Abstract
Edible coatings are an appropriate way to preserve the quality of horticultural crops and reduce post-harvest losses. In this study, treatments with proline (Pro), chitosan (CTS) and proline-coated chitosan nanoparticles (CTS-Pro NPs) to maintain quality and reduce the decay of strawberry fruit were examined during storage at 4 °C for 12 days. The strawberries were treated with Pro 1 and 5 mM, CTS at 0.1% (w/v), CTS-Pro NPs at 0.1% (w/v) and distilled water (control) at 20 °C for 5 min. Following 3, 6, 9 and 12 days of cold storage, the fruits were removed from refrigeration, and some traits were evaluated one day after storage under shelf-life conditions. The results indicated that the fruit coated with CTS and CTS-Pro NPs showed reduced malondialdehyde and hydrogen peroxide content and less decay and weight loss compared to control and proline. CTS-Pro NPs also preserved fruit quality by conserving higher levels of ascorbic acid, total soluble solids, total phenolic content, and antioxidant capacity and enzymes. These results confirmed the benefit of using chitosan and CTS-Pro NP coatings to maintain fruit quality and increase the shelf life of strawberries by enhancing their antioxidant system and their ability to eliminate free radicals under cold storage.
1. Introduction
The strawberry (Fragaria × ananassa) is a non-climacteric fruit rich in polyphenols and many bioactive compounds, including flavonoids, minerals and vitamins [1,2]. Strawberries are very perishable and have unusually demanding post-harvest handling requirements. The susceptibility of the fruit to deterioration and post-harvest diseases increases during storage, and as a result, biochemical and physiological characteristics change [3]. Different post-harvest treatments (spraying, coating, or dipping) contribute to maintaining quality [4].
Today, several methods to increase the shelf life of fresh strawberries have been investigated, such as modified atmosphere packaging [5], moisture absorbance [6], ethylene absorbance [7], chemical treatments [8,9], edible coatings [10] and radiation [11]. Edible films, or coatings, are an innovative, environmentally friendly, non-toxic method of preserving food. They create a physical barrier on the surface of vegetables and fruit to maintain post-harvest quality and increase shelf life [12]. Various studies have shown that the beneficial effects of edible coatings make them the preferred preservation choice [10,13,14]. Their main advantages are their lower respiration and tissue-softening ratio, longer post-harvest life, biodegradability and less microbial contamination [12]. Chitosan is one of the best materials for edible coatings. It is obtained from the chitin shells of shrimp and other crustaceans after deacetylation [15]. Chitosan coatings have been shown to reduce weight loss, firmness and decay and increase antioxidant activity and total soluble solids (TSS) in fresh fruit [16]. Its use as a composite together with nanomaterials has been found to be very useful for maintaining the quality and shelf life of post-harvest crops [17]. Chitosan as an edible coating in combination with aloe vera has been used successfully to delay the ripening of tomatoes for up to 42 days after harvest [18].
It has been shown that an edible chitosan coating maintained the post-harvest quality of strawberries [19,20], ber fruit [21], apples, tomatoes, cucumbers [22] and figs [16]. Dam et al. [23] found that the combined application of calcium gluconate and chitosan maintained the quality of strawberries for up to 10 days, while more than 60% of untreated fruit turned rotten. Quaternary chitosan films combined with carboxymethylcellulose (CMC) were used to increase the shelf life of bananas, which showed that films contained a high percentage of chitosan and postponed decay [24]. The chitosan treatment, in combination with nanosilicon, maintained the quality of jujubes by reducing the red index, respiration rate, decay percentage and weight loss compared to control during storage at room temperature [25]. Shi et al. [26] indicated that chitosan/nanosilica hybrid films significantly increased the shelf life of fresh longan fruit by reducing pericarp browning, weight loss and malondialdehyde (MDA) accumulation and preventing polyphenoloxidase activity while maintaining TSS, titratable acidity (TA) and ascorbic acid content.
Proline, an amino acid, is synthesized in plants via the glutamate pathway by the enzymes pyrroline-5-carboxylate (P5CS) and pyrroline-5-carboxylate reductase (P5CR) [27]. Recent studies showed that proline plays a crucial role not only in increasing cellular osmolarity and stabilizing membrane structures but also in alleviating oxidative damage caused by ROS and protecting proteins during abiotic stress [28,29]. Several studies have been performed on its enhanced ROS-scavenging enzyme activity, which reduces the effects of oxidative stress [30,31]. Mohammadrezakhani et al. [32] also showed that exogenous proline improves ROS-scavenging enzyme activity, including by ascorbate peroxidase (APX) and catalase (CAT).
Recently, the application of chitosan and nanochitosan coatings has gained much attention for extending the storage life of crops [17]. There are few studies on the use of nanostructured chitosan coatings with other compounds for the quality preservation of fruit during storage [23,33,34]. However, to the best of our knowledge, there is no literature on the impact of proline-coated chitosan nanoparticles (CTS-Pro NPs) on the post-harvest quality characteristics of strawberries. Thus, the objective of the present study was to evaluate the potential for proline (Pro), chitosan (CTS) and CTS-Pro NPs to control the decay of strawberry fruit by increasing shelf life and antioxidant enzyme activity as well as improving nutritional quality during low-temperature storage.
2. Materials and Methods
2.1. Chemical Materials
All chemicals, including CTS (molecular weight = 110 kDa, deacetylation degree = 84%, purity = 99%) sodium tripolyphosphate (TPP) (molecular weight = 367.847 g mol−1), and Pro (molecular weight = 115.13 g mol−1), were purchased from Sigma-Aldrich, USA.
2.2. Fruit Materials
The experiments were performed on strawberries (Fragaria × ananassa Duch. Cv. Camarosa), planted in 2020, in the strawberry greenhouse in the Zanjan county of Zanjan province, Iran. Healthy fruit with uniform shape and size and without visual defects or physical damage were harvested at commercial ripeness (3/4 of the surface showing red) and then transported to the laboratory. The strawberries were washed with tap water to remove external impurities and then drained for 10 min after washing and before coating. They were randomly allocated into five groups of 240 for each treatment in 3 replications (80 per replication). The strawberries were placed in a fabric mesh and dipped completely into Pro 1 and 5 mM, CTS at 0.1% (w/v), and CTS-Pro NPs at 0.1% (w/v) solutions and deionized water (control) at 20 °C for 5 min. They were then removed, drained and left on a filter paper for 30 min at room temperature (20 °C) to remove excess surface coating solution. Finally, the coated and control samples were placed in transparent folding PET boxes with 4 to 5 holes (7–8 mm) to maintain the composition of air within the container. Plastic containers were stored in the refrigerator at 4 °C with 90% relative humidity for 12 days. From each replication, 20 strawberries were randomly taken from cold storage. Tests were done on both the control and coated samples every three days (days 3, 6, 9 and 12) and then held at 20 °C for 24 h (shelf-life) and subjected to physiological and biochemical analysis.
2.3. Preparation of Coating Treatment Solutions
In this work, CTS biopolymer nanoparticles were used to load Pro. To prepare a clear solution of CTS, 0.1 g of low-molecular-weight CTS powder was added to 25 mL of a 1% acetic acid solution (w/v) and stirred at 70 °C for 2 h at 300 rpm. Pro (0.1 g) was dissolved in 15 mL of distilled water and added slowly to the CTS solution, then stirred vigorously for 1 h. The amount of tripolyphosphate (TPP) to cross-link the CTS nanocarrier was calculated based on the amount of CTS used. For this purpose, the ratio of TPP to CTS was 1:2.5, so 0.04 g of TPP was dissolved in 5 mL of distilled water and slowly added to the CTS solution. The addition of TPP resulted in cross-linking the CTS nanoparticles in the form of coagulation, which was continued by stirring the nanocarriers overnight and then rinsing with distilled water to remove unreacted material in the supernatant. Then, the nanocarriers that were prepared by freeze-drying were dried using a vacuum pump [35].
2.4. Measurement of Weight Loss
Fruit weight loss was measured using a digital scale to an accuracy of 0.01 g. The fruits were weighed before entering storage and on the sampling day according to the following formula:
Fruit weight loss (%) = weight before storage – weight on sampling stage/weight before storage × 100
2.5. Decay Incidence
The incidence of fruit rot was measured by dividing the number of rotten fruit by the total number of fruit per replication expressed as a percentage [36].
2.6. Firmness
Fruit firmness was determined by a hardness tester (FT011, Facchini srl, Alfonsine (Ra), Italy) with a 5 mm diameter plunger. Firmness was taken on each side of the strawberry, and the mean values were expressed in newtons (N).
2.7. Total Soluble Solids (TSS) and Titratable Acidity (TA)
TSS was measured using a digital refractometer (PAL-1; Atago Co, Tokyo, Japan). The fruits were homogenized using a blender, and a few drops of the strawberry filtrate were placed on the prism glass of the refractometer. The TSS content as a percentage on the Brix scale was assayed after 3, 6, 9 and 12 days.
TA content was analyzed by titration of 10 mL of fresh juice by 0.1 N NaOH. The endpoint of the titration occurred when the pH of the extract reached 8.1 and was expressed as citric acid percentage [37].
2.8. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Contents
MDA contents were measured according to Liu et al. [3]. Briefly, fresh fruit tissue (1 g) was extracted with 5 mL of 10% trichloroacetic acid (TCA) and centrifuged at 10,000 rpm for 15 min. Then, 2 mL of the supernatant was mixed with 2 mL of 10% TCA-containing 0.6 g thiobarbituric acid (TBA). The mixture was placed in a water bath at 100 °C for 20 min, then quickly cooled in an ice bath and centrifuged at 6000 rpm for 10 min. MDA concentration was evaluated according to the following formula:
where A532 is the absorbance at 532 nm, A600 is the absorbance at 600 nm, W is the sample weight, and V is the TCA volume.
MDA= (A532 − A600) × W × V/155 × 1000
To measure the concentration of hydrogen peroxide (H2O2), 1 g of fresh fruit tissue was homogenized with 5 mL of 1% trichloroacetic acid (TCA) solution and centrifuged for 15 min at 12,000 rpm. Then, 0.5 mL of the supernatant was mixed with 0.5 mL of 10 mmol potassium phosphate buffer (pH 7.0) and 1 mL of 1 mol potassium iodide (KI). The H2O2 concentration of the supernatant was evaluated by comparing its absorbance at 390 nm with a standard calibration curve [38].
2.9. Determination of Ascorbic Acid Content
The ascorbic acid content of the strawberry fruit was determined by KI titration [39]. The end of the titration occurred when the color of the fruit extract darkened to blue and the color remained stable for a few seconds. The volume of iodine solution in the KI was read, and then the amount of ascorbic acid was calculated from the standard curve and expressed as mg 100 g−1 [39].
2.10. Measurement of Total Phenolic, Flavonoids and Anthocyanin Content
Total phenolics, anthocyanin, antioxidant capacity and flavonoid content were measured according to the method of Pineli et al. [40]. To prepare the fruit extracts, 1 g of strawberry fruit tissue was homogenized with 10 mL of 80% methanol. The homogenates were then centrifuged at 10,000 rpm for 10 min at 4 °C.
To measure total phenolics, 100 μL of supernatant was added to 100 μL of 50% Folin–Ciocalteau reagent, and after 2 min, the reaction was stopped with 2 mL of 2% sodium carbonate. After adding sodium carbonate, the mixture was kept at room temperature for 30 min, and the absorbance was measured at 720 nm. Total phenolic content was expressed as mg of gallic acid per 100 g of fresh fruit weight.
The total anthocyanin (TAC) content was measured using the pH differential method. First, 200 μL of fruit extract was added to 1800 μL of potassium chloride (KCl) buffer (pH 1.0), and then the same amount was added to 1800 μL of sodium acetate (NaCH3COO) buffer, pH 4.5. The diluted solutions were allowed to stand for 15 min to equilibrate. Finally, the absorbance of each solution was measured at 510 and 700 nm. TAC was calculated in mg per 100 g of fresh weight.
To measure total flavonoids, 250 μL of fruit extract was mixed with 75 μL of 5% sodium nitrite (NaNO2) and 150 μL of 10% aluminum chloride (AlCl3). Then, 0.5 mL of 1 mM sodium hydroxide (NaOH) was added and finally adjusted to 2.5 mL with distilled water. Then absorption was measured at 507 nm with a spectrophotometer, and the result was expressed as mg of rutin per 100 g of fresh fruit weight.
2.11. Enzyme Assay
2.11.1. Extraction of Samples
All enzymes were extracted at 4 °C. The extraction of samples for analysis by homogenizing 1 g of fruit tissue in 3 mL of 50 mM potassium phosphate (KH2PO4) buffer (pH 7.8) containing 0.2 mM EDTA disodium salt (Na2EDTA) and 2% (w/v) polyvinyl-polypyrrolidone (PVP) was performed. The homogenate was centrifuged at 4 °C for 20 min at 12,000 rpm, and the supernatant was collected for enzyme activity measurements of catalase (CAT) and superoxide dismutase (SOD).
2.11.2. Superoxide Dismutase (SOD) Activity
For SOD enzyme activity evaluation, 2 mL of 67 mM phosphate buffer (pH 7.8), 200 μL EDTA, 100 μL of aqueous nitroblue tetrazolium (NBT), 50 μL of riboflavin and 50 μL of enzymatic extract were mixed in a test tube, which was exposed to light for 10 min. Absorbance was measured at 560 nm. One unit of SOD activity was defined as the amount of enzyme that provided 50% inhibition of NBT photoreduction under assay conditions and expressed as U g −1 of fresh weight [41].
2.11.3. CAT Activity
Catalase activity was determined according to the method by Ali et al. [42]: 100 µL of enzyme extract was added to 2 mL KH2PO4 buffer (pH 7.0) and 50 µL H2O2 to initiate the reaction. The blank was prepared according to the above steps and without samples. A decrease in H2O2 absorbance was recorded at a wavelength of 240 nm for 2 min and expressed as U g −1 of fresh weight.
2.12. Measurement of Antioxidant Capacity
Total antioxidant capacity was measured according to the method of Pineli et al. [40]. To prepare fruit extracts, 1 g of strawberry fruit tissue was homogenized with 10 mL of 80% methanol. Then, the homogenates were centrifuged at 10,000 rpm for 10 min at 4 °C. The antioxidant capacity was assessed using DPPH free radical scavenging activity. To perform the reaction, 50 μL of fruit sample extract was added to 950 μL of 0.1 mM DPPH reagent. The samples were incubated in the dark at 25 °C for 30 min, and then their absorption was measured at 517 nm in a spectrophotometer. The DPPH inhibition percentage was calculated according to the following formula:
DPPH scavenging capacity (%): Ac − As/Ac × 10.
Where Ac is the absorbance of the control, and As is the absorbance of the sample.
2.13. Statistical Analysis
The experiment was performed as a factorial experiment in a completely randomized design with 3 replications. Significant differences between mean values were compared using Duncan’s multiple range test at a level of 5%. Two-way analysis of variance (ANOVA) was performed using the SPSS statistical software package program version 18.0 (SPSS Inc., Chicago, IL, USA), and data were expressed as the mean ± standard error (SE).
3. Results and Discussion
3.1. Characterization of CTS-Pro NPs
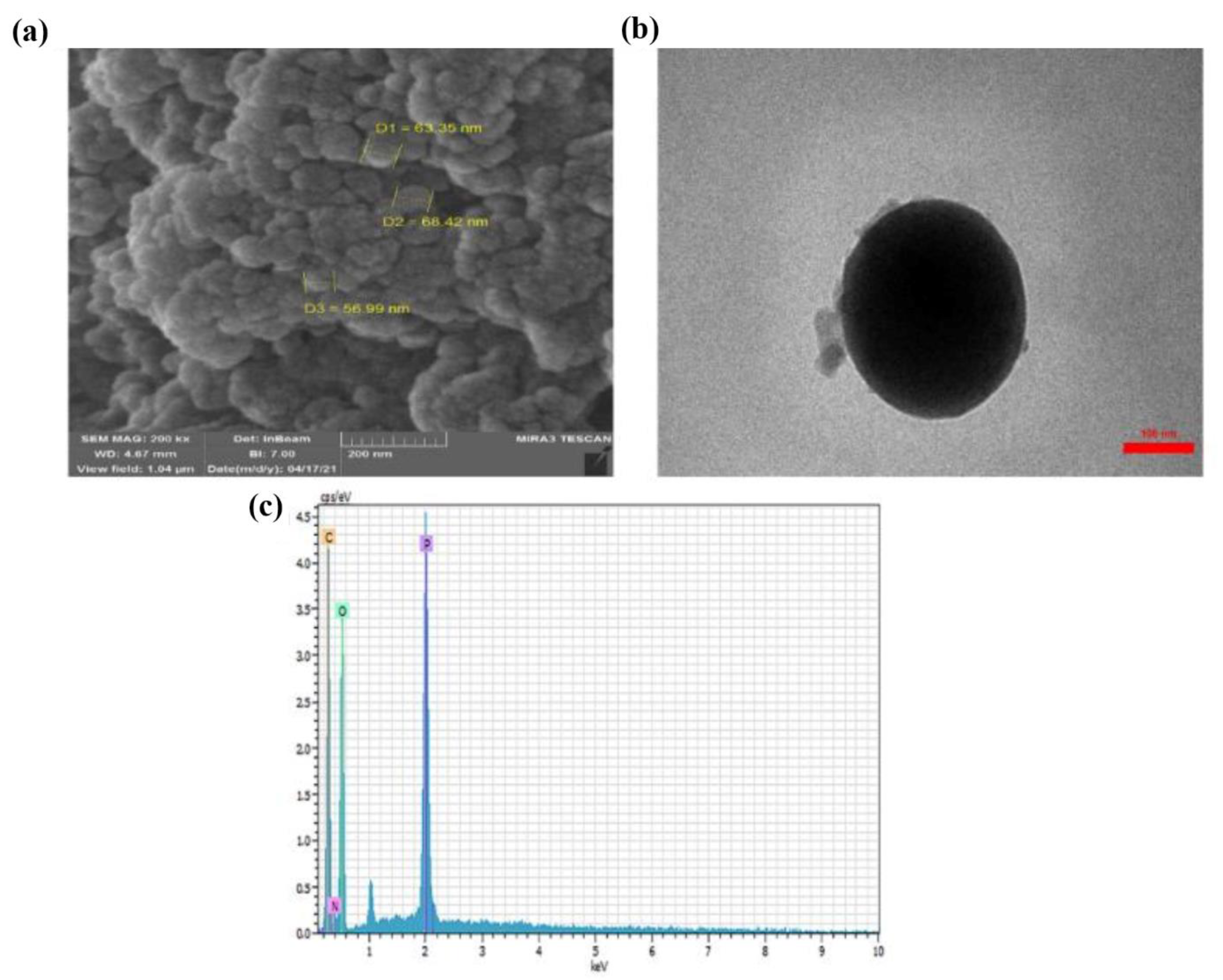
Using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images, the morphology of the CTS-Pro NPs is shown in Figure 1. The SEM image showed that the CTS-Pro surface had spherical nanoparticles with no porosity, which was observed with a suitable dispersion on the carrier surface. The TEM image also confirmed the spherical shape of the CTS-Pro NPs and estimated the size of the nanoparticles at 250 nm. According to energy-dispersive X-ray spectroscopy (EDS) analysis, the elements in the CTS-Pro NPs were identified. The EDS analysis showed peaks of C, O, N, and P in Figure 1c. Due to the elements in the CTS structure, the C, O, and N peaks confirmed the presence of CTS within the CTS-Pro NPs. In addition, the presence of peak P indicated an interaction between CTS and TPP.
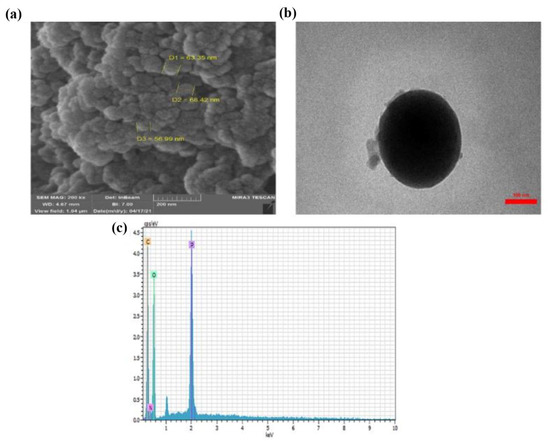
Figure 1.
SEM (a) TEM (b), and EDS (c) analysis of CTS-Pro NPs.
3.2. Weight Loss and Decay Percentage
The weight loss and percentage of rot are two important parameters in determining not only the post-harvest quality of strawberries but also the success of post-harvest storage. Weight loss during storage is the result of respiration and water loss caused by transpiration. Therefore, rapid water loss through the skin is among the main factors negatively affecting the shelf life of strawberries [43]. The results of the present study showed that the percentage of weight loss for the treated fruit was lower than for the control samples (Table 1). In all treatments, the percentage of weight loss significantly (p < 0.05) increased with storage time. After 12 days, the control treatment had the highest percentage of weight loss (16%), and strawberries treated with CTS had the lowest (8%). The 0.1% CTS and CTS-Pro NP coatings formed a smooth, semi-permeable layer on the fruit surface and served as a protective barrier to reduce transpiration [22].

Table 1.
Effect of post-harvest treatments of strawberries with Pro, CTS and CTS-P NPs on decay, weight loss, firmness, TSS, TA and ascorbic acid content during 12 days of storage at 4 °C. Data shown are mean values of n = 3.
The percentage of decayed fruit throughout the storage period is shown in Table 1. The first symptoms of fungal decay in control and 1 mM proline-treated fruit were detected after 6 days, then improved significantly with increasing storage time and attained the highest value after 12 days. At the end of storage, the highest percentage of fruit rot was related to control (57.5%), whereas the decay of the fruit treated with CTS and CTS-Pro NPs was only 15.83%. Our results showed that the CTS and CTS-Pro NP treatments reduced decay by preventing fungal growth on the fruit surface during the 12 days of storage. Previous reports confirmed the antimicrobial properties of chitosan, which had the potential to control the decay of some fruit, including strawberries, sweet cherries, table grapes [44], apples [45], mangos [46], and ber [21]. The low growth of bacteria and fungi in samples treated with nanocomposites and chitosan indicates that the growth rate of pathogens was lower in these treatments [47].
3.3. Firmness, TSS and TA
The change in the firmness of both control and treated fruit during storage is presented in Table 1. According to the results, with increasing storage time, the amount of fruit tissue firmness in all treatments was significantly reduced, but this decrease was significantly delayed by the CTS and CTS-Pro NP treatments. CTS and CTS-Pro NP treatments were more effective in preserving firmness than other treatments, although there was no significant difference between these treatments until day 12. Compared to control, all treatments significantly preserved fruit firmness until 12 d of storage (p < 0.05). Tissue firmness is one of the most important physical parameters used to evaluate the quality of fruit in the ripening and storage stages. Tissue softness is the result of changes in the cell wall structure, including reduction of hemicellulose, galactose and dissolution of pectin, and is the result of the activity of enzymes hydrolyzing the cell wall [48]. Studies have revealed that strawberry tissue softens because of metabolic changes and loss of moisture, which in turn diminishes firmness during storage [49]. In the present study, the retention of firmness in the strawberries coated with CTS and CTS-Pro NPs could be because of the selective permeability of the coating material to gas and water transfer, thus decreasing the respiration ratio and enzyme activities and most metabolic changes and postponing the ripening and over-softening of strawberries. Previous studies have reported the retardation of fruit softening in response to chitosan treatment in the Indian jujube [50], papayas [51], ber fruit [21] and strawberries [37,52].
As shown in Table 2, total soluble solids (TSS) at harvest time was 4.8%. The results displayed that both coating and storage time had a significant effect on the TSS of strawberries (Table 1). The TSS level increased significantly from the third to the sixth day of storage irrespective of treatments and then decreased slowly in both control and cover treatments until the end of the storage period. The concentrations of TSS were affected by Pro 1 mM compared with control. The initial rise in total soluble solids could be due to the conversion of starch to soluble sugar forms, and its subsequent reduction at the end storage could be attributed to the rapid use of reducing sugars and other organic metabolites [10]. In confirmation of the findings of this study, similar results have been reported for strawberries [10,53], berry fruit [21] and mangos [54]. A decrease in TSS content at the end of the experiment can be an important indicator of fruit ripening and senescence. The edible coating has been shown to reduce the rates of carbohydrate breakdown, thereby delaying maturation [55].

Table 2.
Qualitative attributes of the camarosa strawberry cultivar at harvest time.
As shown in Table 1, the interaction effect of coating and storage time on TA was significant (p < 0.05), and the amount decreased with increasing storage time. Strawberries coated with CTS and CTS-Pro NPs showed a delay in TA decrease. Organic acids can usually be considered a source of fruit energy, but during ripening, the increased ratio of respiration or the conversion of organic acids to sugar reduces their amount in fruit extract [56]. However, the edible coating reduced the loss of citric acid over the 12 days of storage by reducing oxygen diffusion and respiration rates, which caused citric acid retention [20]. In a previous study, treatment with chitosan and calcium chloride separately or in combination had no significant effect on the TA of the strawberries during 7 days of storage [37].
3.4. Ascorbic Acid Content
Ascorbic acid is very sensitive to decomposition compared to other nutrients during storage due to oxidation [57]. In the present study, the concentration of ascorbic acid decreased during storage in both control and treated fruit (Table 1). The results showed that after day 12, the highest content of ascorbic acid was related to the treatment of CTS-Pro NPs and CTS and the lowest was related to the control and Pro 5 mM treatments. The differences between CTS-Pro NPs and CTS-0.1% and control and Pro 5 mM were not significant. Ascorbic acid is a water-soluble vitamin that, as a non-enzymatic antioxidant, can reduce ROS and is involved in the detoxification of ROS, especially hydrogen peroxide [58]. Other researchers attributed the cause to the oxidation of ascorbic acid as an electron donor to oxidants to neutralize free radicals and ascorbic acid as an important natural antioxidant in fruit [59].
Coating the fruit with substances such as chitosan increases cytochrome oxidase activity by reducing the internal oxygen of the fruit, and this enzyme can greatly reduce the rate of decomposition of ascorbic acid [60]. Similar results of reduction in ascorbic acid concentration during storage of strawberry fruit were observed by Khodaei et al. [61] and Belal Abu Salha and Gedanken [62].
3.5. Hydrogen Peroxide (H2O2) and Malondialdehyde (MDA) Content
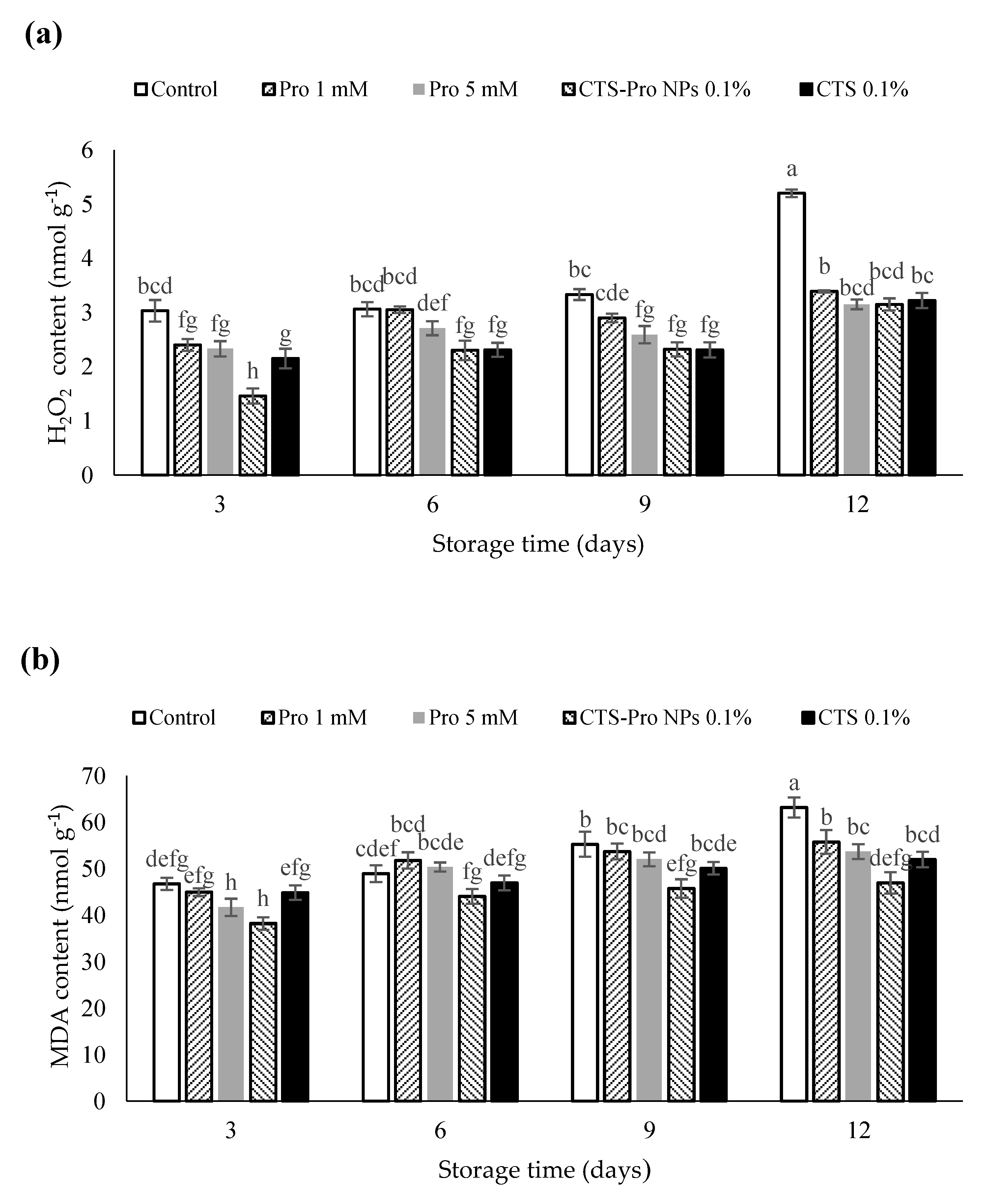
Hydrogen peroxide (H2O2) is a toxic compound produced in plants under oxidative stress and is a strong oxidizer. It increases during cold storage, along with free radicals and reactive oxygen species, when the low temperature causes a change in the fatty acids in the skin membrane [63]. The effect of different coatings on the H2O2 content of strawberries in different storage periods is presented in Figure 2a. The results showed it increased in all samples. However, the H2O2 in CTS- and CTS-Pro NP-coated fruit remained lower than that of control fruit during the 12 days (p < 0.05). The difference between the CTS and CTS-Pro NP coatings was not significant (p > 0.05).
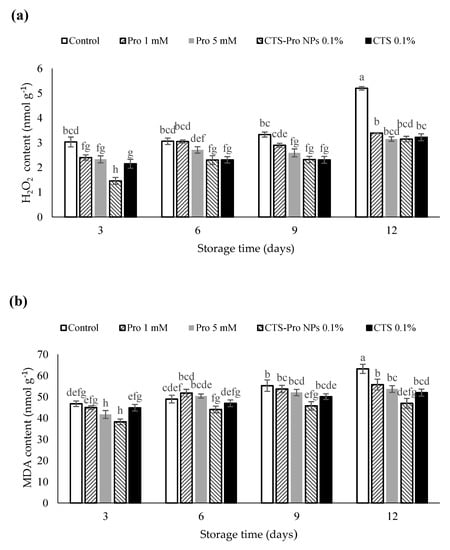
Figure 2.
Effect of post–harvest treatments of strawberries with Pro, CTS and CTS–P NPs on (a) H2O2 and (b) MDA accumulation during 12 days of storage at 4 °C. Data presented are mean ± standard error (SE) of three replications. Different letters (a–h) over bars indicate they are significantly different (p < 0.05) by Duncan’s test.
As shown in Figure 2b, the amount of MDA increased in all fruit with storage time. The control had significantly more, whereas the levels in CTS-Pro NP fruit were significantly lower (p < 0.05). MDA is a breakdown product of unsaturated fatty acids and hydroxides and is used as a suitable marker for lipid peroxide [64]. The CTS-Pro NP coating might have acted as a semi-permeable barrier against the O2 responsible for lipid peroxidation, thereby reducing oxidative damage during storage [20]. Chitosan and its nanocomposites reduce malondialdehyde accumulation in strawberries [53], guavas [65] and plums [66] by preserving membrane structure and reducing free radicals.
3.6. Total Phenolic Content
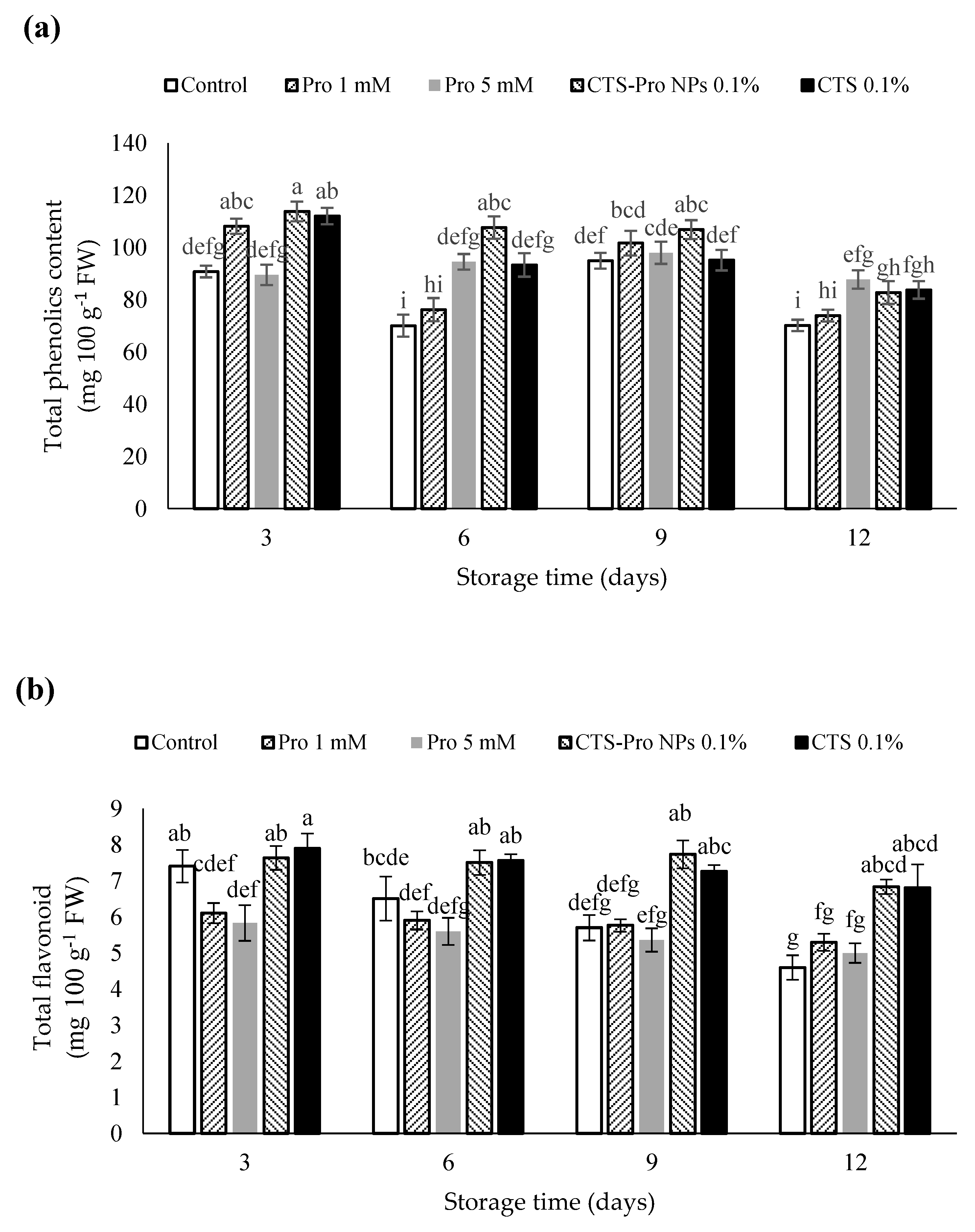
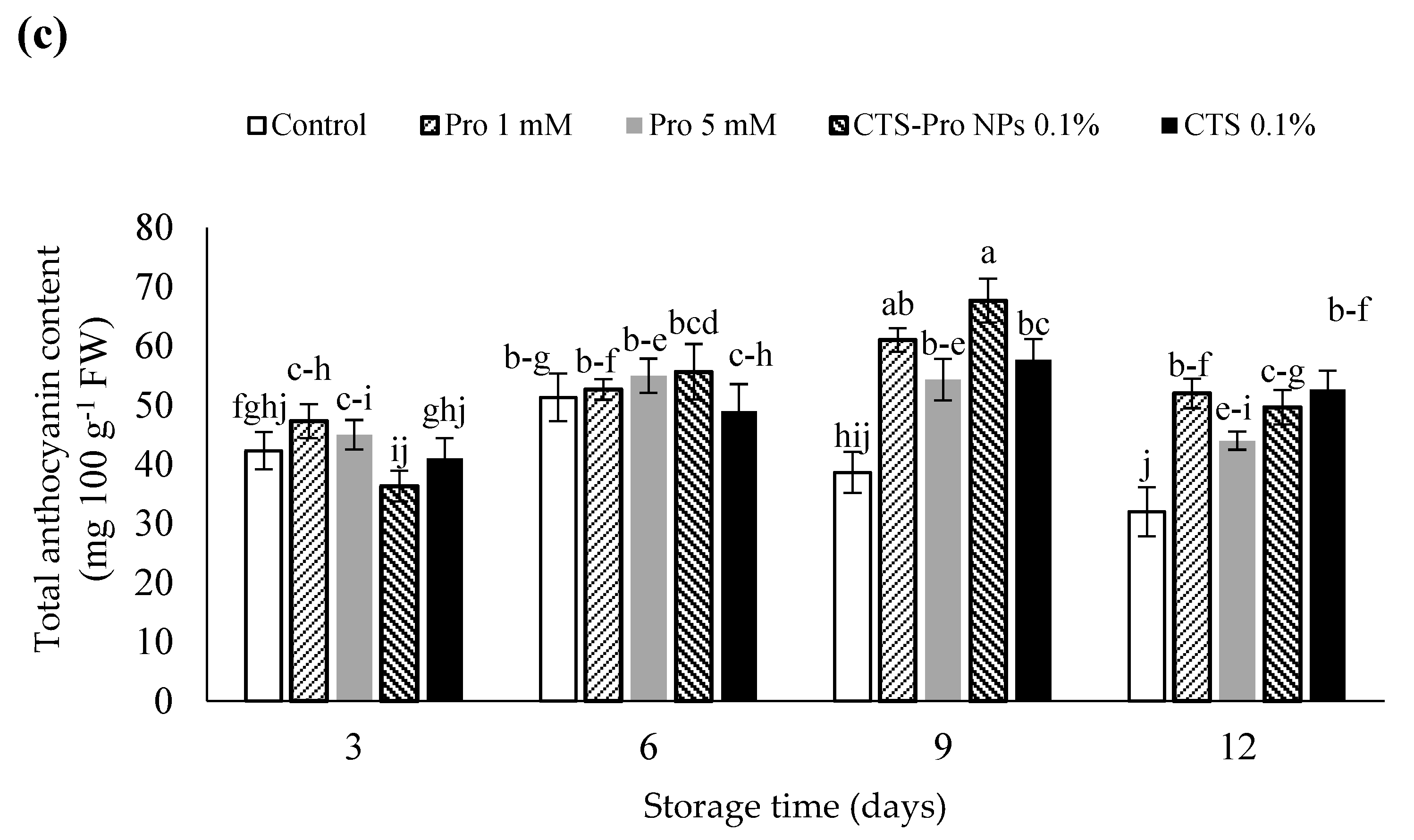
The effect of different treatments on the total phenolic content (TPC) of strawberries in different storage periods is presented in Figure 3a. The results showed that the amount gradually declined in all treatments. The coated strawberries had the highest total phenolic content compared to uncoated fruit. The highest total phenolic content was in fruit with CTS-Pro NPs, which was significantly different from the control treatment by the end of storage but not from any other treatment. However, the decrease in total phenolic accumulation may be the result of the destruction of the cellular structure during fruit senescence. The coatings protected the fruit by providing a barrier to O2 and a moisture supply for the enzymatic oxidation of phenolic compounds [67]. This finding was similar to that of Khodaei et al. [61], Khalifa et al. [68] and Gol et al. [67]. The phenolic compounds interacted with chitosan nanocomposite coatings through hydrogen, which caused the slow, regulated release of phenols into the environment [69]. Jongsri et al. [70] reported that chitosan-coated mangoes showed higher phenol content during storage.
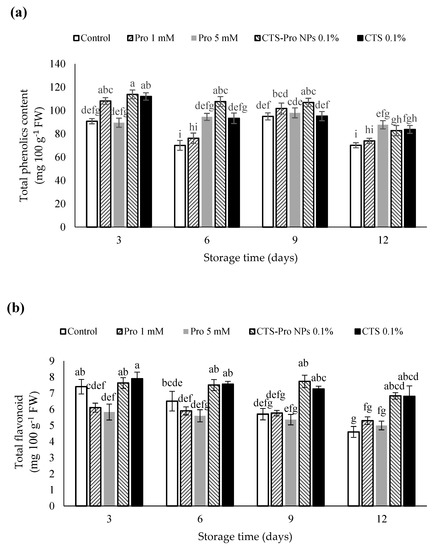
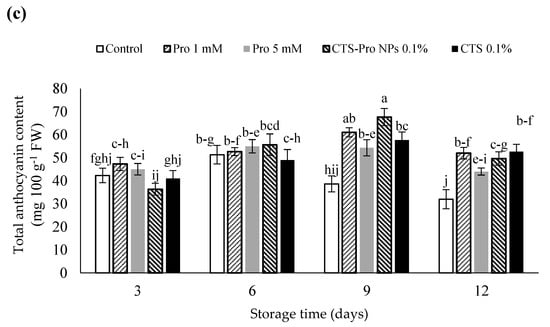
Figure 3.
Effect of post–harvest treatments of strawberries with Pro, CTS and CTS–P NPs on (a) total phenolics, (b) total flavonoids, and (c) total anthocyanins during 12 days of storage at 4 °C. Data presented are mean ± SE of three replications. Different letters (a–j) over bars indicate they are significantly different (p < 0.05) by Duncan’s test.
3.7. Total Flavonoids
The amount of total flavonoids in strawberries at harvest was 6.8 mg 100 g−1 FW in this experiment (Table 1). The results showed that the amount of total flavonoids in uncoated and coated fruit gradually declined until the end of storage time (Figure 3b). Strawberries treated with CTS and CTS-Pro NPs indicated significantly higher flavonoid levels compared to the control fruit (p < 0.05), whereas no significant difference was detected between the control and 1 mM Pro treatment or between the 1 and 5 mM Pro treatment. These findings are in agreement with results from other studies, in which different coatings were applied, and adequate levels of flavonoids in papayas [71], tomatoes [72] and strawberries [73] were obtained.
3.8. Total Anthocyanin Content
Changes in the total anthocyanin (TAC) content of control and coated fruit during 12 days of storage are shown in Figure 3c. The results showed that until the ninth day of storage, anthocyanin content in the treated fruit gradually increased and was higher than in the control fruit. The increase in anthocyanin may have been due to the activation of related enzymes because anthocyanins are the major phenolic compounds synthesized in mature fruit [74]. Afterwards, the anthocyanin content gradually declined until the end of the storage period. The strawberries treated with CTS-Pro NPs recorded the highest anthocyanin content. The initial increase was probably due to ripening, increased sugar, and phenylalanine ammonia-lyase activity during storage; however, a gradual decrease in this index after this period could have been due to increased polyphenol oxidase activity [75].
In this study, the slower rate of anthocyanin depletion in coated fruit compared to control could have been due to lower enzymatic activity and ascorbic acid retention. Indeed, the edible coating acted as a gas barrier during cold storage to decrease the O2 and CO2 exchange. It also inhibited anthocyanin oxidation upon decomposition of the cell wall. The results in this study are consistent with the findings of other researchers [67,76].
3.9. Antioxidant Enzyme Activities
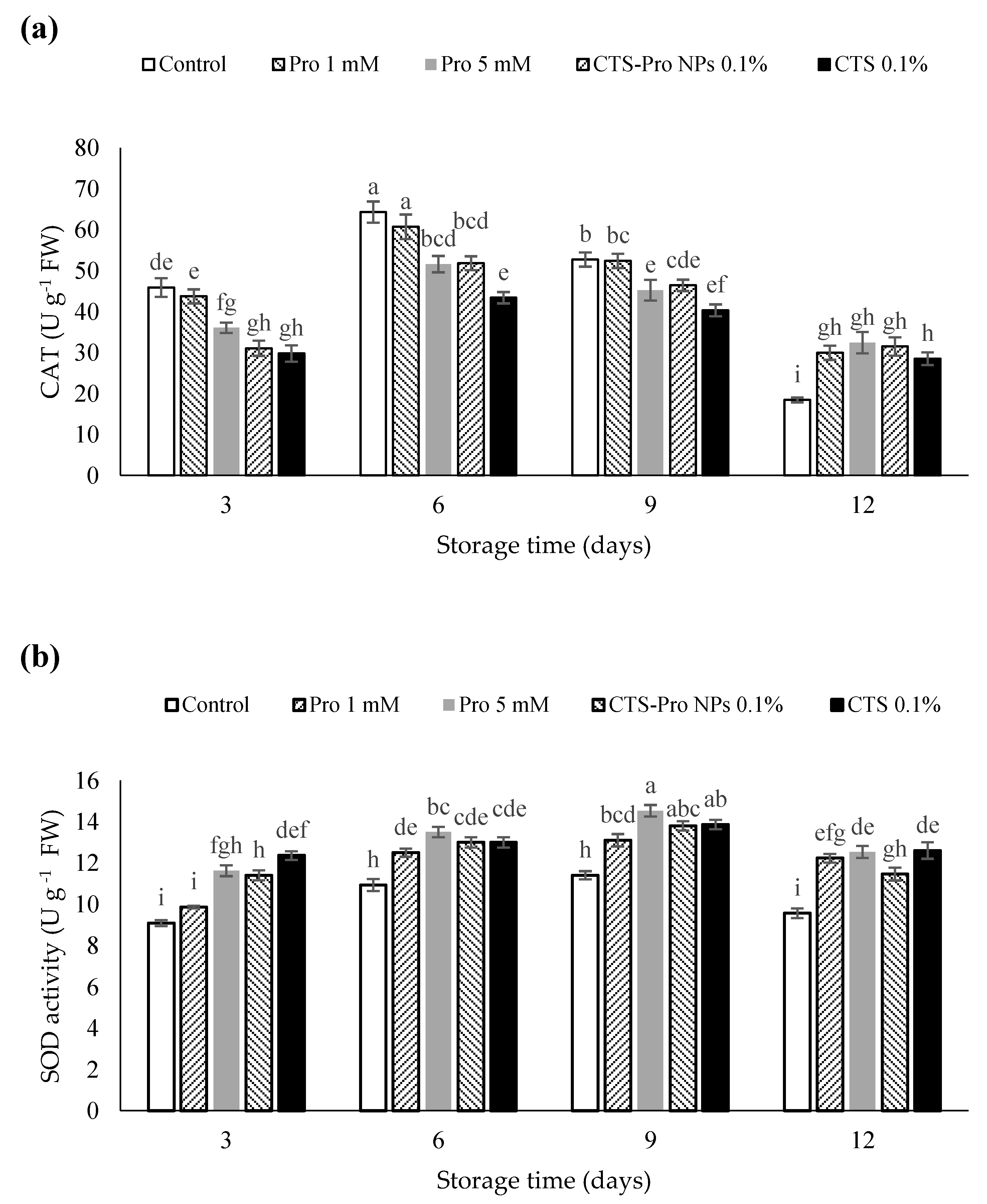
There are several antioxidant enzymes, including SOD, POD, CAT and APX that catalyze reactions to neutralize ROS. The reactive oxygen species could be scavenged by these enzymes and prevent the destructive effects of H2O2 in plant structures [77]. As shown in Figure 4a, catalase activity in all strawberries increased gradually during the initial 6 days of storage and then reduced steadily at the end of the storage period, but this reduction was higher in control than in the coated fruit. The samples treated with Pro 1 mM and control had the highest catalase activity on the sixth day. CAT is an ROS-scavenging enzyme present in all plants, where it functions to catalyze the hydrogen peroxide into H2O and O2 in an energy-efficient manner [78] by preventing excessive H2O2 build-up and allows important cellular processes to occur.
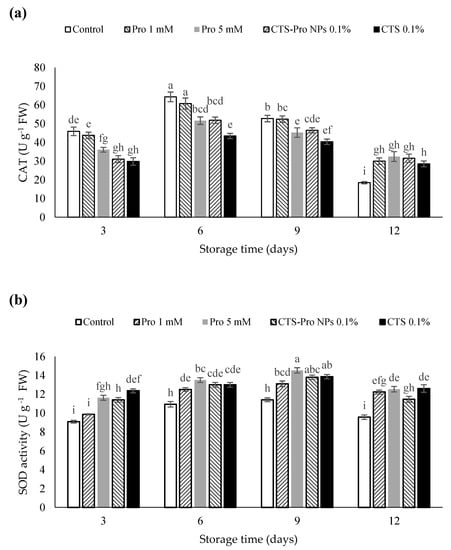
Figure 4.
Effect of post–harvest treatments of strawberries with Pro, CTS and CTS–P NPs on activities of CAT (a) and SOD (b) during 12 days of storage at 4 °C. Data presented are mean ± SE of three replications. Different letters (a–i) over bars indicate they are significantly different (p < 0.05) by Duncan’s test.
The activity of SOD increased in all fruit until the ninth day of storage and then diminished slightly at the end of storage time (Figure 4b). The highest SOD activity was related to fruit treated with 5 mM proline and chitosan and had a significant difference from the control (Figure 3b). Under plant stress conditions, antioxidant compounds such as catalase and superoxide dismutase naturally increase [79]. These compounds prevent damage to plant tissues by removing free radicals [64].
The results showed that CAT and SOD activities were significantly reduced during the 12-day storage period, regardless of the treatments. However, the highest reduction in SOD and CAT activity was observed in the control as compared to the different coatings. In the current study, the use of CTS and Pro were significantly effective in maintaining the activity of higher antioxidant enzymes. Post-harvest chitosan coating has shown to be effective in enhancing activities of CAT, SOD and POD in guava [65] and fresh in-hull pistachio fruit [80]. These results are in agreement with previous studies that have reported the beneficial effect of proline in increasing ROS scavenging enzyme activities as found in rose [81], citrus [32] and flat peach [82]. In the present study, when compared with uncoated fruit, the CTS- and Pro-treated strawberries showed lower H2O2 accumulation and O2−2 production as a result of higher SOD and CAT activity during cold storage. These results suggested that the use of Pro, CTS and CTS-Pro NPs improved the activity of ROS-scavenging enzymes, including CAT and SOD, which prevented oxidation damage; therefore, they increased shelf life and retained fruit quality during storage.
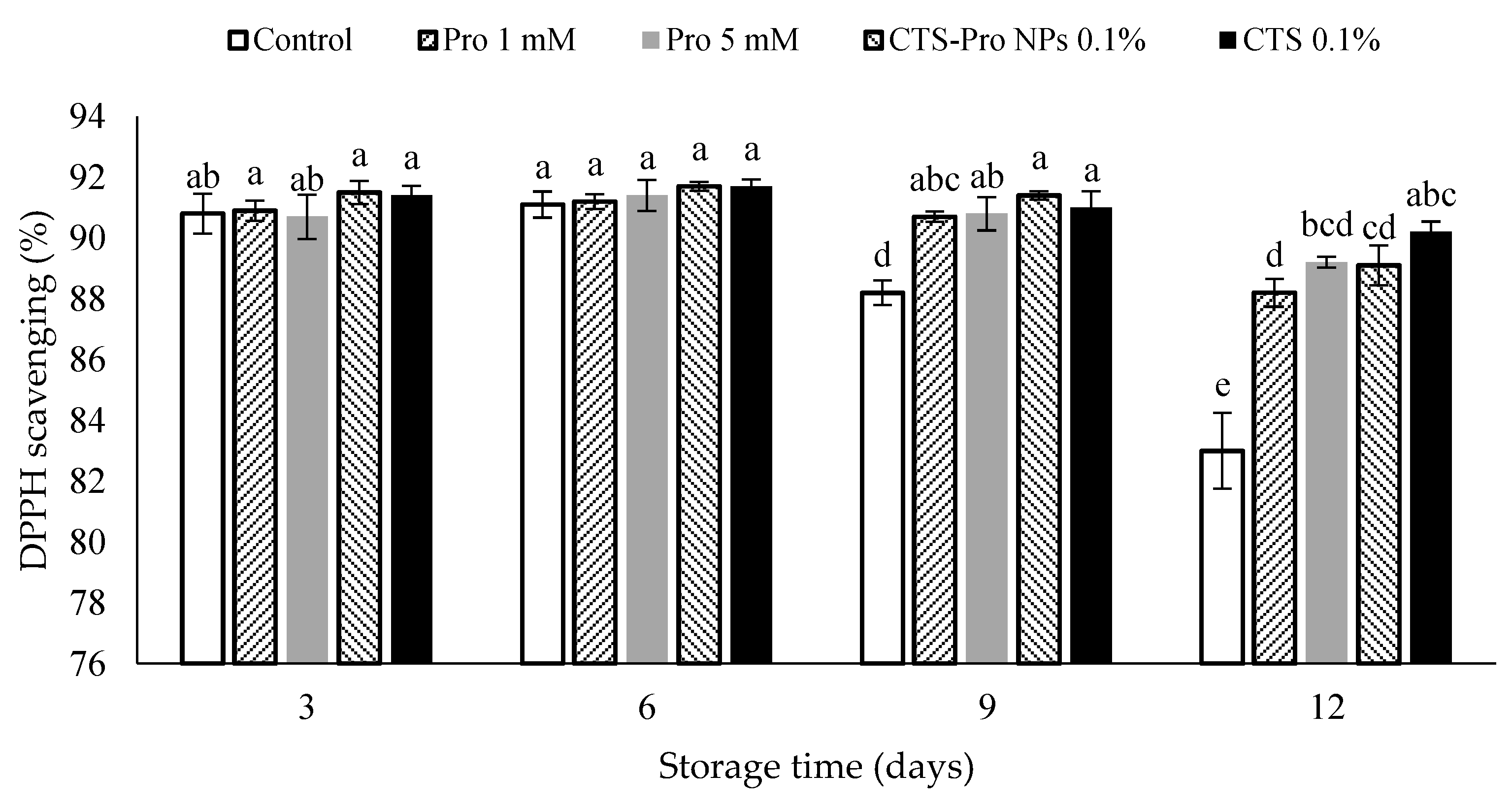
3.10. Antioxidant Capacity
In Figure 5, the antioxidant capacity of control and coated strawberries was constant until the sixth day of storage. Afterward, the antioxidant capacity decreased, especially in the control samples, which decreased rapidly until the end of storage, whereas after the twelfth day, there was significantly higher antioxidant capacity in the coated fruit. Therefore, coating strawberries with CTS and CTS-Pro NPs could be said to have maintained antioxidant capacity. Decreased antioxidant activity may have been due to cell protection against free radical damage. In addition, reduced ascorbic acid and anthocyanin were another reason for decreased antioxidant activity. It has been known that strawberries coated with CTS and CTS-Pro NPs had more antioxidant activity compared to control, which could have been due to higher maintenance of ascorbic acid and anthocyanins [67]. The levels of phenylpropanoid compounds, oxygen-radical scavengers and antioxidant activity increased in strawberries after chitosan treatment [73].
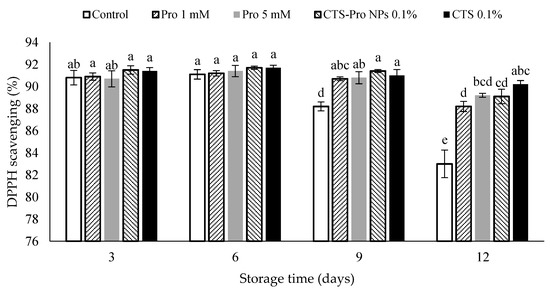
Figure 5.
Effect of post–harvest treatments of strawberries with Pro, CTS and CTS–P NPs on antioxidant capacity (%) during 12 days of cold storage at 4 °C. Data presented are mean ± SE of three replications. Different letters (a–e) over bars indicate they are significantly different (p < 0.05) by Duncan’s test.
4. Conclusions
Strawberries are rich in antioxidant compounds, including phenolic compounds and ascorbic acid, but, similar to other fruit, their nutritional value and quality decline post-harvest. The use of CTS as a nature-friendly and non-chemical substance can greatly preserve the nutritional value of strawberries. In general, as shown in this experiment, fruit treated with CTS, Pro and CTS-Pro NPs had higher antioxidant and enzymatic activity than untreated fruit. The present study showed that the CTS coatings, Pro and CTS-Pro NPs protected strawberries against fungal decay and improved their physiochemical characteristics during storage at 4 °C for 12 days. Overall, fruit coated with CTS and CTS-Pro NPs showed lower weight loss, decay, and MDA and H2O2 accumulation as well as preserved ascorbic acid, anthocyanins and total phenols. Thus, chitosan and proline coated with chitosan nanocomposite coatings are good candidates for maintaining the nutritional quality and increasing the post-harvest life of strawberries.
Author Contributions
Conceptualization, F.R. and R.B.; methodology, F.R., R.B., A.J.-M. and G.G.; software, R.B.; validation, S.N.M. and G.G.; formal analysis, R.B. and G.G.; investigation, R.B., S.N.M. and F.R.; resources, S.N.M. and F.R.; data collection, R.B. and S.N.M.; writing—original draft preparation, R.B., G.G. and F.R.; writing—review and editing, F.R., A.J.-M. and G.G.; visualization, R.B. and S.N.M.; supervision, F.R., A.J.-M. and G.G.; funding acquisition, F.R. and A.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aaby, K.; Ekeberg, D.; Skrede, G. Characterization of phenolic compounds in strawberry (Fragaria x ananassa) fruit by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J. Agric. Food. Chem. 2007, 55, 4395–4406. [Google Scholar] [CrossRef] [PubMed]
- Buendia, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.B.; Tomás-Barberán, F.A. HPLC-MS analysis of proanthocyanin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, H.; Sheng, K.; Liu, W.; Zheng, L. Effects of melatonin treatment on the postharvest quality of strawberry fruit. Postharvest Biol. Technol. 2018, 139, 47–55. [Google Scholar] [CrossRef]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruit: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Rux, G.; Mahajan, P.V.; Linke, M.; Pant, A.; Sangerlaub, S.; Caleb, O.J.; Geyer, M. Humidity-regulating trays: Moisture absorption kinetics and applications for fresh produce packaging. Food. Bioprocess Technol. 2016, 9, 709–716. [Google Scholar] [CrossRef]
- Nguyen, L.P.L.; Visy, A.; Baranyai, L.; Friedrich, L.; Mahajan, P.V. Application of hue spectra fingerprinting during cold storage and shelf-life of packaged sweet cherry. J. Food. Meas. Charact. 2002, 14, 2689–2702. [Google Scholar] [CrossRef]
- Nguyen, L.P.L.; Szabo, G.; Hitka, G.; Zsom, T.; Toth, A.; Nemeth, C.; Kokai, Z. Effect of ethylene absorber on banana during storage. Acta Hortic. 2018, 1216, 55–58. [Google Scholar] [CrossRef]
- Nguyen, L.P.L.; Zsom, T.; Sao Dam, M.; Baranyai, L.; Hitka, G. Comparison of 1-MCP treatment on four melon cultivars using different temperatures. J. Appl. Bot. Food Qual. 2020, 93, 122–129. [Google Scholar]
- Baranyai, L.; Nguyen, L.L.P.; Sao Dam, M.; Zsom, T.; Hitka, G. Evaluation of precooling temperature and 1-MCP treatment on quality of ‘Golden Delicious’ apple. J. Appl. Bot. Food Qual. 2020, 93, 130–135. [Google Scholar]
- Hazarika, T.K.; Lalrinfeli, L.; Lalchhnmawia, J.; Mandal, D. Alteration of quality attributes and shelf-life in strawberry (Fragaria x ananssa) fruit during storage as influence by edible coating. Indian. J. Agri. Sci. 2019, 89, 28–34. [Google Scholar]
- Jesus Filho, M.D.; Scolforo, C.Z.; Saraiva, S.H.; Pinheiro, C.J.G.; Silva, P.I.; Della Lucia, S.M. Physicochemical, microbiological and sensory acceptance alterations of strawberries caused by gamma radiation and storage time. Sci. Hortic. 2018, 238, 187–194. [Google Scholar] [CrossRef]
- Arnon, H.; Zaitsev, Y.; Porat, R. Effects of carboxymethyl cellulose and chitosan bilayer edible coating on postharvest quality of citrus fruit. Postharvest. Biol. Technol. 2014, 87, 21–26. [Google Scholar] [CrossRef]
- Zambrano-Zaragoza, M.L.; Mercado-Silva, E.; Del Real, L.A.; Gutierrez-Cortez, E.; Cornejo-Villegas, M.A.; Quintanar-Guerrero, D. The effect of nano coatings with a-tocopherol and xanthan gum on shelf-life and browning index of fresh-cut “red delicious” apples. Innov. Food. Sci. Emerg. Technol. 2014, 22, 188–196. [Google Scholar] [CrossRef]
- Salvia-Trujillo, L.; Rojas-Graü, M.A.; Soliva-Fortuny, R.; Martín-Belloso, O. Use of antimicrobial nanoemulsions as edible coatings: Impact on safety and quality attributes of fresh-cut fuji apples. Postharvest Biol. Technol. 2015, 105, 8–16. [Google Scholar] [CrossRef]
- George, M.; Abraham, T.E. Polyionic hydrocolloids for the intestinal delivery of proteins drug: Alginate and chitosan—A review. J. Control Release 2006, 114, 1–14. [Google Scholar] [CrossRef]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan coating to preserve the qualitative traits and improve antioxidant system in fresh figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Xing, Y.; Li, W.; Wang, Q.; Li, X.; Xu, Q.; Guo, X. Antimicrobial nanoparticles incorporated in edible coatings and films for the preservation of fruit and vegetables. Molecules 2019, 24, 1695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatri, D.; Panigrahi, J.; Prajapati, A.; Bariya, H. Attributes of Aloe vera gel and chitosan treatments on the quality and biochemical traits of post-harvest tomatoes. Sci. Hortic. 2020, 259, 108837. [Google Scholar] [CrossRef]
- Rahman, M.; Akter Mukta, J.; As Sabir, A.; Rani Gupta, D.; Mohi-Ud-Din, M.; Hasanuzzaman, M.; Miah, M.G.; Rahman, M.; Islam, M.T. Chitosan biopolymer promotes yield and stimulates accumulation of antioxidants in strawberry fruit. PLoS ONE 2018, 13, 1–14. [Google Scholar] [CrossRef]
- Petriccione, M.; Mastrobuoni, F.; Pasquariello, M.S.; Zampella, L.; Nobis, E.; Capriolo, G.; Scortichini, M. Effect of chitosan coating on the postharvest quality and antioxidant enzyme system response of strawberry fruit during cold storage. Foods 2015, 4, 501–523. [Google Scholar] [CrossRef] [Green Version]
- Hesami, A.; Kavoosi, S.; Khademi, R.; Sarikhani, S. Effect of chitosan coating and storage temperature on shelf-life and fruit quality of Ziziphus mauritiana. Intern. J. Fruit Sci. 2021, 21, 509–518. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Meng, J.; Khan, M.I.H.; Dai, L.; Khan, A.; An, X.; Zhang, J.; Huq, T.; Ni, Y. Chitosan as a preservative for fruit and vegetables: A review on chemistry and antimicrobial properties. J. Bioresour. Bioprod. 2019, 4, 11–21. [Google Scholar] [CrossRef]
- Dam, M.S.; To, X.T.; Le, Q.T.P.; Nguyen, L.L.P.; Friedrich, L.; Hitka, G.; Zsom, T.; Thi Nguyen, T.C.; Huynh, C.Q.; Thitran, M.D.; et al. Postharvest quality of hydroponic strawberry coated with chitosan-calcium gluconate. Prog. Agric. Engin. Sci. 2020, 16, 141–151. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, S.; Ren, Y.; Li, H.; Zhang, X.; Di, J. Jujube preservation using chitosan film with nano-silicon dioxide. J. Food Eng. 2012, 113, 408–414. [Google Scholar] [CrossRef]
- Shi, S.; Wang, W.; Liu, L.; Wu, S.; Wei, Y.; Li, W. Effect of chitosan/nano-silica coating on the physicochemical characteristics of longan fruit under ambient temperature. J. Food Eng. 2013, 118, 125–131. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. N. Y. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Signorelli, S. The fermentation analogy: A point of view for understanding the intriguing role of proline accumulation in stressed plants. Frontiers. Front. Plant Sci. 2016, 7, 1339. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho, K.; de Campos, M.K.; Domingues, D.S.; Pereira, L.F.; Vieira, L.G. The accumulation of endogenous proline induces changes in gene expression of several antioxidant enzymes in leaves of transgenic Swingle citrumelo. Mol. Biol. Rep. 2013, 40, 3269–3279. [Google Scholar] [CrossRef]
- Hoque, M.A.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycine betaine enhance antioxidant defense and methylglyoxal detoxification systems and reduce NaCl-induced damage in cultured tobacco cells. J. Plant Physiol. 2008, 165, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of exogenous application of proline on antioxidant compounds in three Citrus species under low temperature stress. J. Plant Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Lopez, G.; Ventura-Aguilar, R.I.; Correa-Pacheco, Z.N.; Bautista-Banos, S.; Barrera-Necha, L.L. Nanostructured chitosan edible coating loaded with a-pinene for the preservation of the postharvest quality of Capsicum annum L. and Alternaria alternate control. Inter. J. Biol. Macro. 2020, 165, 1881–1888. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yang, H. Effect of preharvest chitosan-g-salicylic acid treatment on postharvest table grape quality, shelf life, and resistance to Botrytis cinerea-induced spoilage. Sci. Hortic. 2017, 224, 367–373. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Gohari, G.; Palou, L. Application of Glycine betaine coated chitosan nanoparticles alleviate chilling injury and maintain quality of plum (Prunus domestica L.) fruit. Intern. J. Biol. Macromol. 2022, 207, 965–977. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruit (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Muñoz, P.; Almenar, E.; Ocio, M.J.; Gavara, R. Effect of calcium dips and chitosan coatings on postharvest life of strawberries (Fragaria x ananassa). Postharvest Biol. Technol. 2006, 39, 247–253. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, T.; Zhang, P.; Wang, Z.Y. Melatonin attenuates postharvest physiological deterioration of cassava storage roots. J. Pineal. Res. 2016, 60, 424–434. [Google Scholar] [CrossRef]
- Saini, R.S.; Sharma, K.D.; Kaushik, R.A.; Dhankhar, O.P. Laboratory manual analytical techniques in horticulture. Agrobios (India). IST Edition 2006, 2, 12–26. [Google Scholar]
- Pineli, L.O.; Moretti, C.L.; Dos Santos, M.S.; Campos, A.B.; Brasileiro, A.V.; Cordova, A.C.; Chiarello, M.D. Antioxidants and other chemical and physical characteristics of two strawberry cultivars at different ripeness stages. J. Food Comp. Anal. 2011, 24, 11–16. [Google Scholar] [CrossRef]
- Chen, L.; Han, Y.; Jiang, H.; Korpelainen, H.; Li, C. Nitrogen nutrient status induces sexual differences in responses to cadmium in Populus yunnanensis. J. Exp. Bot. 2011, 62, 5037–5050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, S.; Khan, A.S.; Malik, A.U.; Shahid, M. Effect of controlled atmosphere storage on pericarp browning, bioactive compounds and antioxidant enzymes of litchi fruit. Food Chem. 2016, 206, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Dhital, R.; Mora, N.B.; Watson, D.G.; Kohli, P.; Choudhary, R. Efficacy of limonene nano coatings on post-harvest shelf life of strawberries. LWT-Food. Sci. Technol. 2018, 97, 124–134. [Google Scholar] [CrossRef]
- Romanazzi, G. Chitosan treatment for the control of postharvest decay of table grapes, strawberries and sweet cherries. Fresh Prod. 2010, 4, 111–115. [Google Scholar]
- Li, H.Y.; Wang, F.; Liu, Y.; Yang, Z.; Wu, H.; Cai, Q.; Zhang, Y.; Li, P. Effects of chitosan on control of postharvest blue mold decay of apple fruit and the possible mechanisms involved. Sci. Hort. 2015, 186, 77–83. [Google Scholar] [CrossRef]
- Camatari, F.O.D.S.; Santana, L.C.L.D.A.; Carnelossi, M.A.G.; Alexandre, A.P.S.; Nunes, M.L.; Goulart, M.O.F.; Narain, N.; Silva, M.A.A.P.D. Impact of edible coatings based on cassava starch and chitosan on the post-harvest shelf life of mango (Mangifera indica) ‘Tommy Atkins’ fruit. Food Sci. Technol. 2018, 38, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Youssef, K.; Hashim, A.F. Inhibitory Effect of Clay/Chitosan Nanocomposite against Penicillium digitatum on Citrus and Its Possible Mode of Action. Jordan J. Biol. Sci. 2020, 13, 349–355. [Google Scholar]
- Pasquariello, M.S.; Rega, P.; Migliozzi, T.; Capuano, L.R.; Scortichini, M.; Petriccione, M. Effect of cold storage and shelf life on physiological and quality traits of early ripening pear cultivars. Sci. Hortic. 2013, 162, 341–350. [Google Scholar] [CrossRef]
- Ahmadi-Afzadi, M.; Tahir, I.; Nybom, H. Impact of harvesting time and fruit firmness on the tolerance to fungal storage diseases in an apple germplasm collection. Postharvest. Biol. Technol. 2013, 82, 51–58. [Google Scholar] [CrossRef]
- Zhong, Q.; Xia, W. Effect of 1-methylcyclopropene and/or chitosan coating treatments on storage life and quality maintenance of Indian jujube fruit. Food Sci. Technol. 2007, 40, 404–411. [Google Scholar]
- Aleryani-Raqeeb, A.; Mahmud, T.M.M.; Omar, S.R.S.; Zaki, A.R.M. Effect of calcium infiltration and chitosan coating on storage life and quality characteristics during storage of papaya (Carica papaya L.). Int. J. Agric. Res. 2008, 3, 296–306. [Google Scholar]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. LWT-Food. Sci. Technol. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Nguyen, H.V.H.; Nguyen, D.H.H. Effects of nano-chitosan and chitosan coating on the postharvest quality, polyphenol oxidase activity and malondialdehyde content of strawberry (Fragaria x ananassa Duch.). J. Hort. Postharvest Res. 2020, 3, 11–24. [Google Scholar]
- Kittur, F.S.; Saroja, N.; Tharanathan, H.R.N. Polysaccharide-based composite coating formulations for shelf-life extension of fresh banana and mango. Eur. Food Res. Technol. 2001, 213, 306–311. [Google Scholar] [CrossRef]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Li, L. The effect of the layer-bylayer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Sogvar, O.B.; Saba, M.K.; Emamifar, A. Aloe vera and ascorbic acid coatings maintain postharvest quality and reduce microbial load of strawberry fruit. Postharvest Biol. Technol. 2016, 114, 29–35. [Google Scholar] [CrossRef]
- Davey, M.W.; Van Montagu, M.; Inze, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Suekawa, M.; Fujikawa, Y.; Inoue, A.; Kondo, T.; Uchida, E.; Koizumi, T.; Esaka, M. High levels of expression of multiple enzymes in the Smirnoff-Wheeler pathway are important for high accumulation of ascorbic acid in acerola fruit. Biosci. Biotech. Biochem. 2019, 83, 1713–1716. [Google Scholar] [CrossRef]
- Gao, S.P.; Hu, K.D.; Hu, L.Y.; Li, Y.H.; Han, Y.; Wang, H.L.; Ly, K.; Liu, Y.S.; Zhang, H. Hydrogen sulfide delays postharvest senescence and plays an antioxidative role in fresh-cut kiwifruit. Hort. Sci. 2013, 48, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Amal, S.; Atress, M.M.; El-Mogy, H.E.; Aboul-Anean, B.W. Improving strawberry fruit storability by edible coating as a carrier of thymol or calcium chloride. J. Hortic. Sci. Ornam. Plants 2010, 2, 88–97. [Google Scholar]
- Khodaei, D.; Zohreh Hamidi-Esfahani, Z.; Edris Rahmati, E. Effect of edible coatings on the shelf-life of fresh strawberries: A comparative study using TOPSIS-Shannon entropy method. NFS J. 2021, 23, 17–23. [Google Scholar] [CrossRef]
- Belal Abu Salha, B.; Gedanken, A. Extending the Shelf Life of Strawberries by the Sonochemical Coating of their Surface with Nanoparticles of an Edible Anti-Bacterial Compound. Applied Nano. 2021, 2, 2. [Google Scholar] [CrossRef]
- Mandal, D.; Sahu, C.; Bagchi, S.; Das, A. KKinetics and mechanism of the tropospheric oxidation of vinyl acetate initiated by OH radical: A theoretical study. J. Phys. Chem. 2013, 117, 3739–3750. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Peng, X.; Luo, Y.; Wang, J.; Guo, X.; Huang, K. Physiological and biochemical responses of grapefruit seed extract dip on ‘redglobe’ grape. LWT-Food Sci. Technol. 2009, 42, 471–476. [Google Scholar] [CrossRef]
- Hong, K.; Xie, J.; Zhang, L.; Sun, D.; Gong, D. Effects of chitosan coating on postharvest life and quality of guava (Psidium guajava L.) fruit during cold storage. Sci. Hortic. 2012, 144, 172–178. [Google Scholar] [CrossRef]
- Kumar, P.; Sethia, S.; Sharma, R.; Srivastav, M.; Varghesec, E. Effect of chitosan coating on postharvest life and quality of plum during storage at low temperature. Sci. Horticulturae 2017, 226, 104–109. [Google Scholar] [CrossRef]
- Gol, N.B.; Patel, P.R.; Rao, T.V.R. Improvement of quality and shelf-life of strawberries with edible coatings enriched with chitosan. Postharvest Biol. Technol. 2013, 85, 185–195. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-mansy, H.A.; Soliman, S.A. Effect of chitosan–olive oil processing residues coatings on keeping quality of cold-storage strawberry (Fragaria ananassa var. festival). J. Food Qual. 2016, 39, 504–515. [Google Scholar] [CrossRef]
- Resende, N.S.; Gonçalves, G.A.S.; Reis, K.C.; Tonoli, G.H.D.; Boas, E. Chitosan/Cellulose Nanofibril Nanocomposite and Its Effect on Quality of Coated Strawberries. J. Food Qual. 2018, 2018, 233–254. [Google Scholar] [CrossRef] [Green Version]
- Jongsri, P.; Wangsomboondee, T.; Rojsitthisak, P.; Seraypheap, K. Effect of molecular weights of chitosan coating on postharvest quality and physicochemical characteristics of mango fruit. LWT-Food Sci. Technol. 2016, 73, 28–36. [Google Scholar] [CrossRef]
- Addai, Z.R.; Abdullah, A.; Mutalib, S.A. Influence of ripening stages on antioxidant properties of papaya fruit (Carica papaya L.). In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2013; Volume 1571, pp. 696–701. [Google Scholar]
- Firdous, N.; Khan, M.R.; Butt, M.S.; Shahid, M. Application of Aloe vera gel based edible coating to maintain postharvest quality of tomatoes. Pak. J. Agric. Sci. 2020, 57, 245–249. [Google Scholar]
- Wang, S.Y.; Gao, H. Effect of chitosan-based edible coating on antioxidants, antioxidant enzyme system, and postharvest fruit quality of strawberries (fragaria x aranassa duch.). LWT-Food Sci. Technol. 2013, 52, 71–79. [Google Scholar] [CrossRef]
- Ananga, A.; Georgiev, V.; Ochieng, J.; Phills, B.; Tsolova, V. Production of anthocyanins in grape cell cultures: A potential source of raw material for pharmaceutical, food, and cosmetic industries. Mediterr. Genet. Code-Grapevine Olive 2013, 1, 247–287. [Google Scholar]
- Vargas, M.; Albors, A.; Chiralt, A.; GonzalezMartinez, C. Quality of cold-stored strawberries as affected by chitosan-oleic acid edible coatings. Postharvest Biol. Technol. 2006, 41, 164–171. [Google Scholar] [CrossRef]
- Van, T.B.; Duyen, H.H.; Ha, V.H. Combination effects of calcium chloride and nano-chitosan on the postharvest quality of strawberry (Fragaria x ananassa Duch.). Postharvest Biol. Technol. 2020, 162, 103–111. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends. Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Chelikani, P.; Fita, I.; Loewen, P.C. Diversity of structures and properties among catalyses. Cellular. Molecul. Life Sci. 2004, 61, 192–208. [Google Scholar] [CrossRef]
- Nasr, F.; Pateiro, M.; Rabiei, V.; Razavi, F.; Formaneck, S.; Gohari, G.; Lorenzo, J.M. Chitosan-phenylalanine nanoparticles (Cs-Phe Nps) extend the postharvest life of persimmon (Diospyros kaki) fruits under chilling stress. Coatings 2021, 11, 819. [Google Scholar] [CrossRef]
- Molamohammadi, H.; Pakkish, Z.; Akhavan, H.R.; Saffari, V.R. Effect of salicylic acid incorporated chitosan coating on shelf life extension of fresh in-hull pistachio fruit. Food Bioprocess Technol. 2020, 13, 121–131. [Google Scholar] [CrossRef]
- Kumar, N.; Pal, M.; Singh, A.; SaiRam, R.K.; Srivastava, G.C. Exogenous proline alleviates oxidative stress and increase vase life in rose (Rosa hybrida L.‘Grand Gala’). Sci. Hortic. 2010, 127, 79–85. [Google Scholar] [CrossRef]
- Gohari, G.; Molaei, S.; Kheiry, A.; Ghafouri, M.; Razavi, F.; Lorenzo, J.M.; Juárez-Maldonado, A. Exogenous application of proline and L-cysteine alleviates internal browning and maintains eating quality of cold stored flat ‘Maleki’ peach fruit. Horticulturae 2021, 7, 469. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).