Dwarfing Rootstock ‘Yunnan’ Quince Promoted Fruit Sugar Accumulation by Influencing Assimilate Flow and PbSWEET6 in Pear Scion
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
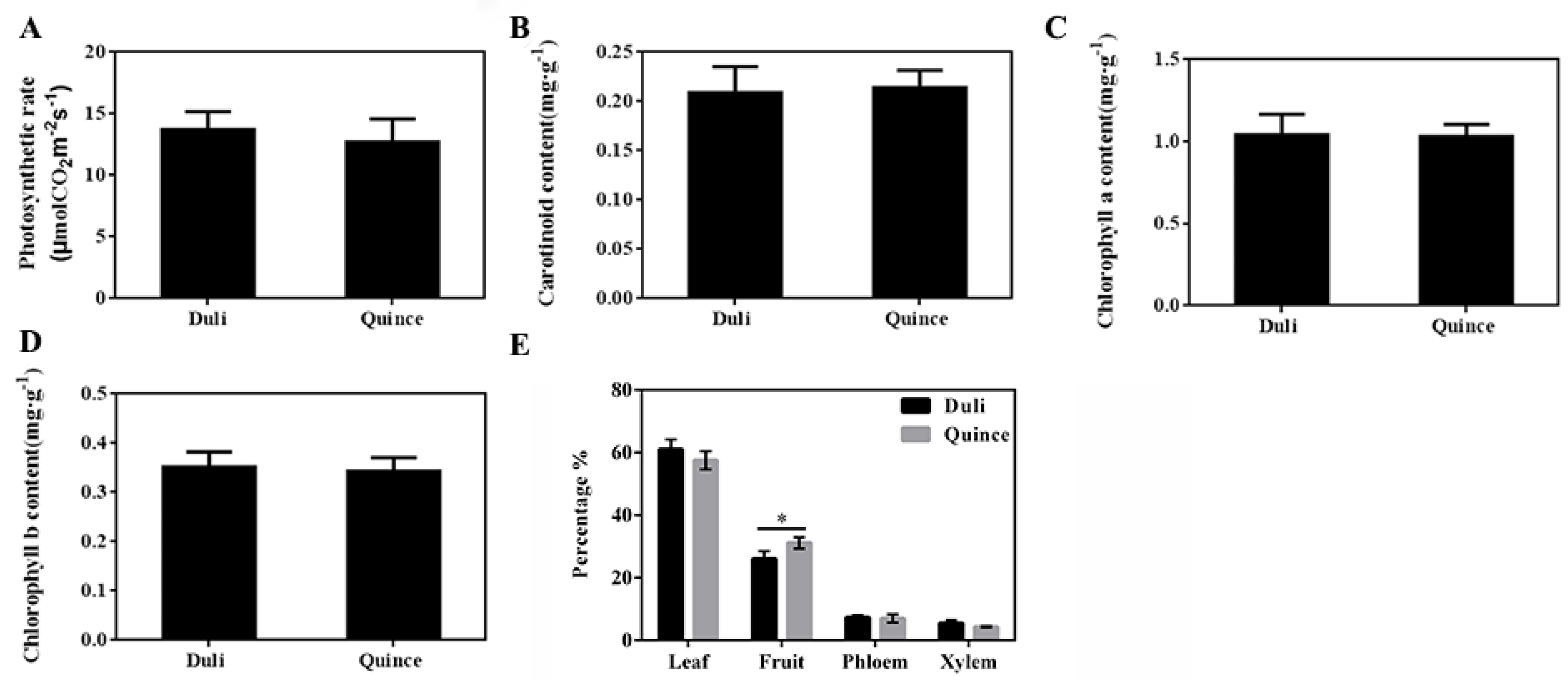
2.2. Photosynthetic Capacity and Assimilate Distribution
2.3. Measurements of Soluble Solids and Sugar Contents
2.4. Sequence Analysis of PbSWEET6
2.5. Gene Cloning and Quantitative Real-Time PCR
2.6. Subcellular Location of PbSWEET6
2.7. Agrobacterium-Mediated Tomato and Pear Fruit Calli Transformation
2.8. Statistical Analysis
3. Results
3.1. ‘Yunnan’ Quince Increased the Sugar Content of Scion Fruit
3.2. ‘Yunnan’ Quince Promoted the Transport of Photoassimilates to Fruit
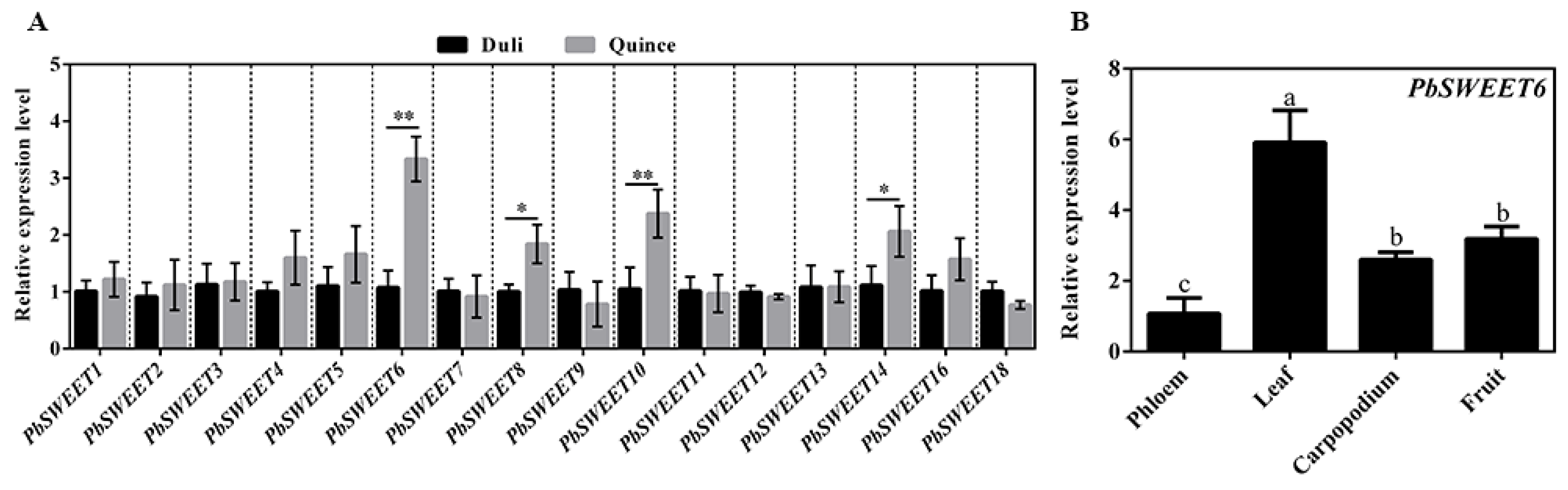
3.3. PbSWEET6 May Participate in the Transport of Photoassimilates
3.4. Phylogenetic Analysis and Subcellular Localization of PbSWEET6
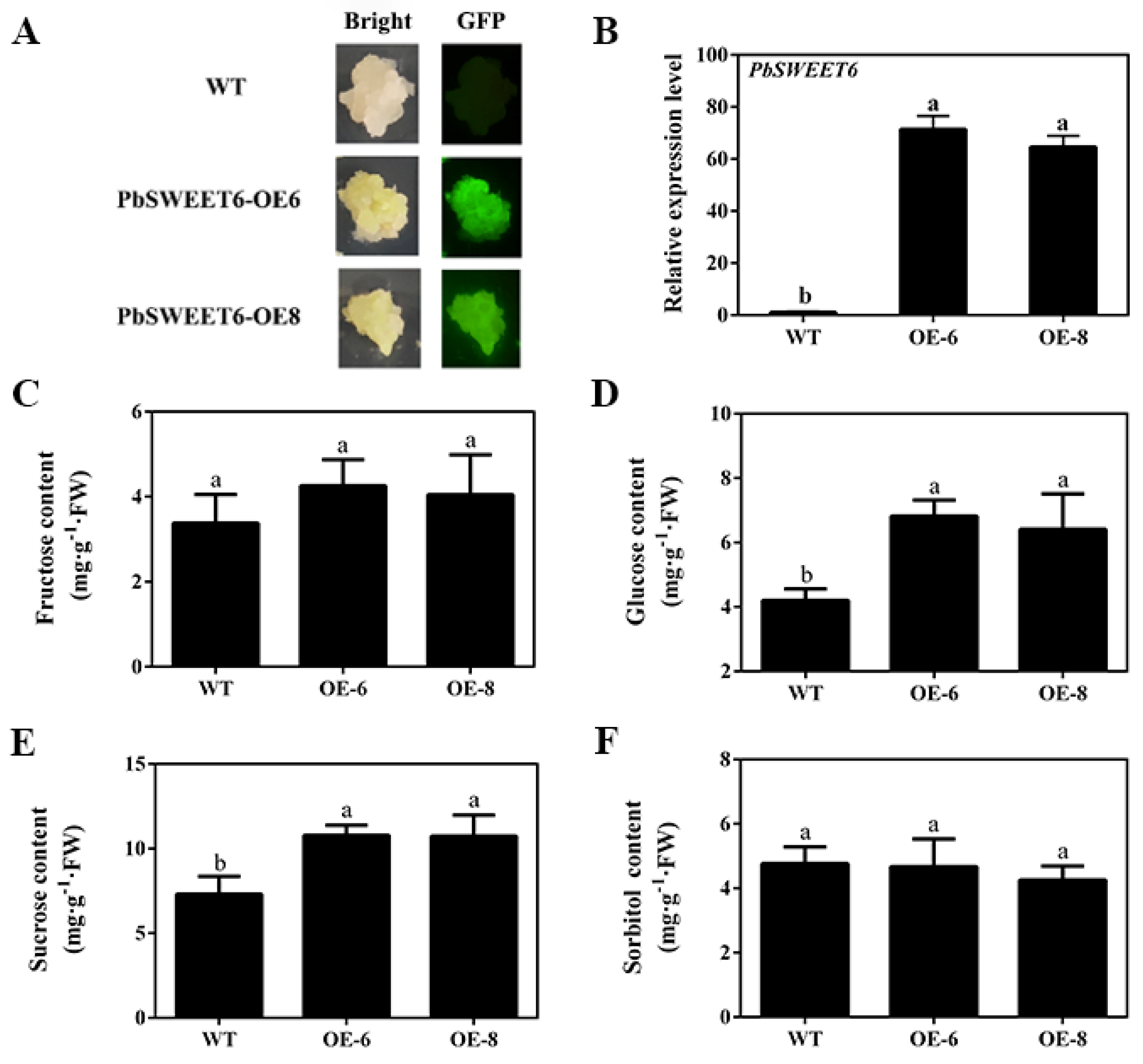
3.5. PbSWEET6 Increased the Glucose and Sucrose Content, in Tomato and Pear Fruit Calli
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Einhorn, T.C. A review of recent Pyrus, Cydonia and Amelanchier rootstock selections for high-density pear plantings. Acta Hortic. Sin. 2021, 60, 185–196. [Google Scholar] [CrossRef]
- Watson, A.E.; Seleznyova, A.N.; Dayatilake, G.A.; Tustin, D.S. Rootstocks affect pear (Pyrus communis) tree growth through extent of node neoformation and flowering wisth key differences to apple. Funct. Plant Biol. 2012, 39, 493–502. [Google Scholar] [CrossRef]
- Donadio, L.C.; Lederman, I.E.; Roberto, S.R.; Stucchi, E.S. Dwarfing-canopy and rootstock cultivars for fruit trees. Rev. Bras. Frutic. 2019, 41, e997. [Google Scholar] [CrossRef]
- McClymont, L.; Goodwin, I.; Whitfield, D.; O’Connell, M.; Turpin, S. Effects of rootstock, tree density and training system on early growth, yield and fruit quality of blush pear. HortScience 2021, 56, 1408–1415. [Google Scholar] [CrossRef]
- Ou, C.; Wang, F.; Wang, J.; Li, S.; Zhang, Y.; Fang, M.; Ma, L.; Zhao, Y.; Jiang, S. A de novo genome assembly of the dwarfing pear rootstock Zhongai 1. Sci. Data 2019, 6, 281. [Google Scholar] [CrossRef] [PubMed]
- Du Plooy, P.; van Huyssteen, P. Effect of BP1, BP3 and Quince A rootstocks, at three planting densities, on precocity and fruit quality of ‘Forelle’ pear (Pyrus communis L.). S. Afr. J. Plant 2000, 17, 57–59. [Google Scholar] [CrossRef]
- Zhang, C.M.; Bian, Y.; Hou, S.H.; Li, X.G. Sugar transport played a more important role than sugar biosynthesis in fruit sugar accumulation during Chinese jujube domestication. Planta 2018, 248, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.M.; Wang, L.; Ruan, Y.L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef]
- Tao, Y.; Cheung, L.S.; Li, S.; Eom, J.S.; Chen, L.Q.; Xu, Y.; Perry, K.; Frommer, W.B.; Feng, L. Structure of a eukaryotic SWEET transporter in a homotrimeric complex. Nature 2015, 527, 259–263. [Google Scholar] [CrossRef]
- Xuan, Y.H.; Hu, Y.B.; Chen, L.Q.; Sosso, D.; Ducat, D.C.; Hou, B.H.; Frommer, W.B. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Natl. Acad. Sci. USA 2013, 110, E3685–E3694. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Q.L.; Fang, T.; Peng, Q.; Liao, L.; Zhao, L.; Owiti, A.; Han, Y.P. Developing gene-tagged molecular markers for evaluation of genetic association of apple SWEET genes with fruit sugar accumulation. Hortic. Res. 2018, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.H.; Klemens, P.A.W.; Neuhaus, H.E.; Ko, H.Y.; Hsieh, S.Y.; Guo, W.J. SlSWEET1a is involved in glucose import to young leaves in tomato plants. J. Exp. Bot. 2019, 70, 3241–3254. [Google Scholar] [CrossRef]
- Eom, J.S.; Chen, L.Q.; Sosso, D.; Julius, B.T.; Lin, I.W.; Qu, X.Q.; Braun, D.M.; Frommer, W.B. SWEETs, transporters for intracellular and intercellular sugar translocation. Curr. Opin. Plant Biol. 2015, 25, 53–62. [Google Scholar] [CrossRef]
- Li, J.M.; Zheng, D.M.; Li, L.T.; Qiao, X.; Wei, S.W.; Bai, B.; Zhang, S.L.; Wu, J. Genome-wide function, evolutionary characterization and expression analysis of sugar transporter family genes in pear (Pyrus bretschneideri Rehd). Plant Cell Physiol. 2015, 56, 1721–1737. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Chen, L.Q.; Qu, X.Q.; Hou, B.H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.L.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef]
- Chardon, F.; Bedu, M.; Calenge, F.; Klemens, P.A.W.; Spinner, L.; Clement, G.; Chietera, G.; Leran, S.; Ferrand, M.; Lacombe, B.; et al. Leaf fructose content is controlled by the vacuolar transporter SWEET17 in Arabidopsis. Curr. Biol. 2013, 23, 697–702. [Google Scholar] [CrossRef]
- Klemens, P.A.W.; Patzke, K.; Deitmer, J.; Spinner, L.; Le Hir, R.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef]
- Valifard, M.; Le Hir, R.; Muller, J.; Scheuring, D.; Neuhaus, H.E.; Pommerrenig, B. Vacuolar fructose transporter SWEET17 is critical for root development and drought tolerance. Plant Physiol. 2021, 187, 2716–2730. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; He, Y.; Zhu, Z.; Patrick, J.W.; Ruan, Y.L. Integrating sugar metabolism with transport: Elevation of endogenous cell wall invertase activity up-regulates SlHT2 and SlSWEET12c expression for early fruit development in tomato. Front. Genet. 2020, 11, 592596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.S.; Feng, C.Y.; Wang, M.N.; Li, T.L.; Liu, X.; Jiang, J. Plasma membrane-localized SlSWEET7a and SlSWEET14 regulate sugar transport and storage in tomato fruits. Hortic. Res. 2021, 8, 186. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, L.; Ren, C.; Ren, F.; Wang, Y.; Fan, P.; Li, S.; Liang, Z. VvSWEET10 mediates sugar accumulation in grapes. Genes 2019, 10, 255. [Google Scholar] [CrossRef]
- Li, J.M.; Qin, M.F.; Qiao, X.; Cheng, Y.S.; Li, X.L.; Zhang, H.P.; Wu, J. A new insight into the evolution and functional divergence of SWEET transporters in Chinese white pear (Pyrus bretschneideri). Plant Cell Physiol. 2017, 58, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Guo, W.; Li, J.C.; Yue, P.T.; Bu, H.D.; Jiang, J.; Liu, W.T.; Xu, Y.X.; Yuan, H.; Li, T.; et al. Histone acetylation at the promoter for the transcription factor PuWRKY31 affects sucrose accumulation in pear fruit(1)(OPEN). Plant Physiol. 2020, 182, 2035–2046. [Google Scholar] [CrossRef]
- Lalonde, S.; Boles, E.; Hellmann, H.; Barker, L.; Patrick, J.W.; Frommer, W.B.; Ward, J.M. The dual function of sugar carriers: Transport and sugar sensing. Plant Cell 1999, 11, 707–726. [Google Scholar] [CrossRef]
- Morales, J.; Bermejo, A.; Navarro, P.; Forner-Giner, M.A.; Salvador, A. Rootstock effect on fruit quality, anthocyanins, sugars, hydroxycinnamic acids and flavanones content during the harvest of blood oranges ‘Moro’ and ‘Tarocco Rosso’ grown in Spain. Food Chem. 2021, 342, 128305. [Google Scholar] [CrossRef]
- Lu, Y.H.; Watanabe, A.; Kimura, M. Input and distribution of photosynthesized carbon in a flooded rice soil. Glob. Biogeochem. Cycles 2002, 16, 1085. [Google Scholar] [CrossRef]
- Sha, J.C.; Wang, F.; Xu, X.X.; Chen, Q.; Zhu, Z.L.; Jiang, Y.M.; Ge, S.F. Studies on the translocation characteristics of C-13-photoassimilates to fruit during the fruit development stage in ‘Fuji’ apple. Plant Physiol. Biochem. 2020, 154, 636–645. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Han, W.; Wang, H.B.; Cong, L.; Zhai, R.; Yang, C.Q.; Wang, Z.G.; Xu, L.F. Downstream of GA(4), PbCYP78A6 participates in regulating cell cycle-related genes and parthenogenesis in pear (Pyrus bretshneideri Rehd.). BMC Plant Biol. 2021, 21, 292. [Google Scholar] [CrossRef]
- Cantín, C.M.; Pinochet, J.; Gogorcena, Y.; Moreno, M.Á. Growth, yield and fruit quality of ‘Van’ and ‘Stark Hardy Giant’ sweet cherry cultivars as influenced by grafting on different rootstocks. Sci. Hortic. 2010, 123, 329–335. [Google Scholar] [CrossRef]
- Kviklys, D.; Lanauskas, J.; Uselis, N.; Viskelis, J.; Viskeliene, A.; Buskiene, L.; Staugaitis, G.; Mazeika, R.; Samuoliene, G. Rootstock vigour and leaf colour affect apple tree nutrition. Zemdirb.-Agric. 2017, 104, 185–190. [Google Scholar] [CrossRef]
- Ou, C.; Jiang, S.; Wang, F.; Tang, C.; Hao, N. An RNA-Seq analysis of the pear (Pyrus communis L.) transcriptome, with a focus on genes associated with dwarf. Plant Gene 2015, 4, 69–77. [Google Scholar] [CrossRef][Green Version]
- Prassinos, C.; Ko, J.H.; Lang, G.; Iezzoni, A.F.; Han, K.H. Rootstock-induced dwarfing in cherries is caused by differential cessation of terminal meristem growth and is triggered by rootstock-specific gene regulation. Tree Physiol. 2009, 29, 927–936. [Google Scholar] [CrossRef]
- Irisarri, P.; Binczycki, P.; Errea, P.; Martens, H.J.; Pina, A. Oxidative stress associated with rootstock-scion interactions in pear/quince combinations during early stages of graft development. J. Plant Physiol. 2015, 176, 25–35. [Google Scholar] [CrossRef]
- Okumura, M.; Inoue, S.; Kuwata, K.; Kinoshita, T. Photosynthesis activates plasma membrane H+-ATPase via sugar accumulation. Plant Physiol. 2016, 171, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Nadakuduti, S.S.; Holdsworth, W.L.; Klein, C.L.; Barry, C.S. KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J. 2014, 78, 1022–1033. [Google Scholar] [CrossRef]
- An, H.S.; Luo, F.X.; Wu, T.; Wang, Y.; Xu, X.F.; Zhang, X.Z.; Han, Z.H. Effect of rootstocks or interstems on dry matter allocation in apple. Eur. J. Hortic. Sci. 2017, 82, 225–231. [Google Scholar] [CrossRef]
- Teo, G.; Suziki, Y.; Uratsu, S.L.; Lampinen, B.; Ormonde, N.; Hu, W.K.; DeJong, T.M.; Dandekar, A.M. Silencing leaf sorbitol synthesis alters long-distance partitioning and apple fruit quality. Proc. Natl. Acad. Sci. USA 2006, 103, 18842–18847. [Google Scholar] [CrossRef]
- Selvana, B.; Yu, Y.C.; Chen, L.Q.; Shukla, D. Molecular basis of the glucose transport mechanism in plants. ACS Cent. Sci. 2019, 5, 1085–1096. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Cong, L.; Pang, J.; Chen, Y.; Wang, Z.; Zhai, R.; Yang, C.; Xu, L. Dwarfing Rootstock ‘Yunnan’ Quince Promoted Fruit Sugar Accumulation by Influencing Assimilate Flow and PbSWEET6 in Pear Scion. Horticulturae 2022, 8, 649. https://doi.org/10.3390/horticulturae8070649
Wang X, Cong L, Pang J, Chen Y, Wang Z, Zhai R, Yang C, Xu L. Dwarfing Rootstock ‘Yunnan’ Quince Promoted Fruit Sugar Accumulation by Influencing Assimilate Flow and PbSWEET6 in Pear Scion. Horticulturae. 2022; 8(7):649. https://doi.org/10.3390/horticulturae8070649
Chicago/Turabian StyleWang, Xiaoli, Liu Cong, Jianwen Pang, Yu Chen, Zhigang Wang, Rui Zhai, Chengquan Yang, and Lingfei Xu. 2022. "Dwarfing Rootstock ‘Yunnan’ Quince Promoted Fruit Sugar Accumulation by Influencing Assimilate Flow and PbSWEET6 in Pear Scion" Horticulturae 8, no. 7: 649. https://doi.org/10.3390/horticulturae8070649
APA StyleWang, X., Cong, L., Pang, J., Chen, Y., Wang, Z., Zhai, R., Yang, C., & Xu, L. (2022). Dwarfing Rootstock ‘Yunnan’ Quince Promoted Fruit Sugar Accumulation by Influencing Assimilate Flow and PbSWEET6 in Pear Scion. Horticulturae, 8(7), 649. https://doi.org/10.3390/horticulturae8070649




