Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Sampling
2.1.1. Experiment 1—Defense Hormone Analysis after Broad Mite Infestation under Controlled Conditions in Two Cultivars of Pot Azalea Shortly after Infestation (Up to 11 Days)
2.1.2. Experiment 2—Defense Hormone Analysis under Natural Infestation in Three Cultivars of Pot Azalea during the Growing Season (Up to 32 Weeks)
2.1.3. Experiment 3—Defense Hormone Analysis under Natural Infestation in 18 Cultivars of Pot Azalea and Occurrence of Mite Species during the Growing Season (30 Weeks)
2.2. JA and SA Analysis
2.3. Statistical Data Analysis
3. Results
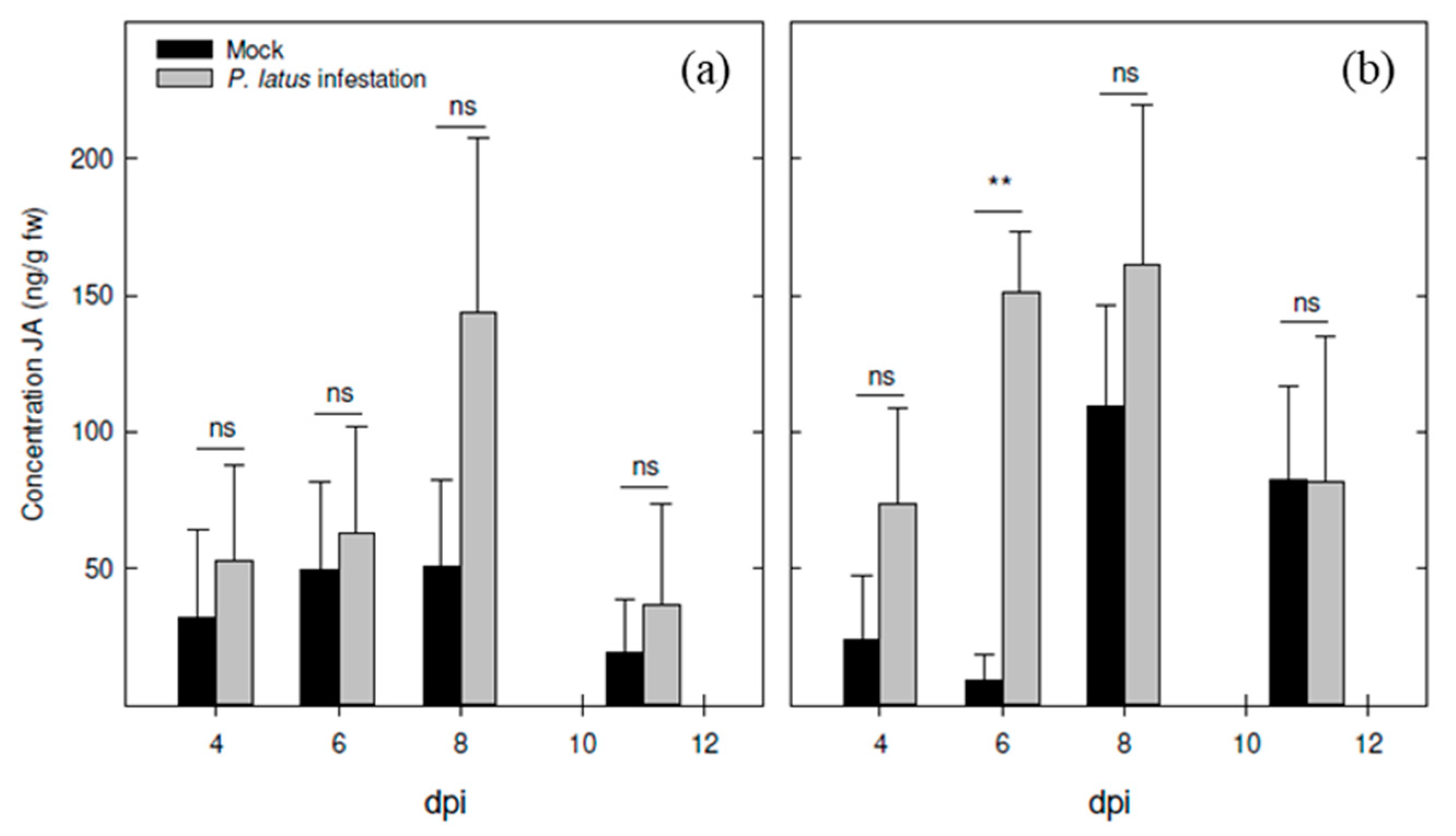
3.1. Experiment 1— Defense Hormone Analysis after Broad Mite Infestation under Controlled Conditions in Two Cultivars of Pot Azalea Shortly after Infestation (Up to 11 Days)
3.2. Experiment 2—Defense Hormone Analysis under Natural Infestation in Three Cultivars of Pot Azalea during the Growing Season (Up to 32 Weeks)
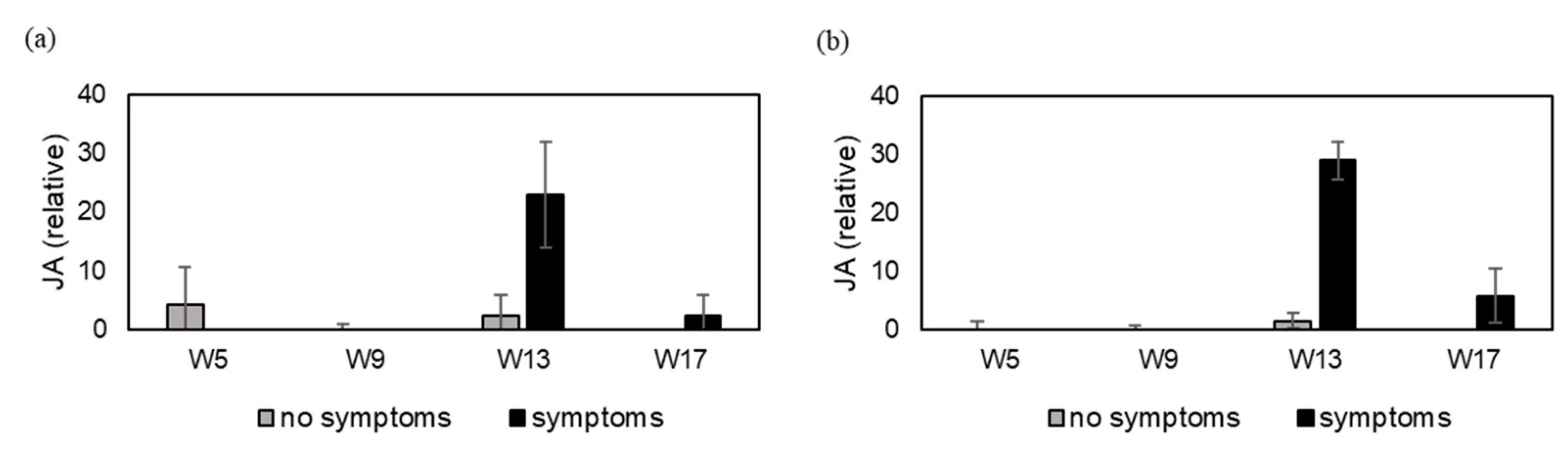
3.3. Experiment 3—Defense Hormone Analysis under Natural Infestation in 18 Cultivars of Pot Azalea and Occurrence of Mite Species during the Growing Season (30 Weeks)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheerlinck, H.; Baumann, J.; De Somer, F.; Haerens, A.; Van Laere, A. Tuinbouwencyclopedie I. De Azalea Indica L. De Sikkel; Wereldbibliotheek: Amsterdam, The Netherlands, 1938; 527p. [Google Scholar]
- Heursel, J.; Lootens, P.; Mertens, M.; Saverwijns, A. Azalea’s. Oorsprong, Veredeling en Cultivars; Lannoo: Tielt, Belgium, 1999; p. 192. [Google Scholar]
- Van Trier, H. Gentse Azalea; Stichting Kunstboek: Oostkamp, Belgium, 2012; 144p. [Google Scholar]
- VLAM 2020. Belgische Export Van Azalea’s en Rhododendrons (2011–2020)—Duitsland Sierteelt 2021—Belgische Sierteeltoppervlakte (2011–2020). Available online: https://www.vlaanderen.be/vlam/kennisbank?f%5B0%5D=facet_sector%3A58 (accessed on 25 February 2022).
- VLAM 2021. Belgische Export Van Azalea’s en Rhododendrons (2011–2020)—Duitsland Sierteelt 2021—Belgische Sierteeltoppervlakte (2011–2020). Available online: https://www.vlaanderen.be/vlam/kennisbank?f%5B0%5D=facet_sector%3A58 (accessed on 25 February 2022).
- Navajas, M.; Migeon, A.; Estrada-Pena, A.; Mallieux, A.C.; Servigne, P.; Petanovic, R. Chapter 7.4: The Acari. Invasive terrestrial invertebrates in Europe. BioRisk 2010, 4, 149–192. [Google Scholar] [CrossRef]
- Heungens, A. Soft-skinned mites in azalea culture and comparable control results on other host plants. Verb. Voor De Belg. Sierteelt 1986, 30, 257–269. [Google Scholar]
- De Coss-Romero, M.; Peña, J.E. Relationship of broad mite (Acari: Tarsonemidae) to host phenology and injury levels in Capsicum annuum. Fla. Entomol. 1998, 81, 515–526. [Google Scholar] [CrossRef]
- Grinberg, M.; Perl-Treves, R.; Palevsky, E.; Shomer, I.; Soroker, V. Interaction between cucumber plants and the broad mite, Polyphagotarsonemus latus: From damage to defense gene expression. Entomol. Exp. Appl. 2005, 115, 135–144. [Google Scholar] [CrossRef]
- Peña, J.E. Relationships of broad mite (Acari: Tarsonemidae) density to lime damage. J. Econ. Entomol. 1990, 83, 2008–2015. [Google Scholar] [CrossRef]
- Dhooria, J.C.; Bindra, O.S. Polyphagotarsonemus latus (Banks) a mite pest of chilli and potato in Punjab. Acarol. Newsl. 1977, 4, 7–9. [Google Scholar]
- Luypaert, G.; Van Huylenbroeck, J.; De Riek, J.; De Clercq, P. Screening for broad mite susceptibility in Rhododendron simsii hybrids. J. Plant Dis. Protect. 2014, 121, 260–269. [Google Scholar] [CrossRef]
- Gerson, U. Biology and control of the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Exp. Appl. Acarol. 1992, 13, 163–178. [Google Scholar] [CrossRef]
- Van Huylenbroeck, J.; Calsyn, E.; De Keyser, E.; Luypaert, G. Breeding for biotic stress resistance in Rhododendron simsii. Acta Hort. 2015, 1104, 375–379. [Google Scholar] [CrossRef]
- Nasrin, M.; Amin, M.R.; Miah, M.R.U.; Akanda, A.M.; Miah, M.G.; Kwon, O.; Suh, S.J. Occurrence and severity of mite Polyphagotarsonemus latus (Tarsonemidae) on chili plants: Analysis of pest-weather and host plant characteristics. Entomol. Res. 2020, 51, 273–281. [Google Scholar] [CrossRef]
- Wang, T.; Jia, Q.; Wang, W.; Hussain, S.; Ahmed, S.; Adnan; Zhou, D.X.; Ni, Z.; Wang, S. GCN5 modulates trichome initiation in Arabidopsis by manipulating histone acetylation of core trichome initiation regulator genes. Plant Cell Rep. 2019, 38, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Luypaert, G.; Van Huylenbroeck, J.; De Riek, J.; Mechant, E.; Pauwels, E.; De Clercq, P. Opportunities to breed for broad mite resistance in Rhododendron simsii hybrids. In Proceedings of the XXV International EUCARPIA Symposium Section Ornamentals: Crossing Borders 1087, Melle, Belgium, 28 June 2015; pp. 479–484. [Google Scholar]
- Pieterse, C.M.; Van Der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlot, A.C.; D’Maris, A.D.; Klessig, D.F. Salicylic acid, a multifaced hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Glazebrook, J. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 2005, 43, 205–227. [Google Scholar] [CrossRef]
- Howe, G.A.; Jander, G. Plant immunity to insect herbivores. Annu. Rev. Plant Biol. 2008, 59, 41–66. [Google Scholar] [CrossRef]
- Blaazer, C.J.H.; Villacis-Perez, E.A.; Chafi, R.; Van Leeuwen, T.; Kant, M.R.; Schimmel, B.C. Why do herbivorous mites suppress plant defenses? Front. Plant Sci. 2018, 9, 1057. [Google Scholar] [CrossRef]
- Grinberg, M.; Alagarmalai, J.; Sharon, L.; Perl-Treves, R.; Soroker, V. The effect of jasmonic acid signalling pathway on tomato defense to broad mite behaviour. Isr. J. Entomol. 2007, 37, 389–390. [Google Scholar]
- Alagarmalai, J.; Grinberg, M.; Reneh, S.; Sharon, L.; Perl-Treves, R.; Soroker, V. Host selection behavior of broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). IOBC-WPRS Bull. 2007, 30, 1–7. [Google Scholar]
- Luypaert, G.; Witters, J.; Van Huylenbroeck, J.; De Clercq, P.; De Riek, J.; De Keyser, E. Induced expression of selected plant defence related genes in pot azalea, Rhododendron simsii hybrid. Euphytica 2017, 231, 227. [Google Scholar] [CrossRef]
- Mechant, E.; Luypaert, G.; Van Delsen, B.; Pauwels, E.; Witters, J.; Van Huylenbroeck, J.; Gobin, B. Development and validation of a three-step detection protocol for broad mites (Polyphagotarsonemus latus) in pot azalea (Rhododendron simsii hybrids). Entomol. Exp. Appl. 2015, 156, 99–104. [Google Scholar] [CrossRef]
- Vacante, V. The Handbook of Mites of Economic Plants: Identification, Bio-Ecology and Control; CABI: Wallingford, UK, 2015. [Google Scholar]
- Krantz, G.W.; Walter, D.E. A Manual of Acarology; Texas Tech University Press: Lubbock, TX, USA, 2009; 807p. [Google Scholar]
- Zhang, Z.Q.; Bejakovich, D.; Martin, N.A. Key to Tarsonemidae of New Zealand; Final report to MAF Science Policy for Project FMA 102; Ministry of Business, Innovation and Employment: Wellington, New Zealand, 2000; pp. 1–35.
- Bosco, R.; Daeseleire, E.; Van Pamel, E.; Scariot, V.; Leus, L. Development of an Ultrahigh-Performance Liquid Chromatography−Electrospray Ionization−Tandem Mass Spectrometry Method for the Simultaneous Determination of Salicylic Acid, Jasmonic Acid, and Abscisic Acid in Rose Leaves. J. Agric. Food Chem. 2014, 62, 6278–6284. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bel, P.; Sanmartín, N.; Pastor, V.; Mateu, D.; Cerezo, M.; Vidal-Albalat, A.; Pastor-Fernández, J.; Pozo, M.J.; Flors, V. Mycorrhizal tomato plants fine tunes the growth-defence balance upon N depleted root environments. Plant Cell Environ. 2018, 41, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Agut, B.; Pastor, V.; Jaques, J.A.; Flors, V. Can plant defence mechanisms provide new approaches for the sustainable control of the two-spotted spider mite Tetranychus urticae? Int. J. Mol. Sci. 2018, 19, 614. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, D.; Wang, Y.; Xie, D. Jasmonate action in plant defense against insects. J. Exp. Bot. 2019, 70, 3391–3400. [Google Scholar] [CrossRef]
- Arena, G.D.; Ramos-González, P.L.; Rogerio, L.A.; Ribeiro-Alves, M.; Casteel, C.L.; Freitas-Astúa, J.; Machado, M.A. Making a Better Home: Modulation of Plant Defensive Response by Brevipalpus Mites. Front. Plant Sci. 2018, 9, 1147. [Google Scholar] [CrossRef]
- Luypaert, G. The Broad Mite, Polyphagotarsonemus latus (Acari: Tarsonemidae), and Its Interactions with Pot Azalea, Rhododendron simsii Hybrid. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2015. [Google Scholar]
- Heil, M.; Ton, J. Long-distance signalling in plant defence. Trends Plant Sci. 2008, 13, 264–272. [Google Scholar] [CrossRef]
- Lim, G.-H.; Sine, M.B.; de Lorenzo, L.; Yu, K.; Cui, W.; Navarre, D.; Hunt, A.G.; Lee, J.Y.; Kachroo, A.; Kachroo, P. Plasmodesmata localizing proteins regulate transport and signaling during systematic acquired immunity in plants. Cell Host Microbe 2016, 19, 541–549. [Google Scholar] [CrossRef]
- Grinberg-Yaari, M.; Alagarmalai, J.; Lewinsohn, E.; Perl-Treves, R.; Soroker, V. Role of jasmonic acid signaling in tomato defense against broad mite, Polyphagotarsonemus latus (Acari: Tarsonemidae). Arthropod-Plant Interact. 2015, 9, 361–372. [Google Scholar] [CrossRef]
- Cabedo-López, M.; Cruz-Miralles, J.; Peris, D.; Ibáñez-Gual, M.V.; Flors, V.; Jaques, J.A. The response of citrus plants to the broad mite Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Agric. For. Entomol. 2021, 23, 411–419. [Google Scholar] [CrossRef]
- De Jonge, R.; Bolton, M.D.; Thomma, B.P. How filamentous pathogens co-opt plants: The ins and outs of fungal effectors. Curr. Opin. Plant Biol. 2011, 4, 400–406. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Bos, J.I.B. Toward understanding the role of aphid effectors in plant infestation. Mol. Plant-Microbe Interact. 2013, 26, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Haegeman, A.; Mantelin, S.; Jones, J.T.; Gheysen, G. Functional roles of effectors of plant-parasitic nematodes. Gene 2012, 492, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Alba, J.M.; Glas, J.J.; Schimmel, B.C.J.; Kant, M.R. Avoidance and suppression of plant defenses by herbivores and pathogens. J. Plant Interact. 2011, 6, 221–227. [Google Scholar] [CrossRef]
- Alba, J.M.; Schimmel, B.C.; Glas, J.J.; Ataide, L.M.; Pappas, M.L.; Villarroel, C.A.; Schuurink, R.C.; Sabelis, M.W.; Kant, M.R. Spider mites suppress tomato defenses downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 2015, 205, 828–840. [Google Scholar] [CrossRef] [PubMed]
- Kant, M.R.; Sabelis, M.W.; Haring, M.A.; Schuurink, R.C. Intraspecific variation in a generalist herbivore accounts for differential induction and impact of host plant defences. P. Roy. Soc. B Biol. Sci. 2008, 752, 443–452. [Google Scholar] [CrossRef]
- Sarmento, R.A.; Lemos, F.; Bleeker, P.M.; Schuurink, R.C.; Pallini, A.; Oliveira, M.G.A.; Lima, E.R.; Kant, M.; Sabelis, M.W.; Janssen, A. A herbivore that manipulates plant defence. Ecol. Lett. 2011, 14, 229–236. [Google Scholar] [CrossRef]
- Grbić, M.; Van Leeuwen, T.; Clark, R.M.; Rombauts, S.; Rouzé, P.; Grbić, V.; Osborne, E.J.; Dermauw, W.; Thi Ngoc, P.C.; Ortego, F.; et al. The genome of Tetranychus urticae reveals herbivorous pest adaptations. Nature 2011, 479, 487–492. [Google Scholar] [CrossRef]
- Higa, S.Y.; Namba, R. Vectors of the papaya mosaic virus in Hawaii. Proc. Hawaii. Entomol. Soc. 1971, 21, 93–96. [Google Scholar]
- Aubert, B.; Lossois, P.; Marchal, J. Mise en evidence des dégâts causés par Polyphagotarsonemus latus (Banks) sur papayers à l’ile de la Réunion. Fruits 1981, 36, 9–24. [Google Scholar]
- Zarate, S.I.; Kempema, L.A.; Walling, L.L. Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 2007, 143, 866–875. [Google Scholar] [CrossRef]
- Zhang, P.J.; Li, W.D.; Huang, F.; Zhang, J.M.; Xu, F.C.; Lu, Y.B. Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 2013, 39, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.-J.; He, Y.-C.; Zhao, C.; Ye, Z.-H.; Yu, X.-P. Jasmonic Acid-Dependent Defenses Play a Key Role in Defending Tomato Against Bemisia tabaci Nymphs, but Not Adults. Front. Plant Sci. 2018, 20, 1065. [Google Scholar] [CrossRef] [PubMed]
- Schimmel, B.C.J.; Alba, J.M.; Wybouw, N.; Glas, J.J.; Meijer, T.T.; Schuurink, R.C.; Kant, M.R. Distinct Signatures of Host Defense Suppression by Plant-Feeding Mites. Int. J. Mol. Sci. 2018, 19, 3265. [Google Scholar] [CrossRef]
- Omer, A.D.; Granett, J.; Kerban, R.; Villa, E.M. Chemically-induced resistance against multiple pests in cotton. Int. J. Pest Man. 2001, 47, 49–54. [Google Scholar] [CrossRef]
- Li, C.; Williams, M.M.; Loh, Y.T.; Lee, G.I.; Howe, G.A. Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 2002, 130, 494–503. [Google Scholar] [CrossRef]
- Warabieda, W.; Miszczak, A.; Olszak, R.W. The influence of methyl jasmonate (JA-Me) and βglucosidase on introduction of resistance mechanisms of strawberry against two-spotted spider mite (Tetranychus urticae Koch). Commun. Agric. Appl. Biol. Sci. 2005, 70, 829–836. [Google Scholar]
- Warabieda, W.; Olszak, R.W. Aspects of the influence of exogenous methyl jasmonate on strawberry plants and on the population size of the two-spotted spider mite (Tetranychus urticae Koch). IOBC-WPRS Bull. 2012, 83, 7–13. [Google Scholar]
- Rohwer, C.L.; Erwin, J.E. Spider mites (Tetranychus utricae) perform poorly on and disperse from plants exposed to methyl jasmonate. Entomol. Exp. Et Appl. 2010, 137, 143–152. [Google Scholar] [CrossRef]
- Agut, B.; Gamir, J.; Jacas, J.A.; Hurtado, M.; Flors, V. Different metabolic and genetic responses in citrus may explain relative susceptibility to Tetranychus urticae. Pest Manag. Sci. 2014, 70, 1728–1741. [Google Scholar] [CrossRef]
- Choh, Y.; Ozawa, R.; Takabayashi, J. Effects of exogenous jasmonic acid and benzo (1,2,3) thiadiazole7-carbothioic acid S-methyl ester (BTH), a functional analogue of salicylic acid, on the egg production of a herbivorous mite Tetranychus urticae (Acari: Tertanychidae). Appl. Entomol. Zool. 2004, 39, 311–314. [Google Scholar] [CrossRef]
- Androcioli, H.G.; Hoshino, A.T.; Ventura, M.U.; Hata, F.T.; Brugnerotto, M.d.R.; Constantino, L.V.; Marques, F.d.A. Resistance of Common Bean Genotypes to the Broad Mite, Polyphagotarsonemus latus (Banks, 1904) (Acari: Tarsonemidae): Offspring Development and Biochemical Basis. Insects 2021, 12, 910. [Google Scholar] [CrossRef] [PubMed]


| Cultivar Name | Parentage / Background | * Used in Luypaert et al. [12] |
|---|---|---|
| ‘Amélie’ | sport from ‘Thesla’ y | |
| ‘Elien’ | sport from ‘Mistral’ x | * |
| ‘Fluostern’ | sport from ‘Sachsenstern’ y | |
| ‘Franziska R.’ | sport from ‘Michelle Marie’ x | |
| ‘Huelsten’ | sport from ‘Helmutt Vogel’ x | |
| ‘Inka’ | sport from a ‘Helmutt Vogel’ sport x | |
| ‘Leonardo’ | seedling with unknown parents y | |
| ‘Luntera’ | sport from a ‘Helmutt Vogel’ sport x | |
| ‘Mevrouw Edmond Troch’ | sport from a ‘Helmutt Vogel’ sport x | |
| ‘Mevrouw Gerard Kint’ (picotee) | sport from ‘Glaser Nummer 10’ x | * |
| ‘Mevrouw Gerard Kint’ (red) | sport from ‘Mevrouw Gerard Kint’ y | |
| ‘Michelle Marie’ | seedling x ‘Rosali’ x | * |
| ‘Nordlicht’ | sport from ‘Helmutt Vogel’ x | * |
| ‘Otto’ | ‘Friedhelm Scherrer’ x seedling x | * |
| ‘Renato’ | derived from ‘Glaser Nummer 10’ x | |
| ‘Sachsenstern’ | Sport from unknown seedling x | * |
| ‘Tamira’ | Seedling with unknown parents x | * |
| ‘Thesla’ | Seedling ‘Sankt Valentin’ x ‘Mevrouw Gerard Kint’x | * |
| Genotype | Time Point (dpi) | Treatment | Area Analyte/Area Internal Standard | Relative Difference of SA Content | Level of Significance (p-Value) |
|---|---|---|---|---|---|
| ‘Nordlicht’ | 4 | Mock | 0.11 | 1.6 | 0.09 |
| P. latus infestation | 0.16 | ||||
| 6 | Mock | 0.08 | 10.4 | 0.01 | |
| P. latus infestation | 0.83 | ||||
| 8 | Mock | 0.10 | 6.3 | 0.09 | |
| P. latus infestation | 0.60 | ||||
| 11 | Mock | 0.12 | 18.7 | 0.01 | |
| P. latus infestation | 2.17 | ||||
| ‘Elien’ | 4 | Mock | 0.10 | 1.48 | 0.19 |
| P. latus infestation | 0.15 | ||||
| 6 | Mock | 0.16 | 1.21 | 0.69 | |
| P. latus infestation | 0.19 | ||||
| 8 | Mock | 0.15 | 0.99 | 1.00 | |
| P. latus infestation | 0.14 | ||||
| 11 | Mock | 0.13 | 1.62 | 0.31 | |
| P. latus infestation | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leus, L.; Luypaert, G.; Dhooghe, E.; Witters, J.; Pauwels, E.; Van Poucke, C.; Van Pamel, E.; Van Huylenbroeck, J.; Audenaert, J. Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus). Horticulturae 2022, 8, 840. https://doi.org/10.3390/horticulturae8090840
Leus L, Luypaert G, Dhooghe E, Witters J, Pauwels E, Van Poucke C, Van Pamel E, Van Huylenbroeck J, Audenaert J. Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus). Horticulturae. 2022; 8(9):840. https://doi.org/10.3390/horticulturae8090840
Chicago/Turabian StyleLeus, Leen, Gil Luypaert, Emmy Dhooghe, Johan Witters, Els Pauwels, Christof Van Poucke, Els Van Pamel, Johan Van Huylenbroeck, and Joachim Audenaert. 2022. "Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus)" Horticulturae 8, no. 9: 840. https://doi.org/10.3390/horticulturae8090840
APA StyleLeus, L., Luypaert, G., Dhooghe, E., Witters, J., Pauwels, E., Van Poucke, C., Van Pamel, E., Van Huylenbroeck, J., & Audenaert, J. (2022). Jasmonic Acid and Salicylic Acid Levels in Defense Response of Azalea (Rhododendron simsii Hybrid) to Broad Mite (Polyphagotarsonemus latus). Horticulturae, 8(9), 840. https://doi.org/10.3390/horticulturae8090840






