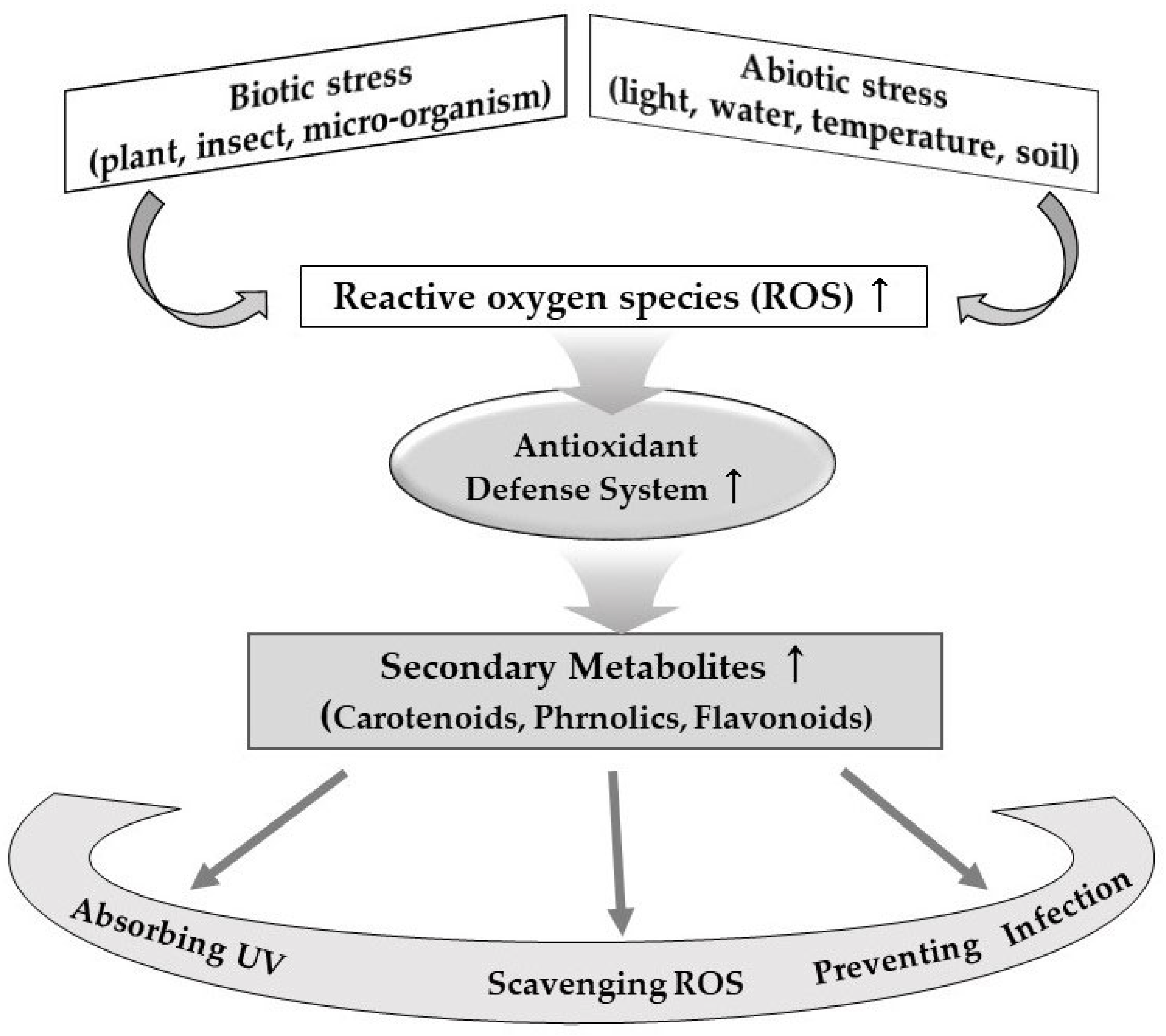
2.2. Mechanisms of Biological Production of Bioactive Compounds in Plant Foods
2.3. Major Bioactive Compounds in Plant Foods
3. Strategies to Improve Bioactive Compounds in Plant Foods by Urban Agriculture
3.1. Ground-Based, Uncontrolled Urban Agriculture
3.2. Ground-Based, Controlled Urban Agriculture
3.3. Building-Integrated, Uncontrolled Urban Agriculture
3.4. Building-Integrated, Controlled Urban Agriculture
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crane, M.; Wehner, T.C.; Naegele, R.P. Cucumber Cultivars for Container Gardening and the Value of Field Trials for Predicting Cucumber Performance in Containers. Hortscience 2018, 53, 16–22. [Google Scholar] [CrossRef]
- Wielemaker, R.; Oenema, O.; Zeeman, G.; Weijma, J. Fertile cities: Nutrient management practices in urban agriculture. Sci. Total Environ. 2019, 668, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.A.; Bonnett, G.D.; McIntyre, C.L.; Hochman, Z.; Wasson, A.P. Strategies to improve the productivity, product diversity and profitability of urban agriculture. Agric. Syst. 2019, 174, 133–144. [Google Scholar] [CrossRef]
- Siegner, A.; Sowerwine, J.; Acey, C. Does Urban Agriculture Improve Food Security? Examining the Nexus of Food Access and Distribution of Urban Produced Foods in the United States: A Systematic Review. Sustainability 2018, 10, 2988. [Google Scholar] [CrossRef]
- Patil, B.S.; Jayaprakasha, G.K.; Vikram, A. Indigenous Crops of Asia and Southeast Asia: Exploring Health-promoting Properties. Hortscience 2012, 47, 821–827. [Google Scholar] [CrossRef]
- Ross, K.; Neilsen, G.; Neilsen, D. The Effect of Irrigation Frequency, Phosphorus Fertigation, and Cultivar on Levels of Phenolic Compounds in Sweet Cherries. Hortscience 2018, 53, 1507–1512. [Google Scholar] [CrossRef]
- Durmic, Z.; Blache, D. Bioactive plants and plant products: Effects on animal function, health and welfare. Anim. Feed Sci. Tech. 2012, 176, 150–162. [Google Scholar] [CrossRef]
- Fathy, S.M.; El-Dash, H.A.; Said, N.I. Neuroprotective effects of pomegranate (Punica granatum L.) juice and seed extract in paraquat-induced mouse model of Parkinson’s disease. BMC Complement. Med. 2021, 21, 130. [Google Scholar] [CrossRef]
- Rutledge, G.A.; Sandhu, A.K.; Miller, M.G.; Edirisinghe, I.; Burton-Freeman, B.B.; Shukitt-Hale, B. Blueberry phenolics are associated with cognitive enhancement in supplemented healthy older adults. Food Funct. 2021, 12, 107–118. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.L. The role of flavonoids in the prevention and management of cardiovascular complications: A narrative review. Ann. Palliat. Med. 2021, 10, 8254–8263. [Google Scholar] [CrossRef]
- do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston-Green, K.; et al. Food anthocyanins decrease concentrations of TNF-alpha in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Vitucci, D.; Amoresano, A.; Nunziato, M.; Muoio, S.; Alfieri, A.; Oriani, G.; Scalfi, L.; Frusciante, L.; Rigano, M.M.; Pucci, P.; et al. Nutritional Controlled Preparation and Administration of Different Tomato Purees Indicate Increase of beta-Carotene and Lycopene Isoforms, and of Antioxidant Potential in Human Blood Bioavailability: A Pilot Study. Nutrients 2021, 13, 1336. [Google Scholar] [CrossRef] [PubMed]
- Shokri-Mashhadi, N.; Tahmasebi, M.; Mohammadi-Asl, J.; Zakerkish, M.; Mohammadshahi, M. The antioxidant and anti-inflammatory effects of astaxanthin supplementation on the expression of miR-146a and miR-126 in patients with type 2 diabetes mellitus: A randomised, double-blind, placebo-controlled clinical trial. Int. J. Clin. Pract. 2021, 75, e14022. [Google Scholar] [CrossRef]
- Nurzynska-Wierdak, R.; Zawislak, G. Herb Yield and Bioactive Compounds of Tarragon (Artemisia dracunculus L.) as Influenced by Plant Density. Acta Sci. Pol.-Hortoru. 2014, 13, 207–221. [Google Scholar]
- Niazian, M.; Howyzeh, M.S.; Sadat-Noori, S.A. Integrative effects of stress- and stress tolerance-inducing elicitors on in vitro bioactive compounds of ajowan [Trachyspermum ammi (L.) Sprague] medicinal plant. Plant Cell Tissue Organ Cult. 2021, 146, 589–604. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant Phenylpropanoids as Emerging Anti-Inflammatory Agents. Mini Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef]
- Croge, C.P.; Cuquel, F.L.; Pintro, P.T.M.; Biasi, L.A.; De Bona, C.M. Antioxidant Capacity and Polyphenolic Compounds of Blackberries Produced in Different Climates. Hortscience 2019, 54, 2209–2213. [Google Scholar] [CrossRef]
- Frias-Moreno, M.N.; Parra-Quezada, R.A.; Gonzalez-Aguilar, G.; Ruiz-Canizales, J.; Molina-Corral, F.J.; Sepulveda, D.R.; Salas-Salazar, N.; Olivas, G.I. Quality, Bioactive Compounds, Antioxidant Capacity, and Enzymes of Raspberries at Different Maturity Stages, Effects of Organic vs. Conventional Fertilization. Foods 2021, 10, 953. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, M.M. Short-term Low Temperature Increases Phenolic Antioxidant Levels in Kale. Hortic. Environ. Biotechnol. 2015, 56, 588–596. [Google Scholar] [CrossRef]
- Wei, P.; Yang, Y.; Wang, F.; Chen, H.J. Effects of Drought Stress on the Antioxidant Systems in Three species of Diospyros L. Hortic. Environ. Biotechnol. 2015, 56, 597–605. [Google Scholar] [CrossRef]
- Proz, M.D.; da Silva, M.A.S.; Rodrigues, E.; Bender, R.J.; Rios, A.D. Effects of indoor, greenhouse, and field cultivation on bioactive compounds from parsley and basil. J. Sci. Food Agric. 2021, 101, 6320–6330. [Google Scholar] [CrossRef]
- Llorach, R.; Martinez-Sanchez, A.; Tomas-Barberan, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.J.; Niu, G.H.; Gu, M.M.; Masabni, J. Morphological and Physiological Responses in Basil and Brassica Species to Different Proportions of Red, Blue, and Green Wavelengths in Indoor Vertical Farming. J. Am. Soc. Hortic. Sci. 2020, 145, 267–278. [Google Scholar] [CrossRef]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Du, S.K.; Wang, H.X.; Cai, M. In vitro antioxidant activity of extracts from common legumes. Food Chem. 2014, 152, 462–466. [Google Scholar] [CrossRef]
- Yuan, Y.; Simplaceanu, V.; Ho, N.T.; Ho, C. An Investigation of the Distal Histidyl Hydrogen Bonds in Oxyhemoglobin: Effects of Temperature, pH, and Inositol Hexaphosphate. Biochemistry 2010, 49, 10606–10615. [Google Scholar] [CrossRef]
- Tulipani, S.; Mezzetti, B.; Capocasa, F.; Bompadre, S.; Beekwilder, J.; De Vos, C.H.R.; Capanoglu, E.; Bovy, A.; Battino, M. Antioxidants, phenolic compounds, and nutritional quality of different strawberry genotypes. J. Agric. Food Chem. 2008, 56, 696–704. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radical. Bio. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Weidner, T.; Yang, A.D.; Hamm, M.W. Consolidating the current knowledge on urban agriculture in productive urban food systems: Learnings, gaps and outlook. J. Clean. Prod. 2019, 209, 1637–1655. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Azevedo, L.; Ribeiro, P.F.D.; Oliveira, J.A.D.; Correia, M.G.; Ramos, F.M.; de Oliveira, E.B.; Barros, F.; Stringheta, P.C. Camu-camu (Myrciaria dubia) from commercial cultivation has higher levels of bioactive compounds than native cultivation (Amazon Forest) and presents antimutagenic effects in vivo. J. Sci. Food Agric. 2019, 99, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Stopar, M.; Bolcina, U.; Vanzo, A.; Vrhovsek, U. Lower crop load for Cv. Jonagold apples (Malus × domestica Borkh.) increases polyphenol content and fruit quality. J. Agric. Food Chem. 2002, 50, 1643–1646. [Google Scholar] [CrossRef] [PubMed]
- Ionica, M.E.; Nour, V.; Trandafir, I. Bioactive compounds and antioxidant activity of hot pepper fruits at different stages of growth and ripening. J. Appl. Bot. Food Qual. 2017, 90, 232–237. [Google Scholar]
- Kwon, Y.S.; Kim, C.W.; Kim, J.; Moon, J.; Yoo, K.S. Effects of bolting and flower stem removal on the growth and chemical qualities of onion bulbs. Hortic. Environ. Biotechnol. 2016, 57, 310. [Google Scholar] [CrossRef]
- Ghiasy-Oskoee, M.; AghaAlikhani, M.; Sefidkon, F.; Mokhtassi-Bidgoli, A.; Ayyari, M. Blessed thistle agronomic and phytochemical response to nitrogen and plant density. Ind. Crop Prod. 2018, 122, 566–573. [Google Scholar] [CrossRef]
- Salehi, A.; Fallah, S.; Zitterl-Eglseer, K.; Kaul, H.P.; Surki, A.A.; Mehdi, B. Effect of Organic Fertilizers on Antioxidant Activity and Bioactive Compounds of Fenugreek Seeds in Intercropped Systems with Buckwheat. Agronomy 2019, 9, 367. [Google Scholar] [CrossRef]
- Jedrszczyk, E.; Kopec, A.; Bucki, P.; Ambroszczyk, A.M.; Skowera, B. The Enhancing Effect of Plants Growth Biostimulants in Garlic Cultivation on the Chemical Composition and Level of Bioactive Compounds in the Garlic Leaves, Stems and Bulbs. Not. Bot. Horti Agrobot. 2019, 47, 81–91. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef]
- Pek, Z.; Daood, H.; Nagyne, M.G.; Nemenyi, A.; Helyes, L. Effect of environmental conditions and water status on the bioactive compounds of broccoli. Cent. Eur. J. Biol. 2013, 8, 777–787. [Google Scholar]
- Vlaic, R.A.; Socaci, S.A.; Muresan, A.E.; Muresan, C.; Moldovan, O.P.; Muste, S.; Muresan, V. Bioactive Compounds and Volatile Profile Dynamics During Fruit Growth of Several Plums Cultivars. J. Agric. Sci. Tech.-Iran 2017, 19, 1565–1576. [Google Scholar]
- Choi, H.G.; Moon, B.Y.; Bekhzod, K.; Park, K.S.; Kwon, J.K.; Lee, J.H.; Cho, M.W.; Kang, N.J. Effects of Foliar Fertilization Containing Titanium Dioxide on Growth, Yield and Quality of Strawberries During Cultivation. Hortic. Environ. Biotechnol. 2015, 56, 575–581. [Google Scholar] [CrossRef]
- Schmitz-Eiberger, M.A.; Blanke, M.M. Bioactive components in forced sweet cherry fruit (Prunus avium L.), antioxidative capacity and allergenic potential as dependent on cultivation under cover. LWT-Food Sci. Technol. 2012, 46, 388–392. [Google Scholar] [CrossRef]
- Blando, F.; Gerardi, C.; Renna, M.; Castellano, S.; Serio, F. Characterisation of bioactive compounds in berries from plants grown under innovative photovoltaic greenhouses. J. Berry Res. 2018, 8, 55–69. [Google Scholar] [CrossRef]
- An, X.J.; Liang, Y.L.; Gao, D.K.; Zhu, S.M.; Kong, F.C. Response of health-promoting bioactive compounds and related enzyme activities of table grape (Vitis vinifera L.) to deficit irrigation in greenhouse. J. Hortic. Sci. Biotech. 2018, 93, 573–584. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Ionica, M.E. Evolution of antioxidant activity and bioactive compounds in tomato (Lycopersicon esculentum Mill.) fruits during growth and ripening. J. Appl. Bot. Food Qual. 2014, 87, 97–103. [Google Scholar]
- Shams, M.; Yildirim, E.; Ekinci, M.; Turan, M.; Dursun, A.; Parlakova, F.; Kul, R. Exogenously applied glycine betaine regulates some chemical characteristics and antioxidative defence systems in lettuce under salt stress. Hortic. Environ. Biotechnol. 2016, 57, 225–231. [Google Scholar] [CrossRef]
- Goicoechea, N.; Garmendia, I.; Fabbrin, E.G.; Bettoni, M.M.; Palop, J.A.; Sanmartin, C. Selenium fertilization and mycorrhizal technology may interfere in enhancing bioactive compounds in edible tissues of lettuces. Sci. Hortic. 2015, 195, 163–172. [Google Scholar] [CrossRef]
- Guo, L.P.; Zhu, Y.L.; Wang, F.W. Calcium sulfate treatment enhances bioactive compounds and antioxidant capacity in broccoli sprouts during growth and storage. Postharvest Biol. Tec. 2018, 139, 12–19. [Google Scholar] [CrossRef]
- Tavallali, V.; Jandoust, S.; Mehrjerdi, A.A. Foliar application of 5-aminolevulinic acid promotes bioactive compounds and nutritional value of purslane, a potential vegetable for the future. J. Appl. Bot. Food Qual 2019, 92, 25–32. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Xiccato, G.; Borin, M.; Sambo, P. Composition and quality traits of vegetables grown in a low-tech aquaponic system at different fish stocking densities. J. Sci. Food Agric. 2020, 100, 4310–4318. [Google Scholar] [CrossRef] [PubMed]
- Saleh, H.A.R.; El-Nashar, Y.I.; Serag-El-Din, M.F.; Dewir, Y.H. Plant growth, yield and bioactive compounds of two culinary herbs as affected by substrate type. Sci. Hortic. 2019, 243, 464–471. [Google Scholar] [CrossRef]
- Appolloni, E.; Orsini, F.; Specht, K.; Thomaier, S.; Sanye-Mengual, E.; Pennisi, G.; Gianquinto, G. The global rise of urban rooftop agriculture: A review of worldwide cases. J. Clean. Prod. 2021, 296, 126556. [Google Scholar] [CrossRef]
- Arcas-Pilz, V.; Parada, F.; Villalba, G.; Rufi-Salis, M.; Rosell-Mele, A.; Durany, X.G. Improving the Fertigation of Soilless Urban Vertical Agriculture Through the Combination of Struvite and Rhizobia Inoculation in Phaseolus vulgaris. Front. Plant Sci. 2021, 12, 649304. [Google Scholar] [CrossRef] [PubMed]
- Walters, S.A.; Midden, K.S. Sustainability of Urban Agriculture: Vegetable Production on Green Roofs. Agriculture 2018, 8, 168. [Google Scholar] [CrossRef]
- Sinkovic, L.; Hribar, J.; Demsar, L.; Vidrih, R.; Necemer, M.; Kump, P.; Znidarcic, D. Bioactive compounds and macroelements of chicory plants (Cichorium intybus L.) after hydroponic forcing in different nutrient solutions. Hortic. Environ. Biotechnol. 2017, 58, 274–281. [Google Scholar] [CrossRef]
- Song, S.; Arora, S.; Laserna, A.K.C.; Shen, Y.; Thian, B.W.Y.; Cheong, J.C.; Tan, J.K.N.; Chiam, Z.; Fong, S.L.; Ghosh, S.; et al. Biochar for urban agriculture: Impacts on soil chemical characteristics and on Brassica rapa growth, nutrient content and metabolism over multiple growth cycles. Sci. Total Environ. 2020, 727, 138742. [Google Scholar] [CrossRef]
- Nour, V.; Ionica, M.E.; Trandafir, I. Bioactive Compounds, Antioxidant Activity and Color of Hydroponic Tomato Fruits at Different Stages of Ripening. Not. Bot. Horti. Agrobo. 2015, 43, 404–412. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, K.H.; Oh, M.M. Increase in biomass and bioactive compounds in lettuce under various ratios of red to far-red LED light supplemented with blue LED light. Hortic. Environ. Biotechnol. 2016, 57, 139–147. [Google Scholar] [CrossRef]
- Gibson, K.E.; Lamm, A.J.; Masambuka-Kanchewa, F.; Fisher, P.R.; Gomez, C. Identifying Indoor Plant Propagation Research and Education Needs of Specialty Crop Growers. Horttechnology 2020, 30, 519–527. [Google Scholar] [CrossRef]
- Magwaza, S.T.; Magwaza, L.S.; Odindo, A.O.; Mditshwa, A. Hydroponic technology as decentralised system for domestic wastewater treatment and vegetable production in urban agriculture: A review. Sci. Total Environ. 2020, 698, 134154. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.C.; Chen, Y.H.; Chen, Y.H.; Wang, C.F.; Hu, M.C. Food-Energy Interactive Tradeoff Analysis of Sustainable Urban Plant Factory Production Systems. Sustainability 2018, 10, 446. [Google Scholar] [CrossRef]
- Son, K.H.; Lee, J.H.; Oh, Y.; Kim, D.; Oh, M.M.; In, B.C. Growth and Bioactive Compound Synthesis in Cultivated Lettuce Subject to Light-quality Changes. Hortscience 2017, 52, 584–591. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and phenolic compounds of Lactuca sativa L. grown in a closed-type plant production system with UV-A, -B, or -C lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Jeon, Y.M.; Son, K.H.; Kim, S.M.; Oh, M.M. Growth of dropwort plants and their accumulation of bioactive compounds after exposure to UV lamp or LED irradiation. Hortic. Environ. Biotechnol. 2018, 59, 659–670. [Google Scholar] [CrossRef]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.; Yoshihara, T. Blue Light-emitting Diode Light Irradiation of Seedlings Improves Seedling Quality and Growth after Transplanting in Red Leaf Lettuce. Hortscience 2010, 45, 1809–1814. [Google Scholar] [CrossRef] [Green Version]
- Qian, H.M.; Liu, T.Y.; Deng, M.D.; Miao, H.Y.; Cai, C.X.; Shen, W.S.; Wang, Q.M. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Cho, J.Y.; Yoo, K.S.; Kim, J.; Choi, B.J.; Oh, W. Growth and Bioactive Compounds of Lettuce as Affected by Light Intensity and Photoperiod in a Plant Factory Using External Electrode Fluorescent Lamps. Hortic. Sci. Technol. 2020, 38, 645–659. [Google Scholar]
- Lee, M.J.; Lim, S.; Kim, J.; Oh, M.M. Heat Shock Treatments Induce the Accumulation of Phytochemicals in Kale Sprouts. Korean J. Hortic. Sci. 2012, 30, 509–518. [Google Scholar]
- Oh, M.M.; Trick, H.N.; Rajashekara, C.B. Secondary metabolism and antioxidants are involved in environmental adaptation and stress tolerance in lettuce. J. Plant Physiol. 2009, 166, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Park, S.A.; Grusak, M.A.; Oh, M.M. Concentrations of Minerals and Phenolic Compounds in Three Edible Sprout Species Treated with Iron-chelates during Imbibition. Hortic. Environ. Biotechnol. 2014, 55, 471–478. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Iron Biofortification of Red and Green Pigmented Lettuce in Closed Soilless Cultivation Impacts Crop Performance and Modulates Mineral and Bioactive Composition. Agronomy 2019, 9, 290. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.J.; Lee, Y.T. Effect of salinity stress on bioactive compounds and antioxidant activity of wheat microgreen extract under organic cultivation conditions. Int. J. Biol. Macromol. 2019, 140, 631–636. [Google Scholar] [CrossRef]

| Crop | Plant Species | Cultivation Factor | Major Bioactive Compounds | Outcome Effect | Reference |
|---|---|---|---|---|---|
| Fruit | Raspberry cultivar | Organic fertilization | Phenolics, anthocyanins, ascorbic acid | + z | [19] |
| Maturity stages | + | ||||
| Camu-camu | Drought stress | Polyphenols, ascorbic acid | + | [32] | |
| Blackberry cultivar | Temperature | Polyphenols, flavonoids, anthocyanins, ascorbic acid | + | [18] | |
| Humidity | − | ||||
| Sweet cherry | Irrigation | Phenolics, tartaric esters, flavanols, anthocyanins | null | [6] | |
| Phosphorus | null | ||||
| Apple | Crop loads | Phenolics, ascorbic acid | − | [33] | |
| Tomato | Organic cultivation | Phenolics, ascorbic acid | + | [25] | |
| Hot pepper | Stage of growth and ripening | Phenolics, ascorbic acid | Ripening + | [34] | |
| Vegetable | Onion | Bolting and flower stem removal | Phenolics, quercetin | null | [35] |
| Tarragon | Plant density | Carotenoids | + | [14] | |
| Blessed thistle | Nitrogen fertilizer rate | Phenolics | − | [36] | |
| Plant density | + | ||||
| Buckwheat | Intercropping ratio | Phenolics | + | [37] | |
| Garlic | Plant growth biostimulants | Polyphenols, ascorbic acid | + | [38] | |
| Amaranthus | Drought stress | Vitamins phenolics, flavonoids | + | [39] | |
| Broccoli | Harvest season | Phenolics | Spring | [40] | |
| Drought stress | + |
| Crop | Plant Species | Cultivation Factor | Major Bioactive Compounds | Outcome Effect | Reference |
|---|---|---|---|---|---|
| Fruit | Strawberry | Titanium dioxide (TiO2) foliar fertilization | Phenolics | − z | [42] |
| Sweet cherry | Plastic cover | Phenolics | − | [43] | |
| Anthocyanin | + | ||||
| Red raspberry, strawberry, blackberry | Photovoltaic cover | Total anthocyanins, phenolic content | + | [44] | |
| Shading | + | ||||
| Grape | Deficit irrigation | Resveratrol, anthocyanins | + | [45] | |
| Tomato | Stage of growth and ripening | Ascorbic acid, lycopene, beta-carotene, total phenolic contents | ripening + | [46] | |
| Vegetable | Leafy vegetation | Short-term low temperature | Phenolics | null | [20] |
| Lettuce | Exogenous glycine betaine (GB) under salt stress | Phenolics | 25 mM GB + | [47] | |
| Dill Parsley | Substrate type | Phenolics, flavonoids | Germany soil | [37] | |
| Green-leaf lettuce | Selenium fertilization and arbuscular mycorrhizal fungi (AMF) | Carotenoids | + | [48] | |
| Phenolics | − | ||||
| Red-leaf lettuce | Flavanols | + | |||
| Broccoli | Calcium sulfate (CaSO4) fertilization | Phenolics | − | [49] | |
| Purslane | Foliar fertilization | Phenolics, ascorbic acid | + | [50] | |
| Chicory Lettuce Swiss chard | Stocking density of fish | Phenolic acid, caffeic acid | + | [51] |
| Crop | Plant Species | Cultivation Factor | Major Bioactive Compounds | Outcome Effect | Reference |
|---|---|---|---|---|---|
| Fruit | Tomato | Stage of ripening | Phenolics ascorbic acid, lycopene, beta-carotene, total flavonoid content | Ripening + z | |
| Vegetable | Chicory cultivars | Nutrient (nitrogen, potassium, or phosphorus) solutions | Phenolics, total flavonoid | Potassium + | [56] |
| Cabbage | Biochar | Flavonoids, glucosinolates | + z | [57] |
| Crop | Plant Species | Cultivation Factor | Major Bioactive Compounds | Outcome Effect | Reference |
|---|---|---|---|---|---|
| Vegetable | Lettuce | Monochromatic or combined LED light | Phenolics | Blue LED + z | [63] |
| Lettuce | Combined light (ratio of blue + red/far-red LED light) | Phenolics, chlorogenic acid, caffeic acid | O.7 and 1.2 LEDs ratio + | [59] | |
| Lettuce | UV-A irradiation | Phenolics | null | [64] | |
| Dropwort | UV lamp or LED irradiation | Phenolics | + | [65] | |
| Red lettuce | Blue LED | Cartenoid, polyphenol | + | [22,66] | |
| Chinese kale sprout | Light quality | Phenolics anthocyanins | Blue LED+ | [67] | |
| Basil | Red:blue LED ratio | Flavonoid | R1B3 | [22,68] | |
| Lettuce | Light intensity | Phenolics | 150 μ mol m−2 s−1 + | [69] | |
| Photoperiod | 20 h + | ||||
| Parsley | Management practices (indoor, greenhouse, field cultivation) | Carotenoids, flavonoids | null | [22] | |
| Ascorbic acid | Field + | ||||
| Basil | Anthocyanins | null | |||
| Kale | Short-term low temperature | Phenolics | + | [20,22] | |
| Kale | Short-term heat shock | Anti-carcinogenic compounds | + | [70] | |
| Lettuce | Short-term water stress | Phenolics, flavonoids | + | [70,71] | |
| Alfalfa, broccooli, radish | Iron-chelates | Phenolics | + | [72] | |
| Green lettuce | Iron (Fe) biofortification | Phenolic acids, carotenoid | null | [73] | |
| Red lettuce | + | ||||
| Wheat microgreen | Salinity stress | Beta-carotene, phenolic acid, flavonoid, vitamin, anthocyanin | 12.5 and 25 mM + | [74] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, J.-H.; Yoon, Y.-H.; Shin, S.-H.; Ju, S.-Y.; Yeum, K.-J. Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods. Horticulturae 2022, 8, 767. https://doi.org/10.3390/horticulturae8090767
Ju J-H, Yoon Y-H, Shin S-H, Ju S-Y, Yeum K-J. Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods. Horticulturae. 2022; 8(9):767. https://doi.org/10.3390/horticulturae8090767
Chicago/Turabian StyleJu, Jin-Hee, Yong-Han Yoon, So-Hui Shin, Se-Young Ju, and Kyung-Jin Yeum. 2022. "Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods" Horticulturae 8, no. 9: 767. https://doi.org/10.3390/horticulturae8090767
APA StyleJu, J.-H., Yoon, Y.-H., Shin, S.-H., Ju, S.-Y., & Yeum, K.-J. (2022). Recent Trends in Urban Agriculture to Improve Bioactive Content of Plant Foods. Horticulturae, 8(9), 767. https://doi.org/10.3390/horticulturae8090767





