Use of Ethephon and Calcium Acetate to Manipulate the Foliage Retention Rates of Camphor and Golden Shower Trees
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Camphor
3.1.1. LRR
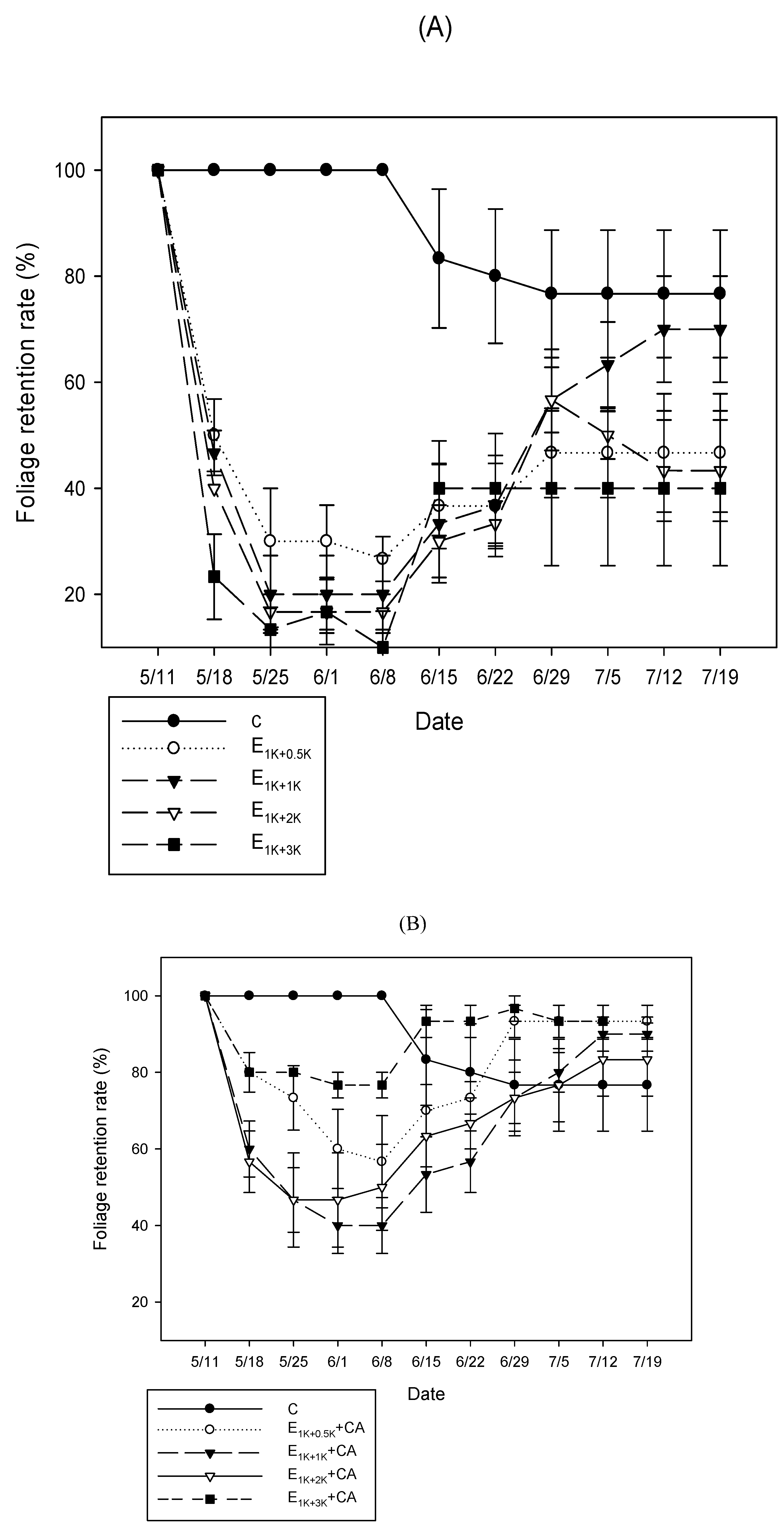
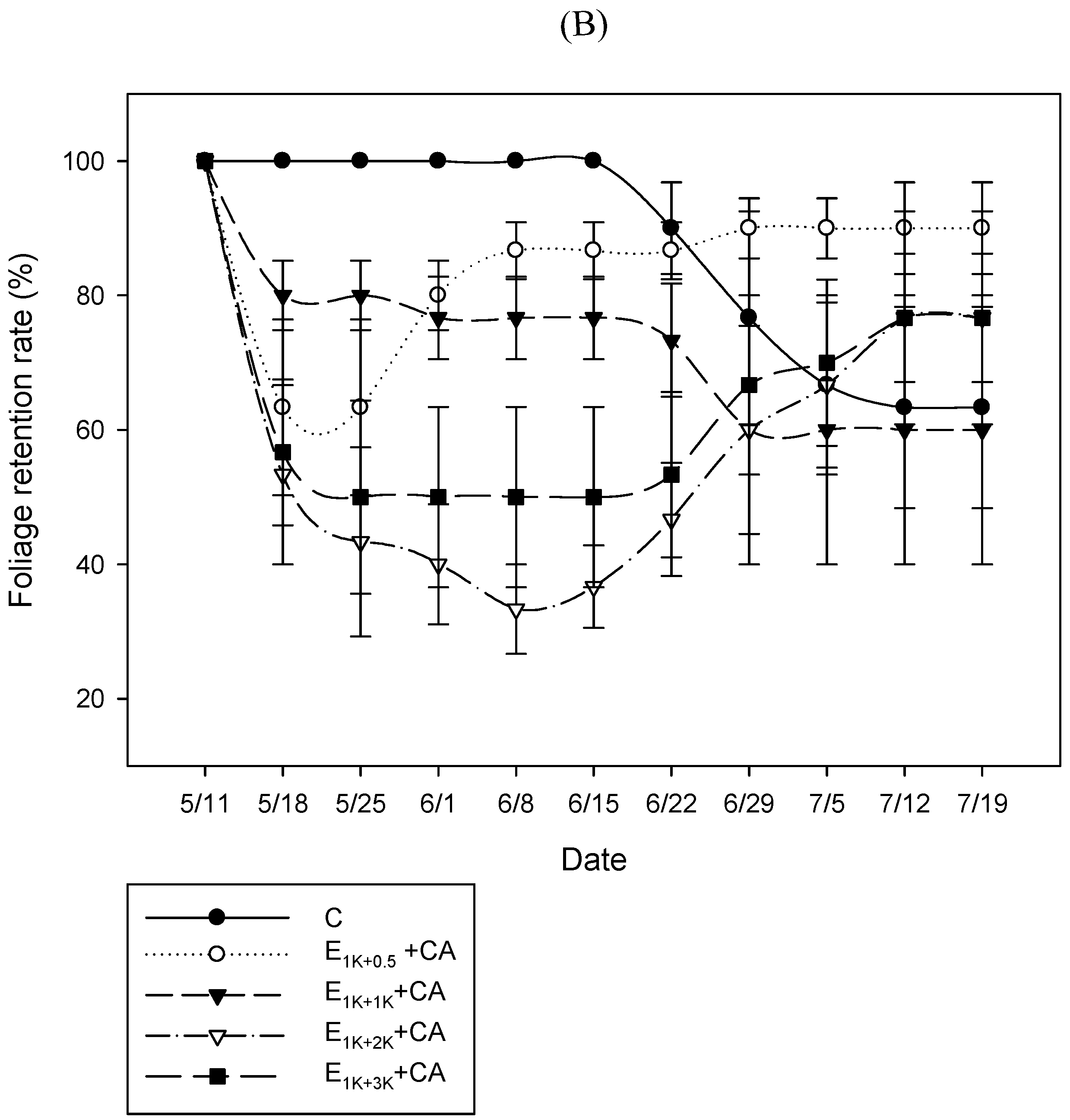
3.1.2. FRR
3.2. Golden Shower
3.2.1. LRR
3.2.2. FRR
3.3. Defoliation Pattern
3.3.1. Camphor
3.3.2. Golden Shower
4. Discussion
4.1. Defoliation Pattern
4.2. LRRs
4.3. FRRs
4.4. Two Sprays versus One Spray
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Levitt, L.K.; Stein, D.B.; Rubinstein, B. Promotion of stomatal opening by indole acetic acid and ethrel in epidermal strips of Vicia faba L. Plant Physiol. 1987, 85, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Allen, G.J.; Hugouvieux, V.; Kwak, J.M.; Waner, D. Guard cell signal transduction. Ann. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 627–658. [Google Scholar] [CrossRef] [PubMed]
- Randolf, W.S.; Wiest, S.C. Relative importance of tractable factors effecting the establishment of transplanted holly. J. Am. Soc. Sci. 1981, 106, 207–210. [Google Scholar]
- Ranney, T.G.; Bassuk, N.L.; Whitlow, T.H. Effect of transplanting practices on growth and water relations of ‘Colt’ cherry trees during reestablishment. J. Environ. Hort. 1989, 7, 41–45. [Google Scholar] [CrossRef]
- Gianfagna, T. Natural and synthetic growth regulators and their use in horticultural and agronomic crops. In Plant Hormones; Davies, P.J., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 751–773. [Google Scholar]
- Dunster, K.W.; Dunlap, R.L.; Gonzales, F.J. Influence of ETHREL plant regulator on boll opening and defoliation of western cotton. In Proceedings of the Plant Growth Regulator Working Group, Longmont, CO, USA, 13–17 July 1980; pp. 5–21. [Google Scholar]
- Abusrewil, G.S.; Larsen, F.E.; Fritts, R., Jr. Prestorage and poststorage starch levels in chemically and hand-defoliated “Delicious” apple nursery stock. J. Am. Soc. Hort. Sci. 1983, 108, 2–23. [Google Scholar] [CrossRef]
- Knight, J.N. Chemical defoliation of nursery stock using chelated forms of copper and iron. J. Hort. Sci. 1983, 58, 471–476. [Google Scholar] [CrossRef]
- Pedersen, M.K.; Burton, J.D.; Coble, H.D. Effect of cyclanilide, ethephon, auxin transport inhibitors, and temperature on whole plant defoliation. Crop. Sci. 2006, 46, 1666–1672. [Google Scholar] [CrossRef]
- Larsen, F.E.; Lowell, G.D. Tree fruit nursery stock defoliation with harvest aid chemical and surfactant mixtures. HortScience 1977, 12, 580–582. [Google Scholar]
- Dozier, W.A.; Gilliam, C.H., Jr.; Knowles, J.W. Chemical defoliation of fig nursery stock using ethephon, harvade, and D-WK surfactant. J. Environ. Hort. 1987, 5, 116–119. [Google Scholar] [CrossRef]
- Lqbal, N.; Khan, N.A.; Ferrante, A.; Trivellini, A.; Francini, A.; Khan, A.I.R. Ethylene role in plant growth development and senescence: Interaction with other phytohormone. Front. Plant Sci. 2017, 8, 475. [Google Scholar] [CrossRef]
- Crisosto, C.H.; Bremer, V.; Norton, M.; Ferguson, L.; Einhorn, T. Preharvest ethephon eliminates first crop figs. HortTech 2010, 20, 173–178. [Google Scholar] [CrossRef]
- Still, S.M. Defoliation of nursery stock for early harvest. Comb. Proc. Ann. Mtg. Intl. Plant Propag. Soc. 1976, 26, 255–259. [Google Scholar]
- Price, C.E. A review of factors influencing the penetration of pesticides through plant leaves. In The Plant Cuticle; Cutler, D.F., Price, C.E., Eds.; Linnaeus Society Symposium Series No. 10; Academic Press: London, UK, 1982; pp. 237–252. [Google Scholar]
- Wang, Y.; Hu, Y. Response to some herbicides of Pharbitis purpurea and Ipomoea cairica. Ecol. Sci. 2000, 19, 77–79. [Google Scholar]
- Burns, J.K.; Ferguson, L.; Glozer, K.; Krueger, W.H.; Rosecrance, R.C. Screening fruit loosening agents for black ripe processed table olives. HortScience 2008, 43, 1449–1453. [Google Scholar] [CrossRef]
- Lashbrook, C.C.; Klee, H.J. Ethylene regulation of abscission competence. In Biology and Biotechnology of the Plant Hormone Ethylene II; Springer: Dordrecht, The Netherlands, 1999; pp. 227–233. [Google Scholar]
- Dhall, R.K. Ethylene in post-harvest quality management of horticultural crops: A review. Res. Rev. J. Crop Sci. Technol. 2013, 2, 9–24. [Google Scholar]
- Sun, Z.Y.; Zhang, T.J.; Su, J.Q.; Chow, W.S.; Liu, J.Q.; Chen, L.L.; Li, W.H.; Peng, S.L.; Peng, C.L. A novel role of ethephon in controlling the noxious weed Ipomoea cairica (Linn.). Sweet. Sci. Rep. 2015, 5, 11372. [Google Scholar] [CrossRef]
- Xin, F.; Zhao, J.; Zhou, Y.T.; Wang, G.B.; Han, X.Q.; Fu, W.; Deng, J.Z.; Lan, Y.B. Effects of dosage and spraying volume on cotton defoliants efficacy: A case study based on application of unmanned aerial vehicles. Agronomy 2018, 8, 85. [Google Scholar] [CrossRef]
- Beaudry, R.M.; Kays, S.J. Application of ethylene-releasing compounds in agriculture. In Plant Growth and Leaf-Applied Chemicals; CRC Press: Boca Raton, FL, USA, 1988; pp. 127–155. [Google Scholar]
- Stewart, A.M.; Edmisten, K.L.; Wells, R. Boll openers in cotton: Effectiveness and environmental influences. Field Crops Res. 2000, 67, 83–90. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; Rasmussen, H.P. Calcium distribution in the abscission zone of bean leaves: Electron microprobe X-ray analysis. Plant Physiol. 1973, 52, 683–684. [Google Scholar] [CrossRef]
- Martin, G.C.; Campbell, R.C.; Carlson, R.M. Effect of calcium in offsetting defoliation induced by ethephon in pecan. J. Am. Soc. Hort. Sci. 1980, 105, 34–37. [Google Scholar]
- Iwahori, S.; Oohata, J.T. Alleviative effects of calcium acetate on defoliation and fruit drop induced by 2-chloroethylphosphonic acid in citrus. Sci. Hort. 1980, 12, 265–271. [Google Scholar] [CrossRef]
- Sterrett, J.P.; Leather, G.R.; Tozer, W.E. Defoliation response of woody seedlings to endothall/ethephon. Hortscience 1973, 8, 387–388. [Google Scholar]
- Smith, E.; Whiting, M. Effect of ethephon on sweet cherry pedicel-fruit retention force and quality is cultivar dependent. Plant Growth Regulat. 2010, 60, 213–223. [Google Scholar] [CrossRef]
- Tyree, M.T.; Ewers, F.W. The hydraulic architecture of trees and other woody plants. New Phytol. 1991, 119, 345–360. [Google Scholar] [CrossRef]
- Li, X.; Blackman, C.J.; Rymer, P.D.; Quintans, D.; Duursma, R.A.; Choat, B.; Medlyn, B.E.; Tissue, D.T. Xylem embolism measured retrospectively is linked to canopy dieback in natural populations of Eucalyptus piperita following drought. Tree Physiol. 2018, 38, 1193–1199. [Google Scholar] [CrossRef]
- Zimmermann, M.H. Pathology of the xylem. In Xylem Structure and the Ascent of sap. Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Tyree, M.T.; Alexander, J.D. Hydraulic conductivity of branch junctions in three temperate tree species. Trees 1993, 7, 156–159. [Google Scholar] [CrossRef]
- Castle, W.S. Antitranspirants and root and canopy pruning effects on mechanically transplanted eight-year-old “Murcott” citrus trees. J. Am. Soc. Hort. Sci. 1983, 108, 981–985. [Google Scholar] [CrossRef]
- Harris, R.; Bassuk, N. Effects of defoliation and antitranspirants treatments on transplant response of scarlet oak, green ash and Turkish hazelnut. J. Arboricult. 1995, 21, 33–36. [Google Scholar] [CrossRef]



| Treatment | First Spray | Second Spray |
|---|---|---|
| C | Water | Water |
| E1K+0.5K z | 1000 mg·L−1 ethephon | 500 mg·L−1 ethephon |
| E1K+1K | 1000 mg·L−1 ethephon | 1000 mg·L−1 ethephon |
| E1K+2K | 1000 mg·L−1 ethephon | 2000 mg·L−1 ethephon |
| E1K+3K | 1000 mg·L−1 ethephon | 3000 mg·L−1 ethephon |
| E1K+0.5K + CA y | 1000 mg·L−1 ethephon | 500 mg·L−1 ethephon + CA |
| E1K+1K + CA | 1000 mg·L−1 ethephon | 1000 mg·L−1 ethephon + CA |
| E1K+2K + CA | 1000 mg·L−1 ethephon | 2000 mg·L−1 ethephon + CA |
| E1K+3K + CA | 1000 mg·L−1 ethephon | 3000 mg·L−1 ethephon + CA |
| Camphor | Golden Shower | |||
|---|---|---|---|---|
| LRR | FRR | LRR | FRR | |
| ethephon treatment Z | ||||
| C (water) | 77 a | 77 a | 63 a | 63 bc |
| E1K+0.5K | 42 a | 70 a | 65 a | 95 a |
| E1K+1K | 30 b | 80 a | 43 ab | 47 c |
| E1K+2K | 32 b | 63 a | 23 b | 75 ab |
| E1K+3K | 43 b | 67 a | 33 b | 88 ab |
| without CA | 30 b | 55 b | 37 b | 74 a |
| with CA | 59 a | 87 a | 54 a | 73 a |
| Significance | ||||
| Ethephon | *** | ns | ** | ** |
| CA | *** | *** | * | ns |
| Ethephon × CA | ** | *** | * | ns |
| Camphor | Golden Shower | |||
|---|---|---|---|---|
| Treatments z | LRR | FRR | LRR | FRR |
| C (water) | 77% a | 77% a | 63% ab | 63% bc |
| E1K+0.5K | 27% cd | 47% bc | 67% a | 100% a |
| E1K+1K | 20% de | 70% ab | 27% cd | 33% c |
| E1K+2K | 17% de | 43% bc | 13% d | 73% ab |
| E1K+3K | 10% e | 40% c | 17% d | 100% a |
| E1K+0.5K + CA | 57% ab | 93% a | 63% ab | 90% ab |
| E1K+1K + CA | 40% bcd | 90% a | 60% ab | 60% bc |
| E1K+2K + CA | 47% bc | 83% a | 33% bcd | 77% ab |
| E1K+3K + CA | 77% a | 93% a | 50% abc | 77% ab |
| Significance | *** | *** | ** | ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, N.; Lo, K.-C.; Chang, Y.-S. Use of Ethephon and Calcium Acetate to Manipulate the Foliage Retention Rates of Camphor and Golden Shower Trees. Horticulturae 2022, 8, 760. https://doi.org/10.3390/horticulturae8090760
Li N, Lo K-C, Chang Y-S. Use of Ethephon and Calcium Acetate to Manipulate the Foliage Retention Rates of Camphor and Golden Shower Trees. Horticulturae. 2022; 8(9):760. https://doi.org/10.3390/horticulturae8090760
Chicago/Turabian StyleLi, Nelson, Kuo-Chin Lo, and Yu-Sen Chang. 2022. "Use of Ethephon and Calcium Acetate to Manipulate the Foliage Retention Rates of Camphor and Golden Shower Trees" Horticulturae 8, no. 9: 760. https://doi.org/10.3390/horticulturae8090760
APA StyleLi, N., Lo, K.-C., & Chang, Y.-S. (2022). Use of Ethephon and Calcium Acetate to Manipulate the Foliage Retention Rates of Camphor and Golden Shower Trees. Horticulturae, 8(9), 760. https://doi.org/10.3390/horticulturae8090760







