In Search of Antifungals from the Plant World: The Potential of Saponins and Brassica Species against Verticillium dahliae Kleb.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sources
2.2. Extraction, Purification, and Characterization of Saponins from Medicago spp.
2.3. Extraction and Characterization of Saponins from Avena Sativa Seeds
2.4. Brassica Sprouts Juices Preparation
2.5. Antifungal Activity In Vitro Test
2.6. Phytotoxicity Tests
2.7. Statistical Analysis
3. Results
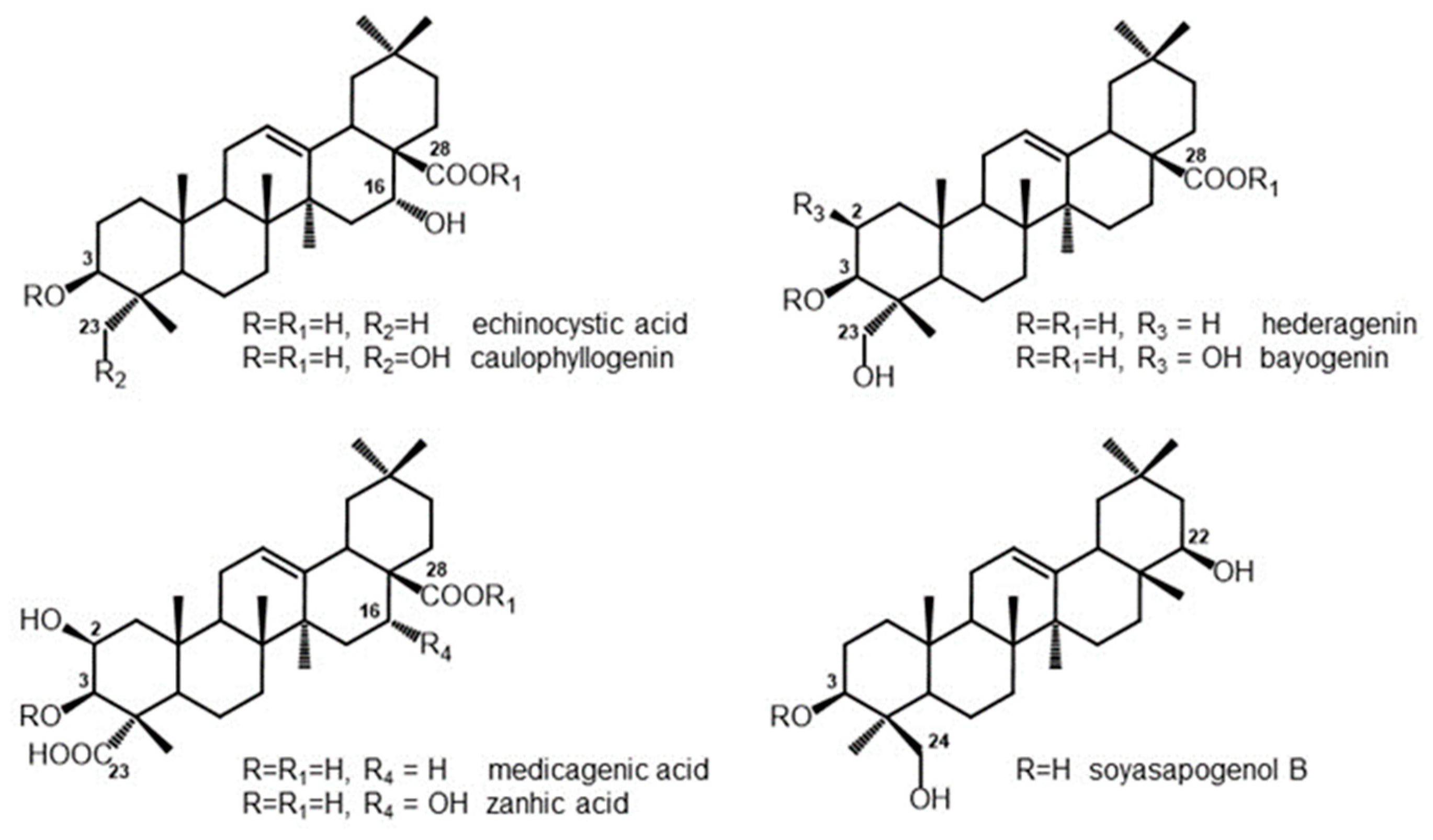
3.1. Composition of Saponins and Related Sapogenins from Medicago spp.
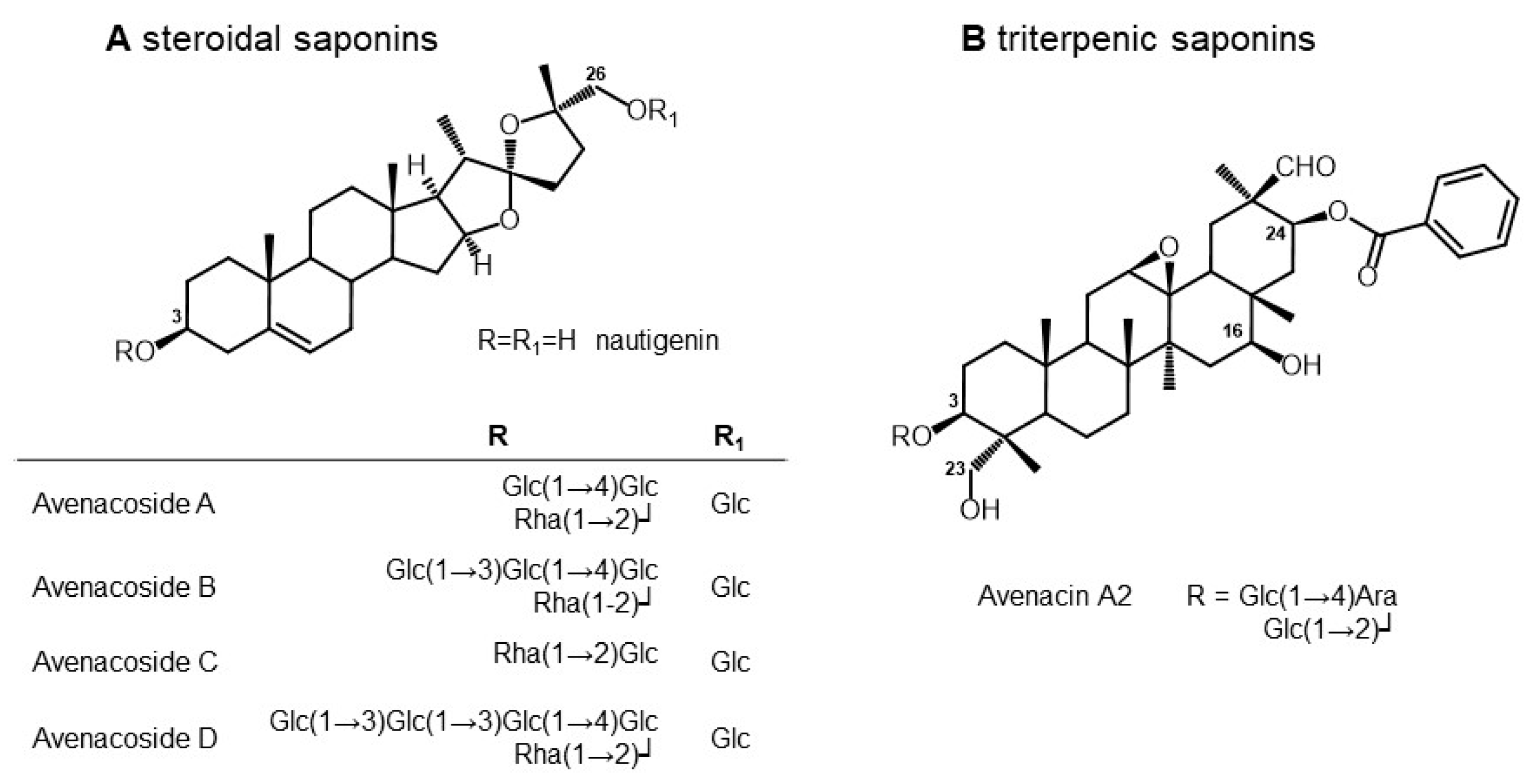
3.2. Composition of Saponins from Oat Seeds
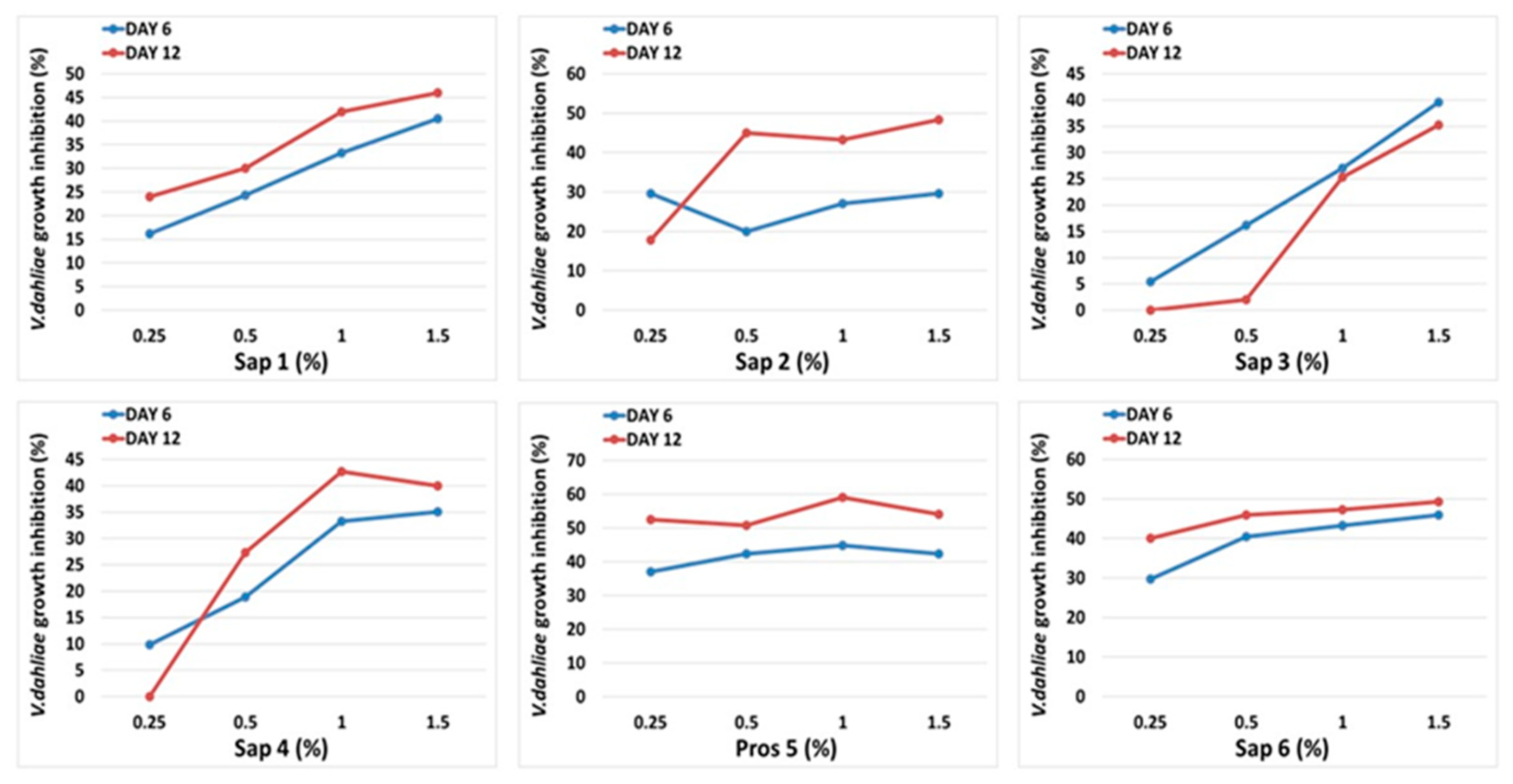
3.3. Medicago Saponins Antifungal Activity
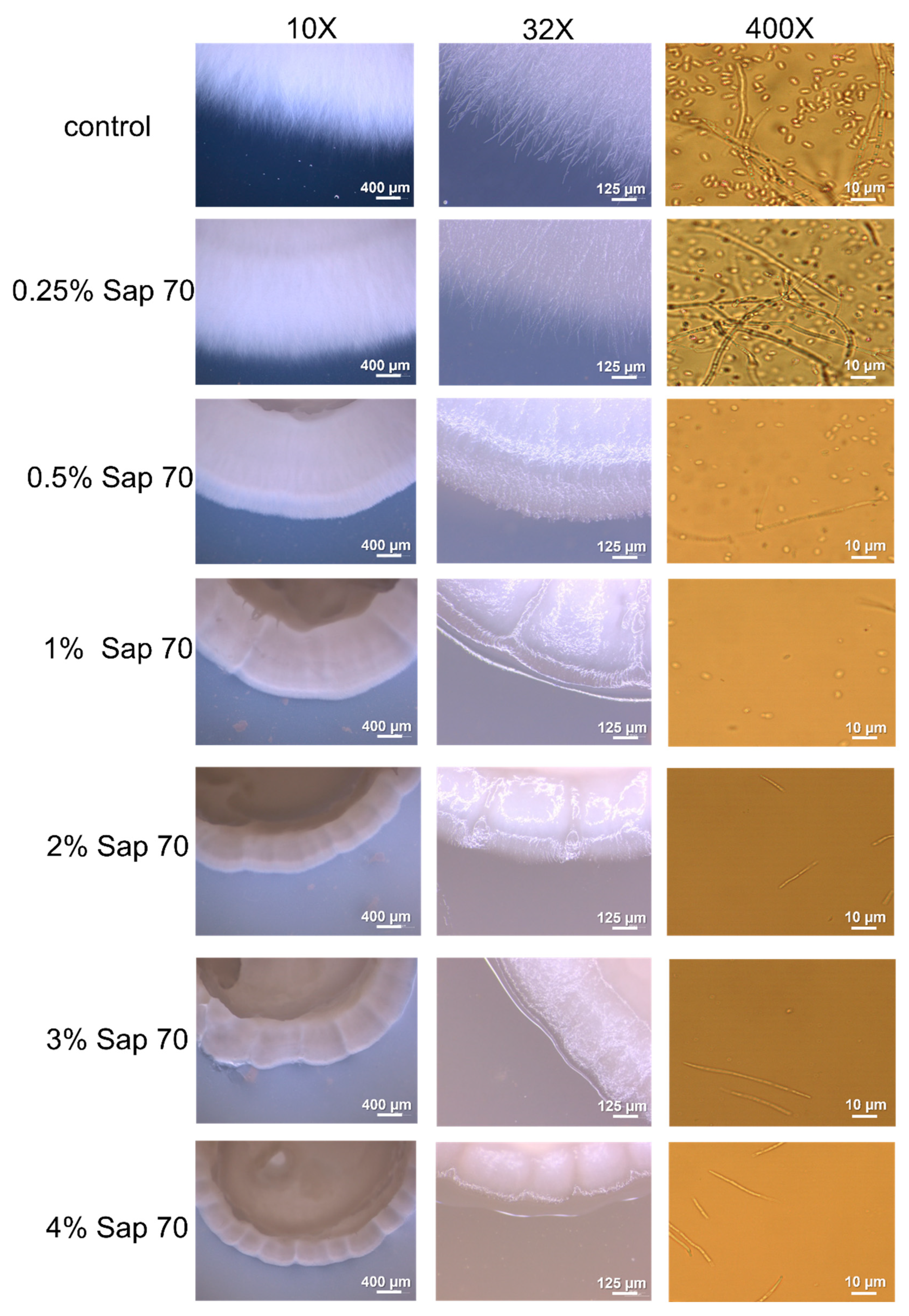
3.4. Oat Saponins Antifungal Activity
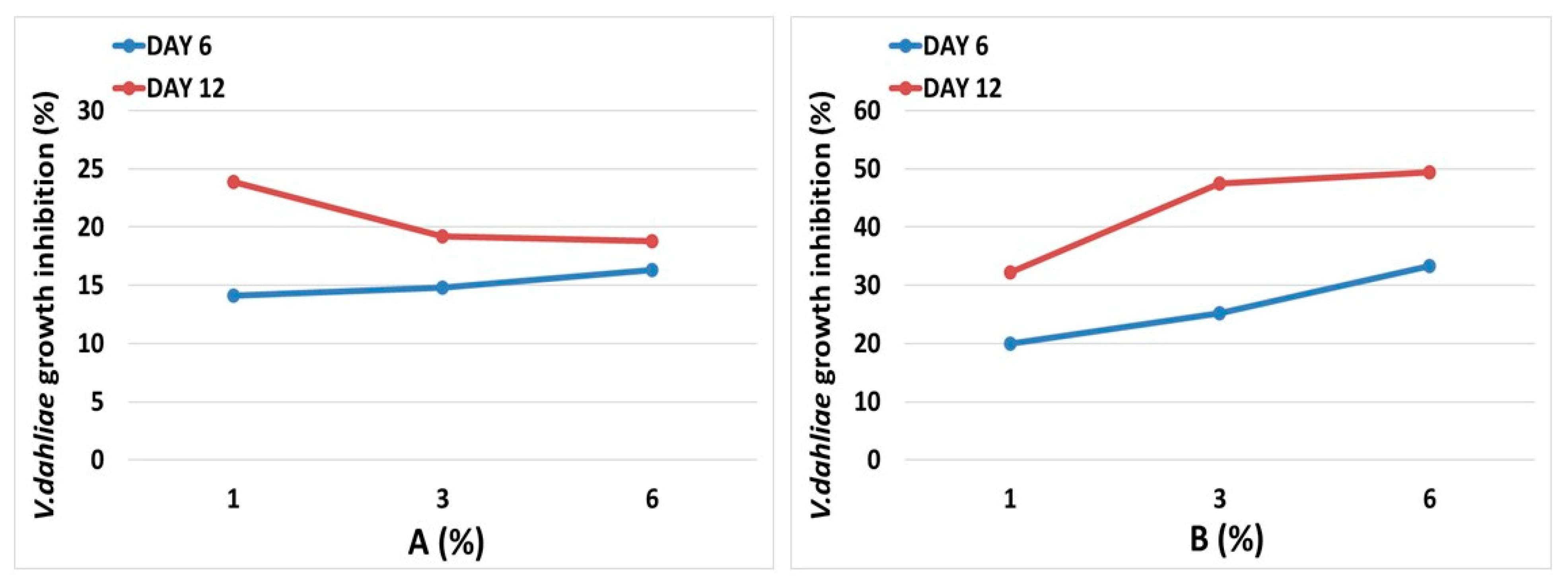
3.5. Brassica Sprouts Juices Antifungal Activity
3.6. Saponins Phytotoxicity
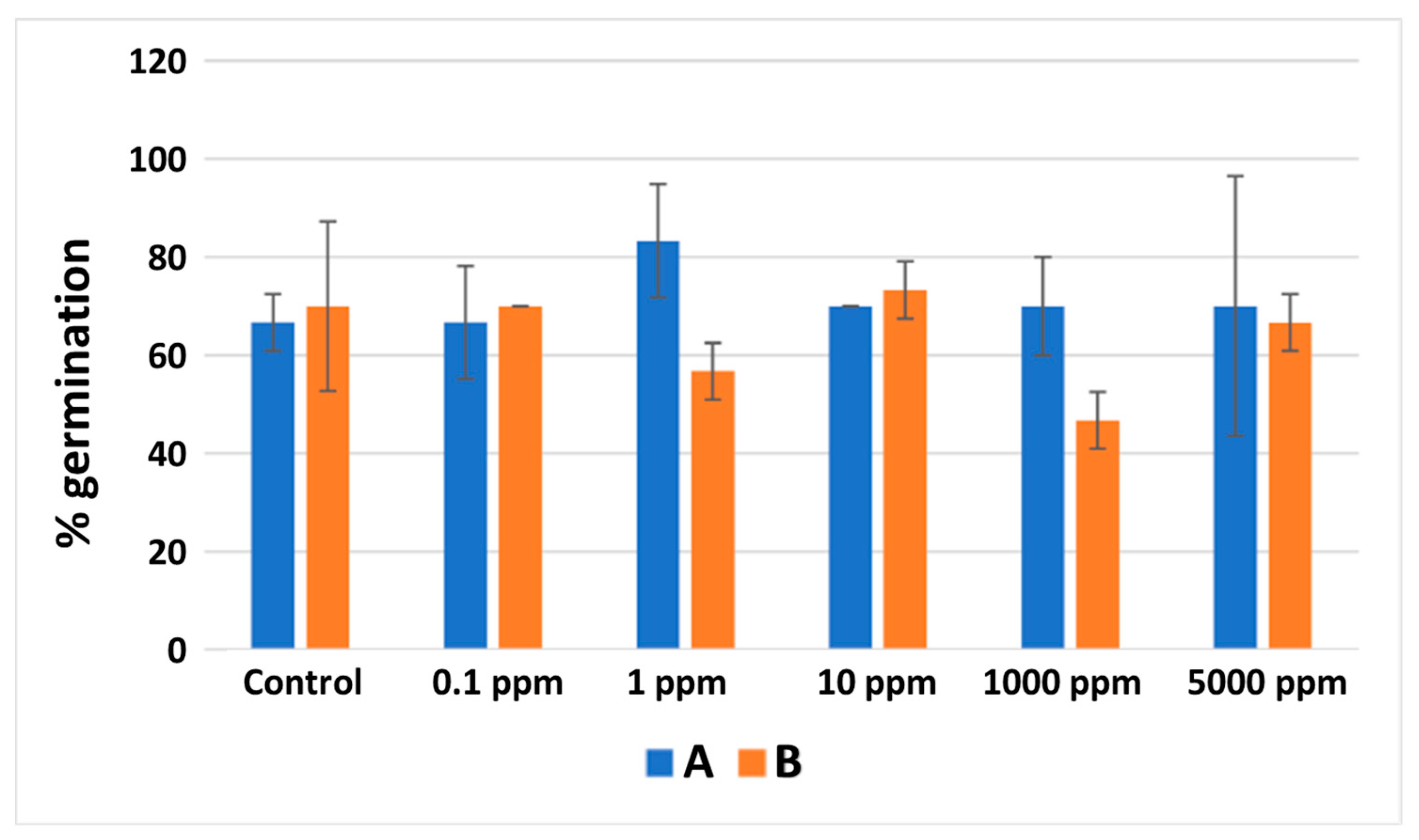
3.7. Brassica Sprouts Juices Phytotoxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The EU Green Deal–A Roadmap to Sustainable Economies 2019. Available online: https://www.switchtogreen.eu/the-eu-green-deal-promoting-a-green-notable-circular-economy/ (accessed on 7 July 2022).
- Alabouvette, C.; Olivain, C.; Steinberg, C. Biological control of plant diseases: The European situation. Eur. J. Plant Pathol. 2006, 114, 329–341. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. In Application and Characterization of Surfactants; Najjar, R., Ed.; InTech: Rijeka, Croatia, 2017; pp. 184–205. [Google Scholar]
- Tava, A.; Pecetti, L. Chemical Investigation of Saponins from Twelve Annual Medicago Species and their Bioassay with the Brine Shrimp Artemia salina. Nat. Prod. Commun. 2012, 7, 837–840. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, H.; Carbajal, Y.; Gonzales, M.; Vásquez, V.; López, A. Determinación de la actividad insecticida de la saponina de la quinua (Chenopodium quinoa) en larvas de Drosophila melanogaster. Sci. Agropecu. 2019, 10, 39–45. [Google Scholar] [CrossRef]
- Zaynab, M.; Sharif, Y.; Abbas, S.; Afzal, M.Z.; Qasim, M.; Khalofah, A.; Ansari, M.J.; Khan, K.A.; Tao, L.; Li, S. Saponin toxicity as key player in plant defense against pathogens. Toxicon 2021, 193, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Acharya-Siwakoti, E.; Kafle, A.; Devkota, H.P.; Bhattarai, A. Plant-Derived Saponins: A Review of Their Surfactant Properties and Applications. Sci 2021, 3, 44. [Google Scholar] [CrossRef]
- Osbourn, A.E. Saponins in cereals. Phytochemistry 2003, 62, 1–4. [Google Scholar] [CrossRef]
- Sparg, S.G.; Luce, M.E.; Van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Blažević, I.; Montaut, S.; Burčul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Possenti, M.; Baima, S.; Raffo, A.; Durazzo, A.; Giusti, A.M.; Natella, F. Glucosinolates in food. In Glucosinolates, Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–132. [Google Scholar]
- Ilahy, R.; Tlili, I.; Pék, Z.; Montefusco, A.; Siddiqui, M.W.; Homa, F.; Hdider, C.; R’Him, T.; Lajos, H.; Lenucci, M.S. Pre- and post-harvest factors affecting glucosinolate content in broccoli. Front. Nutr. 2020, 7, 147. [Google Scholar] [CrossRef]
- Jahangir, M.; Abdel-Farid, I.B.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Healthy and unhealthy plants: The effect of stress on the metabolism of Brassicaceae. Environ. Exp. Bot. 2009, 67, 23–33. [Google Scholar] [CrossRef]
- Baenas, N.; Garcia-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Effect of sucrose and mannitol on the accumulation of health-promoting compounds and the activity of metabolic enzymes in broccoli sprouts. Sci. Hortic. 2011, 128, 159–165. [Google Scholar] [CrossRef]
- Natella, F.; Maldini, M.; Nardini, M.; Azzini, E.; Foddai, M.S.; Giusti, A.M.; Baima, S.; Morelli, G.; Scaccini, C. Improvement of the nutraceutical quality of broccoli sprouts by elicitation. Food Chem. 2016, 201, 101–109. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical improvement increases the protective activity of broccoli sprout juice in a human intestinal cell model of gut inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Mattioli, R.; Costantino, P.; Baima, S.; Morelli, G.; Punzi, P.; Giordano, C.; Pinto, A.; Donini, L.M.; d’Erme, M.; et al. Neuroprotective effect of Brassica oleracea sprouts crude juice in a cellular model of alzheimer’s disease. Oxid. Med. Cell. Longev. 2015, 2015, 781938. [Google Scholar] [CrossRef]
- Rubattu, S.; Di Castro, S.; Cotugno, M.; Bianchi, F.; Mattioli, R.; Baima, S.; Stanzione, R.; Madonna, M.; Bozzao, C.; Marchitti, S.; et al. Protective effects of Brassica oleracea sprouts extract toward renal damage in high-salt-fed SHRSP: Role of AMPK/PPARα/UCP2 axis. J. Hypertens. 2015, 33, 1465–1479. [Google Scholar] [CrossRef]
- Rubattu, S.; Stanzione, R.; Bianchi, F.; Cotugno, M.; Forte, M.; Della Ragione, F.; Fioriniello, S.; D’Esposito, M.; Marchitti, S.; Madonna, M.; et al. Reduced brain UCP2 expression mediated by microRNA-503 contributes to increased stroke susceptibility in the high-salt fed stroke-prone spontaneously hypertensive rat. Cell Death Dis. 2017, 8, 2891. [Google Scholar] [CrossRef]
- Hu, X.; Puri, K.D.; Gurung, S.; Klosterman, S.J.; Wallis, C.M.; Britton, M.; Durbin-Johnson, B.; Phinney, B.; Salemi, M.; Short, D.P.G.; et al. Proteome and metabolome analyses reveal differential responses in tomato-Verticillium dahliae-interactions. J. Proteom. 2019, 207, 103449. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, K. Verticillium dahliae (Verticillium wilt). In Invasive Species Compendium; CABI: Wallingford, UK, 2020. [Google Scholar] [CrossRef]
- Tava, A.; Mella, M.; Avato, P.; Argentieri, M.P.; Bialy, Z.; Jurzysta, M. Triterpenoid glycosides from the leaves of Medicago arborea L. J. Agric. Food Chem. 2005, 53, 9954–9965. [Google Scholar] [CrossRef] [PubMed]
- Tava, A.; Pecetti, L.; Romani, M.; Mella, M.; Avato, P. Triterpenoid glycosides from the leaves of two cultivars of Medicago polymorpha L. J. Agric. Food Chem. 2011, 59, 6142–6149. [Google Scholar] [CrossRef]
- Tava, A.; Avato, P. Chemical and biological activity of triterpene saponins from Medicago species. Nat. Prod. Commun. 2006, 1, 1159–1180. [Google Scholar] [CrossRef]
- Tava, A.; Biazzi, E.; Mella, M.; Quadrelli, P.; Avato, P. Artefact formation during acid hydrolysis of saponins from Medicago spp. Phytochemistry 2017, 238, 116–127. [Google Scholar] [CrossRef]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Crombie, W.M.L.; Crombie, L. Distribution of avenacins A-1, A-2, B-1 and B-2 in oat roots: Their fungicidal activity towards ‘‘take-all’’ fungus. Phytochemistry 1984, 25, 2069–2073. [Google Scholar]
- Hu, C.; Sang, S. Triterpenoid saponins in oat bran and their levels in commercial oat products. J. Agric. Food Chem. 2020, 68, 6381–6389. [Google Scholar] [CrossRef]
- Yang, J.; Wang, P.; Wu, W.; Zhao, Y.; Idehen, E.; Sang, S. Steroidal Saponins in Oat Bran. J. Agric. Food Chem. 2016, 64, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Tschesche, R.; Tauscher, M.; Fehlhaber, H.W.; Wulff, G. Avenacosid A, ein bisdesmosidisches Steroidsaponin aus Avena sativa. Chem. Ber. 1969, 102, 2072–2082. [Google Scholar] [CrossRef]
- Pecio, Ł.; Wawrzyniak-Szołkowska, A.; Oleszek, W.; Stochmal, A. Rapid analysis of avenacosides in grain and husks of oats by UPLC-TQ-MS. Food Chem. 2013, 141, 2300–2304. [Google Scholar] [CrossRef] [PubMed]
- Osbourn, A.; Goss, R.J.M.; Field, R.A. The saponins: Polar isoprenoids with important and diverse biological activities. Nat. Prod. Rep. 2011, 28, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Bucci, R.; Tava, A.; Vitali, C.; Rosato, A.; Bialy, Z.; Jurzysta, M. Antimicrobial activity of saponins from Medicago sp.: Structure activity relationship. Phytother. Res. 2006, 20, 454–457. [Google Scholar] [CrossRef]
- Abbruscato, P.; Tosi, S.; Crispino, L.; Biazzi, E.; Menin, B.; Picco, A.M.; Pecetti, L.; Avato, P.; Tava, A. Triterpenoid glycosides from Medicago sativa as antifungal agents against Pyricularia oryzae. J. Agric. Food Chem. 2014, 62, 11030–11036. [Google Scholar] [CrossRef]
- Raguindin, P.F.; Itodo, O.A.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A systematic review of phytochemicals in oat and buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Kemen, A.C.; Honkanen, S.; Melton, R.E.; Findlay, K.C.; Mugford, S.T.; Hayashi, K.; Haralampidis, K.; Rosser, S.J.; Osbourna, A. Investigation of triterpene synthesis and regulation in oats reveals a role for β-amyrin in determining root epidermal cell patterning. Proc. Natl. Acad. Sci. USA 2014, 111, 8679–8684. [Google Scholar] [CrossRef] [PubMed]
- Leveau, A.; Reed, J.; Qiao, X.; Stephenson, M.J.; Mugford, S.T.; Melton, R.E.; Rant, J.C.; Vickerstaff, R.; Langdon, T.; Osbourn, A. Towards take-all control: A C-21β oxidase required for acylation of triterpene defence compounds in oat. New Phytol. 2019, 221, 1544–1555. [Google Scholar] [CrossRef]
- Coleman, J.J.; Okoli, I.; Tegos, G.P.; Holson, E.B.; Wagner, F.F.; Hamblin, M.R.; Mylonakis, E. Characterization of plant-derived saponin natural products against Candida albicans. ACS Chem. Biol. 2010, 5, 321–332. [Google Scholar] [CrossRef]
- Wojciechowski, K.; Jurek, I.; Góral, I.; Campana, M.; Geue, T.; Gutberlet, T. Surface-active extracts from plants rich in saponins—Effect on lipid mono-and bilayers. Surf. Interfaces 2021, 27, 101486. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef]
- Schaefer, H.M.; Rentzsch, M.; Breuer, M. Anthocyanins reduce fungal growth in fruits. Nat. Prod. Commun. 2008, 3, 1267–1272. [Google Scholar] [CrossRef]
- Acharya, B.; Ingram, T.W.; Oh, Y.; Adhikari, T.B.; Dean, R.A.; Louws, F.J. Opportunities and challenges in studies of host-pathogen interactions and management of Verticillium dahliae in tomatoes. Plants 2020, 9, 1622. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, E.; Muzzalupo, R.; Chiappetta, A.; Muzzalupo, I. Control of the Verticillium Wilt on tomato plants by means of olive leaf extracts loaded on chitosan nanoparticles. Microorganisms 2022, 10, 136. [Google Scholar] [CrossRef] [PubMed]


| Plant Species | Plant Tissue | Main Compound | Code |
|---|---|---|---|
| Medicago arborea | leaves | saponins | Sap1 |
| Medicago polymorpha 22507 | leaves | Sap2 | |
| Medicago polymorpha 155004 | leaves | Sap3 | |
| Medicago sativa | leaves | Sap4 | |
| Medicago sativa | leaves | prosapogenins | Pros5 |
| Medicago sativa | roots | saponins | Sap6 |
| Avena sativa | seeds | saponins | Sap70 |
| Brassica oleracea | etiolated sprouts | Brassica homogenates | A |
| Brassica oleracea | purple sprouts | B |
| Echinocystic Acid | Caulophyllogenin | Hederagenin | Bayogenin | Medicagenic Acid | Zanhic Acid | Soyasapogenol B | ||
|---|---|---|---|---|---|---|---|---|
| M. arborea leaves | Sap1 | - | - | 0.1 | 3.5 | 27.5 | 45.8 | 10.1 |
| M. polymorpha 25570 leaves | Sap2 | 76.5 | 4.6 | 9.7 | 1.5 | - | - | 2.0 |
| M. polymorpha 15504 leaves | Sap3 | 0.3 | 0.1 | 85.9 | 1.2 | - | - | 2.3 |
| M. sativa leaves | Sap4 | - | - | 0.2 | 1.4 | 43.4 | 30.2 | 13.1 |
| M. sativa roots | Sap6 | - | - | 19.3 | 2.3 | 64.7 | 2.9 | 3.1 |
| n | tR | Molecular Formula | Monoisotopic Mass | [M-H]− (m/z) | Metabolite Annotation | Grade | MS2 (m/z) | % of Total Detected Saponins | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.09 | C54H80O21 | 1064.51918 | 1063.7 | Avenacin A2 | C | 16.5 | Crombie et al., 1984 [31] (oat roots); Hu and Sang (2020) (sprouted oat bran) [32] | |
| 2 | 10.29 | C63H102O33 | 1386.6303 | 1385.8 | Avenacoside D (isomer 1) | B | 1223.3 [M-H-Hexose]−; 1061.0 [M-H-2Hexose]− | 1.4 | Yang et al., 2016 [33] (oat bran) |
| 3 | 10.52 | C57H92O28 | 1224.57748 | 1223.7 | Isomer to Avenacoside B | B | 1077.4 [M-H-Rha]−; 1061.5 [M-H-Hex]−; 1043.3 [M-H-Hex-H2O]−; 915.3 [M-H-Hex-Rha]−; 899.4 [M-H-2Hex]− | 2.7 | Yang et al., 2016 [33] (oat bran) |
| 4 | 11.12 | C63H102O33 | 1386.6303 | 1385.8 | Avenacoside D (isomer 2) | B | 1223.3 [M-H-Hex]−; 1061.0 [M-H-2Hex]− | 7.1 | Yang et al., 2016 [33] (oat bran) |
| 5 | 11.34 | C57H92O28 | 1224.57748 | 1223.7 | Avenacoside B | C | 35.3 | Yang et al., 2016 [33] (oat bran) | |
| 6 | 11.57 | C51H82O23 | 1062.52466 | 1061.7 | Avenacoside A | A | 915.3 [M-H-Rha]−; 899.3 [M-H-Hex]−; 753.3 [M-H-Rha-Hex]−; 736.3 [M-H-2Hex]− | 28.9 | Tschesche et al. Chem Ber 1969 [34] (seeds and leaves) |
| 7 | 11.98 | C45H72O18 | 900.47184 | 899.6 | Avenacoside C | B | 753.0 [M-H-Rha]−; 737.3 [M-H-Hex]− | 6.4 | Pecio et al., 2013 [35] (seeds) |
| 8 | 12.52 | C45H72O18 | 900.47184 | 899.6 | Isomer to Avenacoside C | C | 1.9 | Pecio et al., 2013 [35] (seeds) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcia, C.; Piazza, I.; Ghizzoni, R.; Delbono, S.; Felici, B.; Baima, S.; Scossa, F.; Biazzi, E.; Tava, A.; Terzi, V.; et al. In Search of Antifungals from the Plant World: The Potential of Saponins and Brassica Species against Verticillium dahliae Kleb. Horticulturae 2022, 8, 729. https://doi.org/10.3390/horticulturae8080729
Morcia C, Piazza I, Ghizzoni R, Delbono S, Felici B, Baima S, Scossa F, Biazzi E, Tava A, Terzi V, et al. In Search of Antifungals from the Plant World: The Potential of Saponins and Brassica Species against Verticillium dahliae Kleb. Horticulturae. 2022; 8(8):729. https://doi.org/10.3390/horticulturae8080729
Chicago/Turabian StyleMorcia, Caterina, Isabella Piazza, Roberta Ghizzoni, Stefano Delbono, Barbara Felici, Simona Baima, Federico Scossa, Elisa Biazzi, Aldo Tava, Valeria Terzi, and et al. 2022. "In Search of Antifungals from the Plant World: The Potential of Saponins and Brassica Species against Verticillium dahliae Kleb." Horticulturae 8, no. 8: 729. https://doi.org/10.3390/horticulturae8080729
APA StyleMorcia, C., Piazza, I., Ghizzoni, R., Delbono, S., Felici, B., Baima, S., Scossa, F., Biazzi, E., Tava, A., Terzi, V., & Finocchiaro, F. (2022). In Search of Antifungals from the Plant World: The Potential of Saponins and Brassica Species against Verticillium dahliae Kleb. Horticulturae, 8(8), 729. https://doi.org/10.3390/horticulturae8080729







