The New Green Challenge in Urban Planning: The Right Genetics in the Right Place
Abstract
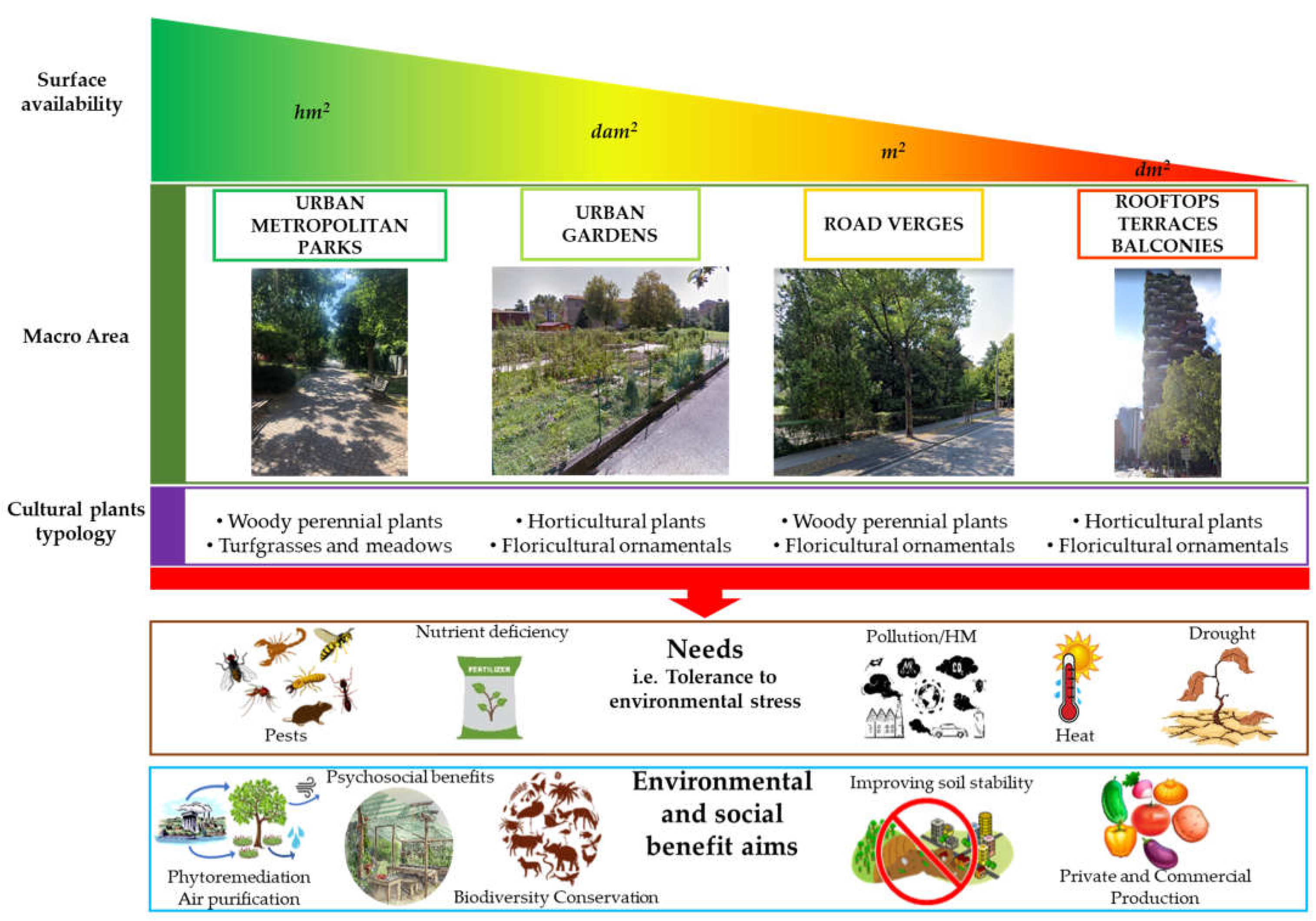
1. Introduction
Processing Criteria
- (i)
- Urban/metropolitan parks: these are green areas in cities, and other incorporated places, that offer recreation and green spaces to residents of and visitors to the municipality;
- (ii)
- Urban gardens: these are areas where urban vegetation is exploited to provide food products, especially employing horticultural species. Urban food production can be carried out by citizens or administrations in private buildings and public spaces for self-consumption, or can be performed by farms with commercial purposes, also using innovative outdoor or indoor growth systems;
- (iii)
- Road verges: these are small, vegetated areas composed of grass or plants and sometimes also trees, mainly located between a roadway and a sidewalk or within roundabouts;
- (iv)
- Roofs/terraces/balconies: these are small green areas located in private or public buildings. They include both surfaces partially or completely covered with vegetation (e.g., green roofs) and container gardens where plants are maintained in pots.
- (a)
- The environment of interest: “urban areas”, “cities”, “green areas, “green gardens” “public green”, “public parks”, “urban agriculture”;
- (b)
- The plant typologies: “plant”, “ornamental”, “flowering”, “horticultural”, “woody”, “trees”, “meadows”, “turfgrasses”;
- (c)
- The genetic subject: “breeding” “molecular markers”, “marker-assisted selection” “marker-assisted breeding” “molecular selection” “genomic selection” “genomics” “genetic improvement” “variety” “cultivar”;
- (d)
- Specific goals: “abiotic stress”, “heat shock”, “biotic stress”, “pathogen stress”, “water stress”, “drought”, “dwarf” “compacted”, “growth habit”, “edible flowers” “food production”, “leafy vegetation”, “baby leaf”, “phytoremediation”, “air purification”, “biodiversity”, “soil erosion”, “soil stability”, “psychosocial”, “ecosystem services”.
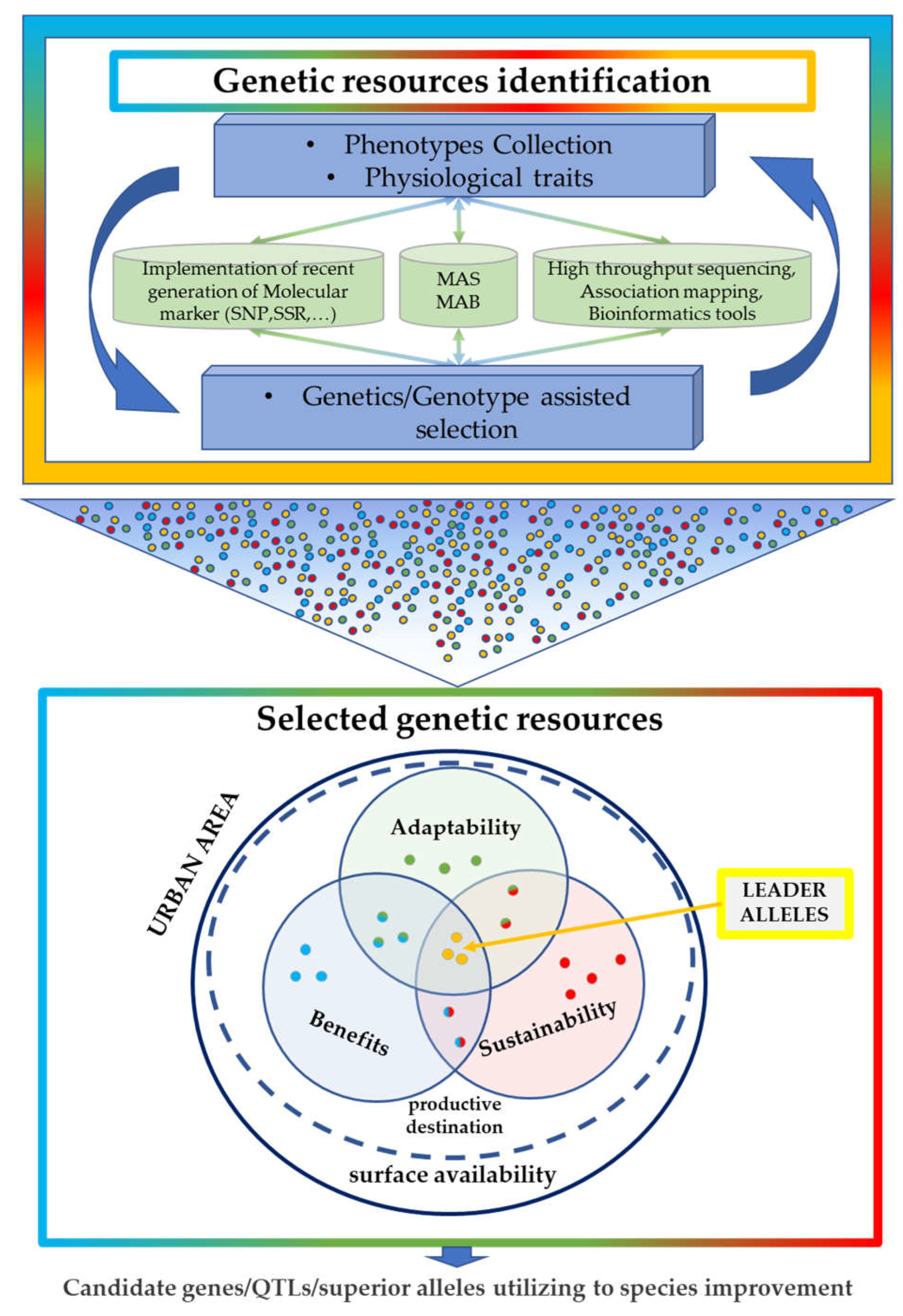
2. Genetic Information as a Genomic Tool That Is Potentially Helpful in Breeding Approaches for Urban Contexts
3. The Role of Genetics in the Adaptability and Sustainability of Plants in Different Urban Contexts
3.1. Abiotic Stresses: Heat and Water Stress
3.2. Pathogen Stress
3.3. Limited Surface Availability
4. What Are the Achievable Goals with the Help of Genetics?
4.1. Phytoremediation
4.2. Air Purification
4.3. Improving Soil Stability
4.4. Food Production
4.4.1. Leafy Vegetables
4.4.2. Edible Flowers
4.5. Biodiversity Conservation
4.6. Psychosocial Benefits
5. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Urban Green Spaces: A Brief for Action; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Donihue, C.M.; Lambert, M.R. Adaptive evolution in urban ecosystems. Ambio 2015, 44, 194–203. [Google Scholar] [CrossRef] [PubMed]
- McKinney, M.L. Urbanization as a major cause of biotic homogenization. Biol. Conserv. 2006, 127, 247–260. [Google Scholar] [CrossRef]
- Seto, K.C.; Sánchez-Rodríguez, R.; Fragkias, M. The New Geography of Contemporary Urbanization and the Environment. Annu. Rev. Environ. Resour. 2010, 35, 167–194. [Google Scholar] [CrossRef]
- Aronson, M.F.; La Sorte, F.A.; Nilon, C.H.; Katti, M.; Goddard, M.A.; Lepczyk, C.A.; Warren, P.S.; Williams, N.S.; Cilliers, S.; Clarkson, B. A global analysis of the impacts of urbanization on bird and plant diversity reveals key anthropogenic drivers. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133330. [Google Scholar] [CrossRef]
- Zhou, Y.; Smith, S.J.; Zhao, K.; Imhoff, M.; Thomson, A.; Bond-Lamberty, B.; Asrar, G.R.; Zhang, X.; He, C.; Elvidge, C.D. A global map of urban extent from nightlights. Environ. Res. Lett. 2015, 10, 054011. [Google Scholar] [CrossRef]
- Panduro, T.E.; Veie, K.L. Classification and valuation of urban green spaces—A hedonic house price valuation. Landsc. Urban Plan. 2013, 120, 119–128. [Google Scholar] [CrossRef]
- Peña-Salmón, C.; Leyva-Camacho, O.; Rojas-Caldelas, R.; Alonso-Navarrete, A.; Iñiguez-Ayón, P. The identification and classification of green areas for urban planning using multispectral images at Baja California, Mexico. WIT Trans. Ecol. Environ. 2014, 191, 611–621. [Google Scholar]
- Xu, Z.; Zhou, Y.; Wang, S.; Wang, L.; Li, F.; Wang, S.; Wang, Z. A Novel Intelligent Classification Method for Urban Green Space Based on High-Resolution Remote Sensing Images. Remote Sens. 2020, 12, 3845. [Google Scholar] [CrossRef]
- Gerullis, M.K.; Heckelei, T.; Rasch, S. Toward understanding the governance of varietal and genetic diversity. Ecol. Soc. 2021, 26, 28. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and Molecular Techniques from Simple Breeding to Speed Breeding in Crop Plants: Recent Advances and Future Outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Xie, C.; Zhang, X.; Prasanna, B.M.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.A.; Hotaling, S.; Frandsen, P.B.; VanBuren, R. Representation and participation across 20 years of plant genome sequencing. Nat. Plants 2021, 7, 1571–1578. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Li, P.; Li, L.; Zhang, Q. Research advances in and prospects of ornamental plant genomics. Hortic. Res. 2021, 8, 65. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.A.; Terol, J.; Ibanez, V.; Lopez-Garcia, A.; Perez-Roman, E.; Borreda, C.; Domingo, C.; Tadeo, F.R.; Carbonell-Caballero, J.; Alonso, R.; et al. Genomics of the origin and evolution of Citrus. Nature 2018, 554, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Mii, M. Production of Interspecific Hybrids in Ornamental Plants. In Plant Cell Culture Protocols; Loyola-Vargas, V.M., Ochoa-Alejo, N., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 233–245. [Google Scholar] [CrossRef]
- Bhattarai, K.; Van Huylenbroeck, J. Breeding, Genetics, and Genomics of Ornamental Plants. Horticulturae 2022, 8, 148. [Google Scholar] [CrossRef]
- Lv, Q.; Li, W.; Sun, Z.; Ouyang, N.; Jing, X.; He, Q.; Wu, J.; Zheng, J.; Zheng, J.; Tang, S.; et al. Resequencing of 1,143 indica rice accessions reveals important genetic variations and different heterosis patterns. Nat. Commun. 2020, 11, 4778. [Google Scholar] [CrossRef]
- Stevens, K.A.; Wegrzyn, J.L.; Zimin, A.; Puiu, D.; Crepeau, M.; Cardeno, C.; Paul, R.; Gonzalez-Ibeas, D.; Koriabine, M.; Holtz-Morris, A.E.; et al. Sequence of the Sugar Pine Megagenome. Genetics 2016, 204, 1613–1626. [Google Scholar] [CrossRef]
- Simko, I.; Jia, M.; Venkatesh, J.; Kang, B.-C.; Weng, Y.; Barcaccia, G.; Lanteri, S.; Bhattarai, G.; Foolad, M.R. Genomics and Marker-Assisted Improvement of Vegetable Crops. Crit. Rev. Plant Sci. 2021, 40, 303–365. [Google Scholar] [CrossRef]
- Bohra, A.; Jha, U.C.; Kishor, P.B.; Pandey, S.; Singh, N.P. Genomics and molecular breeding in lesser explored pulse crops: Current trends and future opportunities. Biotechnol. Adv. 2014, 32, 1410–1428. [Google Scholar] [CrossRef]
- Bhat, J.A.; Ali, S.; Salgotra, R.K.; Mir, Z.A.; Dutta, S.; Jadon, V.; Tyagi, A.; Mushtaq, M.; Jain, N.; Singh, P.K.; et al. Genomic Selection in the Era of Next Generation Sequencing for Complex Traits in Plant Breeding. Front. Genet. 2016, 7, 221. [Google Scholar] [CrossRef]
- Poland, J.A.; Rife, T.W. Genotyping-by-Sequencing for Plant Breeding and Genetics. Plant Genome 2012, 5. [Google Scholar] [CrossRef]
- Edwards, D.; Batley, J. Plant genome sequencing: Applications for crop improvement. Plant Biotechnol. J. 2010, 8, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gong, Z.; Bebber, D.P.; Miao, J.; Zhao, Z.; Jiang, Y.; Xiao, S.; Zhang, G.; Yu, D.; Fang, J.; et al. Phenological matching drives wheat pest range shift under climate change. bioRxiv 2019. [Google Scholar] [CrossRef]
- Xu, X.; Bai, G. Whole-genome resequencing: Changing the paradigms of SNP detection, molecular mapping and gene discovery. Mol. Breed. 2015, 35, 33. [Google Scholar] [CrossRef]
- Elshire, R.J.; Glaubitz, J.C.; Sun, Q.; Poland, J.A.; Kawamoto, K.; Buckler, E.S.; Mitchell, S.E. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS ONE 2011, 6, e19379. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.K.; Weber, J.N.; Kay, E.H.; Fisher, H.S.; Hoekstra, H.E. Double digest RADseq: An inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 2012, 7, e37135. [Google Scholar] [CrossRef]
- Wang, S.; Meyer, E.; McKay, J.K.; Matz, M.V. 2b-RAD: A simple and flexible method for genome-wide genotyping. Nat. Methods 2012, 9, 808–810. [Google Scholar] [CrossRef]
- Toonen, R.J.; Puritz, J.B.; Forsman, Z.H.; Whitney, J.L.; Fernandez-Silva, I.; Andrews, K.R.; Bird, C.E. ezRAD: A simplified method for genomic genotyping in non-model organisms. PeerJ 2013, 1, e203. [Google Scholar] [CrossRef]
- Ostezan, A.; McDonald, S.C.; Tran, D.T.; Souza, R.S.E.; Li, Z. Target region sequencing and applications in plants. JCSB 2020, 24, 13–26. [Google Scholar] [CrossRef]
- Rajcan, I.; Boersma, J.; Shaw, E. Plant genetic techniques: Plant breeder’s toolbox. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier: Burlington, NJ, USA, 2011; Volume 4, pp. 133–147. [Google Scholar]
- Hasan, N.; Choudhary, S.; Naaz, N.; Sharma, N.; Laskar, R.A. Recent advancements in molecular marker-assisted selection and applications in plant breeding programmes. J. Genet. Eng. Biotechnol. 2021, 19, 128. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Huang, L.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Sirohi, M.H.; Wang, F. Applications of Multi-Omics Technologies for Crop Improvement. Front. Plant Sci. 2021, 12, 563953. [Google Scholar] [CrossRef] [PubMed]
- Donald, C. The breeding of crop ideotypes. Euphytica 1968, 17, 385–403. [Google Scholar] [CrossRef]
- Czaja, M.; Kołton, A.; Muras, P. The Complex Issue of Urban Trees—Stress Factor Accumulation and Ecological Service Possibilities. Forests 2020, 11, 932. [Google Scholar] [CrossRef]
- Koyro, H.-W.; Ahmad, P.; Geissler, N. Abiotic stress responses in plants: An overview. In Environmental Adaptations Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- Wang, W.; Vinocur, B.; Altman, A. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta 2003, 218, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef]
- Lobell, D.B.; Burke, M.B.; Tebaldi, C.; Mastrandrea, M.D.; Falcon, W.P.; Naylor, R.L. Prioritizing climate change adaptation needs for food security in 2030. Science 2008, 319, 607–610. [Google Scholar] [CrossRef]
- Renard, D.; Tilman, D. National food production stabilized by crop diversity. Nature 2019, 571, 257–260. [Google Scholar] [CrossRef]
- Husaini, A.M. High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 2022, 128, 460–472. [Google Scholar] [CrossRef]
- Bharadwaj, D.N. Sustainable Agriculture and Plant Breeding. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 3–34. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Lutken, H.; Clarke, J.L.; Muller, R. Genetic engineering and sustainable production of ornamentals: Current status and future directions. Plant Cell Rep. 2012, 31, 1141–1157. [Google Scholar] [CrossRef]
- Baldauf, R. Roadside vegetation design characteristics that can improve local, near-road air quality. Transp. Res. 2017, 52, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, D.I.; Hodgkin, T.; Sthapit, B.R.; Fadda, C.; Lopez-Noriega, I. An Heuristic Framework for Identifying Multiple Ways of Supporting the Conservation and Use of Traditional Crop Varieties within the Agricultural Production System. Crit. Rev. Plant Sci. 2011, 30, 125–176. [Google Scholar] [CrossRef]
- Kumar, A.; Anju, T.; Kumar, S.; Chhapekar, S.S.; Sreedharan, S.; Singh, S.; Choi, S.R.; Ramchiary, N.; Lim, Y.P. Integrating Omics and Gene Editing Tools for Rapid Improvement of Traditional Food Plants for Diversified and Sustainable Food Security. Int. J. Mol. Sci. 2021, 22, 8093. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, M.; Li, S.; Chen, Q.; Teixeira da Silva, J.A.; Wang, A.; Yu, X.; Wang, L. Germplasm resources and genetic breeding of Paeonia: A systematic review. Hortic. Res. 2020, 7, 107. [Google Scholar] [CrossRef]
- Farida Traore, F.; El-Baouchi, A.; En-Nahli, Y.; Hejjaoui, K.; Metougui, M.L.; Hamwieh, A.; Sohail, Q.; Istanbuli, T.; Boughribil, S.; Amri, M. Exploring the Genetic Variability and Potential Correlations Between Nutritional Quality and Agro-Physiological Traits in Kabuli Chickpea Germplasm Collection (Cicer arietinum L.). Front. Plant Sci. 2022, 13, 905320. [Google Scholar] [CrossRef]
- Egorova, A.A.; Chalaya, N.A.; Fomin, I.N.; Barchuk, A.I.; Gerasimova, S.V. De Novo Domestication Concept for Potato Germplasm Enhancement. Agronomy 2022, 12, 462. [Google Scholar] [CrossRef]
- Breider, I.S.; Gaynor, R.C.; Gorjanc, G.; Thorn, S.; Pandey, M.K.; Varshney, R.K.; Hickey, J.M. A multi-part strategy for introgression of exotic germplasm into elite plant breeding programs using genomic selection. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Huang, K.; Jahani, M.; Gouzy, J.; Legendre, A.; Carrere, S.; Lázaro-Guevara, J.M.; González Segovia, E.G.; Todesco, M.; Mayjonade, B.; Rodde, N.; et al. The genomics of linkage drag in sunflower. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wahid, A.; Farooq, M.; Hussain, I.; Rasheed, R.; Galani, S. Responses and Management of Heat Stress in Plants. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 135–157. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef]
- Feller, U.; Vaseva, I.I. Extreme climatic events: Impacts of drought and high temperature on physiological processes in agronomically important plants. Front. Environ. Sci. 2014, 2, 39. [Google Scholar] [CrossRef]
- Young, J.C. Mechanisms of the Hsp70 chaperone system. Biochem. Cell Biol. 2010, 88, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhu, W.; Zhang, H.; Liu, N.; Tian, S. Heat shock factors in tomatoes: Genome-wide identification, phylogenetic analysis and expression profiling under development and heat stress. PeerJ 2016, 4, e1961. [Google Scholar] [CrossRef] [PubMed]
- Aldubai, A.A.; Alsadon, A.A.; Migdadi, H.H.; Alghamdi, S.S.; Al-Faifi, S.A.; Afzal, M. Response of Tomato (Solanum lycopersicum L.) Genotypes to Heat Stress Using Morphological and Expression Study. Plants 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Su, Z.; Zhou, H.; Huang, Q.; Fan, S.; Liu, C.; Han, Y. LsHSP70 is induced by high temperature to interact with calmodulin, leading to higher bolting resistance in lettuce. Sci. Rep. 2020, 10, 15155. [Google Scholar] [CrossRef]
- Kang, Y.; Jang, S.-W.; Lee, H.J.; Barchenger, D.W.; Jang, S. Expression Profiling of Heat Shock Protein Genes as Putative Early Heat-Responsive Members in Lettuce. Horticulturae 2021, 7, 312. [Google Scholar] [CrossRef]
- Yan, J.; Yu, L.; Xuan, J.; Lu, Y.; Lu, S.; Zhu, W. De novo transcriptome sequencing and gene expression profiling of spinach (Spinacia oleracea L.) leaves under heat stress. Sci. Rep. 2016, 6, 19473. [Google Scholar] [CrossRef]
- Li, S.; Yu, J.; Li, Y.; Zhang, H.; Bao, X.; Bian, J.; Xu, C.; Wang, X.; Cai, X.; Wang, Q.; et al. Heat-Responsive Proteomics of a Heat-Sensitive Spinach Variety. Int. J. Mol. Sci. 2019, 20, 3872. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Xia, Y. SMRT sequencing of full-length transcriptome for in-depth understanding of genes under heat stress of Rhododendron hainanense. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone, Torino, Italy, 3 March 2021; pp. 261–268. [Google Scholar]
- Wan, X.L.; Zhou, Q.; Wang, Y.Y.; Wang, W.E.; Bao, M.Z.; Zhang, J.W. Identification of heat-responsive genes in carnation (Dianthus caryophyllus L.) by RNA-seq. Front. Plant Sci. 2015, 6, 519. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.G.; Rafii, M.Y.; Martini, M.Y.; Yusuff, O.A.; Ismail, M.R.; Miah, G. Introgression of heat shock protein (Hsp70 and sHsp) genes into the Malaysian elite chilli variety Kulai (Capsicum annuum L.) through the application of marker-assisted backcrossing (MAB). Cell Stress Chaperones 2018, 23, 223–234. [Google Scholar] [CrossRef]
- Sjöman, H.; Hirons, A.D.; Bassuk, N.L. Urban forest resilience through tree selection—Variation in drought tolerance in Acer. Urban For. Urban Green. 2015, 14, 858–865. [Google Scholar] [CrossRef]
- Miller, D.L.; Wetherley, E.B.; Roberts, D.A.; Tague, C.L.; McFadden, J.P. Vegetation cover change during a multi-year drought in Los Angeles. Urban Clim. 2022, 43, 101157. [Google Scholar] [CrossRef]
- FAO & Water World Council. Towards a Water and Food Secure Future. Critical Perspectives for Policy-Makers; FAO: Rome, Italy, 2015. [Google Scholar]
- Khan, A.; Sovero, V.; Gemenet, D. Genome-assisted Breeding For Drought Resistance. Curr. Genom. 2016, 17, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Eriksen, R.L.; Simko, I.; Mou, B. Molecular Mapping of Water-Stress Responsive Genomic Loci in Lettuce (Lactuca spp.) Using Kinetics Chlorophyll Fluorescence, Hyperspectral Imaging and Machine Learning. Front. Genet. 2021, 12, 634554. [Google Scholar] [CrossRef]
- Grace, O.M. Succulent plant diversity as natural capital. Plants People Planet 2019, 1, 336–345. [Google Scholar] [CrossRef]
- Lewis, E.; Phoenix, G.K.; Alexander, P.; David, J.; Cameron, R.W.F. Rewilding in the Garden: Are garden hybrid plants (cultivars) less resilient to the effects of hydrological extremes than their parent species? A case study with Primula. Urban Ecosyst. 2019, 22, 841–854. [Google Scholar] [CrossRef]
- Komar-Tyomnaya, L.D. Drought resistance of ornamental peach cultivars with a different origin. In Proceedings of the I International Conference and X National Horticultural Science Congress of Iran (IrHC2017), Tehran, Iran, 7 September 2017; pp. 353–358. [Google Scholar]
- Esfahani, R.E.; Paço, T.A.; Martins, D.; Arsénio, P. Increasing the resistance of Mediterranean extensive green roofs by using native plants from old roofs and walls. Ecol. Eng. 2022, 178, 106576. [Google Scholar] [CrossRef]
- Fuertes, A.; Sixto, H.; González, I.; Pérez-Cruzado, C.; Cañellas, I.; Rodríguez-Soalleiro, R.; Oliveira, N. Time-course foliar dynamics of poplar short rotation plantations under Mediterranean conditions. Responses to different water scenarios. Biomass Bioenergy 2022, 159, 106391. [Google Scholar] [CrossRef]
- Ferrante, A.; Toscano, S.; Romano, D.; Vagge, I. Physiological and morpho-anatomical traits used as markers for the selection of drought tolerance of ornamental plants. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone, Torino, Italy, 3 March 2021; pp. 253–260. [Google Scholar]
- Salekdeh, G.H.; Reynolds, M.; Bennett, J.; Boyer, J. Conceptual framework for drought phenotyping during molecular breeding. Trends Plant Sci. 2009, 14, 488–496. [Google Scholar] [CrossRef]
- Altunoglu, Y.C.; Keles, M.; Can, T.H.; Baloglu, M.C. Identification of watermelon heat shock protein members and tissue-specific gene expression analysis under combined drought and heat stresses. Turk. J. Biol. 2019, 43, 404–419. [Google Scholar] [CrossRef]
- Diouf, I.; Albert, E.; Duboscq, R.; Santoni, S.; Bitton, F.; Gricourt, J.; Causse, M. Integration of QTL, Transcriptome and Polymorphism Studies Reveals Candidate Genes for Water Stress Response in Tomato. Genes 2020, 11, 900. [Google Scholar] [CrossRef]
- Zhou, R.; Yu, X.; Ottosen, C.O.; Zhang, T.; Wu, Z.; Zhao, T. Unique miRNAs and their targets in tomato leaf responding to combined drought and heat stress. BMC Plant Biol. 2020, 20, 107. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Gantait, S.; Azizi, P.; Mazumdar, P. Drought tolerance improvement in Solanum lycopersicum: An insight into “OMICS” approaches and genome editing. 3 Biotech 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Sarazin, V.; Duclercq, J.; Guillot, X.; Sangwan, B.; Sangwan, R.S. Water-stressed sunflower transcriptome analysis revealed important molecular markers involved in drought stress response and tolerance. Environ. Exp. Bot. 2017, 142, 45–53. [Google Scholar] [CrossRef]
- Darbani, S.P.; Mehrabi, A.A.; Pordad, S.S.; Maleki, A.; Farshadfar, M. Effect of drought stress on agro-morphological traits in sunflower (Helianthus annuus L.) genotypes and identification of informative ISSR markers. Plant Genet. Resour. Characterisation Util. 2020, 18, 49–62. [Google Scholar] [CrossRef]
- Muhammad Ahmad, H.; Wang, X.; Fiaz, S.; Mahmood Ur, R.; Azhar Nadeem, M.; Aslam Khan, S.; Ahmar, S.; Azeem, F.; Shaheen, T.; Mora-Poblete, F. Comprehensive genomics and expression analysis of eceriferum (CER) genes in sunflower (Helianthus annuus). Saudi J. Biol. Sci. 2021, 28, 6884–6896. [Google Scholar] [CrossRef]
- Yukawa, J.; Kiritani, K.; Kawasawa, T.; Higashiura, Y.; Sawamura, N.; Nakada, K.; Gyotoku, N.; Tanaka, A.; Kamitani, S.; Matsuo, K.; et al. Northward range expansion by Nezara viridula (Hemiptera: Pentatomidae) in Shikoku and Chugoku Districts, Japan, possibly due to global warming. Appl. Entomol. Zoo 2009, 44, 429–437. [Google Scholar] [CrossRef]
- Macfadyen, S.; McDonald, G.; Hill, M.P. From species distributions to climate change adaptation: Knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agric. Ecosyst. Environ. 2018, 253, 208–219. [Google Scholar] [CrossRef]
- Horgan, F.G.; Arida, A.; Ardestani, G.; Almazan, M.L.P. Intraspecific competition counters the effects of elevated and optimal temperatures on phloem-feeding insects in tropical and temperate rice. PLoS ONE 2020, 15, e0240130. [Google Scholar] [CrossRef]
- Bommarco, R.; Kleijn, D.; Potts, S.G. Ecological intensification: Harnessing ecosystem services for food security. Trends Ecol. Evol. 2013, 28, 230–238. [Google Scholar] [CrossRef]
- Forkuoh, F.; Boadi, N.O.; Borquaye, L.S.; Afful, S. Risk Of Human Dietary Exposure To Organochlorine Pesticide Residues In Fruits From Ghana. Sci. Rep. 2018, 8, 16686. [Google Scholar] [CrossRef]
- Jokanovic, M. Neurotoxic effects of organophosphorus pesticides and possible association with neurodegenerative diseases in man: A review. Toxicology 2018, 410, 125–131. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, W.; Bianchi, F. Options for diversifying agricultural systems to reduce pesticide use: Can we learn from nature? Outlook Agric. 2022, 51, 105–113. [Google Scholar] [CrossRef]
- Yin, J.-J.; Xiong, J.; Xu, L.-T.; Chen, X.-W.; Li, W.-T. Recent advances in plant immunity with cell death: A review. J. Integr. Agric. 2022, 21, 610–620. [Google Scholar] [CrossRef]
- Karasov, T.L.; Shirsekar, G.; Schwab, R.; Weigel, D. What natural variation can teach us about resistance durability. Curr. Opin. Plant Biol. 2020, 56, 89–98. [Google Scholar] [CrossRef]
- Sniezko, R.A.; Liu, J.-J. Prospects for developing durable resistance in populations of forest trees. New For. 2021, 1–17. [Google Scholar] [CrossRef]
- Tremblay, V.; McLaren, D.L.; Kim, Y.M.; Strelkov, S.E.; Conner, R.L.; Wally, O.; Belanger, R.R. Molecular Assessment of Pathotype Diversity of Phytophthora sojae in Canada Highlights Declining Sources of Resistance in Soybean. Plant Dis. 2021, 105, 4006–4013. [Google Scholar] [CrossRef]
- Miedaner, T. Breeding strategies for improving plant resistance to diseases. In Advances in Plant Breeding Strategies: Agronomic, Abiotic and Biotic Stress Traits; Springer: Berlin/Heidelberg, Germany, 2016; pp. 561–599. [Google Scholar]
- Peng, T.; Sun, X.; Mumm, R.H. Optimized breeding strategies for multiple trait integration: II. Process efficiency in event pyramiding and trait fixation. Mol. Breed. 2014, 33, 105–115. [Google Scholar] [CrossRef][Green Version]
- Foolad, M.R.; Panthee, D.R. Marker-Assisted Selection in Tomato Breeding. Crit. Rev. Plant Sci. 2012, 31, 93–123. [Google Scholar] [CrossRef]
- Lee, J.M.; Oh, C.-S.; Yeam, I. Molecular Markers for Selecting Diverse Disease Resistances in Tomato Breeding Programs. Plant Breed. Biotech. 2015, 3, 308–322. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Salej, S.; Ashwani, K.; Vanishree, G.; Sanjeev, S.; Sundaresha, S. Efficiency and reliability of marker assisted selection for resistance to major biotic stresses in potato. Potato J. 2019, 46, 56–66. [Google Scholar]
- Meuwissen, T.H.; Hayes, B.J.; Goddard, M.E. Prediction of total genetic value using genome-wide dense marker maps. Genetics 2001, 157, 1819–1829. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X.; Fu, J.; Wang, H.; Wang, J.; Huang, C.; Prasanna, B.M.; Olsen, M.S.; Wang, G.; Zhang, A. Enhancing Genetic Gain through Genomic Selection: From Livestock to Plants. Plant Commun. 2020, 1, 100005. [Google Scholar] [CrossRef]
- Tan, X.; Shibata, S. Factors influencing street tree health in constrained planting spaces: Evidence from Kyoto City, Japan. Urban For. Urban Green. 2022, 67, 127416. [Google Scholar] [CrossRef]
- Xie, X.; Cheng, H.; Hou, C.; Ren, M. Integration of Light and Auxin Signaling in Shade Plants: From Mechanisms to Opportunities in Urban Agriculture. Int. J. Mol. Sci. 2022, 23, 3422. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.; Else, M. Understanding how rootstocks dwarf fruit trees. Compact Fruit Tree 2001, 34, 46–49. [Google Scholar]
- Foster, T.M.; Hooijdonk, B.M.V.; Friend, A.; Seleznyova, A.N.; McLachlan, A.R.G. Apple Rootstock-Induced Dwarfing is Strongly Influenced by Growing Environment. J. Hortic. 2016, 3, 1–8. [Google Scholar] [CrossRef]
- Hollender, C.A.; Hadiarto, T.; Srinivasan, C.; Scorza, R.; Dardick, C. A brachytic dwarfism trait (dw) in peach trees is caused by a nonsense mutation within the gibberellic acid receptor PpeGID1c. New Phytol. 2016, 210, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhao, Y.; Shan, D.; Shi, K.; Wang, L.; Li, Q.; Wang, N.; Zhou, J.; Yao, J.; Xue, Y.; et al. MdWRKY9 overexpression confers intensive dwarfing in the M26 rootstock of apple by directly inhibiting brassinosteroid synthetase MdDWF4 expression. New Phytol. 2018, 217, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Gong, X.; Li, M.; Li, C.; Sun, T.; Ma, F. Overexpression of a Novel Apple NAC Transcription Factor Gene, MdNAC1, Confers the Dwarf Phenotype in Transgenic Apple (Malus domestica). Genes 2018, 9, 229. [Google Scholar] [CrossRef]
- Salojarvi, J.; Smolander, O.P.; Nieminen, K.; Rajaraman, S.; Safronov, O.; Safdari, P.; Lamminmaki, A.; Immanen, J.; Lan, T.; Tanskanen, J.; et al. Genome sequencing and population genomic analyses provide insights into the adaptive landscape of silver birch. Nat. Genet. 2017, 49, 904–912. [Google Scholar] [CrossRef]
- Xu, D.; Qi, X.; Li, J.; Han, X.; Wang, J.; Jiang, Y.; Tian, Y.; Wang, Y. PzTAC and PzLAZY from a narrow-crown poplar contribute to regulation of branch angles. Plant Physiol. Biochem. 2017, 118, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Dardick, C.; Callahan, A.; Horn, R.; Ruiz, K.B.; Zhebentyayeva, T.; Hollender, C.; Whitaker, M.; Abbott, A.; Scorza, R. PpeTAC1 promotes the horizontal growth of branches in peach trees and is a member of a functionally conserved gene family found in diverse plants species. Plant J. 2013, 75, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Q.; Cheng, T.; Yang, W.; Pan, H.; Zhong, J.; Huang, L.; Liu, E. High-density genetic map construction and identification of a locus controlling weeping trait in an ornamental woody plant (Prunus mume Sieb. et Zucc). DNA Res. 2015, 22, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.L., Jr.; Hollender, C.A. Branching out: New insights into the genetic regulation of shoot architecture in trees. Curr. Opin. Plant Biol. 2019, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Guseman, J.M.; Webb, K.; Srinivasan, C.; Dardick, C. DRO1 influences root system architecture in Arabidopsis and Prunus species. Plant J. 2017, 89, 1093–1105. [Google Scholar] [CrossRef]
- Motte, H.; Vanneste, S.; Beeckman, T. Molecular and Environmental Regulation of Root Development. Annu. Rev. Plant Biol. 2019, 70, 465–488. [Google Scholar] [CrossRef]
- Barnes, B.D.; Kopecký, D.; Lukaszewski, A.J.; Baird, J.H. Evaluation of Turf-type Interspecific Hybrids of Meadow Fescue with Perennial Ryegrass for Improved Stress Tolerance. Crop Sci. 2014, 54, 355–365. [Google Scholar] [CrossRef]
- Baldoni, R.; Giardini, L. Coltivazioni Erbacee; Pàtron: Bologna, Italy, 2000; pp. 1–409. [Google Scholar]
- Casler, M.D. Perennial grasses for turf, sport and amenity uses: Evolution of form, function and fitness for human benefit. J. Agric. Sci. 2006, 144, 189–203. [Google Scholar] [CrossRef]
- Sampoux, J.P.; Baudouin, P.; Bayle, B.; Béguier, V.; Bourdon, P.; Chosson, J.F.; de Bruijn, K.; Deneufbourg, F.; Galbrun, C.; Ghesquière, M.; et al. Breeding perennial ryegrass (Lolium perenne L.) for turf usage: An assessment of genetic improvements in cultivars released in Europe, 1974–2004. Grass Forage Sci. 2013, 68, 33–48. [Google Scholar] [CrossRef]
- Beckett, K.P.; Freer-Smith, P.H.; Taylor, G. Urban woodlands: Their role in reducing the effects of particulate pollution. Environ. Pollut. 1998, 99, 347–360. [Google Scholar] [CrossRef]
- Biasioli, M.; Barberis, R.; Ajmone-Marsan, F. The influence of a large city on some soil properties and metals content. Sci. Total Environ. 2006, 356, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Fazekaš, J.; Fazekašova, D.; Chovancová, J. Soil Material Quality and Environmental Potential of Metallically Contaminated Soils. Key Eng. Mater. 2020, 838, 164–169. [Google Scholar] [CrossRef]
- Thomaidi, V.; Petousi, I.; Kotsia, D.; Kalogerakis, N.; Fountoulakis, M.S. Use of green roofs for greywater treatment: Role of substrate, depth, plants, and recirculation. Sci. Total Environ. 2022, 807, 151004. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Holt, A.R.; Warren, P.H.; Evans, K.L. Optimising UK urban road verge contributions to biodiversity and ecosystem services with cost-effective management. J. Environ. Manag. 2017, 191, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.L.; Falagán, N.; Hardman, C.A.; Kourmpetli, S.; Liu, L.; Mead, B.R.; Davies, J.A.C. Ecosystem service delivery by urban agriculture and green infrastructure—A systematic review. Ecosyst. Serv. 2022, 54, 101405. [Google Scholar] [CrossRef]
- Amoroso, G.; Frangi, P.; Piatti, R.; Fini, A.; Ferrini, F.; Faoro, M. Evaluation of Shrubs for Side Slope Greening and Protection in Urban Landscape. HortTechnology 2011, 21, 359–366. [Google Scholar] [CrossRef]
- Garbuzov, M.; Ratnieks, F.L.W.; Thompson, K. Quantifying variation among garden plants in attractiveness to bees and other flower-visiting insects. Funct. Ecol. 2014, 28, 364–374. [Google Scholar] [CrossRef]
- Armson, D.; Stringer, P.; Ennos, A.R. The effect of street trees and amenity grass on urban surface water runoff in Manchester, UK. Urban For. Urban Green. 2013, 12, 282–286. [Google Scholar] [CrossRef]
- Kumar, R.; Kaushik, S.C. Performance evaluation of green roof and shading for thermal protection of buildings. Build. Environ. 2005, 40, 1505–1511. [Google Scholar] [CrossRef]
- Berardi, U. The outdoor microclimate benefits and energy saving resulting from green roofs retrofits. Energy Build. 2016, 121, 217–229. [Google Scholar] [CrossRef]
- Yang, H.S.; Kang, J.; Choi, M.S. Acoustic effects of green roof systems on a low-profiled structure at street level. Build. Environ. 2012, 50, 44–55. [Google Scholar] [CrossRef]
- Li, J.-f.; Wai, O.W.H.; Li, Y.S.; Zhan, J.-m.; Ho, Y.A.; Li, J.; Lam, E. Effect of green roof on ambient CO2 concentration. Build. Environ. 2010, 45, 2644–2651. [Google Scholar] [CrossRef]
- WHO. Urban Green Spaces and Health; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2016. [Google Scholar]
- Garcia, R.; Millán, E. Assessment of Cd, Pb and Zn contamination in roadside soils and grasses from Gipuzkoa (Spain). Chemosphere 1998, 37, 1615–1625. [Google Scholar] [CrossRef]
- Ferro, A.M.; Sims, R.C.; Bugbee, B. Hycrest crested wheatgrass accelerates the degradation of pentachlorophenol in soil. J. Environ. Qual. 1994, 23, 272–279. [Google Scholar] [CrossRef]
- Chekol, T.; Vough, L.R. A Study of the Use of Alfalfa (Medicago sativa L.) for the Phytoremediation of Organic Contaminants in Soil. Remediation 2001, 11, 89–101. [Google Scholar] [CrossRef]
- Alkorta, I.; Garbisu, C. Phytoremediation of organic contaminants in soils. Bioresour. Technol. 2001, 79, 273–276. [Google Scholar] [CrossRef]
- Salt, D.E.; Blaylock, M.; Kumar, N.P.; Dushenkov, V.; Ensley, B.D.; Chet, I.; Raskin, I. Phytoremediation: A novel strategy for the removal of toxic metals from the environment using plants. Biotechnology 1995, 13, 468–474. [Google Scholar] [CrossRef]
- Capuana, M. A review of the performance of woody and herbaceous ornamental plants for phytoremediation in urban areas. iFor.-Biogeosci. For. 2020, 13, 139–151. [Google Scholar] [CrossRef]
- Dadea, C.; Russo, A.; Tagliavini, M.; Mimmo, T.; Zerbe, S.J.A.; Forestry, U. Tree species as tools for biomonitoring and phytoremediation in urban environments: A review with special regard to heavy metals. Arboric. Urban For. 2017, 43, 155–167. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Ishikawa, S. Mechanisms of cadmium accumulation in rice grains and molecular breeding for its reduction. Soil Sci. Plant Nutr. 2020, 66, 28–33. [Google Scholar] [CrossRef]
- Hayashi, S.; Kuramata, M.; Abe, T.; Yamaguchi, N.; Takagi, H.; Tanikawa, H.; Iino, M.; Sugimoto, K.; Ishikawa, S. Deficiency in alcohol dehydrogenase 2 reduces arsenic in rice grains by suppressing silicate transporters. Plant Physiol. 2021, 186, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.A.; Bass, B. Estimates of air pollution mitigation with green plants and green roofs using the UFORE model. Urban Ecosyst. 2008, 11, 409–422. [Google Scholar] [CrossRef]
- Jayasooriya, V.M.; Ng, A.W.M.; Muthukumaran, S.; Perera, B.J.C. Green infrastructure practices for improvement of urban air quality. Urban For. Urban Green. 2017, 21, 34–47. [Google Scholar] [CrossRef]
- Tallis, M.; Taylor, G.; Sinnett, D.; Freer-Smith, P. Estimating the removal of atmospheric particulate pollution by the urban tree canopy of London, under current and future environments. Landsc. Urban Plan. 2011, 103, 129–138. [Google Scholar] [CrossRef]
- Nowak, D.J.; Greenfield, E.J.; Hoehn, R.E.; Lapoint, E. Carbon storage and sequestration by trees in urban and community areas of the United States. Environ. Pollut. 2013, 178, 229–236. [Google Scholar] [CrossRef]
- Saebo, A.; Popek, R.; Nawrot, B.; Hanslin, H.M.; Gawronska, H.; Gawronski, S.W. Plant species differences in particulate matter accumulation on leaf surfaces. Sci. Total Environ. 2012, 427–428, 347–354. [Google Scholar] [CrossRef]
- Weber, F.; Kowarik, I.; Saumel, I. Herbaceous plants as filters: Immobilization of particulates along urban street corridors. Environ. Pollut. 2014, 186, 234–240. [Google Scholar] [CrossRef]
- Tong, Z.; Baldauf, R.W.; Isakov, V.; Deshmukh, P.; Max Zhang, K. Roadside vegetation barrier designs to mitigate near-road air pollution impacts. Sci. Total Environ. 2016, 541, 920–927. [Google Scholar] [CrossRef]
- Shrestha, S.; Baral, B.; Dhital, N.B.; Yang, H.-H. Assessing air pollution tolerance of plant species in vegetation traffic barriers in Kathmandu Valley, Nepal. Sustain. Environ. Res. 2021, 31, 3. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Solivas, J.L.; Alberto, M.C. Effects of flooding and redox conditions on solute diffusion in soil. Eur. J. Soil Sci. 2003, 54, 617–624. [Google Scholar] [CrossRef]
- Gallegos Reina, A.; Perles Roselló, M.J. Relationships between Peri-Urbanization Processes and Multi-Hazard Increases: Compared Diachronic Analysis in Basins of the Mediterranean Coast. ISPRS Int. J. Geo-Inf. 2021, 10, 759. [Google Scholar] [CrossRef]
- Bartens, J.; Day, S.D.; Harris, J.R.; Dove, J.E.; Wynn, T.M. Can urban tree roots improve infiltration through compacted subsoils for stormwater management? J. Environ. Qual. 2008, 37, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil 2009, 324, 91–102. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Hollender, C.A.; Pascal, T.; Tabb, A.; Hadiarto, T.; Srinivasan, C.; Wang, W.; Liu, Z.; Scorza, R.; Dardick, C. Loss of a highly conserved sterile alpha motif domain gene (WEEP) results in pendulous branch growth in peach trees. Proc. Natl. Acad. Sci. USA 2018, 115, E4690–E4699. [Google Scholar] [CrossRef]
- De Baets, S.; Poesen, J.; Reubens, B.; Muys, B.; De Baerdemaeker, J.; Meersmans, J. Methodological framework to select plant species for controlling rill and gully erosion: Application to a Mediterranean ecosystem. Earth Surf. Process. Landf. 2009, 34, 1374–1392. [Google Scholar] [CrossRef]
- Ghestem, M.; Sidle, R.C.; Stokes, A. The Influence of Plant Root Systems on Subsurface Flow: Implications for Slope Stability. BioScience 2011, 61, 869–879. [Google Scholar] [CrossRef]
- Fattet, M.; Fu, Y.; Ghestem, M.; Ma, W.; Foulonneau, M.; Nespoulous, J.; Le Bissonnais, Y.; Stokes, A. Effects of vegetation type on soil resistance to erosion: Relationship between aggregate stability and shear strength. Catena 2011, 87, 60–69. [Google Scholar] [CrossRef]
- Francini, A.; Toscano, S.; Romano, D.; Ferrini, F.; Ferrante, A. Biological Contribution of Ornamental Plants for Improving Slope Stability along Urban and Suburban Areas. Horticulturae 2021, 7, 310. [Google Scholar] [CrossRef]
- Farnham, M.W.; Grusak, M.A. Assessing nutritional changes in a vegetable over time: Issues and considerations. HortScience 2014, 49, 128–132. [Google Scholar] [CrossRef]
- Bumgarner, N.; Dorn, S.; McGinnis, E.; Bennett, P.; Bauske, E.; Krishnan, S.; Bradley, L. Consumer horticulture advancement: Identifying critical research areas and cultivating collaborations. HortTechnology 2019, 29, 769–776. [Google Scholar] [CrossRef]
- Damerum, A.; Chapman, M.A.; Taylor, G. Innovative breeding technologies in lettuce for improved post-harvest quality. Postharvest Biol. Technol. 2020, 168, 111266. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.M.; Ricaurte, J.; Tovar, E.; Cajiao, C.; Teran, H.; Grajales, M.; Polania, J.; Rao, I.; Beebe, S.; Raatz, B. QTL analyses for tolerance to abiotic stresses in a common bean (Phaseolus vulgaris L.) population. PLoS ONE 2018, 13, e0202342. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, F.; Galla, G.; Vitulo, N.; Barcaccia, G. First draft genome sequencing of fennel (Foeniculum vulgare Mill.): Identification of simple sequence repeats and their application in marker-assisted breeding. Mol. Breed. 2018, 38, 1–17. [Google Scholar] [CrossRef]
- Patella, A.; Palumbo, F.; Galla, G.; Barcaccia, G. The Molecular Determination of Hybridity and Homozygosity Estimates in Breeding Populations of Lettuce (Lactuca sativa L.). Genes 2019, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Gabellini, S.; Scaramuzzi, S. Evolving consumption trends, marketing strategies, and governance settings in ornamental horticulture: A grey literature review. Horticulturae 2022, 8, 234. [Google Scholar] [CrossRef]
- Orsini, F.; Pennisi, G.; Michelon, N.; Minelli, A.; Bazzocchi, G.; Sanyé-Mengual, E.; Gianquinto, G. Features and Functions of Multifunctional Urban Agriculture in the Global North: A Review. Front. Sustain. Food Syst. 2020, 4, 562513. [Google Scholar] [CrossRef]
- Bhattarai, K.; Sharma, S.; Panthee, D.R. Diversity among Modern Tomato Genotypes at Different Levels in Fresh-Market Breeding. Int. J. Agron. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Burbano-Erazo, E.; Pastrana-Vargas, I.J.; Mejía-Salazar, J.R.; Vallejo-Cabrera, F.A. Selection criteria in tomato lines with determinate growth habit. Agron. Mesoam. 2020, 31, 1–11. [Google Scholar] [CrossRef]
- Nascimento, M.V.; Ávila, M.C.R.; de Abreu-Tarazi, M.F.; Nogueira, A.P.O.; Campos, L.F.C.; dos Reis Nascimento, A. Identification of promising tomato breeding lines with determinate growth by selection index. Adv. Hortic. Sci. 2020, 34, 337–347. [Google Scholar]
- Bergougnoux, V. The history of tomato: From domestication to biopharming. Biotechnol. Adv. 2014, 32, 170–189. [Google Scholar] [CrossRef] [PubMed]
- Rothan, C.; Diouf, I.; Causse, M. Trait discovery and editing in tomato. Plant J. 2019, 97, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.; Gisbert, C.; Bishop, G.J.; Dixon, M.S.; Garcia-Martinez, J.L. Genetic and physiological characterization of tomato cv. Micro-Tom. J. Exp. Bot. 2006, 57, 2037–2047. [Google Scholar] [CrossRef]
- Meissner, R.; Jacobson, Y.; Melamed, S.; Levyatuv, S.; Shalev, G.; Ashri, A.; Elkind, Y.; Levy, A. A new model system for tomato genetics. Plant J. 1997, 12, 1465–1472. [Google Scholar] [CrossRef]
- Scott, J.W.; Harbaugh, B.K. Micro-Tom. A miniature dwarf tomato. Circ. Univ. Fla. Agric. Exp. Stn. 1989, 370, 6. [Google Scholar]
- Shikata, M.; Ezura, H. Micro-Tom Tomato as an Alternative Plant Model System: Mutant Collection and Efficient Transformation. In Plant Signal Transduction: Methods and Protocols; Botella, J.R., Botella, M.A., Eds.; Springer: New York, NY, USA, 2016; pp. 47–55. [Google Scholar] [CrossRef]
- Arena, C.; Vitale, E.; Hay Mele, B.; Cataletto, P.R.; Turano, M.; Simoniello, P.; De Micco, V. Suitability of Solanum lycopersicum L. ‘Microtom’ for growth in Bioregenerative Life Support Systems: Exploring the effect of high-LET ionising radiation on photosynthesis, leaf structure and fruit traits. Plant Biol. 2019, 21, 615–626. [Google Scholar] [CrossRef]
- Marzioli, P.; Gugliermetti, L.; Santoni, F.; Delfini, A.; Piergentili, F.; Nardi, L.; Metelli, G.; Benvenuto, E.; Massa, S.; Bennici, E. CultCube: Experiments in autonomous in-orbit cultivation on-board a 12-Units CubeSat platform. Life Sci. Space Res. 2020, 25, 42–52. [Google Scholar] [CrossRef]
- Scott, J.; Harbaugh, B.; Baldwin, E. Micro-Tina’andMicro-Gemma’Miniature Dwarf Tomatoes. HortScience 2000, 35, 774–775. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 724–727. [Google Scholar] [CrossRef]
- Al-Kodmany, K. The Vertical Farm: Exploring Applications for Peri-urban Areas. In Smart Village Technology; Patnaik, S., Sen, S., Mahmoud, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 203–232. [Google Scholar] [CrossRef]
- Folta, K.M. Breeding new varieties for controlled environments. Plant Biol. 2019, 21 (Suppl. 1), 6–12. [Google Scholar] [CrossRef] [PubMed]
- Stagnari, F.; Di Mattia, C.; Galieni, A.; Santarelli, V.; D’Egidio, S.; Pagnani, G.; Pisante, M. Light quantity and quality supplies sharply affect growth, morphological, physiological and quality traits of basil. Ind. Crops Prod. 2018, 122, 277–289. [Google Scholar] [CrossRef]
- Nogler, G.; Johri, B. Embryology of angiosperms. In Gametophytic Embryogenesis; Johri, B., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1984; pp. 475–518. [Google Scholar]
- Palumbo, F.; Draga, S.; Vannozzi, A.; Lucchin, M.; Barcaccia, G. Trends in Apomixis Research: The 10 Most Cited Research Articles Published in the Pregenomic and Genomic Eras. Front. Plant Sci. 2022, 13, 878074. [Google Scholar] [CrossRef]
- Kozai, T. Resource use efficiency of closed plant production system with artificial light: Concept, estimation and application to plant factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Touliatos, D.; Dodd, I.C.; McAinsh, M. Vertical farming increases lettuce yield per unit area compared to conventional horizontal hydroponics. Food Energy Secur. 2016, 5, 184–191. [Google Scholar] [CrossRef]
- Nicola, S.; Fontana, E. Fresh-Cut Produce Quality. In Postharvest Handling; Florkowski, W.J., Shewfelt, R.L., Brueckner, B., Prussia, S.E., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 217–273. [Google Scholar] [CrossRef]
- Ferrante, A. Ortaggi per la IV gamma [Produce for pre-cut salads]. In La Concimazione Azotata Degli Ortaggi; Incrocci, L., Santamaria, P., Pardossi, A., Eds.; Barone e Bella & C: Ragusa, Italy, 2013; pp. 165–180. [Google Scholar]
- Saini, R.K.; Ko, E.Y.; Keum, Y.-S. Minimally processed ready-to-eat baby-leaf vegetables: Production, processing, storage, microbial safety, and nutritional potential. Food Rev. Int. 2016, 33, 644–663. [Google Scholar] [CrossRef]
- Halgamuge, M.N.; Bojovschi, A.; Fisher, P.M.J.; Le, T.C.; Adeloju, S.; Murphy, S. Internet of Things and autonomous control for vertical cultivation walls towards smart food growing: A review. Urban For. Urban Green. 2021, 61, 127094. [Google Scholar] [CrossRef]
- Gil, M.I. Preharvest factors and fresh-cut quality of leafy vegetables. In Proceedings of the III International Conference on Fresh-Cut Produce: Maintaining Quality and Safety, Davis, CA, USA, 13 September 2015; pp. 57–64. [Google Scholar]
- Jasper, J.; Elmore, J.S.; Wagstaff, C. Determining the quality of leafy salads: Past, present and future. Postharvest Biol. Technol. 2021, 180, 111630. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Luna, M.C.; Selma, M.V.; Tudela, J.A.; Abad, J.; Gil, M.I. Baby-leaf and multi-leaf of green and red lettuces are suitable raw materials for the fresh-cut industry. Postharvest Biol. Technol. 2012, 63, 1–10. [Google Scholar] [CrossRef]
- Pires, E.O., Jr.; Di Gioia, F.; Rouphael, Y.; Ferreira, I.; Caleja, C.; Barros, L.; Petropoulos, S.A. The Compositional Aspects of Edible Flowers as an Emerging Horticultural Product. Molecules 2021, 26, 6940. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Copetta, A.; Marchioni, I.; Ruffoni, B. The edible flowers from woody ornamental plants. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone, Torino, Italy, 3 March 2021; pp. 195–204. [Google Scholar]
- Yagi, M. Recent Progress in Genomic Analysis of Ornamental Plants, with a Focus on Carnation. Hortic. J. 2015, 84, 3–13. [Google Scholar] [CrossRef]
- Kuligowska, K.; Lutken, H.; Muller, R. Towards development of new ornamental plants: Status and progress in wide hybridization. Planta 2016, 244, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Debener, T. Molecular Markers for Ornamental Plant Genetics, Genomics and Breeding. In Proceedings of the XXIV International Eucarpia Symposium Section Ornamentals: Ornamental Breeding Worldwide, Warsaw, Poland, 2 September 2012; pp. 193–200. [Google Scholar]
- Nowak, M.D.; Russo, G.; Schlapbach, R.; Huu, C.N.; Lenhard, M.; Conti, E. The draft genome of Primula veris yields insights into the molecular basis of heterostyly. Genome Biol. 2015, 16, 12. [Google Scholar] [CrossRef]
- Vukosavljev, M.; Arens, P.; Voorrips, R.E.; van’t Westende, W.P.; Esselink, G.D.; Bourke, P.M.; Cox, P.; van de Weg, W.E.; Visser, R.G.; Maliepaard, C.; et al. High-density SNP-based genetic maps for the parents of an outcrossed and a selfed tetraploid garden rose cross, inferred from admixed progeny using the 68k rose SNP array. Hortic. Res. 2016, 3, 16052. [Google Scholar] [CrossRef]
- Yagi, M. Recent progress in whole genome sequencing, high-density linkage maps, and genomic databases of ornamental plants. Breed Sci. 2018, 68, 62–70. [Google Scholar] [CrossRef]
- Scariolo, F.; Palumbo, F.; Vannozzi, A.; Sacilotto, G.B.; Gazzola, M.; Barcaccia, G. Genotyping Analysis by RAD-Seq Reads Is Useful to Assess the Genetic Identity and Relationships of Breeding Lines in Lavender Species Aimed at Managing Plant Variety Protection. Genes 2021, 12, 1656. [Google Scholar] [CrossRef]
- Wan, H.; Yu, C.; Han, Y.; Guo, X.; Ahmad, S.; Tang, A.; Wang, J.; Cheng, T.; Pan, H.; Zhang, Q. Flavonols and Carotenoids in Yellow Petals of Rose Cultivar (Rosa ‘Sun City’): A Possible Rich Source of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 4171–4181. [Google Scholar] [CrossRef] [PubMed]
- Krzyminska, A.; Gasecka, M.; Magdziak, Z. Content of Phenolic Compounds and Organic Acids in the Flowers of Selected Tulipa gesneriana Cultivars. Molecules 2020, 25, 5627. [Google Scholar] [CrossRef]
- Marchioni, I.; Pistelli, L.; Copetta, A.; Dimita, R.; Descamps, S.; Cambournac, L.; Ruffoni, B. Edible roses as novel food with healthy value. In Proceedings of the IV International Symposium on Woody Ornamentals of the Temperate Zone, Torino, Italy, 3 March 2021; pp. 239–244. [Google Scholar]
- Goddard, M.A.; Dougill, A.J.; Benton, T.G. Scaling up from gardens: Biodiversity conservation in urban environments. Trends Ecol. Evol. 2010, 25, 90–98. [Google Scholar] [CrossRef]
- Spotswood, E.N.; Beller, E.E.; Grossinger, R.; Grenier, J.L.; Heller, N.E.; Aronson, M.F.J. The Biological Deserts Fallacy: Cities in Their Landscapes Contribute More than We Think to Regional Biodiversity. Bioscience 2021, 71, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, S.L.; Petermann, J.S.; Rolff, J. Wild bees as pollinators of city trees. Insect Conserv. Divers. 2016, 9, 97–107. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.B.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L.; et al. Biodiversity and Resilience of Ecosystem Functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Tylianakis, J.M. Ecology. The global plight of pollinators. Science 2013, 339, 1532–1533. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination—A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Pellmyr, O. Pollination by animals. In Plant–Animal Interactions, an Evolutionary Approach; Herrera, C.M., Pellmyr, O., Eds.; Blackwell Science: Oxford, UK, 2002; pp. 157–184. [Google Scholar]
- Payne, A.; Schildroth, D.A.; Starks, P.T. Nest site selection in the European wool-carder bee, Anthidium manicatum, with methods for an emerging model species. Apidologie 2011, 42, 181–191. [Google Scholar] [CrossRef]
- Rahimi, E.; Barghjelveh, S.; Dong, P. A review of diversity of bees, the attractiveness of host plants and the effects of landscape variables on bees in urban gardens. Agric. Food Secur. 2022, 11, 6. [Google Scholar] [CrossRef]
- Slate, M.L.; Matallana-Mejia, N.; Aromin, A.; Callaway, R.M. Nitrogen addition, but not pulse frequency, shifts competitive interactions in favor of exotic invasive plant species. Biol. Invasions 2022. [Google Scholar] [CrossRef]
- Rojas-Botero, S.; Kollmann, J.; Teixeira, L.H. Competitive trait hierarchies of native communities and invasive propagule pressure consistently predict invasion success during grassland establishment. Biol. Invasions 2021, 24, 107–122. [Google Scholar] [CrossRef]
- Adler, P.B.; Smull, D.; Beard, K.H.; Choi, R.T.; Furniss, T.; Kulmatiski, A.; Meiners, J.M.; Tredennick, A.T.; Veblen, K.E. Competition and coexistence in plant communities: Intraspecific competition is stronger than interspecific competition. Ecol. Lett. 2018, 21, 1319–1329. [Google Scholar] [CrossRef]
- Drenovsky, R.E.; Grewell, B.J.; D’Antonio, C.M.; Funk, J.L.; James, J.J.; Molinari, N.; Parker, I.M.; Richards, C.L. A functional trait perspective on plant invasion. Ann. Bot. 2012, 110, 141–153. [Google Scholar] [CrossRef]
- Kaushik, P.; Pati, P.K.; Khan, M.L.; Khare, P.K. Plant functional traits best explain invasive species’ performance within a dynamic ecosystem—A review. Trees For. People 2022, 8, 100260. [Google Scholar] [CrossRef]
- Ellstrand, N.C.; Schierenbeck, K.A. Hybridization as a stimulus for the evolution of invasiveness in plants? Proc. Natl. Acad. Sci. USA 2000, 97, 7043–7050. [Google Scholar] [CrossRef]
- Takakura, K.; Matsumoto, T.; Nishida, T.; Nishida, S. Effective range of reproductive interference exerted by an alien dandelion, Taraxacum officinale, on a native congener. J. Plant Res. 2011, 124, 269–276. [Google Scholar] [CrossRef]
- Tang, J.; Mao, K.; Zhang, H.; Xu, X.; Xu, X.; Guo, H.; Li, B. Multiple introductions and genetic admixture facilitate the successful invasion of Plantago virginica into China. Biol. Invasions 2022, 24, 2261–2272. [Google Scholar] [CrossRef]
- Uemura, R.; Asakawa, A.; Fujii, S.; Matsuo, A.; Suyama, Y.; Maki, M. Can Rumex madaio (Polygonaceae) be threatened by natural hybridization with an invasive species in Japan. Nord. J. Bot. 2022, 2022, e03543. [Google Scholar] [CrossRef]
- Deng, Z.; Wilson, S.B.; Ying, X.; Chen, C.; Freyre, R.; Zayas, V.; Czarnecki, D.M. ‘UF-1013-1’: An Infertile Cultivar of Lantana camara. HortScience 2020, 55, 953–958. [Google Scholar] [CrossRef]
- Henderson, I.R.; Salt, D.E. Natural genetic variation and hybridization in plants. J. Exp. Bot. 2017, 68, 5415–5417. [Google Scholar] [CrossRef]
| Techniques | General Description | To Learn More about | |
|---|---|---|---|
| Whole Genome Sequencing (WGS) | The genome of a species is assembled for the first time into chromosomes with high coverage and it is functionally annotated to produce a reference assembly and to predict hundreds of loci underlying agronomic traits | [24] | |
| RNA-seq analysis | RNA-seq can be used to examine the RNA sequences that are present in a sample (transcriptome). This is crucial for linking the information contained within the genome with the functional proteins that are expressed. RNA-seq can be used to elucidate which genes are turned on or off within a cell under specific conditions | [25] | |
| Whole genome resequencing (WGR) | The genome is fully sequenced with low or modest coverage and is aligned against the reference genome assembly to predict allelic variants | [26] | |
| Reduced Representation Sequencing (RRS) | GBS | A fraction of the genome is sequenced and aligned against the reference genome assembly to predict allelic variants. For GBS, ddRAD-seq, 2bRAD-seq and ezRAD-seq the regions to be sequenced are randomly chosen using restriction enzymes, for target-seq the regions to be sequenced are selected through PCR | [27] |
| ddRAD-seq | [28] | ||
| 2bRAD-seq | [29] | ||
| ezRAD-seq | [30] | ||
| target-seq | [31] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farinati, S.; Betto, A.; Palumbo, F.; Scariolo, F.; Vannozzi, A.; Barcaccia, G. The New Green Challenge in Urban Planning: The Right Genetics in the Right Place. Horticulturae 2022, 8, 761. https://doi.org/10.3390/horticulturae8090761
Farinati S, Betto A, Palumbo F, Scariolo F, Vannozzi A, Barcaccia G. The New Green Challenge in Urban Planning: The Right Genetics in the Right Place. Horticulturae. 2022; 8(9):761. https://doi.org/10.3390/horticulturae8090761
Chicago/Turabian StyleFarinati, Silvia, Angelo Betto, Fabio Palumbo, Francesco Scariolo, Alessandro Vannozzi, and Gianni Barcaccia. 2022. "The New Green Challenge in Urban Planning: The Right Genetics in the Right Place" Horticulturae 8, no. 9: 761. https://doi.org/10.3390/horticulturae8090761
APA StyleFarinati, S., Betto, A., Palumbo, F., Scariolo, F., Vannozzi, A., & Barcaccia, G. (2022). The New Green Challenge in Urban Planning: The Right Genetics in the Right Place. Horticulturae, 8(9), 761. https://doi.org/10.3390/horticulturae8090761








